Carbon/Attapulgite Composites as Recycled Palm Oil-Decoloring and Dye Adsorbents
Abstract
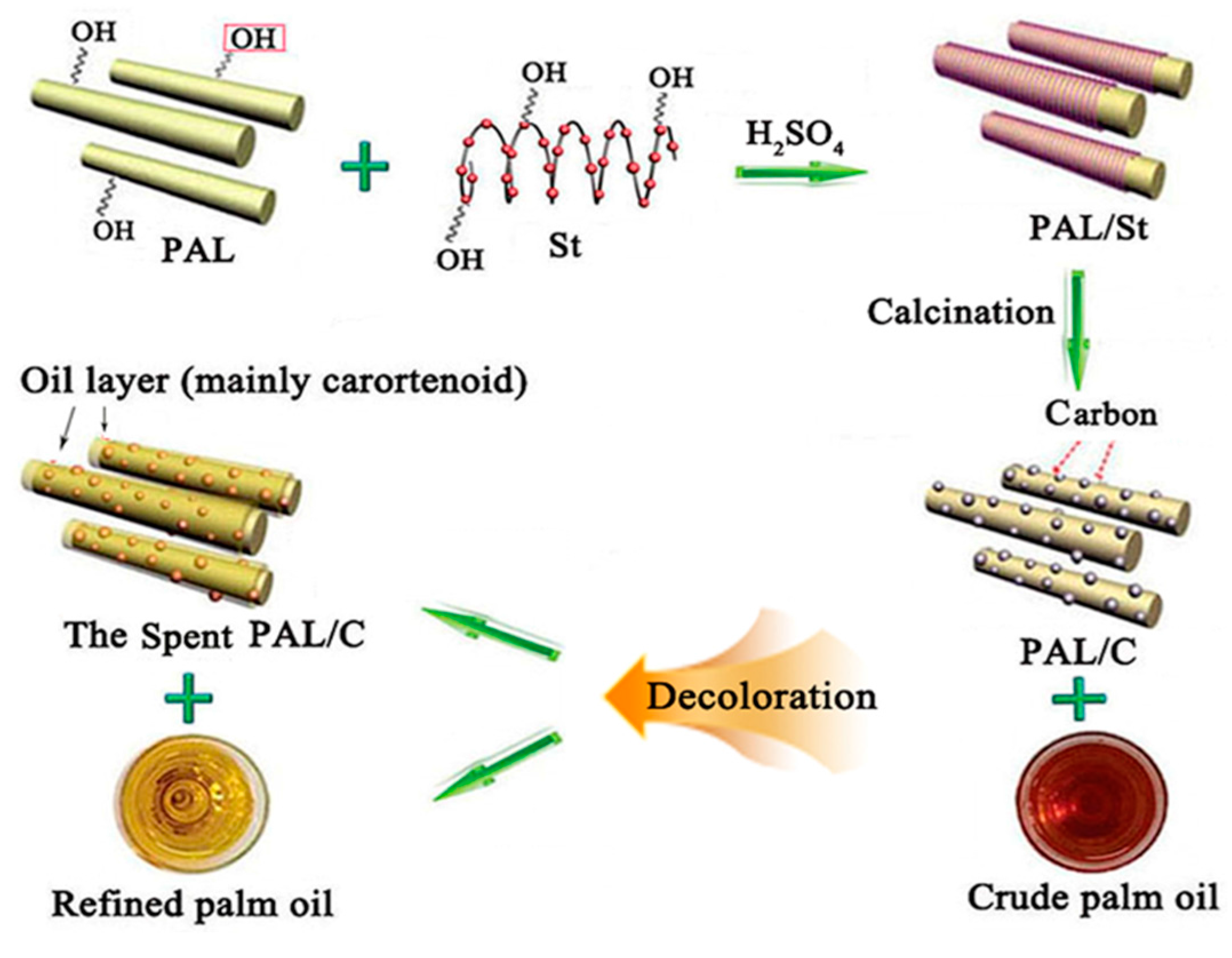
1. Introduction
2. Results and Discussions
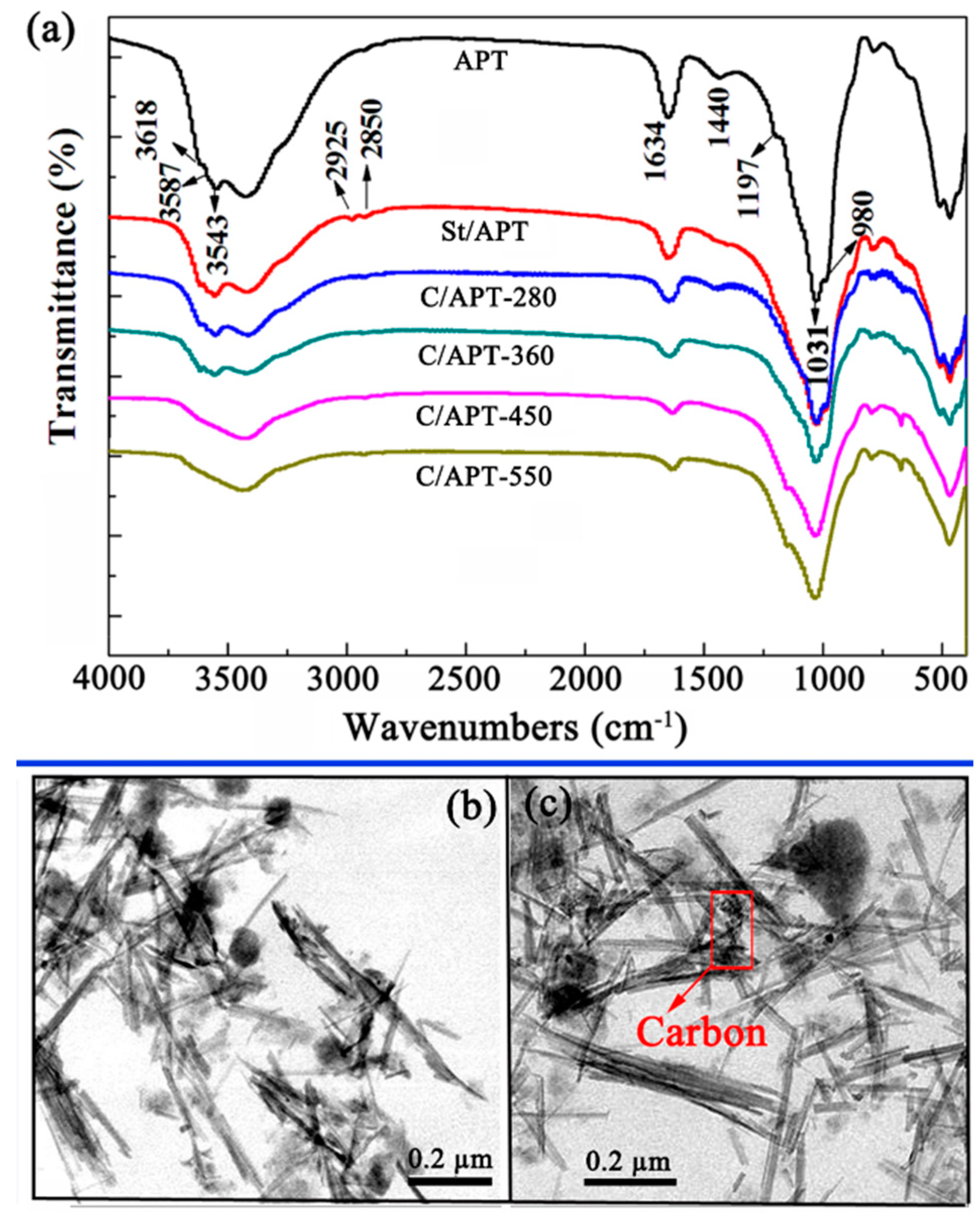
2.1. Structure and Characteristics of C/APT Composite
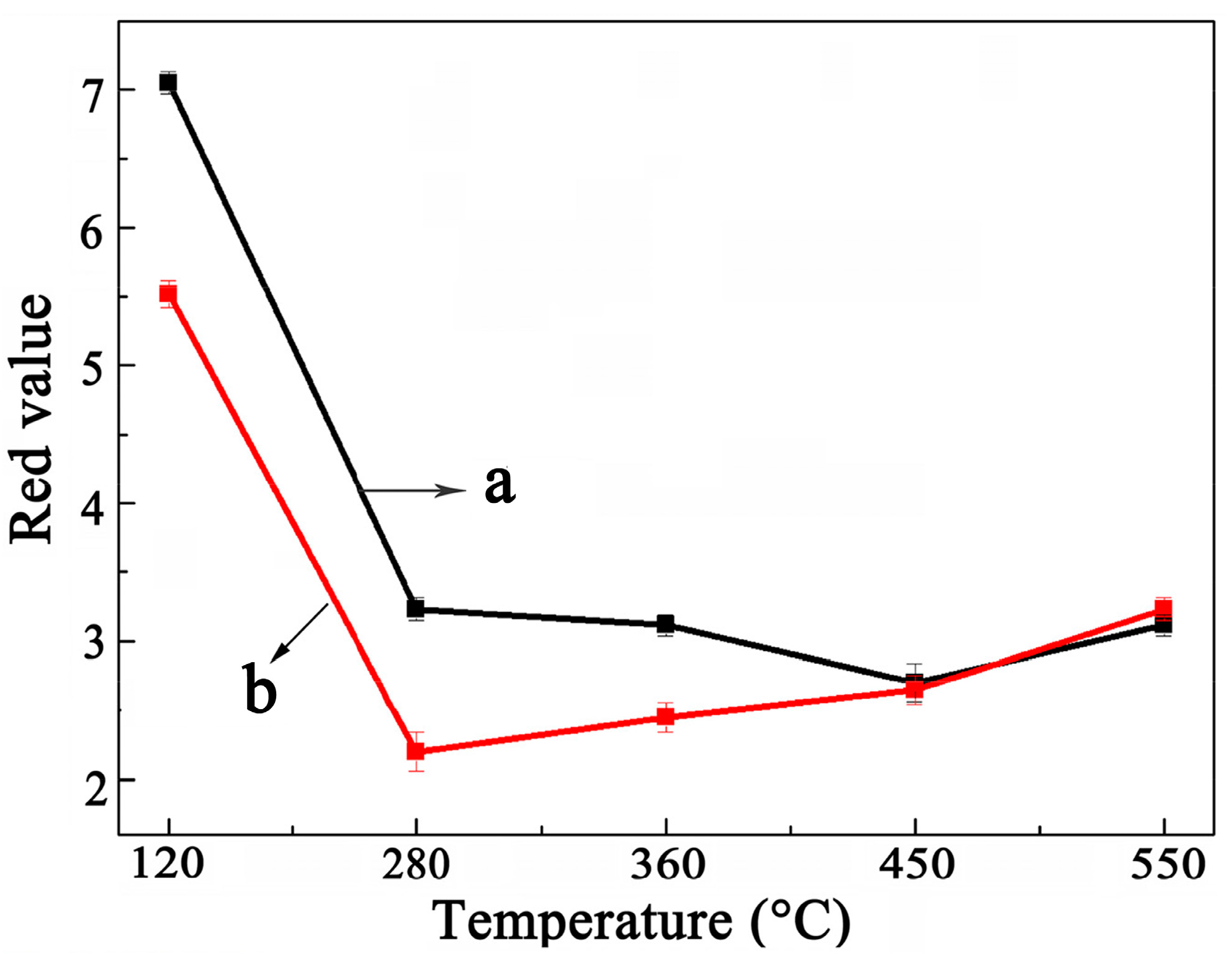
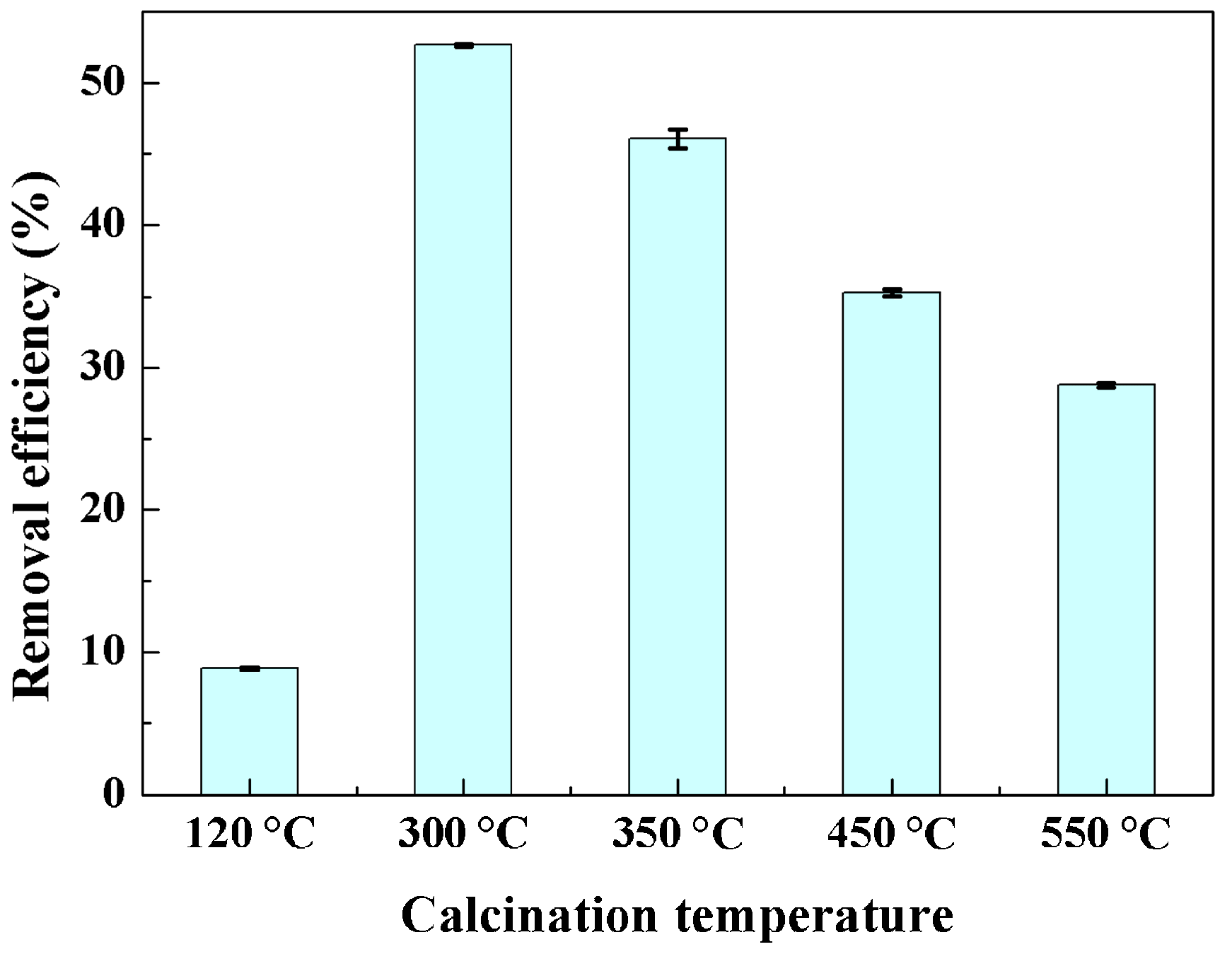
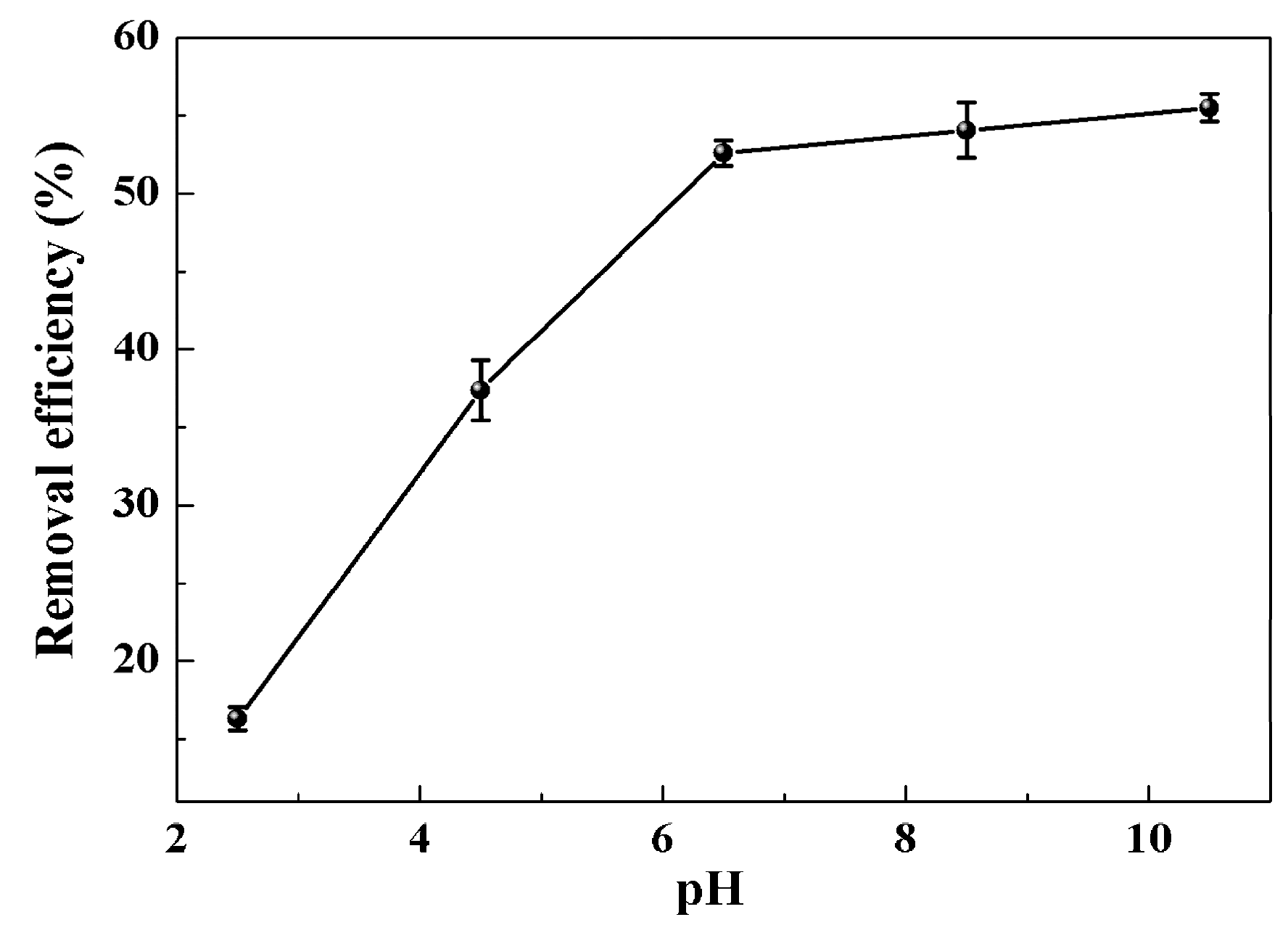
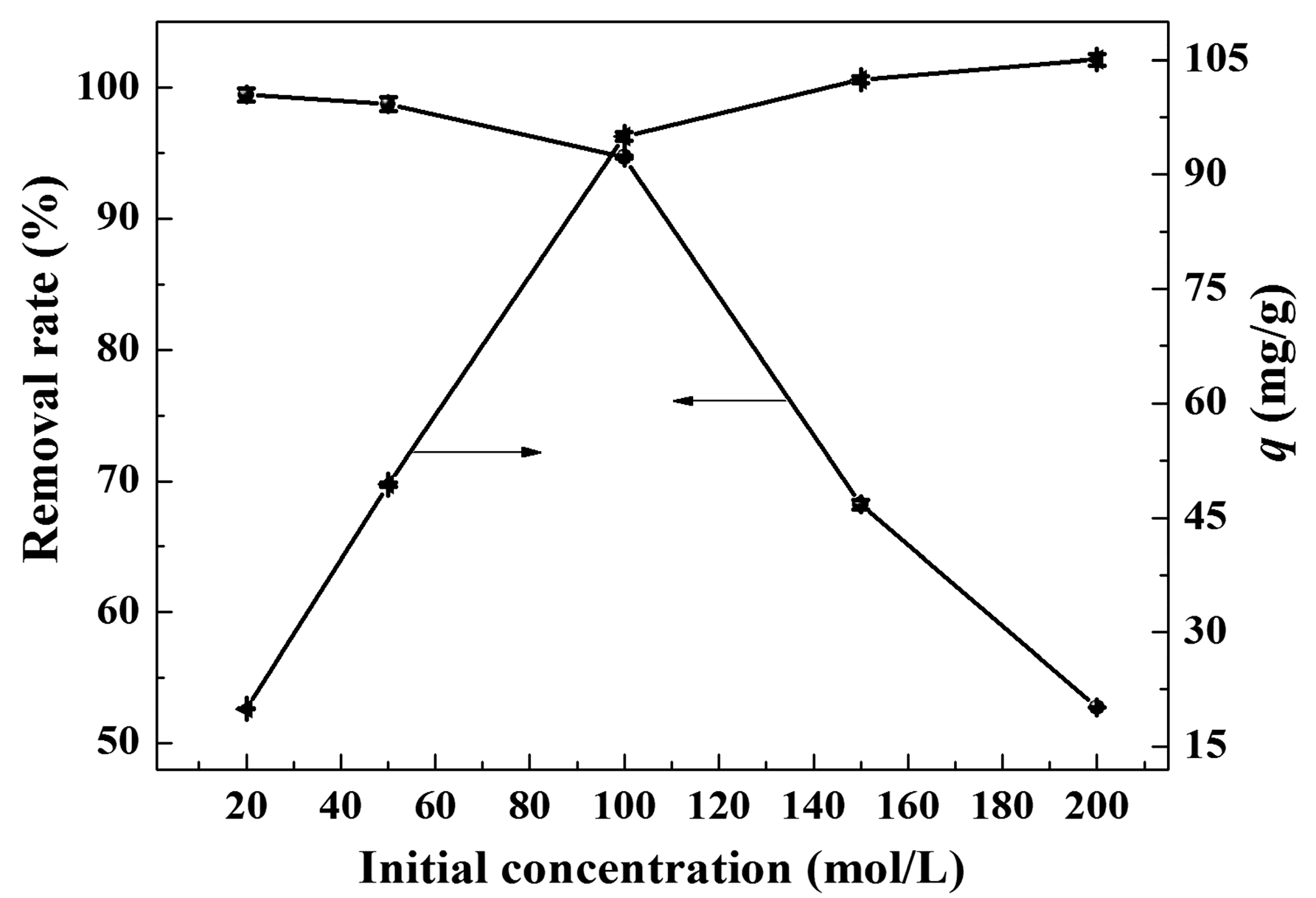
2.2. Decoloring Efficiency
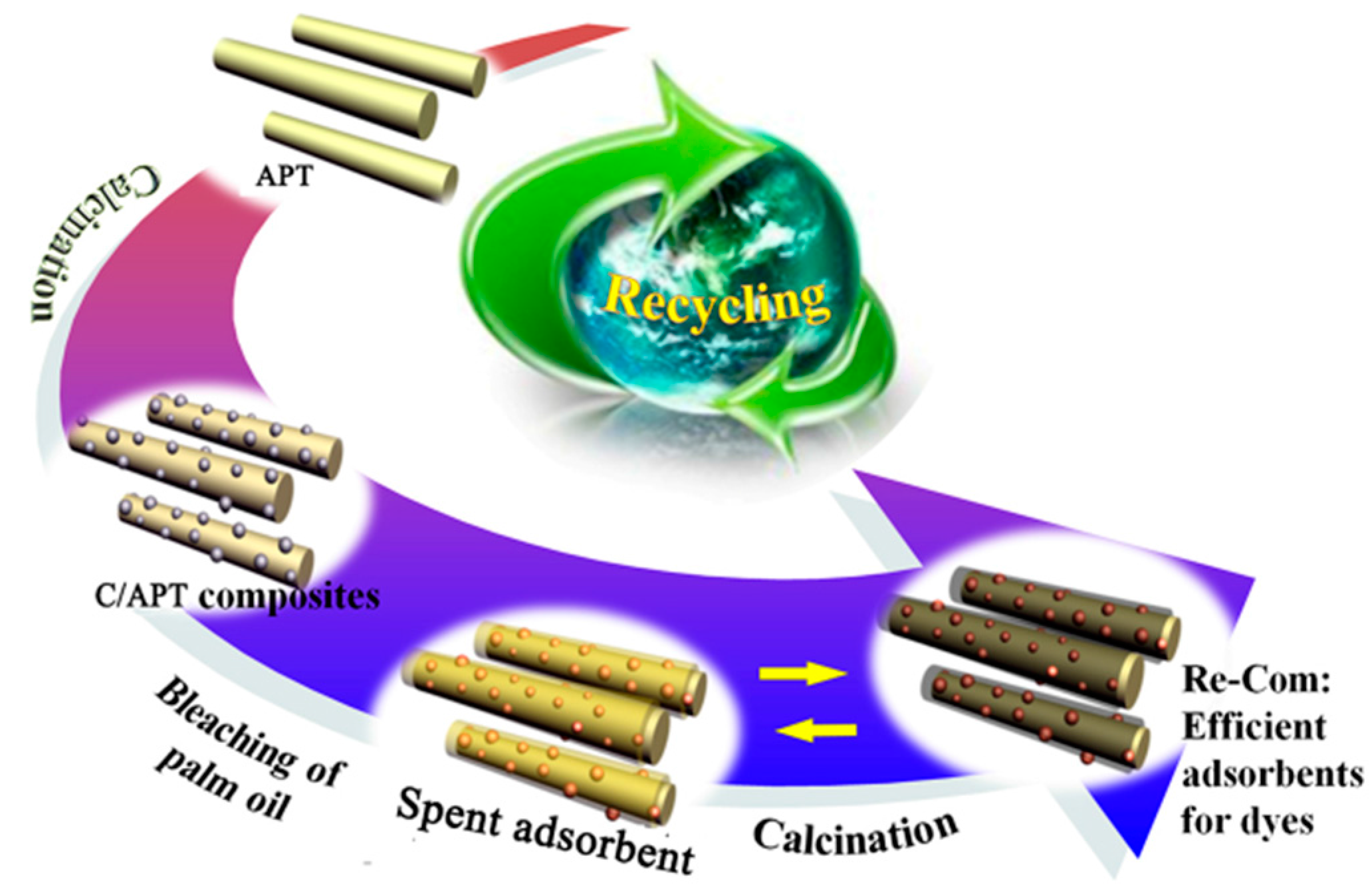
2.3. Cyclic Regeneration of C/APT Composites
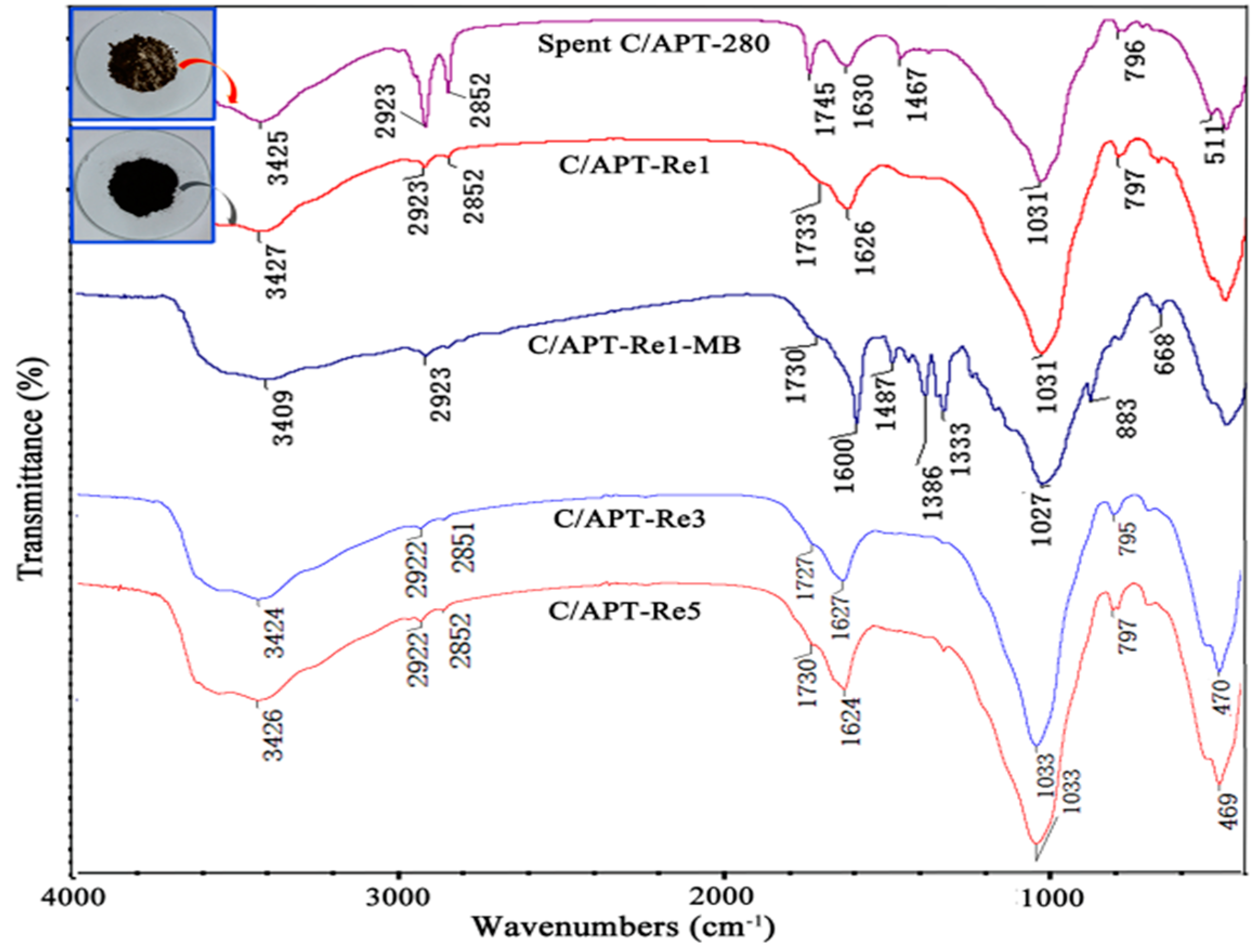
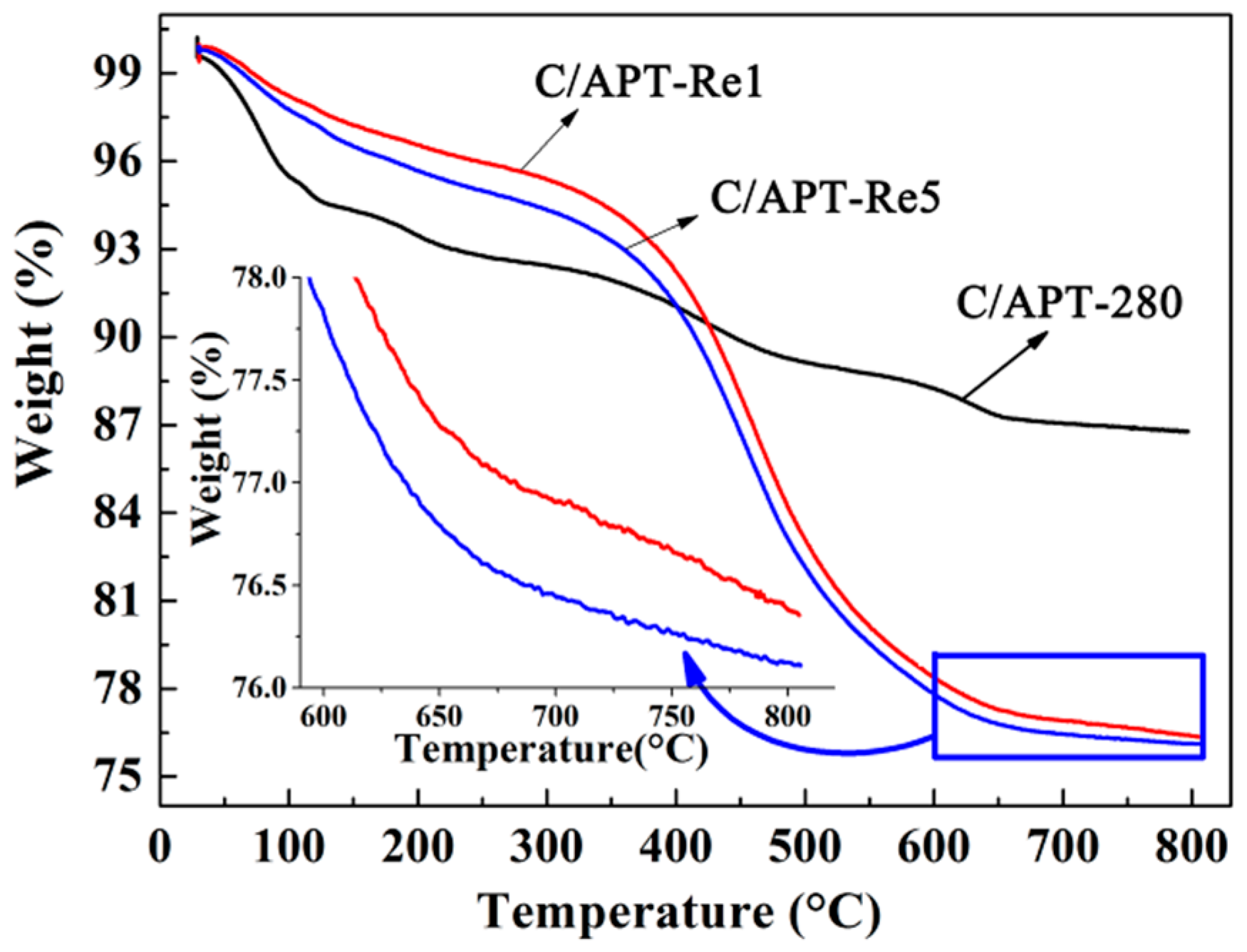
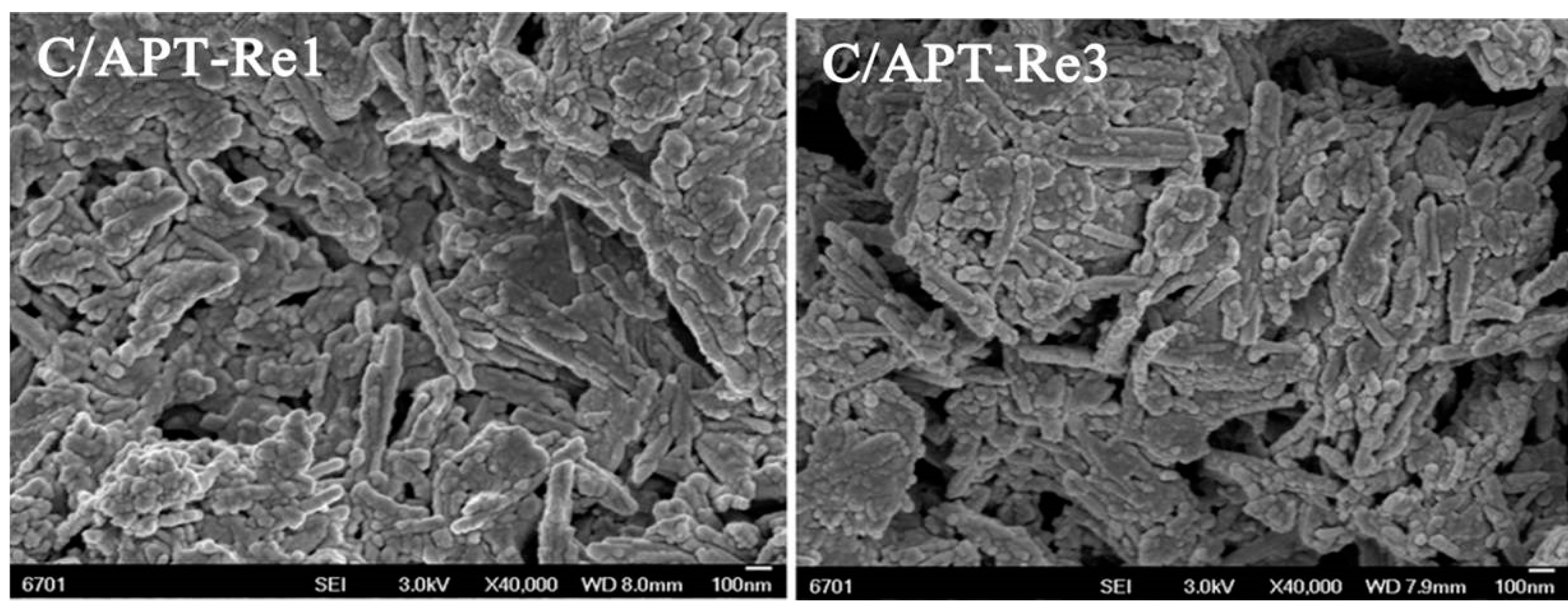
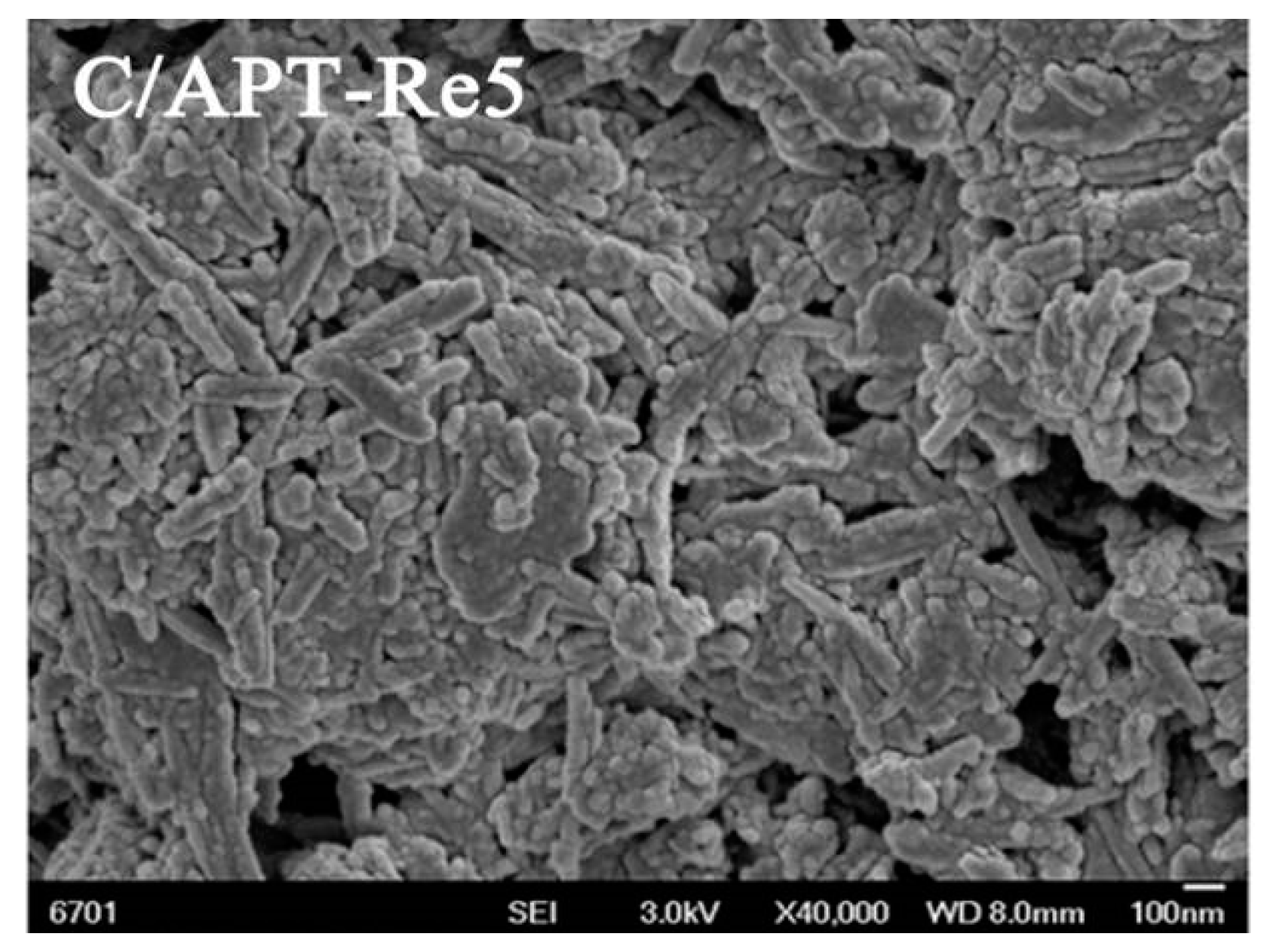
2.3.1. Structure Characteristic of C/APT-Re Composite
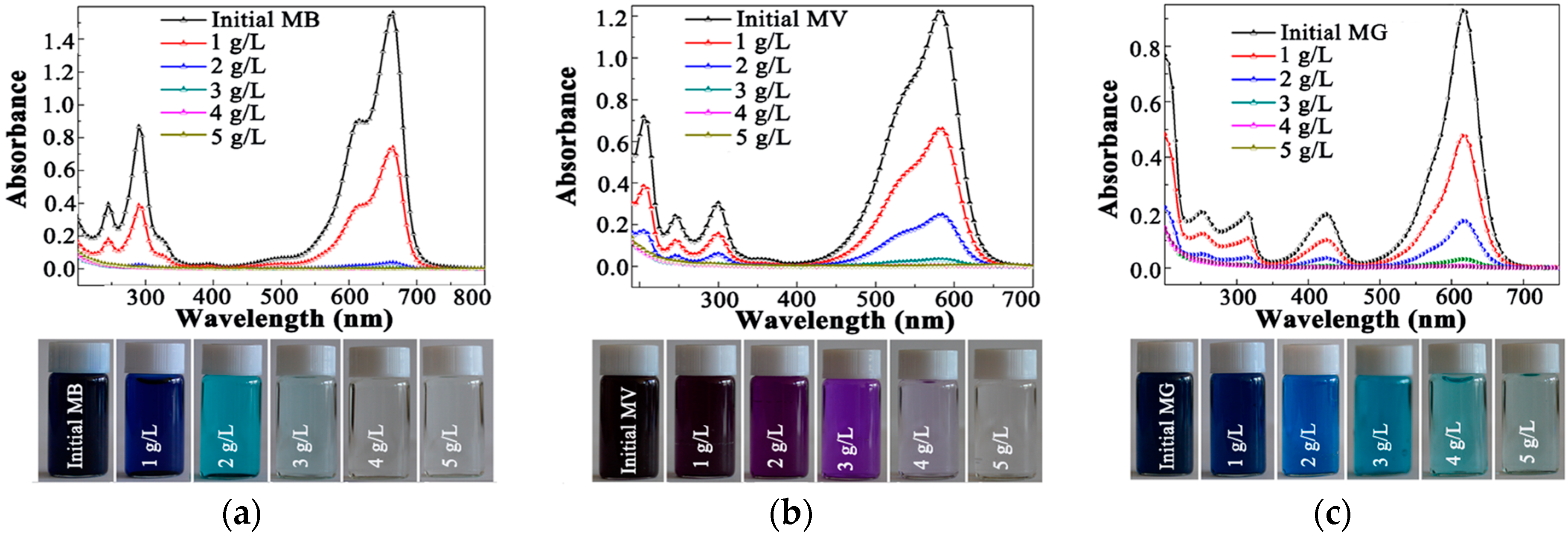
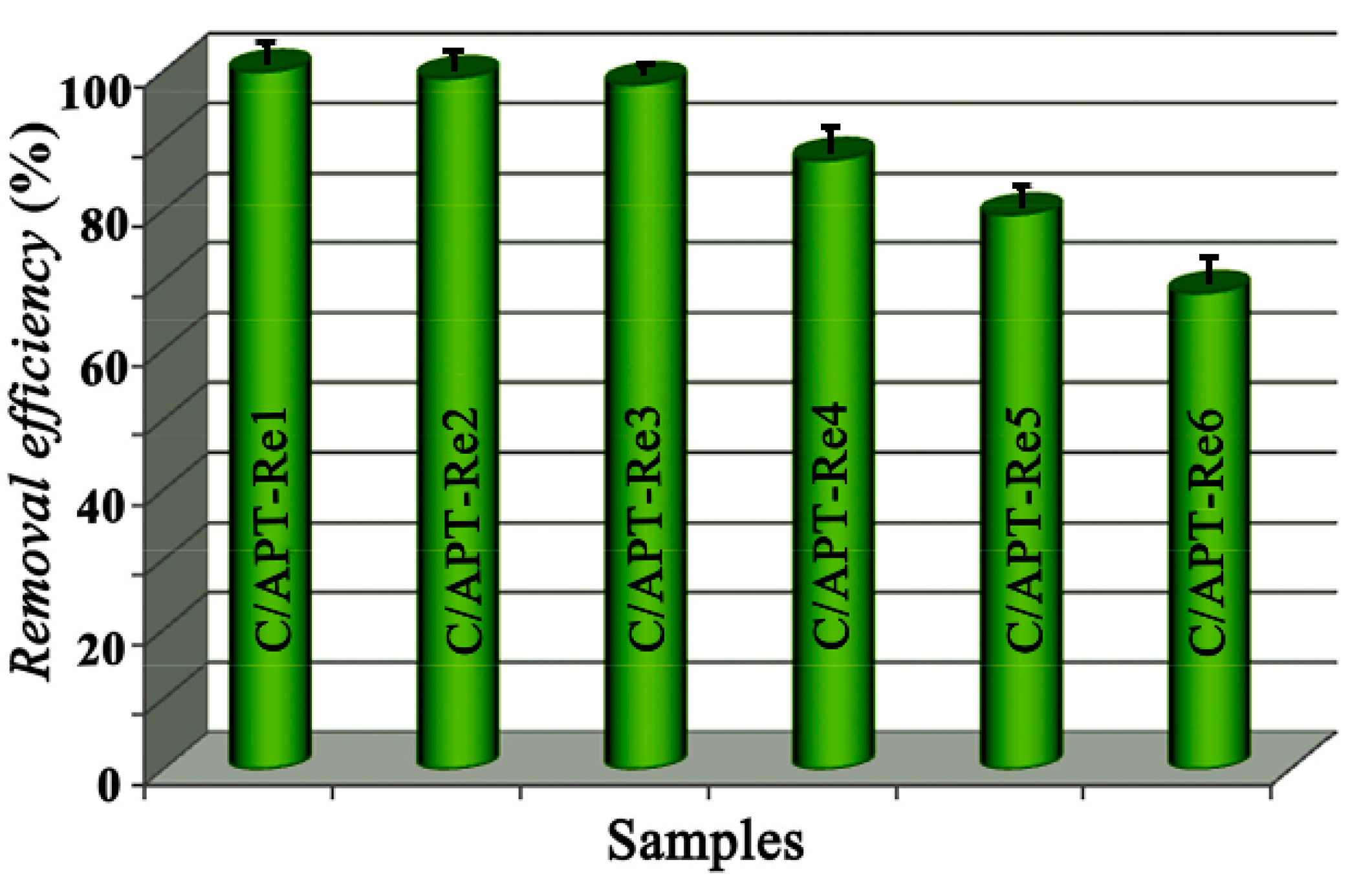
2.3.2. Adsorption Efficiency for Dyes
3. Materials and Methods
3.1. Materials
3.2. Preparation of C/APT Adsorbent from St and APT
3.3. Regeneration of the Spent C/APT Composites
3.4. Decoloring Test of Crude Palm Oil and Evaluation of Decoloring Capacity
3.5. Batch Adsorption Experiments
3.6. Characterizations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De Vries, R.J. Utilization of Malaysian palm oil and palm kernel oil for fatty acids and derivatives. J. Am. Oil Chem. Soc. 1984, 61, 404–407. [Google Scholar] [CrossRef]
- Traitler, H.; Dieffenbacher, A. Palm oil and palm kernel oil in food products. J. Am. Oil Chem. Soc. 1985, 62, 417–421. [Google Scholar] [CrossRef]
- Mana, M.; Ouali, M.S.; de Menorval, L.C.; Zajac, J.J.; Charnay, C. Regeneration of spent bleaching earth by treatment with cethyltrimethylammonium bromide for application in elimination of acid dye. Chem. Eng. J. 2011, 174, 275–280. [Google Scholar] [CrossRef]
- Vincent, C.J.; Shamsudin, R.; Baharuddin, A.S. Pre-treatment of oil palm fruits: A review. J. Food Eng. 2014, 143, 123–131. [Google Scholar] [CrossRef]
- Sabah, E.; Çelik, M.S. Sepiolite: An effective bleaching adsorbent for the physical refining of degummed rapeseed oil. J. Am. Oil Chem. Soc. 2005, 82, 911–916. [Google Scholar] [CrossRef]
- Kheok, S.C.; Lim, E.E. Mechanism of palm oil bleaching by montmorillonite clay activated at various acid concentrations. J. Am. Oil Chem. Soc. 1982, 59, 129–131. [Google Scholar] [CrossRef]
- Hussin, F.; Aroua, M.K.; Daud, W.M.A.W. Textural characteristics, surface chemistry and activation of bleaching earth: A review. Chem. Eng. J. 2011, 170, 90–106. [Google Scholar] [CrossRef]
- Singh, R.P.; Embrandiri, A.; Ibrahima, M.H.; Esa, N. Management of biomass residues generated from palm oil mill: Vermicomposting a sustainable option. Resour. Conserv. Rec. 2011, 55, 423–434. [Google Scholar] [CrossRef]
- Mohammad, N.; Alam, M.Z.; Kabbashi, N.A.; Ahsan, A. Effective composting of oil palm industrial waste by filamentous fungi: A review. Resour. Conserv. Rec. 2012, 58, 69–78. [Google Scholar] [CrossRef]
- Mana, M.; Ouali, M.S.; de Menorval, L.C. Removal of basic dyes from aqueous solutions with a treated spent bleaching earth. J. Colloid Interface Sci. 2007, 307, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Mana, M.; Oualia, M.S.; Lindheimer, M.; de Menorval, L.C. Removal of lead from aqueous solutions with a treated spent beaching earth. J. Hazard. Mater. 2008, 159, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Anadão, P.; Pajolli, I.L.R.; Hildebrando, E.A.; Wiebeck, H. Preparation and characterization of carbon/montmorillonite composites and nanocomposites from waste bleaching sodium montmorillonite clay. Adv. Powder Technol. 2014, 25, 926–932. [Google Scholar] [CrossRef]
- Zhu, R.; Chen, Q.; Zhou, Q.; Xi, Y.F.; Zhu, J.X.; He, H.P. Adsorbents based on montmorillonite for contaminant removal from water: A review. Appl. Clay Sci. 2016, 123, 239–258. [Google Scholar] [CrossRef]
- Komadel, P. Acid activated clays: Materials in continuous demand. Appl. Clay Sci. 2016, 131, 84–99. [Google Scholar] [CrossRef]
- Drits, V.A.; Sokolova, G.V. Structure of palygorskite. Sov. Phys. Crystallogr. 1971, 16, 183–185. [Google Scholar]
- Chishoim, J.E. An X-ray powder-diffraction study of palygorskite. Can. Mineral. 1990, 28, 329–339. [Google Scholar]
- Wang, W.B.; Wang, A.Q. Recent progress in dispersion of palygorskite crystal bundles for nanocomposites. Appl. Clay Sci. 2016, 119, 18–30. [Google Scholar] [CrossRef]
- Zhou, C.H.; Zhao, L.Z.; Wang, A.Q. Current fundamental and applied research into clay minerals in China. Appl. Clay Sci. 2016, 119, 3–7. [Google Scholar] [CrossRef]
- Wang, W.B.; Wang, F.F.; Kang, Y.R.; Wang, A.Q. Nanoscale dispersion crystal bundles of palygorskite by associated modification with phytic acid and high-pressure homogenization for enhanced colloidal properties. Powder Technol. 2015, 269, 85–92. [Google Scholar] [CrossRef]
- Zhuang, G.Z.; Wu, H.; Zhang, H.X.; Zhang, Z.P.; Zhang, X.M.; Liao, L.B. Rheological properties of organo-palygorskite in oil-based drilling fluids aged at different temperatures. Appl. Clay Sci. 2017, 137, 50–58. [Google Scholar] [CrossRef]
- Álvarez-Ayuso, E.; García-Sánchez, A. Removal of cadmium from aqueous solutions by palygorskite. J. Hazard. Mater. 2007, 147, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Frost, R.L.; Xi, Y.F.; He, H.P. Synthesis, characterization of palygorskite supported zero-valent iron and its application for methylene blue adsorption. J. Colloid Interface Sci. 2010, 341, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.B.; Tian, G.Y.; Zhang, Z.F.; Wang, A.Q. A simple hydrothermal approach to modify palygorskite for high-efficient adsorption of methylene blue and Cu(II) ions. Chem. Eng. J. 2015, 265, 228–238. [Google Scholar] [CrossRef]
- Middea, A.; Fernandes, T.L.A.P.; Neumann, R.; da, F.M.; Gomes, O.; Spinelli, L.S. Evaluation of Fe(III) adsorption onto palygorskite surfaces. Appl. Surf. Sci. 2013, 282, 253–258. [Google Scholar] [CrossRef]
- Moreira, M.A.; Ciuffi, K.J.; Rives, V.; Vicente, M.A.; Trujillano, R.; Gil, A.; Korili, S.A.; de Faria, E.H. Effect of chemical modification of palygorskite and sepiolite by 3-aminopropyltriethoxisilane on adsorption of cationic and anionic dyes. Appl. Clay Sci. 2017, 135, 394–404. [Google Scholar] [CrossRef]
- Stathatos, E.; Papoulis, D.; Aggelopoulos, C.A.; Panagiotaras, D.; Nikolopoulou, A. TiO2/palygorskite composite nanocrystalline films prepared by surfactant templating route: Synergistic effect to the photocatalytic degradation of an azo-dye in water. J. Hazard. Mater. 2012, 211–212, 68–76. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Fu, L.J.; Yang, H.M. Insight into the nature of Au-Au2O3 functionalized palygorskite. Appl. Clay Sci. 2014, 100, 118–122. [Google Scholar] [CrossRef]
- Lu, H.L.; Wang, W.B.; Wang, A.Q. Ethanol-NaOH solidification method intensify chitosan/poly(vinyl alcohol)/attapulgite composite film. RSC Adv. 2015, 5, 17775–17781. [Google Scholar] [CrossRef]
- Tang, Q.G.; Wang, F.; Liu, X.D.; Tang, M.R.; Zeng, Z.G.; Liang, J.S.; Guan, X.Y.; Wang, J.; Mu, X.Z. Surface modified palygorskite nanofibers and their applications as reinforcement phase in cis-polybutadiene rubber nanocomposites. Appl. Clay Sci. 2016, 132–133, 175–181. [Google Scholar] [CrossRef]
- Zhu, L.X.; Guo, J.S.; Liu, P.; Zhao, S.B. Novel strategy for palygorskite/poly(acrylic acid) nanocomposite hydrogels from bi-functionalized palygorskite nanorods as easily separable adsorbent for cationic basic dye. Appl. Clay Sci. 2016, 121–122, 29–35. [Google Scholar] [CrossRef]
- Chalvatzi, S.; Arsenos, G.; Tserveni-Goussi, A.; Fortomaris, P. Tolerance and efficacy study of palygorskite incorporation in the diet of laying hens. Appl. Clay Sci. 2014, 101, 643–647. [Google Scholar] [CrossRef]
- Papadopoulos, G.A.; Kanoulas, V.; Arsenos, G.; Janssens, G.P.J.; Buyse, J.; Tzika, E.D.; Fortomaris, P.D. Effects of palygorskite dietary supplementation on back fat mobilization, leptin levels and oxidative stress parameters in sows. Appl. Clay Sci. 2016, 132–133, 535–541. [Google Scholar] [CrossRef]
- Chen, Y.P.; Cheng, Y.F.; Yang, W.L. An evaluation of palygorskite inclusion on the growth performance and digestive function of broilers. Appl. Clay Sci. 2016, 129, 1–6. [Google Scholar] [CrossRef]
- Tian, G.Y.; Wang, W.B.; Zong, L.; Kang, Y.R.; Wang, A.Q. A functionalized hybrid silicate adsorbent derived from naturally abundant low-grade palygorskite clay for highly efficient removal of hazardous antibiotics. Chem. Eng. J. 2016, 293, 376–385. [Google Scholar] [CrossRef]
- Tian, G.Y.; Wang, W.B.; Wang, D.D.; Wang, Q.; Wang, A.Q. Novel environment friendly inorganic red pigments based on attapulgite. Powder Technol. 2017, 315, 60–67. [Google Scholar] [CrossRef]
- Foletto, E.L.; Paz, D.S.; Gündel, A. Acid-activation assisted by microwave of a Brazilian bentonite and its activity in the bleaching of soybean oil. Appl. Clay Sci. 2013, 83–84, 63–67. [Google Scholar] [CrossRef]
- Kuuluvainen, V.; Mäki-Arvela, P.; Rautio, A. Properties of adsorbents used for bleaching of vegetable oils and animal fats. J. Chem. Technol. Biotechnol. 2015, 90, 1579–1591. [Google Scholar] [CrossRef]
- Xavier, K.C.M.; Santos, M.S.F.; Osajima, J.A. Thermally activated palygorskites as agents to clarify soybean oil. Appl. Clay Sci. 2016, 119, 338–347. [Google Scholar] [CrossRef]
- Sarkar, B.; Liu, E.M.; McClure, S. Biomass derived palygorskite–carbon nanocomposites: Synthesis, characterisation and affinity to dye compounds. Appl. Clay Sci. 2015, 114, 617–626. [Google Scholar] [CrossRef]
- Tian, G.Y.; Wang, W.B.; Mu, B.; Kang, Y.R.; Wang, A.Q. Facile fabrication of carbon/attapulgite composite for bleaching of palm oil. J. Taiwan Inst. Chem. Eng. 2015, 50, 252–258. [Google Scholar] [CrossRef]
- Fabian, C.; Ayucitra, A.; Ismadji, S.; Ju, Y.H. Isolation and characterization of starch from defatted rice bran. J. Taiwan Inst. Chem. Eng. 2011, 42, 86–91. [Google Scholar] [CrossRef]
- Hsu, S.H.; Hsu, W.C.; Chung, T.W.; Liao, C.C. Dynamic adsorption study for water removal from ethanol solution by using a novel immobilized starch sorbent. J. Taiwan Inst. Chem. Eng. 2013, 44, 952–956. [Google Scholar] [CrossRef]
- Guo, J.; Gui, B.; Xiang, S.X.; Bao, X.T.; Zhang, H.J.; Lua, A.C. Preparation of activated carbons by utilizing solid wastes from palm oil processing mills. J. Porous Mater. 2008, 15, 535–540. [Google Scholar] [CrossRef]
- Tian, G.Y.; Wang, W.B.; Zong, L.; Kang, Y.R.; Wang, A.Q. From spent dye-loaded palygorskite to a multifunctional palygorskite/carbon/Ag nanocomposite. RSC Adv. 2016, 6, 41696–41706. [Google Scholar] [CrossRef]
- Deng, Y.H.; Gao, Z.Q.; Liu, B.Z.; Hu, X.B.; Wei, Z.B.; Sun, C. Selective removal of lead from aqueous solutions by ethylenediamine-modified attapulgite. Chem. Eng. J. 2013, 223, 91–98. [Google Scholar] [CrossRef]
- Yan, W.C.; Liu, D.; Tan, D.Y.; Yuan, P.; Chen, M. FTIR spectroscopy study of the structure changes of palygorskite under heating. Spectrochim. Acta Part A 2012, 97, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Suárez, M.; García-Romero, E. FTIR spectroscopic study of palygorskite: Influence of the composition of the octahedral sheet. Appl. Clay Sci. 2006, 31, 154–163. [Google Scholar] [CrossRef]
- Frini-Srasra, N.; Srasra, E. Effect of heating on palygorskite and acid treated palygorskite properties. Surf. Eng. Appl. Electrochem. 2008, 44, 43–49. [Google Scholar] [CrossRef]
- Michot, A.; Smith, D.S.; Degot, S. Thermal conductivity and specific heat of kaolinite: Evolution with thermal treatment. J. Eur. Ceram. Soc. 2008, 28, 2639–2644. [Google Scholar] [CrossRef]
- Yılmaz, M.; Kalpaklı, Y.; Pişkin, S. Thermal behavior and dehydroxylation kinetics of naturally occurring sepiolite and bentonite. J. Therm. Anal. Calorim. 2013, 114, 1191–1199. [Google Scholar] [CrossRef]
- Huang, J.H.; Liu, Y.F.; Liu, Y.; Wang, X.G. Effect of attapulgite pore size distribution on soybean oil bleaching. J. Am. Oil Chem. Soc. 2007, 84, 687–692. [Google Scholar] [CrossRef]
- Sabah, E.; Çinar, M.; Çelik, M.S. Decolorization of vegetable oils: Adsorption mechanism of β-carotene on acid-activated sepiolite. Food Chem. 2007, 100, 1661–1668. [Google Scholar] [CrossRef]
- Lamas, D.L.; Crapiste, G.H.; Constenla, D.T. Changes in quality and composition of sunflower oil during enzymatic degumming process. LWT-Food Sci. Technol. 2014, 58, 71–76. [Google Scholar] [CrossRef]
- Chiavaro, E.; Rodriguez-Estrada, M.T.; Vittadini, E.; Pellegrini, N. Microwave heating of different vegetable oils: Relation between chemical and thermal parameters. LWT-Food Sci. Technol. 2010, 43, 1104–1112. [Google Scholar] [CrossRef]
- Liu, P.; Jiang, L.P.; Zhu, L.X.; Wang, A.Q. Novel approach for attapulgite/poly(acrylic acid) (ATP/PAA) nanocomposite microgels as selective adsorbent for Pb(II) ion. React. Funct. Polym. 2014, 74, 72–80. [Google Scholar] [CrossRef]
- Tian, G.Y.; Wang, W.B.; Kang, Y.R.; Wang, A.Q. Ammonium sulfide-assisted hydrothermal activation of palygorskite for enhanced adsorption of methyl violet. J. Environ. Sci. 2016, 41, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.Q.; Yu, J.G.; Cheng, B.; Su, B.L.; Jaroniec, M. Synthesis of boehmite hollow core/shell and hollow microspheres via sodium tartrate-mediated phase transformation and their enhanced adsorption performance in water treatment. J. Phys. Chem. C 2009, 113, 14739–14746. [Google Scholar] [CrossRef]
- Yu, F.; Wu, Y.Q.; Ma, J. Influence of the pore structure and surface chemistry on adsorption of ethylbenzene and xylene isomers by KOH-activated multi-walled carbon nanotubes. J. Hazard. Mater. 2012, 237–238, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Sheikhhosseini, A.; Shirvani, M.; Shariatmadari, H.; Zvomuya, F.; Najafic, B. Kinetics and thermodynamics of nickel sorption to calcium–palygorskite and calcium–sepiolite: A batch study. Geoderma 2014, 217–218, 111–117. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, D.; Zhang, G.; Cai, C.; Zhang, C.; Qiu, G.; Zheng, K.; Wu, Z. Adsorption of methylene blue from aqueous solution onto multiporous palygorskite modified by ion beam bombardment: Effect of contact time, temperature, pH and ionic strength. Appl. Clay Sci. 2013, 83, 137–143. [Google Scholar] [CrossRef]
- Sun, Z.; Li, C.; Wu, D. Removal of methylene blue from aqueous solution by adsorption onto zeolite synthesized from coal fly ash and its thermal regeneration. J. Chem. Technol. Biotechnol. 2010, 85, 845–850. [Google Scholar] [CrossRef]
- Musyoka, S.M.; Mittal, H.; Mishra, S.B.; Ngila, J.C. Effect of functionalization on the adsorption capacity of cellulose for the removal of methyl violet. Int. J. Biol. Macromol. 2014, 65, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Aksu, Z.; Dönmez, G. A comparative study on the biosorption characteristics of some yeasts for Remazol Blue reactive dye. Chemosphere 2003, 50, 1075–1083. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Mondal, D.; Roy, I.; Sarkar, G.; Saha, N.R.; Rana, D.; Ghosh, T.K.; Mandal, D.; Chakraborty, M.; Chattopadhyay, D. Studies of the kinetics and mechanism of the removal process of proflavine dye through adsorption by graphene oxide. J. Mol. Liq. 2017, 230, 696–704. [Google Scholar] [CrossRef]
- Rahchamani, J.; Zavvar Mousavi, H.; Behzad, M. Adsorption of methyl violet from aqueous solution by polyacrylamide as an adsorbent: Isotherm and kinetic studies. Desalination 2011, 267, 256–260. [Google Scholar] [CrossRef]


| Samples * | SBET/m2/g | SD | Smicro/m2/g | SD | Sext./m2/g | SD | Vmicro/cm3/g | SD×104 | Vtotal/cm3/g | SD× 103 | PZ/nm | SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| APT | 192.4 | 1.5 | 49.9 | 0.5 | 142.5 | 2.0 | 0.03 | 3.1 | 0.35 | 8.4 | 7.33 | 0.1 |
| C/APT-280 | 176.8 | 1.2 | 39.5 | 0.6 | 137.3 | 0.7 | 0.02 | 5.6 | 0.37 | 2.0 | 8.33 | 0.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, G.; Wang, W.; Zhu, Y.; Zong, L.; Kang, Y.; Wang, A. Carbon/Attapulgite Composites as Recycled Palm Oil-Decoloring and Dye Adsorbents. Materials 2018, 11, 86. https://doi.org/10.3390/ma11010086
Tian G, Wang W, Zhu Y, Zong L, Kang Y, Wang A. Carbon/Attapulgite Composites as Recycled Palm Oil-Decoloring and Dye Adsorbents. Materials. 2018; 11(1):86. https://doi.org/10.3390/ma11010086
Chicago/Turabian StyleTian, Guangyan, Wenbo Wang, Yongfeng Zhu, Li Zong, Yuru Kang, and Aiqin Wang. 2018. "Carbon/Attapulgite Composites as Recycled Palm Oil-Decoloring and Dye Adsorbents" Materials 11, no. 1: 86. https://doi.org/10.3390/ma11010086
APA StyleTian, G., Wang, W., Zhu, Y., Zong, L., Kang, Y., & Wang, A. (2018). Carbon/Attapulgite Composites as Recycled Palm Oil-Decoloring and Dye Adsorbents. Materials, 11(1), 86. https://doi.org/10.3390/ma11010086








