Temperature Dependent Micro-Structure of KAlF4 from Solid to Molten States
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Synthesis
2.2. Material Characterization
2.3. Computational Details
3. Results and Discussion
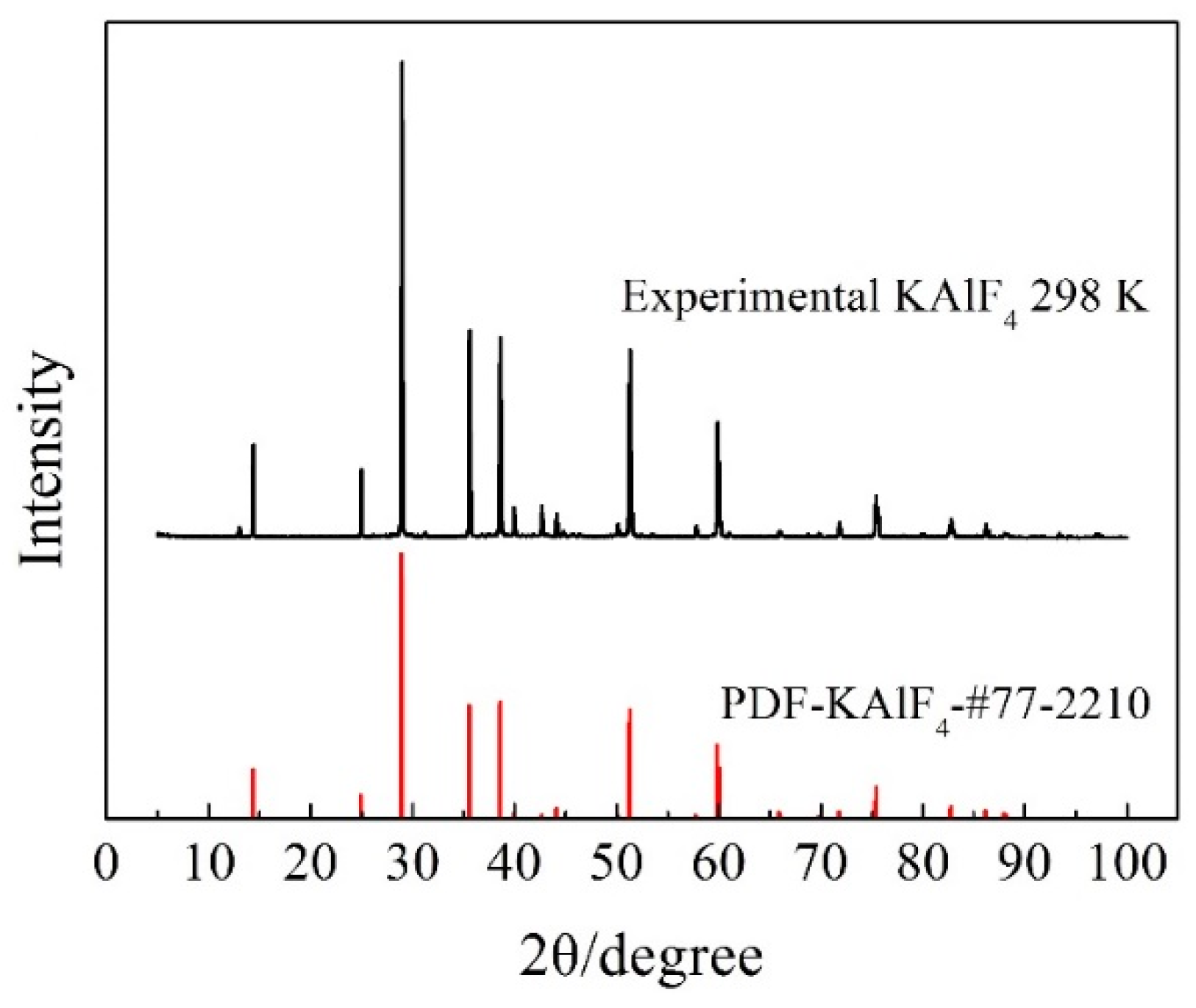
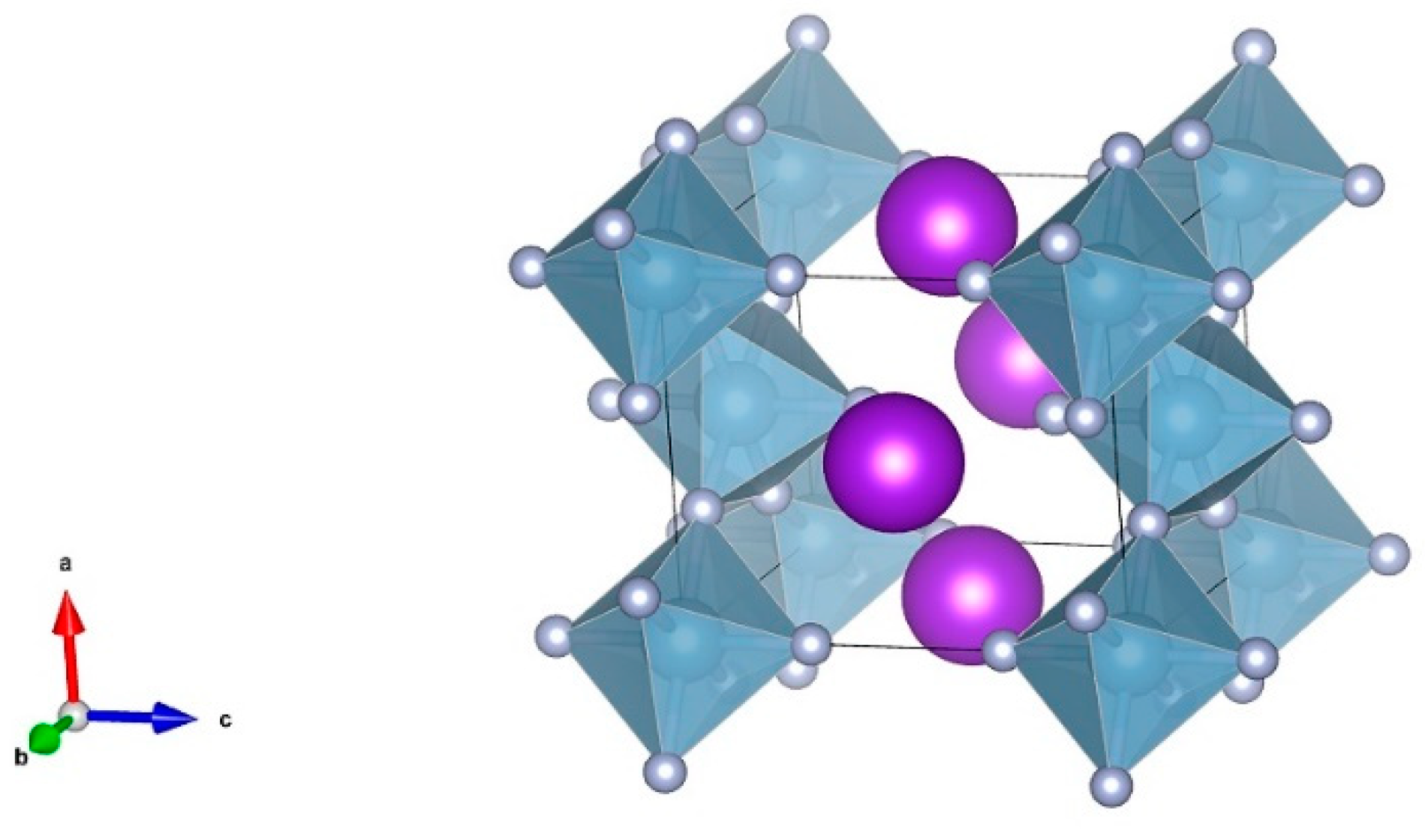
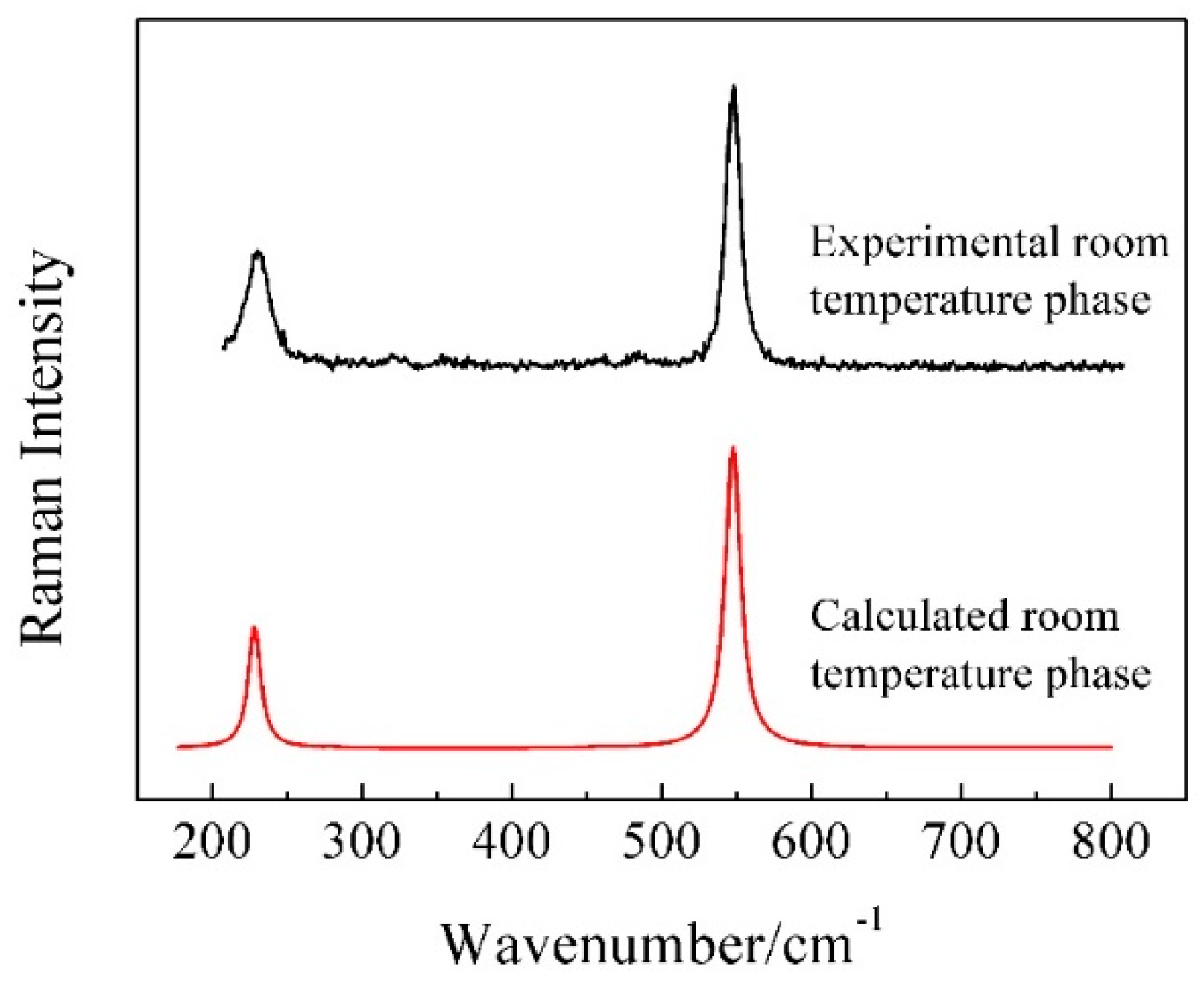
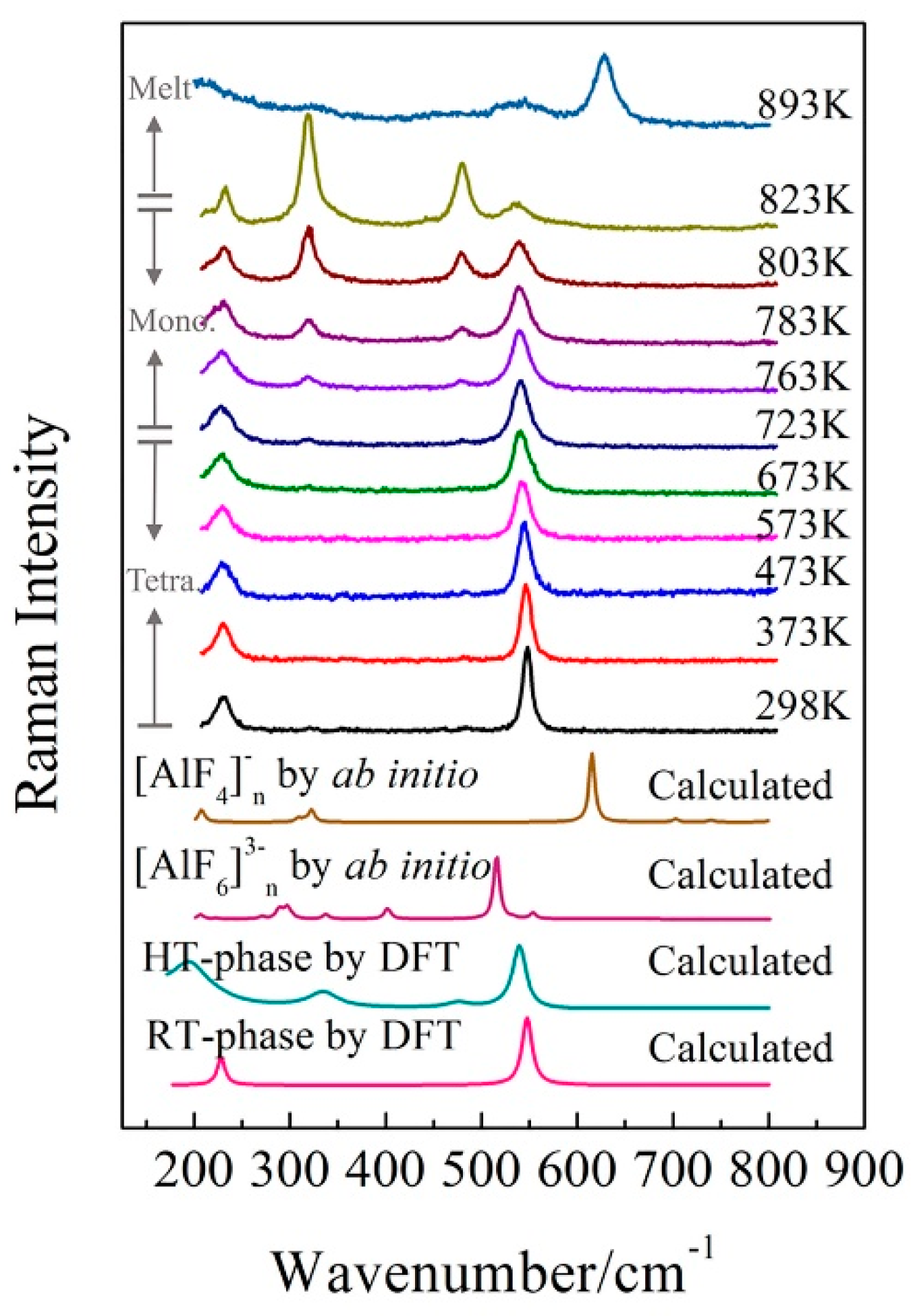
3.1. Room Temperature Characteristics of KAlF4
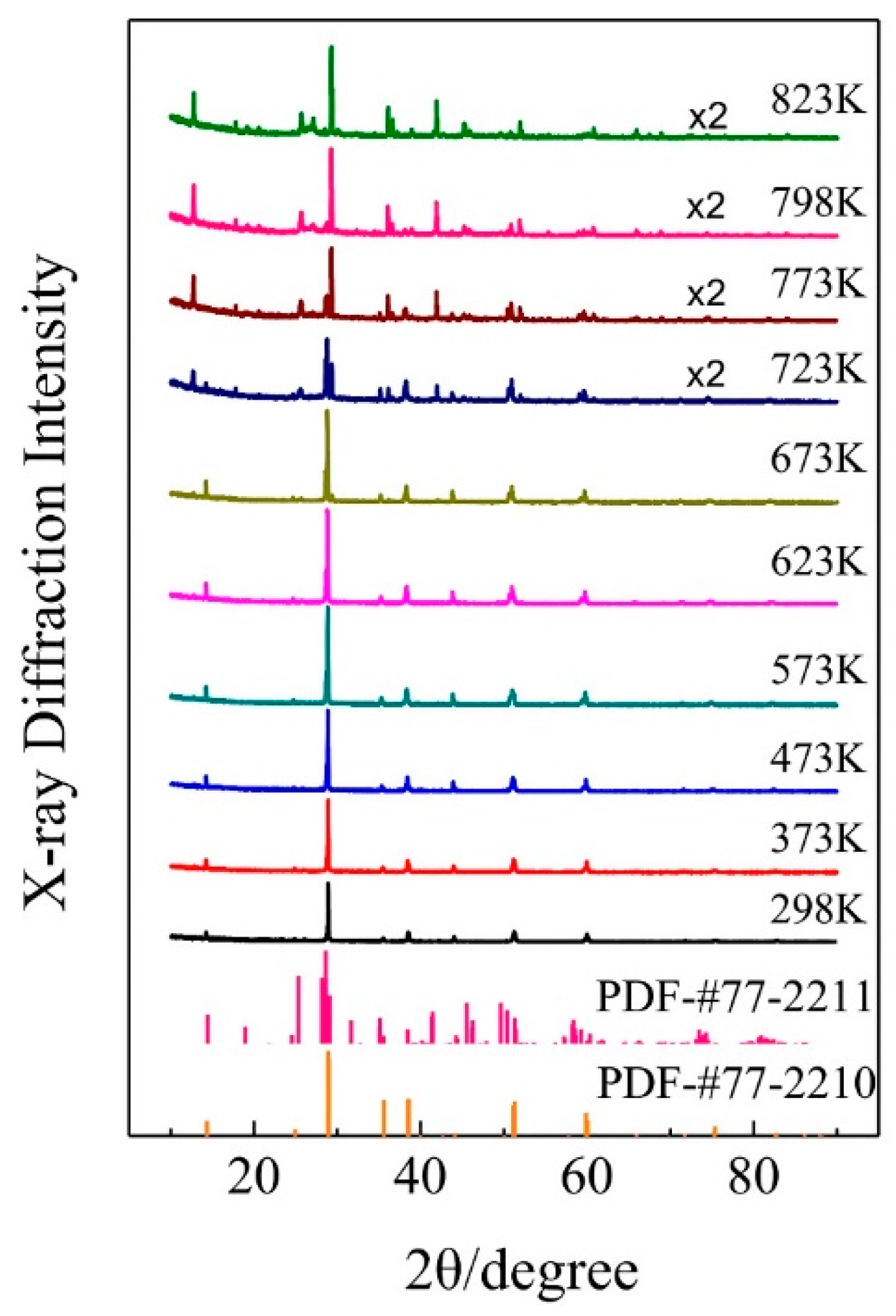
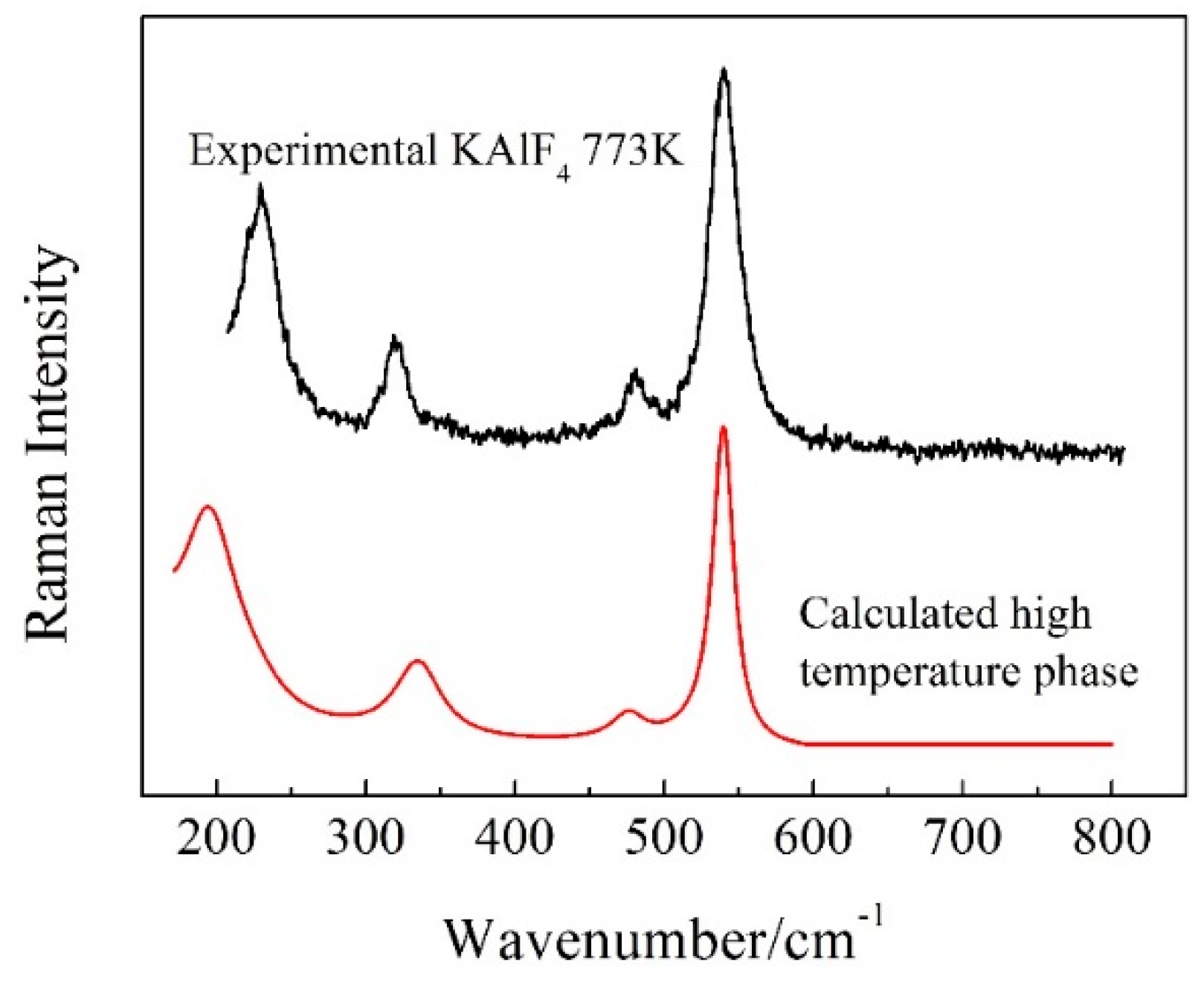
3.2. The Phase Transformation of KAlF4
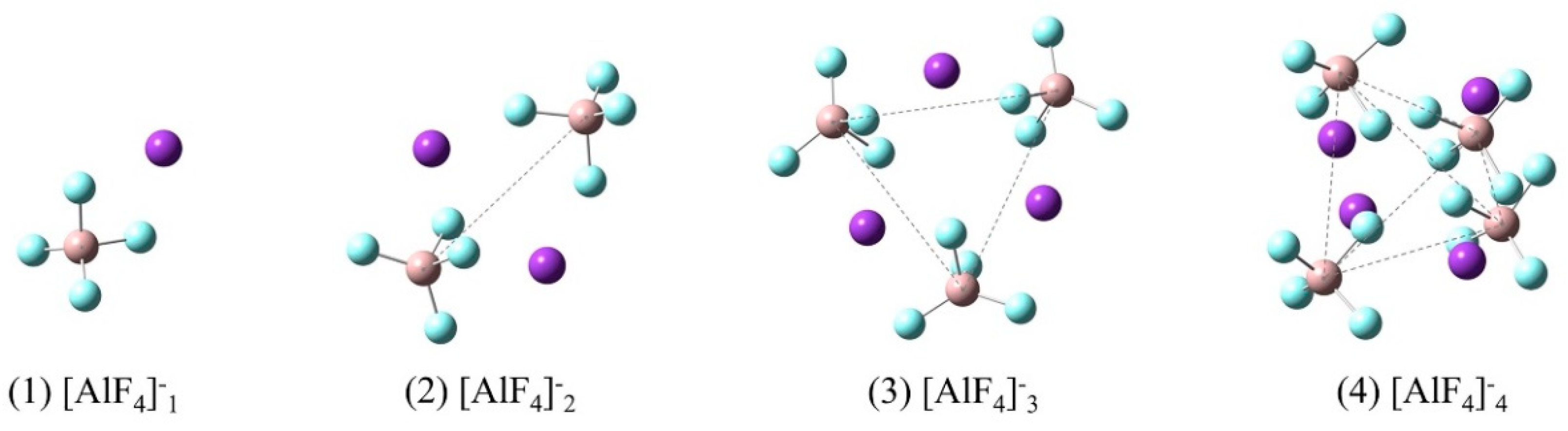
3.3. Characteristics of Molten KAlF4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Danielik, V.; Híveš, J. Low-melting electrolyte for aluminum smelting. J. Chem. Eng. Data 2004, 49, 1414–1417. [Google Scholar] [CrossRef]
- Kawase, H.; Shintani, H.; Miyamoto, M. Flux for Brazing the Aluminum Parts and Preparing Method of the Same. U.S. Patent US4579605A, 1 April 1986. [Google Scholar]
- Nouet, J.; Pannetier, J.; Fourquet, J.L. The room-temperature structure of potassium tetrafluoroaluminate. Acta Crystallogr. 1981, 37, 32–34. [Google Scholar] [CrossRef] [Green Version]
- Bail, A.L. Ab initio structure determination of nanosized θ-KAlF4 with edge-sharing AlF6 octahedra. Powder Diffr. 2009, 24, 185–190. [Google Scholar] [CrossRef]
- Gibaud, A.; Bail, A.L.; Bulou, A. A re-investigation of the room-temperature phase of KAlF4: Evidence of antiphase domains. J. Phys. C Solid State Phys. 1986, 19, 4623–4633. [Google Scholar] [CrossRef]
- Gibaud, A.; Bulou, A.; Bail, A.L.; Nouet, J.; Zeyen, C. A premartensitic phase in KAlF4: Neutron and X-ray scattering evidences. J. Phys. 1987, 48, 1521–1532. [Google Scholar] [CrossRef]
- Launay, J.M.; Bulou, A.; Hewat, A.W. Shear transformation in the layered compound KAlF4: Low temperature phase structure and transformation mechanism. J. Phys. 1985, 46, 771–782. [Google Scholar] [CrossRef]
- Robert, E.; Olsen, J.E.; Gilbert, B.; Østvold, T. Structure and thermodynamics of potassium fluoride-aluminium fluoride melts. Raman spectroscopic and vapour pressure studies. Acta Chem. Scand. 1997, 51, 379–386. [Google Scholar] [CrossRef]
- Chen, R.; Wu, G.H.; Zhang, Q.Y. Phase Diagram of the System KF-AlF3. J. Am. Ceram. Soc. 2000, 83, 3196–3198. [Google Scholar] [CrossRef]
- Chen, R.; Wu, G.H.; Zhang, Q.Y. The preparation and stability of KAlF4 and related fluorides. Chemistry (Chin.) 2000, 9, 50–52. [Google Scholar] [CrossRef]
- Schoonman, J.; Huggins, R. Electrical properties of undoped and doped potassium tetrafluoaluminate: KAlF4. J. Solid State Chem. 1976, 16, 413–422. [Google Scholar] [CrossRef]
- Brosset, C. Herstellung und kristallbau einiger alkalialuminiumfluoride vom typus TlAlF4. Anorg. Allg. Chem. 1938, 239, 301–304. [Google Scholar] [CrossRef]
- Robert, E.; Lacassagne, V.; Bessada, C.; Massiot, D.; Gillbert, B.; Coutures, J.P. Study of NaF-AlF3 melts by high-temperature 27Al NMR spectroscopy comparison with results from Raman spectroscopy. Inorg. Chem. 1999, 38, 214–217. [Google Scholar] [CrossRef]
- Akdeniz, Z.; Cicek, Z.; Pastore, G.; Tosi, M.P. Ionic clusters in aluminium-sodium fluoride melts. Mod. Phys. Lett. B 1998, 12, 995–1002. [Google Scholar] [CrossRef]
- Akdeniz, Z.; Madden, P.A. Raman spectra of ionic liquids: A simulation study of AlF3 and its mixtures with NaF. J. Phys. Chem. B 2006, 110, 6683–6691. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; You, J.L.; Xie, Y.F.; Wang, M.; Wang, J. A New Type Crucible Suitable for High Temperature Raman Spectroscopy on Volatile Molten. China Patent CN 206161531 U, 10 May 2017. Available online: http://dbpub.cnki.net/grid2008/dbpub/detail.aspx?dbcode=SCPD&dbname=SCPD2017&filename=CN206161531U&uid=WEEvREcwSlJHSldRa1FhdXNXa0hFbGhkR3creGlMU0pqcUYvbEoyS0YzUT0=$9A4hF_YAuvQ5obgVAqNKPCYcEjKensW4IQMovwHtwkF4VYPoHbKxJw!! (accessed on 10 May 2017).
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3968. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.G.; Cohen, R.E. More accurate generalized gradient approximation for solids. Phys. Rev. B 2006, 73, 235116. [Google Scholar] [CrossRef]
- Hamann, D.R.; Schlüter, M.; Chiang, C. Norm-conserving pseudopotentials. Phys. Rev. Lett. 1979, 43, 1494–1497. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 2002, 14, 2717. [Google Scholar] [CrossRef]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Kristallogr. 2005, 220, 567–570. [Google Scholar] [CrossRef] [Green Version]
- Troullier, N.; Martins, J.L. Efficient pseudopotentials for plane-wave calculations. Phys. Rev. B 1991, 43, 1993–2006. [Google Scholar] [CrossRef]
- Lee, T.J.; Jayatilaka, D. An open-shell restricted Hartree-Fock perturbation theory based on symmetric spin orbitals. Chem. Phys. Lett. 1993, 201, 1–10. [Google Scholar] [CrossRef]
- Nowak, M.; Rostkowska, H.; Lapinski, L.; Kwiatkowski, J.S.; Leszczynski, J. Experimental matrix isolation and theoretical ab initio HF/6-31G (d, p) studies of infrared spectra of purine, adenine and 2-chloroadenine. Spectrochim. Acta Part A Mol. Spectrosc. 1994, 50, 1081–1094. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Smrčok, L’.; Kucharík, M.; Tovar, M.; Žižak, I. High temperature powder diffraction and solid state DFT study of β-cryolite (Na3AlF6). Cryst. Res. Technol. 2009, 44, 834–840. [Google Scholar] [CrossRef]
- Picard, G.S.; Bouyer, F.C.; Leroy, M.; Bertaud, Y.; Bouvet, S. Structures of oxyfluoroaluminates in molten cryolite-alumina mixtures investigated by DFT-based calculations. Theochem. J. Mol. Struct. 1996, 368, 67–80. [Google Scholar] [CrossRef]
- Ma, N.; You, J.L.; Lu, L.M.; Wang, J.; Wang, M.; Wan, S.M. Micro-structure studies of the molten binary K3AlF6-Al2O3 system by in situ high temperature Raman spectroscopy and theoretical simulation. Inorg. Chem. Front. 2018, 5, 1861–1868. [Google Scholar] [CrossRef]
- Machado, K.; Zanghi, D.; Kanian, V.S.; Cadars, S.; Burbano, M.; Salanne, M.; Bessada, C. Study of NaF-AlF3 melts by coupling molecular dynamics, density functional theory and NMR measurements. J. Phys. Chem. C 2017, 121, 10289–10297. [Google Scholar] [CrossRef]
- Cikit, S.; Akdeniz, Z.; Madden, P.A. Structure and Raman spectra in cryolitic melts: Simulations with an ab initio interaction potential. J. Phys. Chem. B 2014, 118, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Wadt, W.R.; Hay, P.J. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- You, J.L.; Jiang, G.C.; Hou, H.Y.; Chen, H.; Wu, Y.Q.; Xu, K.D. Quantum chemistry study on superstructure and Raman spectra of binary sodium silicates. J. Raman Spectrosc. 2005, 36, 237–249. [Google Scholar] [CrossRef]
- Scott, A.P.; Radom, L. Harmonic vibrational frequencies: An evaluation of Hartree-Fock, Møller-Plesset, quadratic configuration interaction, density functional theory, and semiempirical scale factors. J. Phys. Chem. 1996, 100, 16502–16513. [Google Scholar] [CrossRef]
- Robert, E.; Olsen, J.E.; Danek, V.; Tixhon, E.; Østvold, T.; Gilbert, B. Structure and thermodynamics of alkali fluoride-aluminum fluoride-alumina melts. Vapor pressure, solubility, and Raman spectroscopic studies. J. Phys. Chem. B 1997, 101, 9447–9457. [Google Scholar] [CrossRef]
- Robert, E.; Gilbert, B. Raman spectroscopy study of molten MxAlF3+x-M′xAlF3+x Systems (M, M′= Li, Na, K). Appl. Spectrosc. 2000, 54, 396–401. [Google Scholar] [CrossRef]
- Hu, X.W.; Qu, J.Y.; Gao, B.L.; Shi, Z.N.; Liu, F.G.; Wang, Z.W. Raman spectroscopy and ionic structure of Na3AlF6-Al2O3 melts. Trans. Nonferr. Met. Soc. China 2011, 21, 402–406. [Google Scholar] [CrossRef]



| Wavenumber | Vibrational Modes | Type of Vibration | |
|---|---|---|---|
| υexp (cm−1) | υcal (cm−1) | ||
| 228 | 227.6 | Eg | γ (AlVI-Fnb) 1 |
| 547 | 547.4 | A1g | υs (AlVI-Fnb) 1 |
| Wavenumber | Vibrational Modes | Type of Vibration | |
|---|---|---|---|
| υexp (cm−1) | υcal (cm−1) | ||
| 229 | 193.9 | Bg | γ (AlVI-Fnb) 1 |
| 319 | 335.9 | Ag | γ (AlVI-Fnb) 1 |
| 481 | 474.5 | Ag | γ (AlVI-Fnb) 1 |
| 539 | 539.5 | Ag | υs (AlVI-Fnb) 1 |
| Parameter | Compound | ||
|---|---|---|---|
| Room Temperature Phase | High Temperature Phase | ||
| Crystal System | tetragonal | monoclinic | |
| Space Group | P4/mbm | P21/m | |
| Cell Length/Å | a | 5.122 | 6.542 |
| b | 5.122 | 7.195 | |
| c | 6.288 | 7.177 | |
| Cell Angle/° | α | 90.000 | 90.000 |
| β | 90.000 | 108.478 | |
| γ | 90.000 | 90.000 | |
| Bond Length/Å | AlVI-Fb | 1.733 | 1.829, 1.800 |
| AlVI-Fnb | 1.674 | 1.769, 1.768 | |
| Bond Angle/° | ∠Fb-AlVI-Fb | 90.000 | 89.307, 90.693, 90.307, 89.693 |
| ∠Fb-AlVI-Fnb | 90.000 | 90.958, 91.871, 88.129, 89.042, 91.204, 92.520, 87.480, 88.796 | |
| ∠AlVI-Fb-AlVI | 167.476 | 170.788(2), 159.189, 158.912 | |
| Clusters | Wavenumber (cm−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of Molecules | Exp. | Ref. 10 (Cal.) | Ref. 35 (Exp.) | ||||||
| 1 | 2 | 3 | 4 | … | n = 7 | ||||
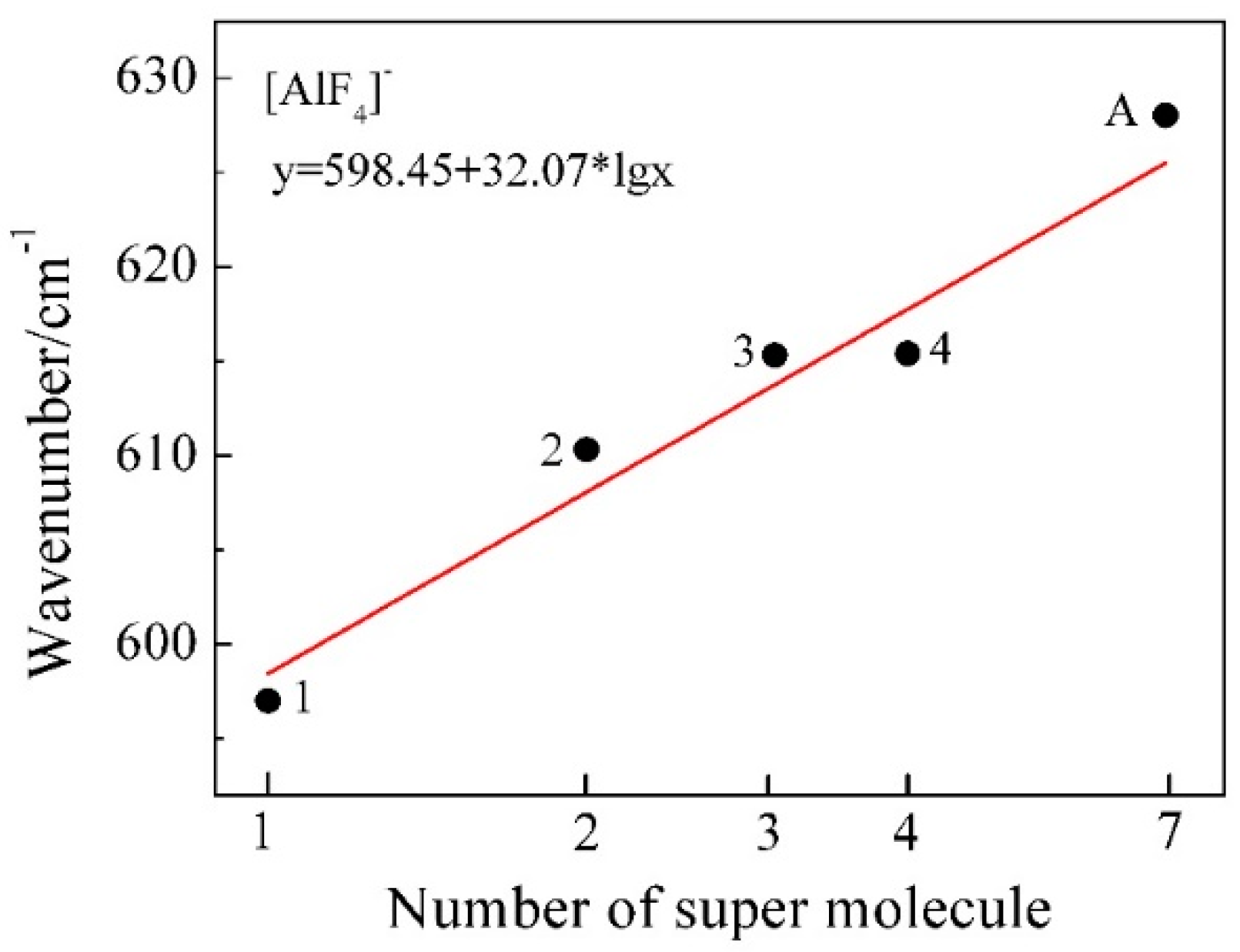
| [AlF4]−n | 597.0 | 610.3 | 615.3 | 615.4 | … | 625.6 | 628 | 631 | 622 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, N.; You, J.; Lu, L.; Wang, J.; Wan, S. Temperature Dependent Micro-Structure of KAlF4 from Solid to Molten States. Materials 2018, 11, 1846. https://doi.org/10.3390/ma11101846
Ma N, You J, Lu L, Wang J, Wan S. Temperature Dependent Micro-Structure of KAlF4 from Solid to Molten States. Materials. 2018; 11(10):1846. https://doi.org/10.3390/ma11101846
Chicago/Turabian StyleMa, Nan, Jinglin You, Liming Lu, Jian Wang, and Songming Wan. 2018. "Temperature Dependent Micro-Structure of KAlF4 from Solid to Molten States" Materials 11, no. 10: 1846. https://doi.org/10.3390/ma11101846
APA StyleMa, N., You, J., Lu, L., Wang, J., & Wan, S. (2018). Temperature Dependent Micro-Structure of KAlF4 from Solid to Molten States. Materials, 11(10), 1846. https://doi.org/10.3390/ma11101846







