Compatibility between Co-Metallized PbTe Thermoelectric Legs and an Ag–Cu–In Brazing Alloy
Abstract
:1. Introduction
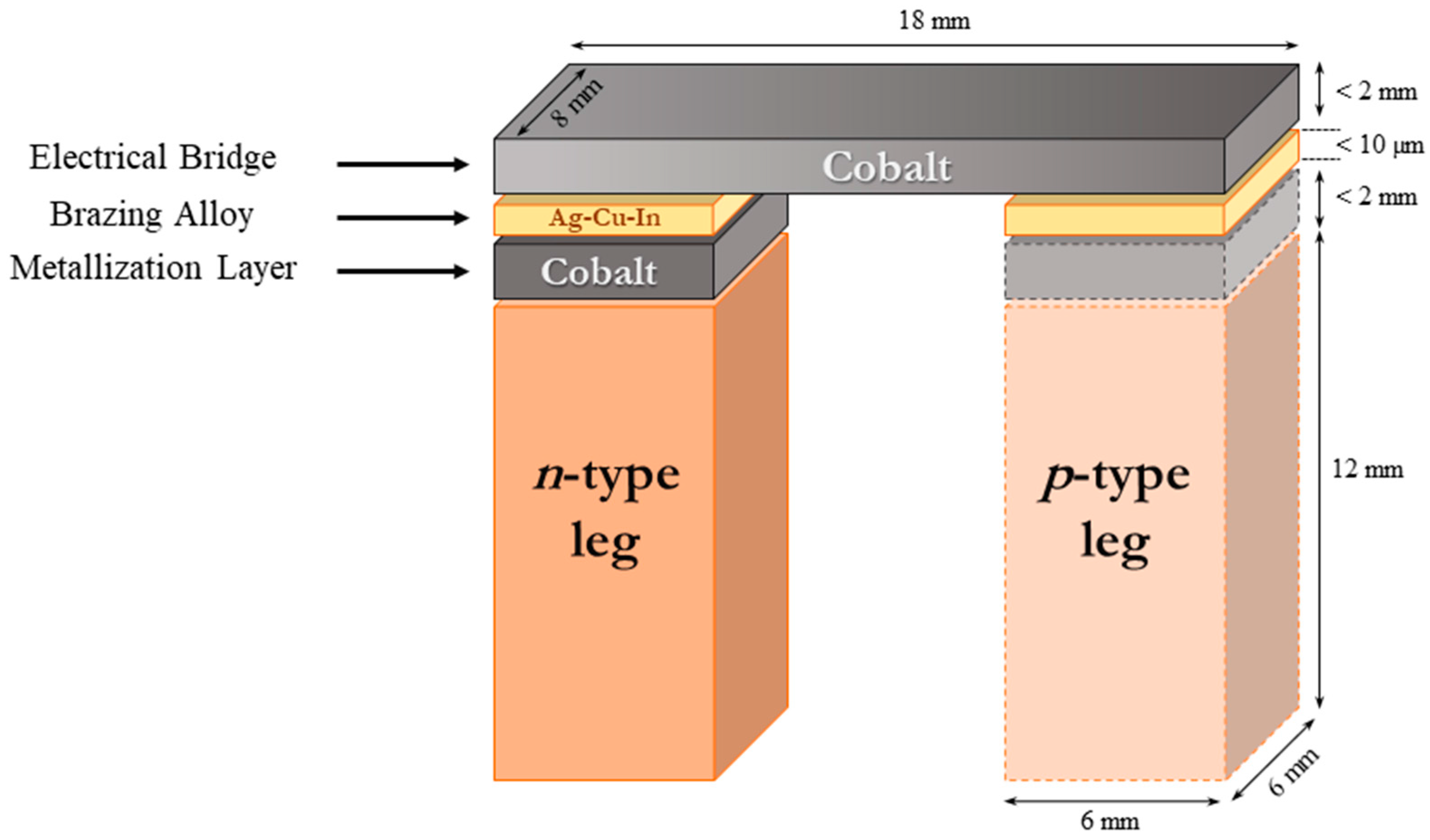
2. Experimental
3. Results and Discussion
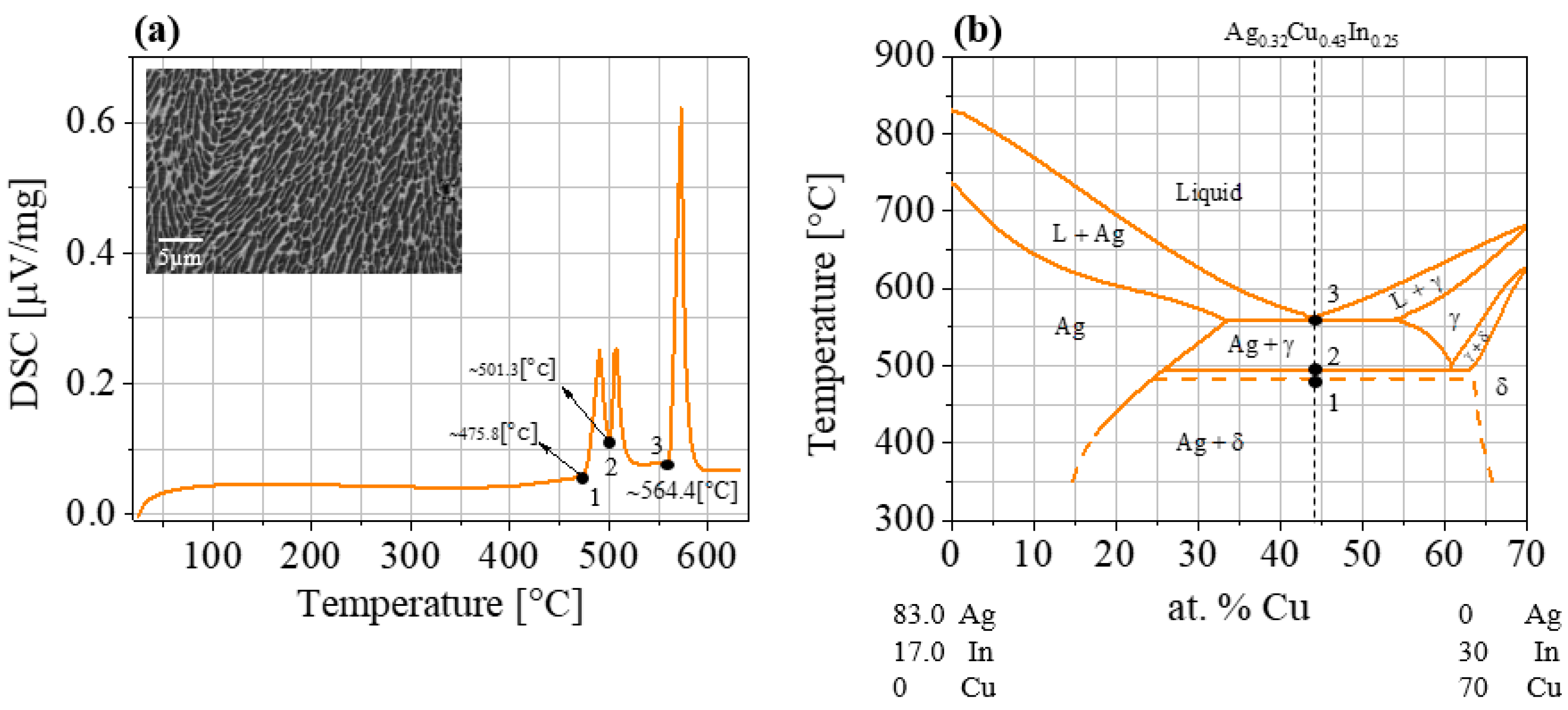
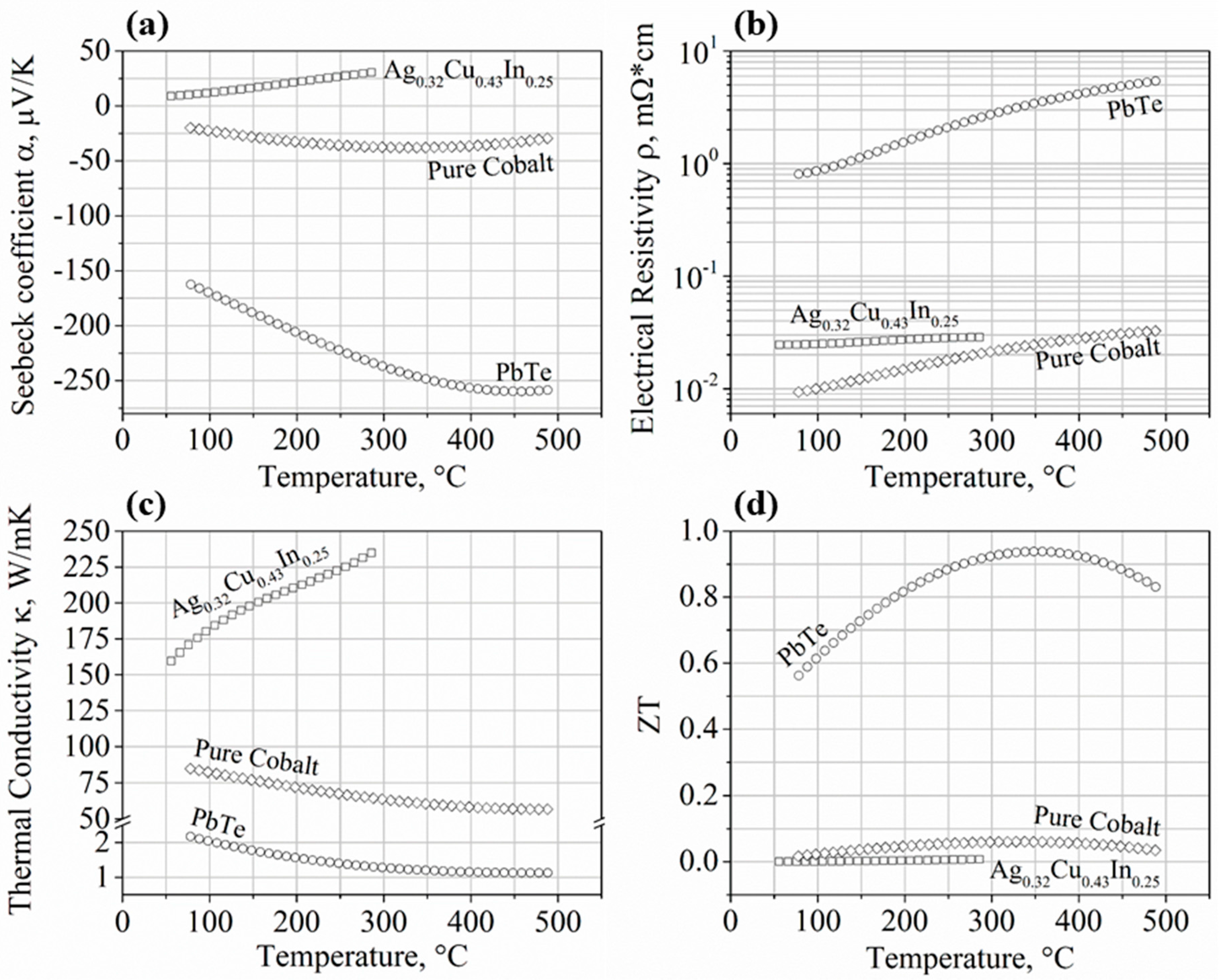
3.1. Synthesized Materials
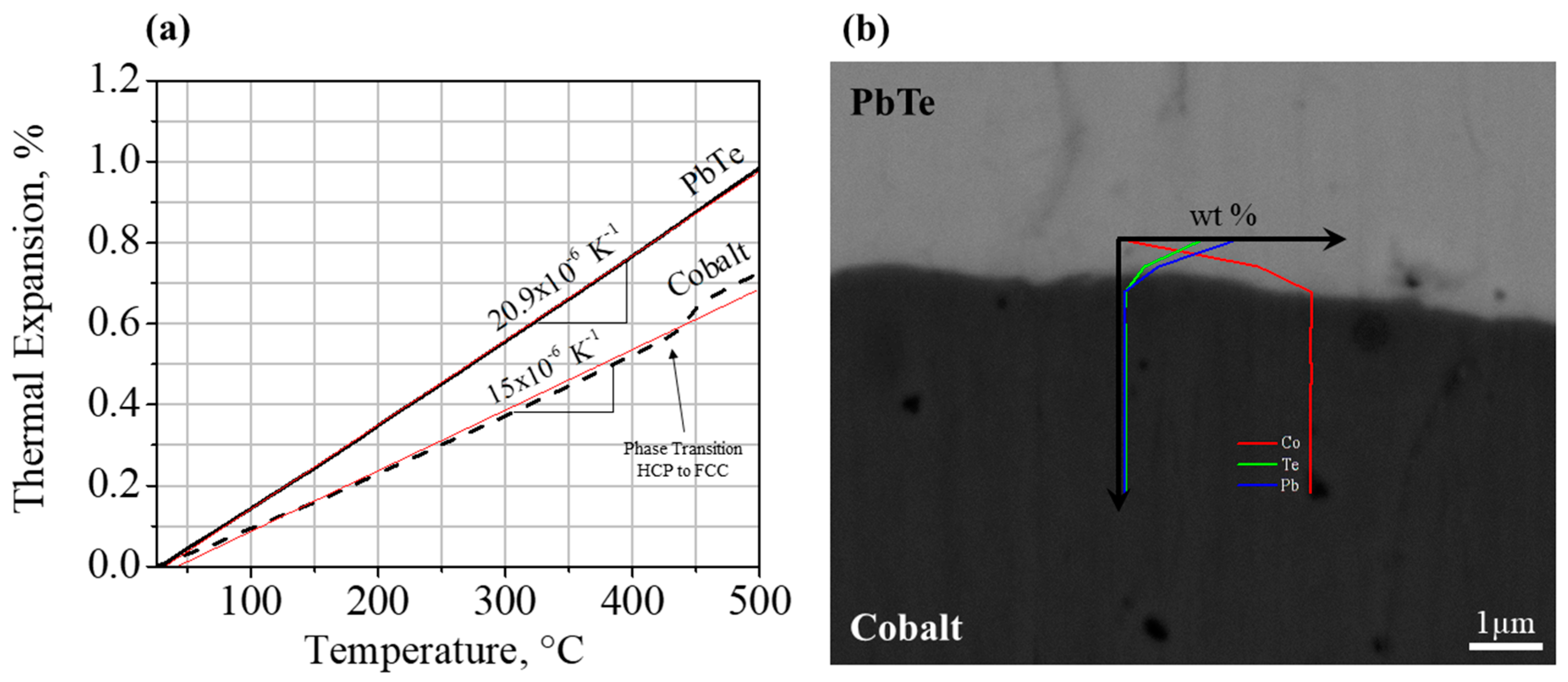
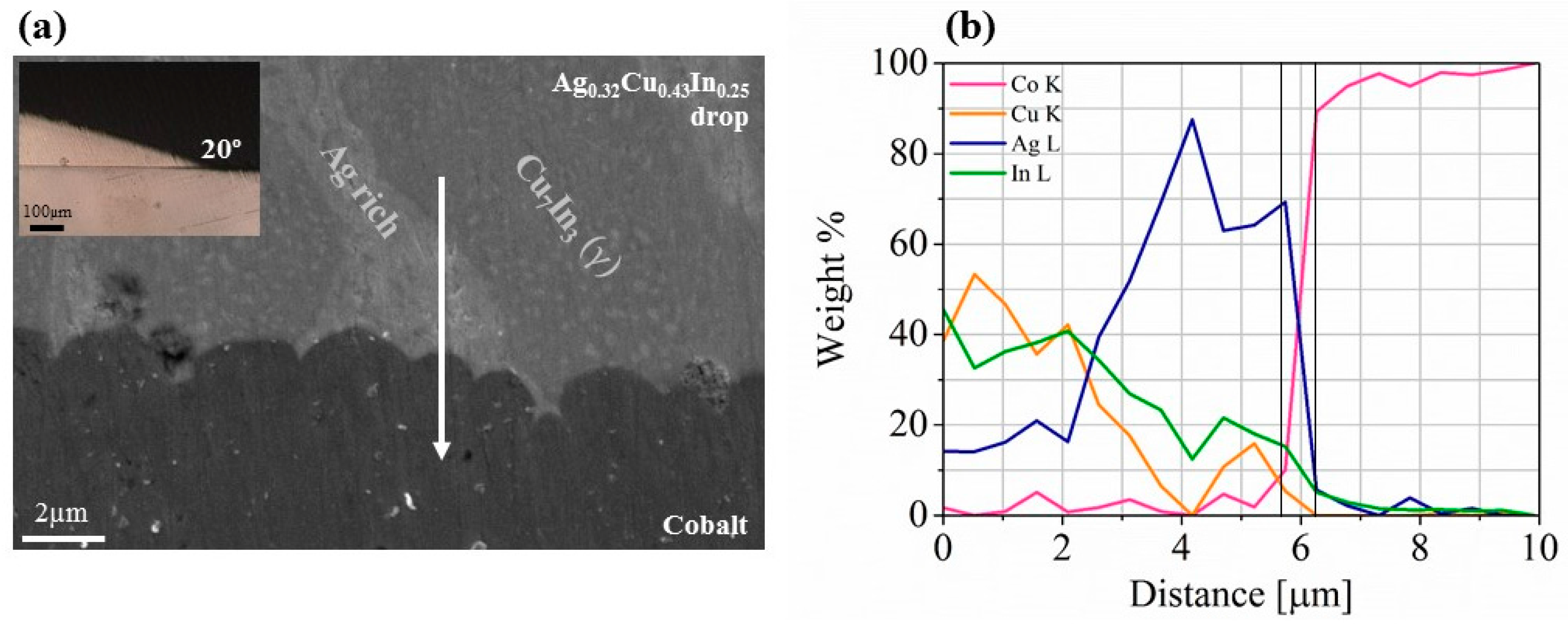
3.2. Thermoelectric Element-Cobalt Interface
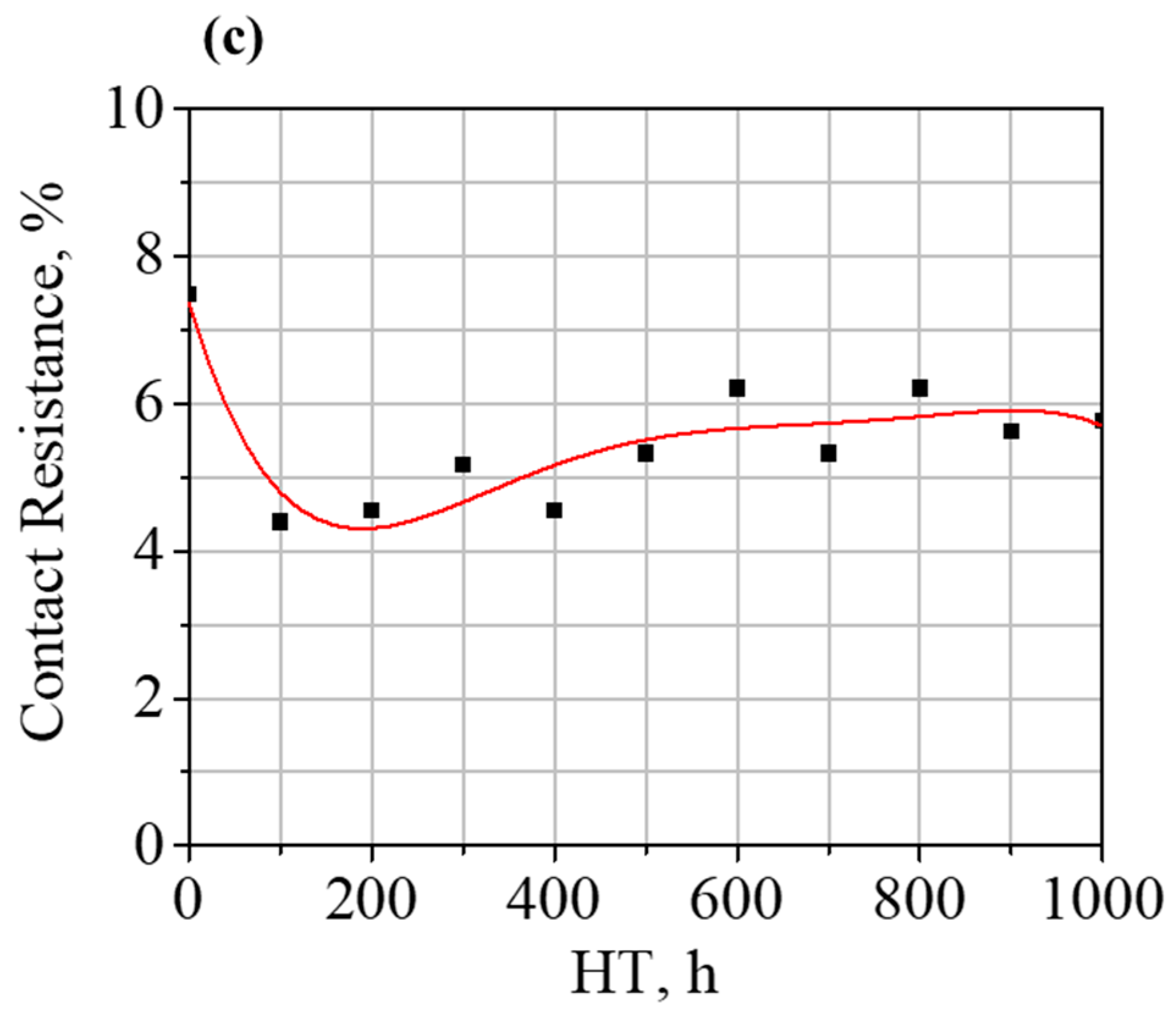
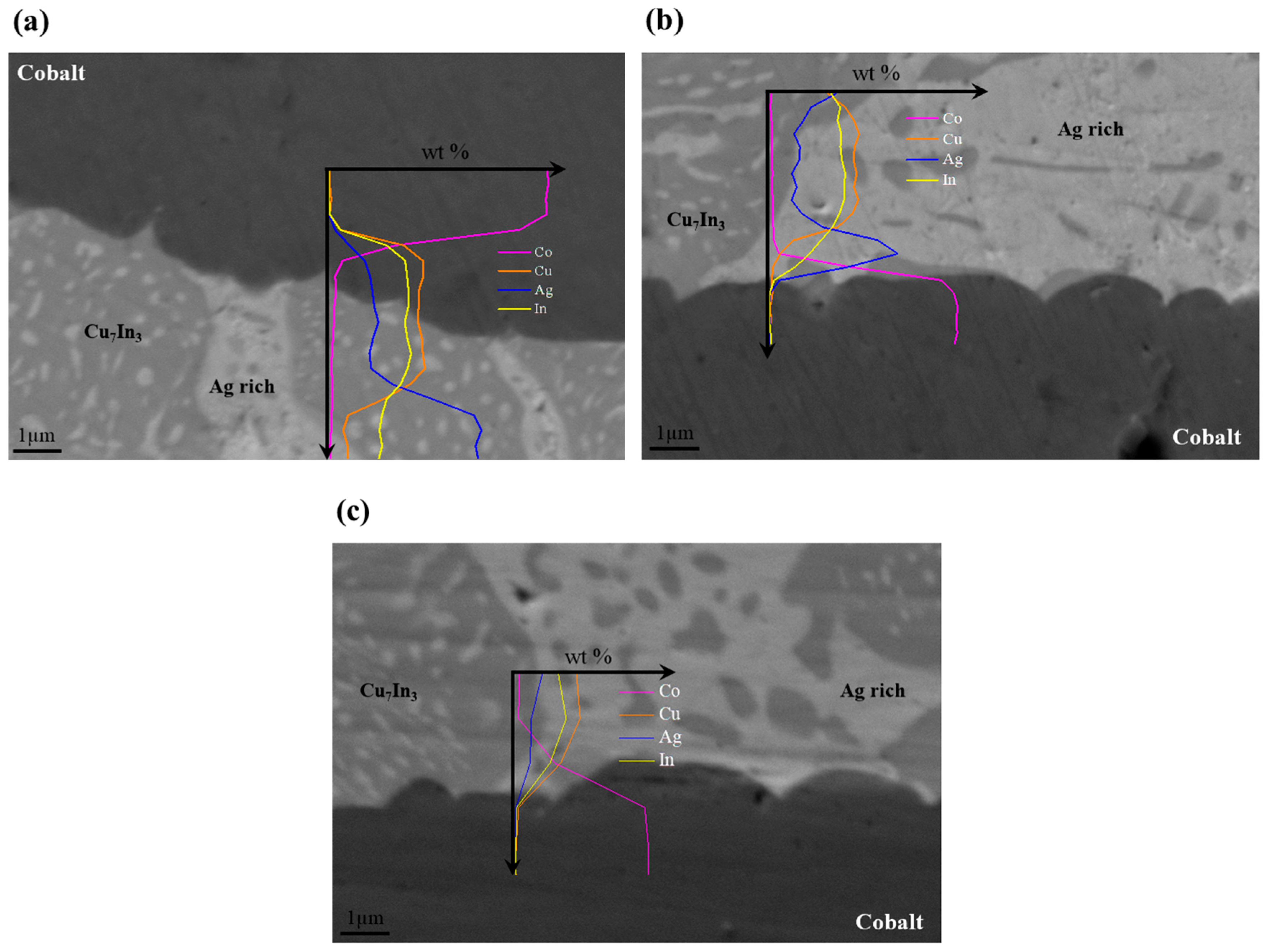
3.3. Cobalt–Brazing Alloy Interface
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Poudel, B.; Hao, Q.; Ma, Y.; Lan, Y.; Minnich, A.; Yu, B.; Yan, X.; Dresselhaus, M.S.; Chen, G.; Ren, Z. High-Thermoelectric Performance of Nanostructured Bismuth Antimony Telluride Bulk Alloys. Science 2008, 320, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Tang, X.; Yan, Y.; Zhang, Q.; Tritt, T.M. Unique nanostructures and enhanced thermoelectric performance of melt-spun BiSbTe alloys. Appl. Phys. Lett. 2009, 94, 102111. [Google Scholar] [CrossRef]
- Bomshtein, N.; Spiridonov, G.; Dashevsky, Z. Thermoelectric, Structural, and Mechanical Properties of Spark-Plasma-Sintered Submicro- and Microstructured p-Type Bi0.5Sb1.5Te3. J. Electron. Mater. 2012, 41, 1546–1553. [Google Scholar] [CrossRef]
- Ben-Yahuda, O.; Shuker, R.; Gelbstein, Y.; Dashevsky, Z.; Dariel, M.P. Highly textured Bi2Te3-based materials for thermoelectric energy conversion. J. Appl. Phys. 2007, 101, 113707. [Google Scholar] [CrossRef]
- Shen, J.J.; Hu, L.P.; Zhu, T.J.; Zhao, X.B. The texture related anisotropy of thermoelectric properties in bismuth telluride based polycrystalline alloys. Appl. Phys. Lett. 2011, 99, 124102. [Google Scholar] [CrossRef]
- Ma, Y.; Hao, Q.; Poudel, B.; Lan, Y.; Yu, B.; Wang, D.; Chen, G.; Ren, Z. Enhanced Thermoelectric Figure-of-Merit in p-Type Nanostructured Bismuth Antimony Tellurium Alloys Made from Elemental Chunks. Nano Lett. 2008, 8, 2580–2584. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, O.; Tomiyoshi, S.; Makita, K. Bismuth telluride compounds with high thermoelectric figures of merit. J. Appl. Phys. 2003, 93, 368–374. [Google Scholar] [CrossRef]
- Liu, W.S.; Zhang, Q.; Lan, Y.; Chen, S.; Yan, X.; Zhang, Q.; Wang, H.; Wang, D.; Chen, G.; Ren, Z. Thermoelectric Property Studies on Cu-Doped n-type CuxBi2Te2.7Se0.3. Adv. Energy Mater. 2011, 1, 577–587. [Google Scholar] [CrossRef]
- Yan, X.; Poudel, B.; Ma, Y.; Liu, W.S.; Joshi, G.; Wang, H.; Lan, Y.; Wang, D.; Chen, G.; Ren, Z.F. Experimental Studies on Anisotropic Thermoelectric Properties and Structures of n-type Bi2Te2.7Se0.3. Nano Lett. 2010, 10, 3373–3378. [Google Scholar] [CrossRef] [PubMed]
- Vasilevskiy, D.; Dawood, M.S.; Masse, J.; Turenne, S. Generation of Nanosized Particles during Mechanical Alloying and Their Evolution through the Hot Extrusion Process in Bismuth-Telluride-Based Alloys. J. Electron. Mater. 2010, 39, 1890–1896. [Google Scholar] [CrossRef]
- Gothard, N.; Ji, X.; He, J.; Tritt, T.M. Thermoelectric and transport properties of n-type nanocomposites. J. Appl. Phys. 2008, 103, 054314. [Google Scholar] [CrossRef]
- Wojciechowski, K.T. Study of transport properties of the Co1−xRhxSb3. J. Alloys Compd. 2007, 439, 18–24. [Google Scholar] [CrossRef]
- Rogl, G.; Grytsiv, A.; Falmbigl, M.; Bauer, E.; Rogl, P.; Zehetbauer, M.; Gelbstein, Y. Thermoelectric properties of p-type didymium (DD) based skutterudites. J. Alloys Compd. 2012, 537, 242–249. [Google Scholar] [CrossRef]
- Kirievsky, K.; Shlimovich, M.; Fuks, D.; Gelbstein, Y. An ab initio study of the thermoelectric enhancement potential in nano-grained TiNiSn. Phys. Chem. Chem. Phys. 2014, 16, 20023–20029. [Google Scholar] [CrossRef] [PubMed]
- Gelbstein, Y.; Tunbridge, J.; Dixon, R.; Reece, M.J.; Ning, H.; Gilchrist, R.; Summers, R.; Agote, I.; Lagos, M.A.; Simpson, K.; et al. Mechanical, and Structural Properties of Highly Efficient Nanostructured n- and p-Silicides for Practical Thermoelectric Applications. J. Electron. Mater. 2014, 43, 1703–1711. [Google Scholar] [CrossRef]
- Scotsman, J.R.; Pcionek, R.J.; Kong, H.; Uher, C.; Kanatzidis, M.G. Strong reduction of thermal conductivity in nanostructured PbTe prepared by matrix encapsulation. Chem. Mater. 2006, 18, 4993–4995. [Google Scholar] [CrossRef]
- Lin, H.; Božin, E.S.; Billinge, S.J.L.; Androulakis, J.; Malliakas, C.D.; Lin, C.H.; Kanatzidis, M.G. Phase separation and nanostructuring in the thermoelectric material PbTe1−xSx studied using the atomic pair distribution function technique. Phys. Rev. B-Condens. 2009, 80, 1–8. [Google Scholar]
- Gorsse, S.; Bellanger, P.; Brechet, Y.; Sellier, E.; Umarji, A.; Ail, U.; Decourt, R. Nanostructuration via solid state transformation as a strategy for improving the thermoelectric efficiency of PbTe alloys. Acta Mater. 2011, 59, 7425–7437. [Google Scholar] [CrossRef]
- He, J.; Girard, S.N.; Kanatzidis, M.G.; Dravid, V.P. Microstructure-lattice thermal conductivity correlation in nanostructured PbTe0.7S0.3 thermoelectric materials. Adv. Funct. Mater. 2010, 20, 764–772. [Google Scholar] [CrossRef]
- Girard, S.N.; He, J.; Zhou, X.; Shoemaker, D.; Jaworski, C.M.; Uher, C.; Dravid, V.P.; Heremans, J.P.; Kanatzidis, M.G. High performance Na-doped PbTe-PbS thermoelectric materials: Electronic density of states modification and shape-controlled nanostructures. J. Am. Chem. Soc. 2011, 133, 16588–16597. [Google Scholar] [CrossRef] [PubMed]
- Heremans, J.P.; Jovovic, V.; Toberer, E.S.; Saramat, A.; Kurosaki, K.; Charoenphakdee, A.; Yamanaka, S.; Snyder, G.J. Enhancement of thermoelectric efficiency in PbTe by distortion of the electronic density of states. Science 2008, 321, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Kanatzidis, M.G. Ultralow thermal conductivity and high thermoelectric figure of merit in SnSe crystals. Nature 2014, 508, 373–377. [Google Scholar]
- Gelbstein, Y.; Rosenberg, Y.; Sadia, Y.; Dariel, M.P. Thermoelectric Properties Evolution of Spark Plasma Sintered (Ge0.6Pb0.3Sn0.1)Te Following a Spinodal Decomposition. J. Phys. Chem. C 2010, 114, 13126–13131. [Google Scholar] [CrossRef]
- Gelbstein, Y.; Davidow, J.; Girard, S.N.; Chung, D.Y.; Kanatzidis, M.G. Controlling Metallurgical Phase Separation Reactions of the Ge0.87Pb0.13Te. Adv. Energy Mater. 2013, 3, 815–820. [Google Scholar] [CrossRef]
- Gelbstein, Y.; Davidow, J. Highly efficient functional GexPb1−xTe based thermoelectric alloys. Phys. Chem. Chem. Phys. 2014, 16, 20120–20126. [Google Scholar] [CrossRef] [PubMed]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nature 2008, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Gelbstein, Y. Phase morphology effects on the thermoelectric properties of Pb0.25Sn0.25Ge0.5Te. Acta Mater. 2013, 61, 1499–1507. [Google Scholar] [CrossRef]
- Rosenberg, Y.; Gelbstein, Y.; Dariel, M.P. Phase separation and thermoelectric properties of the Pb0.25Sn0.25Ge0.5Te compound. J. Alloys Compd. 2012, 526, 31–38. [Google Scholar] [CrossRef]
- Biswas, K.; He, J.; Blum, I.D.; Wu, C.I.; Hogan, T.P.; Seidman, D.N.; Dravid, V.P.; Kanatzidis, M.G. High-performance bulk thermoelectrics with all-scale hierarchical architectures. Nature 2012, 489, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.; He, J.; Zhang, Q.; Wang, G.; Uher, C.; Dravid, V.P.; Kanatzidis, M.G. Strained endotaxial nanostructures with high thermoelectric figure of merit. Nat. Chem. 2011, 3, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Ohta, M.; Biswas, K.; Lo, S.H.; He, J.; Chung, D.Y.; Dravid, V.P.; Kanatzidis, M.G. Enhancement of thermoelectric figure of merit by the insertion of MgTe nanostructures in p-type PbTe doped with Na2Te. Adv. Energy Mater. 2012, 2, 1117–1123. [Google Scholar] [CrossRef]
- Pei, Y.; LaLonde, A.D.; Heinz, N.A.; Snyder, G.J. High thermoelectric figure of merit in PbTe alloys demonstrated in PbTe-CdTe. Adv. Energy Mater. 2012, 2, 670–675. [Google Scholar] [CrossRef]
- Pei, Y.; Shi, X.; LaLonde, A.; Wang, H.; Chen, L.; Snyder, G.J. Convergence of electronic bands for high performance bulk thermoelectrics. Nature 2011, 473, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Gelbstein, Y.; Dashevsky, Z.; Dariel, M.P. In-doped Pb0.5Sn0.5Te p-type samples prepared by powder metallurgical processing for thermoelectric applications. Physica B 2007, 396, 16–21. [Google Scholar] [CrossRef]
- Gelbstein, Y.; Dashevsky, Z.; Dariel, M.P. High performance n-type PbTe-based materials for thermoelectric applications. Phys. B Condens. Matter 2005, 363, 196–205. [Google Scholar] [CrossRef]
- Gelbstein, Y.; Dashevsky, Z.; Dariel, M.P. Powder metallurgical processing of functionally graded p-Pb1−xSnxTe materials for thermoelectric applications. Physica B 22007, 391, 256–265. [Google Scholar] [CrossRef]
- Hazan, E.; Ben-yehuda, O.; Madar, N.; Gelbstein, Y. Functional Graded Germanium-Lead Chalcogenide-Based Thermoelectric Module for Renewable Energy Applications. Adv. Energy Mater. 2015, 5, 1500272. [Google Scholar] [CrossRef]
- Guttmann, G.M.; Dadon, D.; Gelbstein, Y. Electronic tuning of the transport properties of off-stoichiometric PbxSn1−xTe thermoelectric alloys by Bi2Te3 doping. J. Appl. Phys. 2015, 118, 065102. [Google Scholar] [CrossRef]
- Houston, B.; Strakna, R.E.; Belson, H.S. Elastic Constants, Thermal Expansion, and Debye Temperature of Lead Telluride. Phys. Rev. 1961, 123, 2020. [Google Scholar] [CrossRef]
- McAlonan, M.; Patel, K.; Cummer, K. Radioisotope thermoelectric generators based on segmented BiTe/PbTe-BiTe/TAGS/PbSnTe. AIP Conf. Proc. 2006, 813, 573–580. [Google Scholar]
- Lieberman, A.; Leanna, A.; McAlonan, M.; Heshmatpour, B. Small thermoelectric radioisotope power sources. AIP Conf. Proc. 2007, 880, 347–354. [Google Scholar]
- Skrabek, E.A.; Trimmer, D.S. CRC Handbook of Thermoelectrics; Rowe, D.M., Ed.; CRC Press: Boca Raton, FL, USA, 1995; p. 267. [Google Scholar]
- Liu, W.; Jie, Q.; Kim, H.S.; Ren, Z. Current progress and future challenges in thermoelectric power generation: From materials to devices. Acta Mater. 2015, 87, 357–376. [Google Scholar] [CrossRef]
- Gefken, R.M.; Komarek, K.L.; Miller, E.M. Thermodynamic Properties of Cobalt-Tellurium Alloys. J. Solid State Chem. 1972, 4, 153–162. [Google Scholar] [CrossRef]
- Weinstein, M.; Mlavsky, A.I. Bonding of lead telluride to pure iron electrodes. Rev. Sci. Instrum. 1962, 33, 1119–1120. [Google Scholar] [CrossRef]
- Singh, A.; Bhattacharya, S.; Thinaharan, C.; Aswal, D.K.; Gupta, S.K.; Yakhmi, J.V.; Bhanumurthy, K. Development of low resistance electrical contacts for thermoelectric devices based on n-type PbTe and p-type TAGS-85 ((AgSbTe2)0.15(GeTe)0.85). J. Appl. Phys. 2009, 42, 015502. [Google Scholar] [CrossRef]
- Sadia, Y.; Ohaion-Raz, T.; Ben-Yehuda, O.; Korngold, M.; Gelbstein, Y. Criteria for extending the operation periods of thermoelectric converters based on IV-VI compounds. J. Solid State Chem. 2016, 241, 79–85. [Google Scholar] [CrossRef]
- Ben-Ayoun, D.; Sadia, Y.; Gelbstein, Y. High temperature thermoelectric properties evolution of Pb1−xSnxTe based alloys. J. Alloys Compd. 2017, 722, 33–38. [Google Scholar] [CrossRef]
- Woychik, C.G.; Massalski, T.B. Phase Stability Relationships and Glass Formation in the System Cu-Ag-ln. Metall. Trans. A 1988, 19, 13–21. [Google Scholar] [CrossRef]
- Fano, V.; Mondt, J.F. CRC Handbook of Thermoelectrics; Rowe, D.M., Ed.; CRC Press: Boca Raton, FL, USA, 1995; pp. 257, 532. [Google Scholar]
- Orhashi, M.; Noda, Y.; Chen, L.; Kang, Y.; Moro, A.; Hiraj, T. Ni/n-PbTe and Ni/p-Pb0.5Sn0.5Te Joining by Plasma Activated Sintering. In Proceedings of the 17th International Conference on Thermoelectrics, Piscataway, NJ, USA, 28 May 1998; pp. 543–546. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben-Ayoun, D.; Sadia, Y.; Gelbstein, Y. Compatibility between Co-Metallized PbTe Thermoelectric Legs and an Ag–Cu–In Brazing Alloy. Materials 2018, 11, 99. https://doi.org/10.3390/ma11010099
Ben-Ayoun D, Sadia Y, Gelbstein Y. Compatibility between Co-Metallized PbTe Thermoelectric Legs and an Ag–Cu–In Brazing Alloy. Materials. 2018; 11(1):99. https://doi.org/10.3390/ma11010099
Chicago/Turabian StyleBen-Ayoun, Dana, Yatir Sadia, and Yaniv Gelbstein. 2018. "Compatibility between Co-Metallized PbTe Thermoelectric Legs and an Ag–Cu–In Brazing Alloy" Materials 11, no. 1: 99. https://doi.org/10.3390/ma11010099
APA StyleBen-Ayoun, D., Sadia, Y., & Gelbstein, Y. (2018). Compatibility between Co-Metallized PbTe Thermoelectric Legs and an Ag–Cu–In Brazing Alloy. Materials, 11(1), 99. https://doi.org/10.3390/ma11010099




