Control of the Size of Silver Nanoparticles and Release of Silver in Heat Treated SiO2-Ag Composite Powders
Abstract
:1. Introduction
2. Results and Discussion
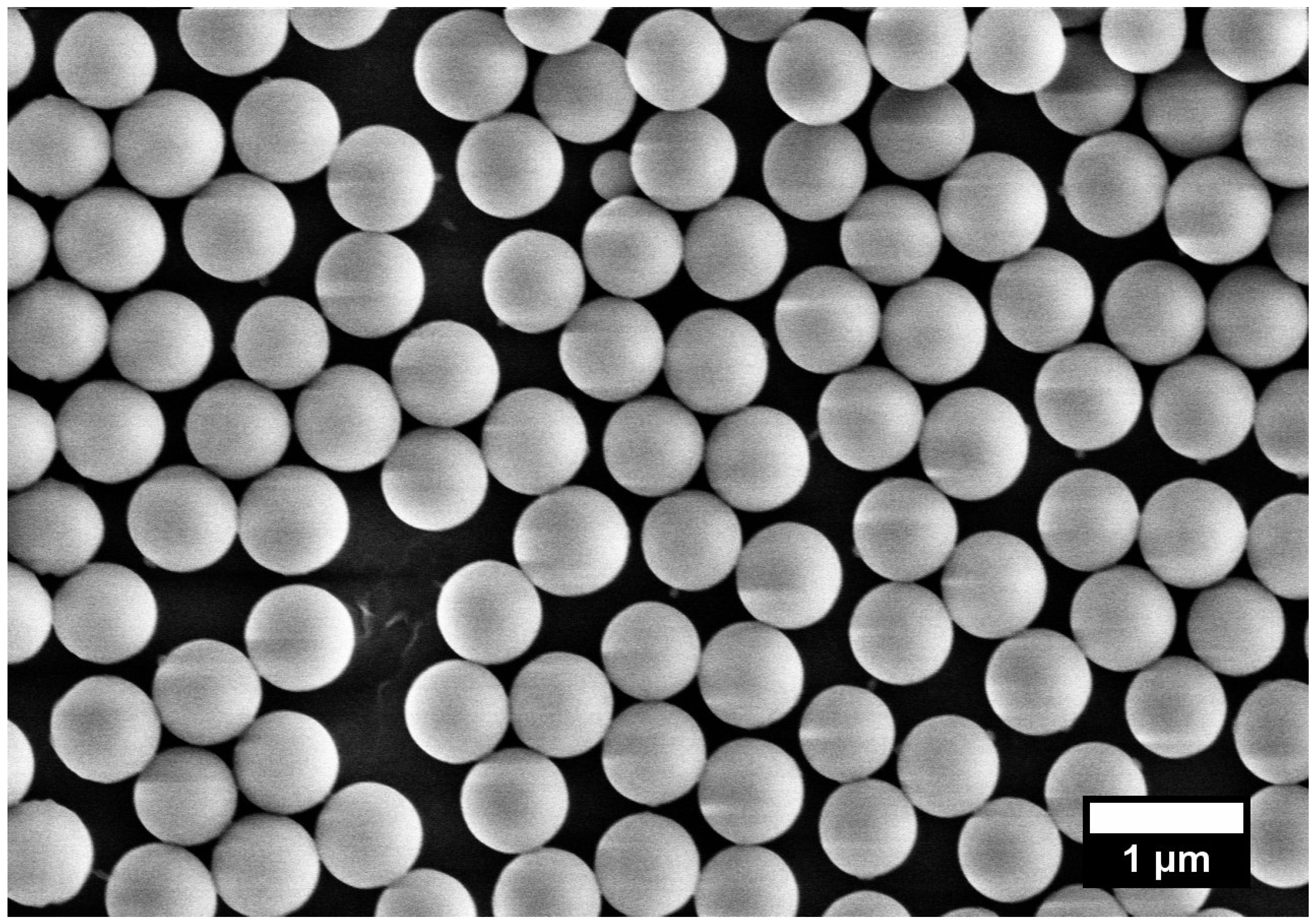
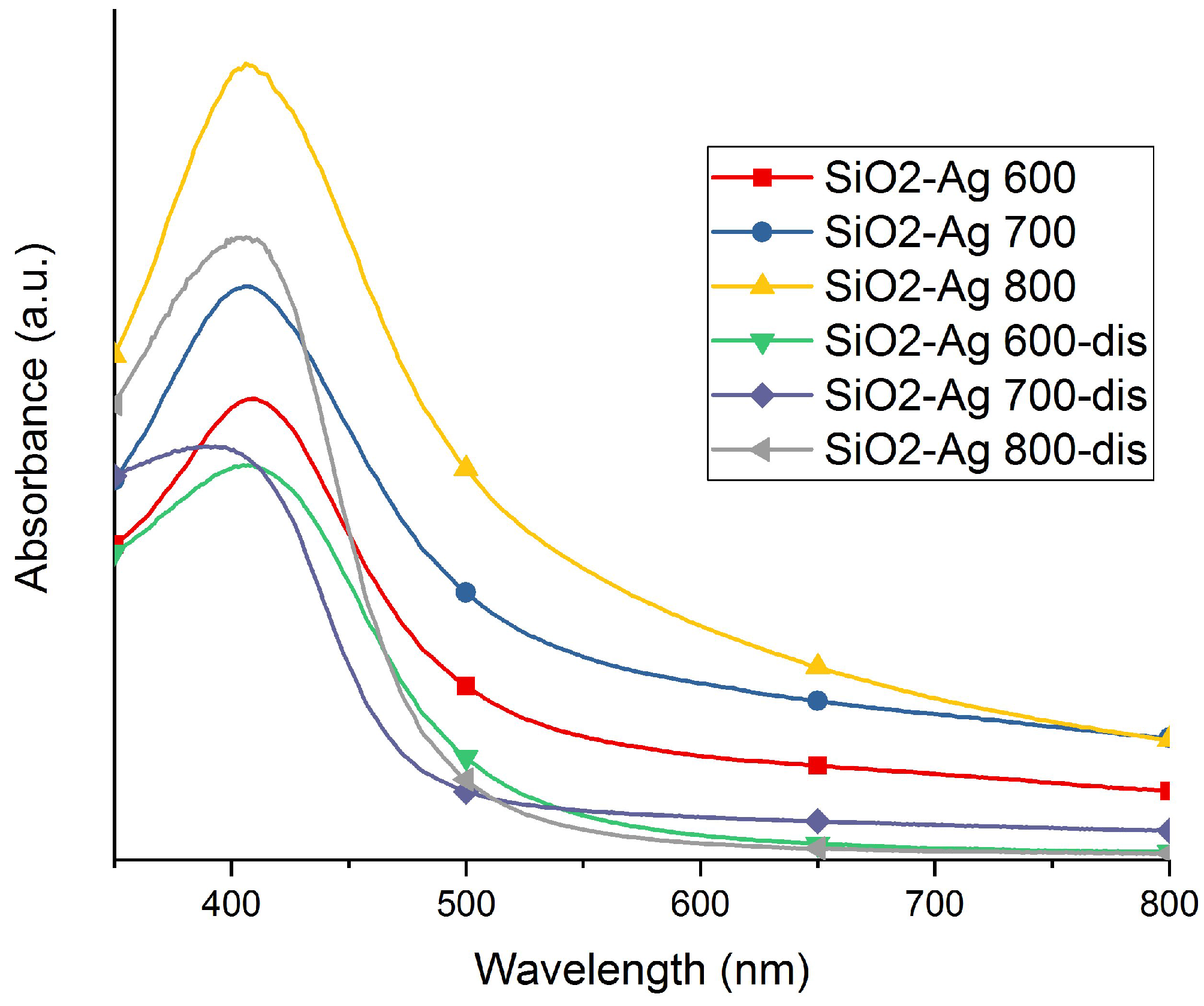
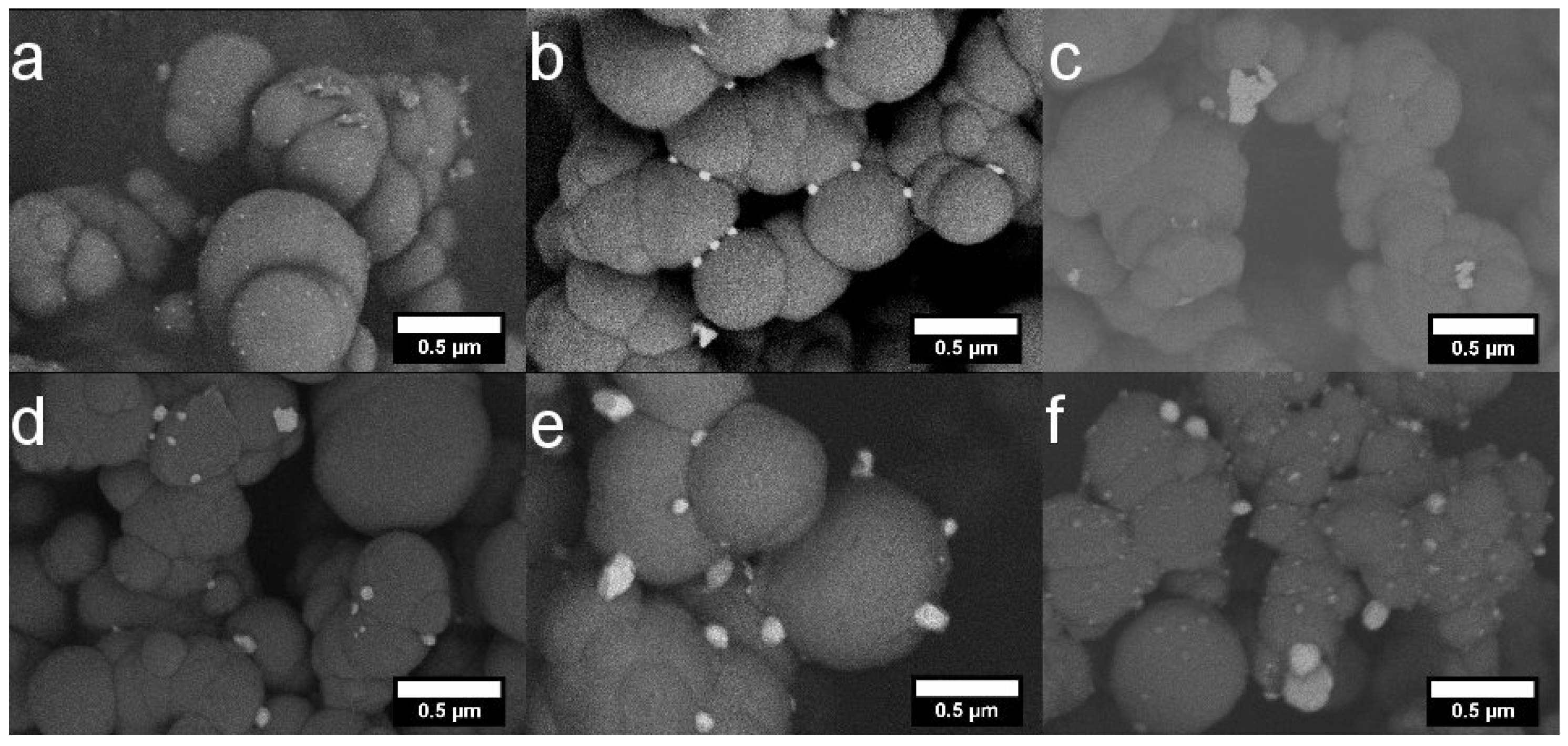
2.1. Morphology and Structure
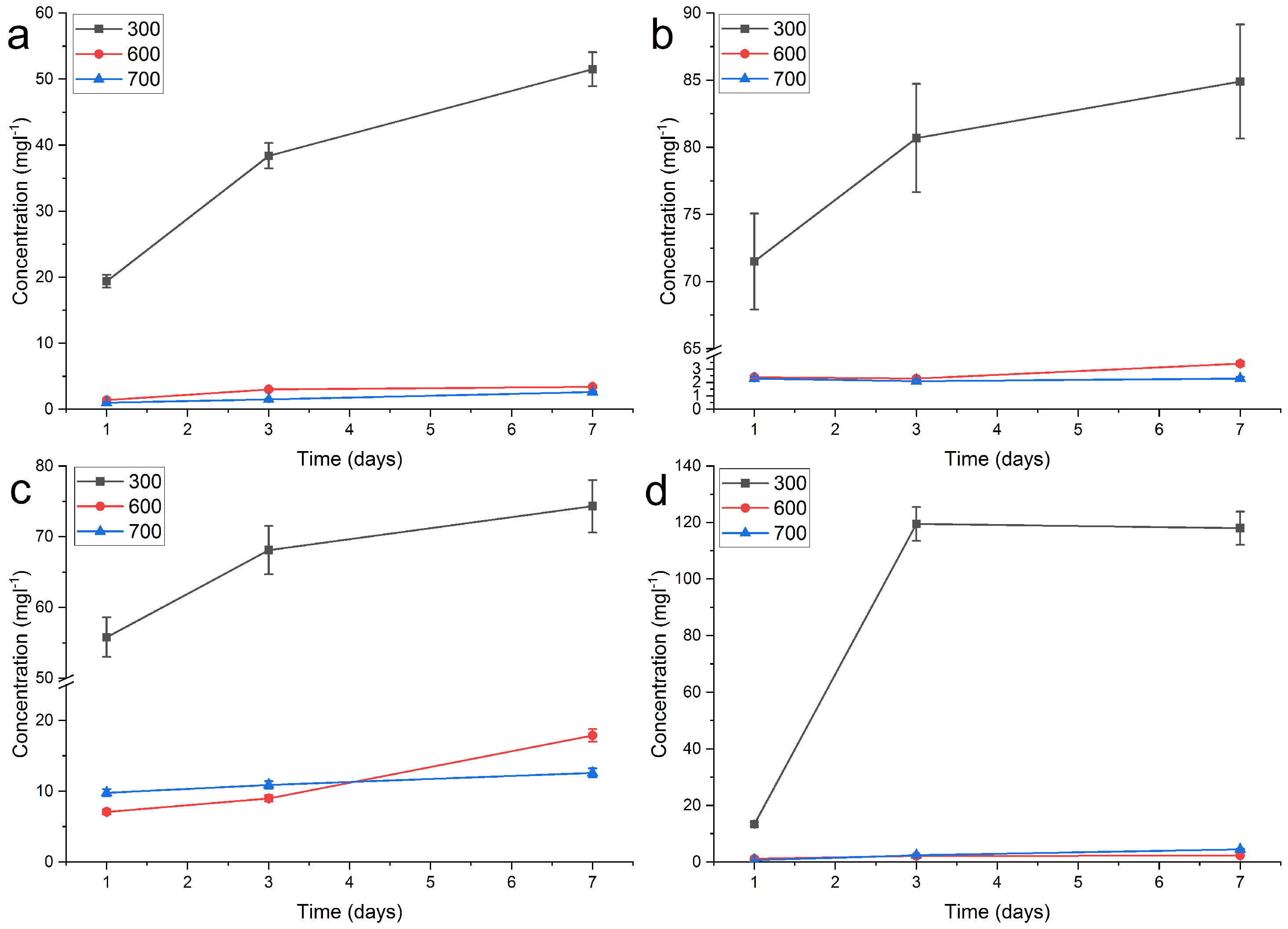
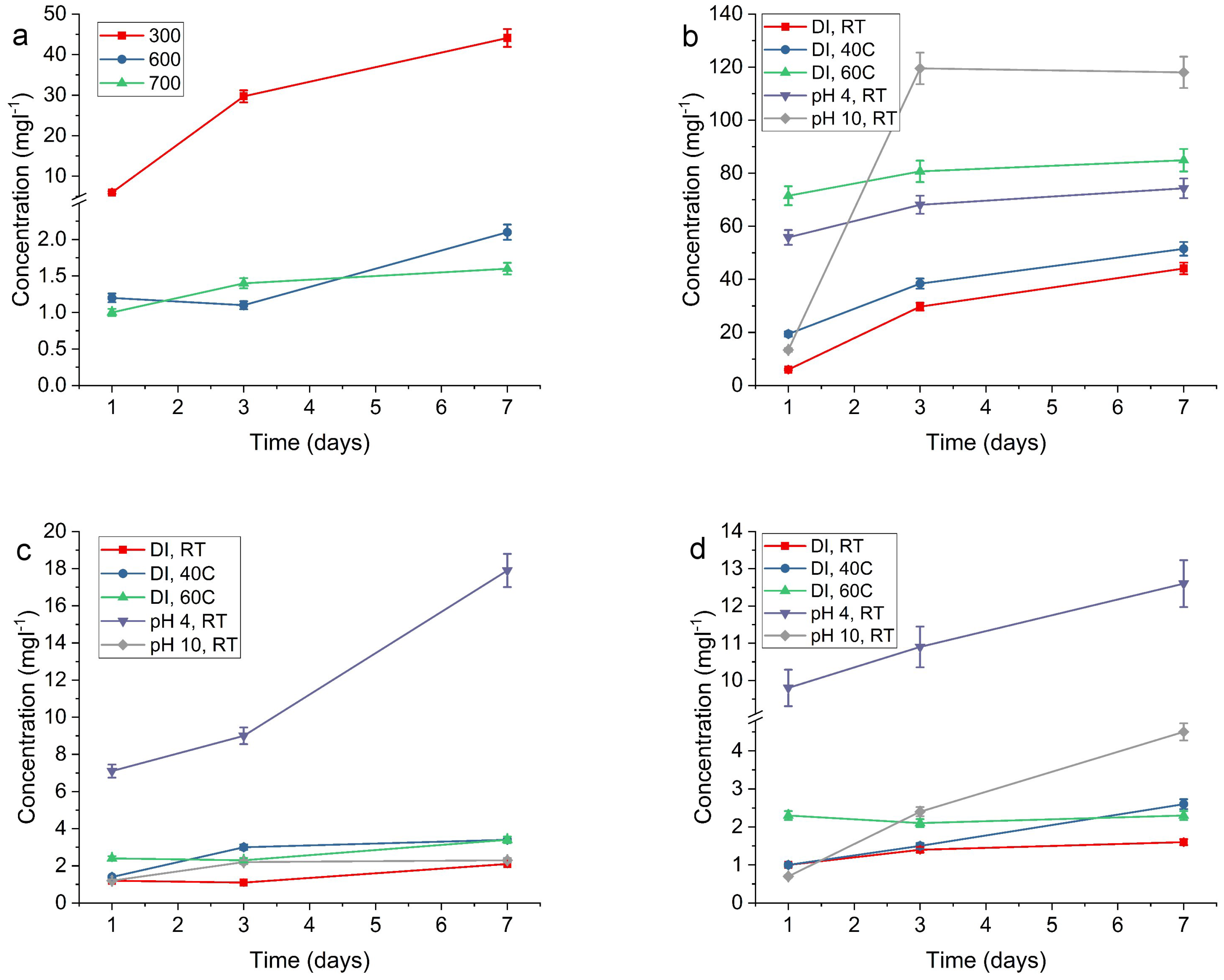
2.2. Release of Silver
3. Materials and Methods
3.1. Preparation of -Ag Composites
3.2. Dissolution of Silver
3.3. Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| APS | 3-aminopropyltrimethoxysilane |
| BET | Brunauer-Emmmet-Teller |
| DI | De-ionized water |
| FEG | Field Emission Gun |
| FTIR | Fourier Transform Infrared |
| ICP-AES | Inductively Coupled Plasma Atomic Emission Spectroscopy |
| NP | Nanoparticle |
| PVP | Polyvinylpyrrolidone |
| RT | Room temperature |
| SD | Standard Deviation |
| SEM | Scanning Electron Microscopy |
| SPR | Surface Plasmon Resonance |
| TEM | Transmission Electron Microscopy |
| TEOS | Tetraethyl orthosilicate |
| XRD | X-Ray Diffraction |
| XPS | X-ray Photoelectron Spectroscopy |
Appendix A. Supporting Information

| Sample | -Ag 300 | -Ag 600 | -Ag 700 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Days | 1 | 3 | 7 | 1 | 3 | 7 | 1 | 3 | 7 |
| RT, DI | 6.0 | 29.7 | 44.1 | 1.2 | 1.1 | 2.1 | 1.0 | 1.4 | 1.6 |
| 40 °C, DI | 19.4 | 38.4 | 51.5 | 1.4 | 3.0 | 3.4 | 1.0 | 1.5 | 2.6 |
| 60 °C, DI | 71.5 | 80.7 | 84.9 | 2.4 | 2.3 | 3.4 | 2.3 | 2.1 | 2.3 |
| pH 4, RT | 55.8 | 68.1 | 74.3 | 7.1 | 9.0 | 17.9 | 9.8 | 10.9 | 12.6 |
| pH 10, RT | 13.4 | 119.5 | 118.0 | 1.2 | 2.2 | 2.3 | 0.7 | 2.4 | 4.5 |
References
- Tzounis, L.; Logothetidis, S. Fe3O4@SiO2 core shell particles as platforms for the decoration of Ag nanoparticles. Mater. Today-Proc. 2017, 4, 7076–7082. [Google Scholar] [CrossRef]
- Tzounis, L.; Contreras-Caceres, R.; Schellkopf, L.; Jehnichen, D.; Fischer, D.; Cai, C.; Uhlmann, P.; Stamm, M. Controlled growth of Ag nanoparticles decorated onto the surface of SiO2 spheres: A nanohybrid system with combined SERS and catalytic properties. RSC Adv. 2014, 4, 17846–17855. [Google Scholar] [CrossRef]
- Niitsoo, O.; Couzis, A. Facile synthesis of silver core – silica shell composite nanoparticles. J. Colloid Interf. Sci. 2011, 354, 887–890. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, N.M.; Furusawa, T.; Sato, M.; Kurayama, F.; Siddiquey, I.A.; Suzuki, N. Fast and facile synthesis of silica coated silver nanoparticles by microwave irradiation. J. Colloid Interf. Sci. 2011, 355, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Tan, F.; Qiao, X.; Wang, W.; Chen, J.; Qiu, X. Room-temperature synthesis of silica supported silver nanoparticles in basic ethanol solution and their antibacterial activity. RSC Adv. 2016, 6, 18407–18412. [Google Scholar] [CrossRef]
- Ju, J.; Liu, W.; Perlaki, C.; Chen, K.; Feng, C.; Liu, Q. Sustained and Cost Effective Silver Substrate for Surface Enhanced Raman Spectroscopy Based Biosensing. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Furno, F.; Morley, K.S.; Wong, B.; Sharp, B.L.; Arnold, P.L.; Howdle, S.M.; Bayston, R.; Brown, P.D.; Winship, P.D.; Reid, H.J. Silver nanoparticles and polymeric medical devices: A new approach to prevention of infection? J. Antimicrob. Chemother. 2004, 54, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, A.; Boyd, D.; Douglas, C.W.I.; Towler, M.R. Antibacterial coatings for medical devices based on glass polyalkenoate cement chemistry. J. Mater. Sci.-Mater. M 2008, 19, 3555–3560. [Google Scholar] [CrossRef] [PubMed]
- Chernousova, S.; Epple, M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Chem. Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef]
- Kim, J.; Kuk, E.; Yu, K.; Kim, J.H.; Park, S.; Lee, H.; Kim, S.; Park, Y.; Park, Y.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomedicine 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Mosselhy, D.; Granbohm, H.; Hynönen, U.; Ge, Y.; Palva, A.; Nordström, K.; Hannula, S.P. Nanosilver–silica composite: Prolonged antibacterial effects and bacterial interaction mechanisms for wound dressings. Nanomaterials (Basel) 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Kittler, S.; Greulich, C.; Diendorf, J.; Köller, M.; Epple, M. Toxicity of Silver Nanoparticles Increases during Storage Because of Slow Dissolution under Release of Silver Ions. Chem. Mater. 2010, 22, 4548–4554. [Google Scholar] [CrossRef]
- Liu, J.; Hurt, R.H. Ion Release Kinetics and Particle Persistence in Aqueous Nano-Silver Colloids. Environ. Sci. Technol. 2010, 44, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sonshine, D.A.; Shervani, S.; Hurt, R.H. Controlled Release of Biologically Active Silver from Nanosilver Surfaces. ACS Nano 2010, 4, 6903–6913. [Google Scholar] [CrossRef] [PubMed]
- Gaiser, B.K.; Fernandes, T.F.; Jepson, M.A.; Lead, J.R.; Tyler, C.R.; Baalousha, M.; Biswas, A.; Britton, G.J.; Cole, P.A.; Johnston, B.D.; et al. Interspecies comparisons on the uptake and toxicity of silver and cerium dioxide nanoparticles. Environ. Toxicol. Chem. 2012, 31, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Miura, N.; Shinohara, Y. Cytotoxic effect and apoptosis induction by silver nanoparticles in HeLa cells. Biochem. Bioph. Res. Commun. 2009, 390, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Gliga, A.R.; Skoglund, S.; Odnevall Wallinder, I.; Fadeel, B.; Karlsson, H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: The role of cellular uptake, agglomeration and Ag release. Part. Fibre Toxicol. 2014, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef]
- Bastus, N.G.; Merkoci, F.; Piella, J.; Puntes, V. Synthesis of Highly Monodisperse Citrate-Stabilized Silver Nanoparticles of up to 200 nm: Kinetic Control and Catalytic Properties. Chem. Mater. 2014, 26, 2836–2846. [Google Scholar] [CrossRef]
- He, D.; Bligh, M.W.; Waite, T.D. Effects of Aggregate Structure on the Dissolution Kinetics of Citrate-Stabilized Silver Nanoparticles. Environ. Sci. Technol 2013, 47, 9148–9156. [Google Scholar]
- Tejamaya, M.; Römer, I.; Merrifield, R.C.; Lead, J.R. Stability of Citrate, PVP, and PEG Coated Silver Nanoparticles in Ecotoxicology Media. Environ. Sci. Technol. 2012, 46, 7011–7017. [Google Scholar] [CrossRef] [PubMed]
- Henglein, A.; Giersig, M. Formation of Colloidal Silver Nanoparticles: Capping Action of Citrate. J. Phys. Chem. B 1999, 103, 9533–9539. [Google Scholar] [CrossRef]
- Romih, T.; Jemec, A.; Kos, M.; Hocevar, S.B.; Kralj, S.; Makovec, D.; Drobne, D. The role of PVP in the bioavailability of Ag from the PVP-stabilized Ag nanoparticle suspension. Environ. Pollut. 2016, 218, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Park, J.E.; Osaka, T.; Park, S.G. The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochim. Acta 2005, 51, 956–960. [Google Scholar] [CrossRef]
- Yin, B.; Ma, H.; Wang, S.; Chen, S. Electrochemical Synthesis of Silver Nanoparticles under Protection of Poly(N-vinylpyrrolidone). J. Phys. Chem. B 2003, 107, 8898–8904. [Google Scholar] [CrossRef]
- He, B.; Tan, J.J.; Liew, K.Y.; Liu, H. Synthesis of size controlled Ag nanoparticles. J. Mol. Catal. A- Chem. 2004, 221, 121–126. [Google Scholar] [CrossRef]
- Elashnikov, R.; Lyutakov, O.; Ulbrich, P.; Svorcik, V. Light-activated polymethylmethacrylate nanofibers with antibacterial activity. Mat. Sci. Eng. C 2016, 64, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Yang, J.; Gong, J.; Zheng, N. Photo- and pH-Triggered Release of Anticancer Drugs from Mesoporous Silica-Coated Pd@Ag Nanoparticles. Adv. Funct. Mater. 2012, 22, 842–848. [Google Scholar] [CrossRef]
- Lyutakov, O.; Goncharova, I.; Rimpelova, S.; Kolarova, K.; Svanda, J.; Svorcik, V. Silver release and antimicrobial properties of PMMA films doped with silver ions, nano-particles and complexes. Mat. Sci. Eng. C 2015, 49, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Turova, N. Inorganic Chemistry in Tables; Springer: Berlin, Germany, 2011. [Google Scholar]
- Wang, J.X.; Wen, L.X.; Wang, Z.H.; Chen, J.F. Immobilization of silver on hollow silica nanospheres and nanotubes and their antibacterial effects. Mater. Chem. Phys. 2006, 96, 90–97. [Google Scholar] [CrossRef]
- Rainville, L.; Dorais, M.C.; Boudreau, D. Controlled synthesis of low polydispersity Ag@SiO2 core-shell nanoparticles for use in plasmonic applications. RSC Adv. 2013, 3, 13953–13960. [Google Scholar] [CrossRef]
- Amendola, V.; Bakr, O.M.; Stellacci, F. A Study of the Surface Plasmon Resonance of Silver Nanoparticles by the Discrete Dipole Approximation Method: Effect of Shape, Size, Structure, and Assembly. Plasmonics 2010, 5, 85–97. [Google Scholar] [CrossRef]
- De, G.; Licciulli, A.; Massaro, C.; Tapfer, L.; Catalano, M.; Battaglin, G.; Meneghini, C.; Mazzoldi, P. Silver nanocrystals in silica by sol-gel processing. J. Non-Cryst. Solids 1996, 194, 225–234. [Google Scholar] [CrossRef]
- Grünert, W.; Schlögl, R.; Karge, H. Investigations of zeolites by photoelectron and ion scattering spectroscopy. 1. New applications of surface spectroscopic methods to zeolites by a high-temperature measurement technique. J. Phys. Chem. 1993, 97, 8638–8645. [Google Scholar] [CrossRef]
- Rodriguez, J.; Hrbek, J. Synergistic interactions in trimetallic bonding: A comparison of the ZnNM Ru(001) (NM = Cu, Ag or Au) systems. Surf. Sci. 1994, 312, 345–360. [Google Scholar] [CrossRef]
- Ramalingam, S.; Devi, L.B.; Raghava Rao, J.; Unni Nair, B. Rapid hydrogenation: Perfect quasi architecture (Ag@SiO2NPs) as a substrate for nitrophenol reduction. RSC Adv. 2014, 4, 56041–56051. [Google Scholar] [CrossRef]
- Peszke, J.; Dulski, M.; Nowak, A.; Balin, K.; Zubko, M.; Sulowicz, S.; Nowak, B.; Piotrowska-Seget, Z.; Talik, E.; Wojtyniak, M.; et al. Unique properties of silver and copper silica-based nanocomposites as antimicrobial agents. RSC Adv. 2017, 7, 28092–28104. [Google Scholar] [CrossRef]
- Racles, C.; Nistor, A.; Cazacu, M. A silica-silver nanocomposite obtained by sol-gel method in the presence of silver nanoparticles. Cent. Eur. J. Chem. 2013, 11, 1689–1698. [Google Scholar] [CrossRef]
- Ekimov, A. Growth and optical properties of semiconductor nanocrystals in a glass matrix. J. Lumin. 1996, 70, 1–20. [Google Scholar] [CrossRef]
- Jiménez, J.; Sendova, M.; Hartsfield, T.; Sendova-Vassileva, M. In situ optical microspectroscopy of the growth and oxidation of silver nanoparticles in silica thin films on soda-lime glass. Mater. Res. Bull. 2011, 46, 158–165. [Google Scholar] [CrossRef]
- Ila, D.; Baglin, J.; Zimmerman, R. Nano-Crystal Formation and Growth from High-Fluence Ion Implantation of Au, Ag or Cu in Silica. Phys. Proc. 2015, 66, 548–555. [Google Scholar] [CrossRef]
- Jimenez, J.; Sendova, M. In situ optical microspectroscopy approach for the study of metal transport in dielectrics via temperature- and time-dependent plasmonics: Ag nanoparticles in SiO2 films. J. Chem. Phys. 2011, 134, 054707. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.; Sendova, M.; Liu, H. Evolution of the optical properties of a silver-doped phosphate glass during thermal treatment. J. Lumin. 2011, 131, 535–538. [Google Scholar] [CrossRef]
- Jimenez, J.; Sendova, M. Diffusion activation energy of Ag in nanocomposite glasses determined by in situ monitoring of plasmon resonance evolution. Chem. Phys. Lett. 2011, 503, 283–286. [Google Scholar] [CrossRef]
- Jimenez, J.; Sendova, M. Kinetics of Ag nanoparticle growth in thick SiO2 films: An in situ optical assessment of Ostwald ripening. Mater. Chem. Phys. 2012, 135, 282–286. [Google Scholar] [CrossRef]
- Jiménez, J.A.; Sendova, M. Unfolding diffusion-based Ag nanoparticle growth in SiO2 nanofilms heat-treated in air via in situ optical microspectroscopy. Opt. Mater. 2013, 35, 968–972. [Google Scholar] [CrossRef]
- Babapour, A.; Akhavan, O.; Azimirad, R.; Moshfegh, A. Physical characteristics of heat-treated nano-silvers dispersed in sol-gel silica matrix. Nanotechnology 2006, 17, 763–771. [Google Scholar] [CrossRef]
- Larismaa, J.; Honkanen, T.; Ge, Y.; Söderberg, O.; Friman, M.; Hannula, S.P. Effect of annealing on Ag-doped submicron silica powder prepared with modified Stöber Method. Mater. Sci. Forum 2011, 695, 449–452. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers—The Scienta ESCA300 Database; Wiley: Hoboken, NJ, USA, 1993. [Google Scholar]
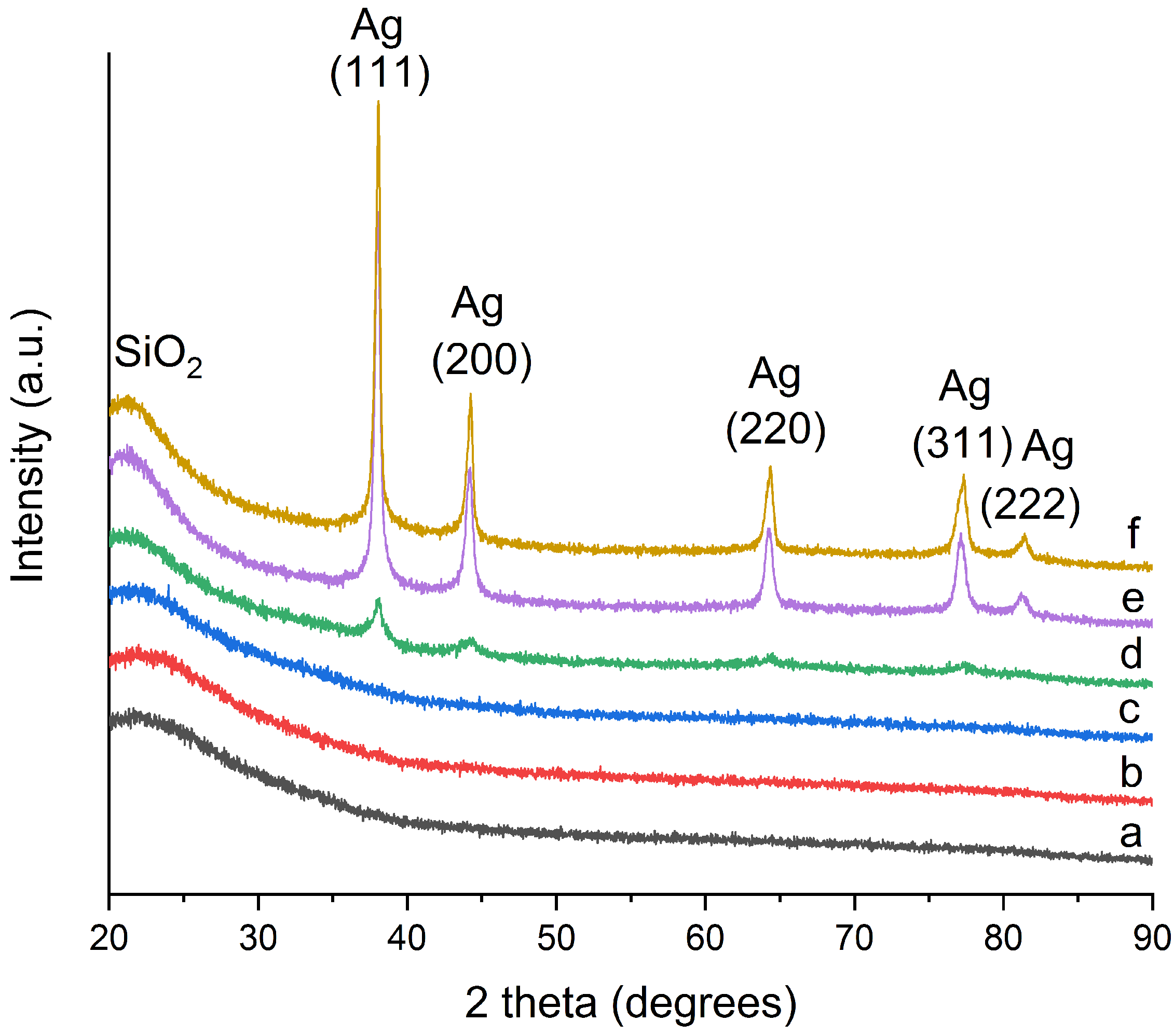
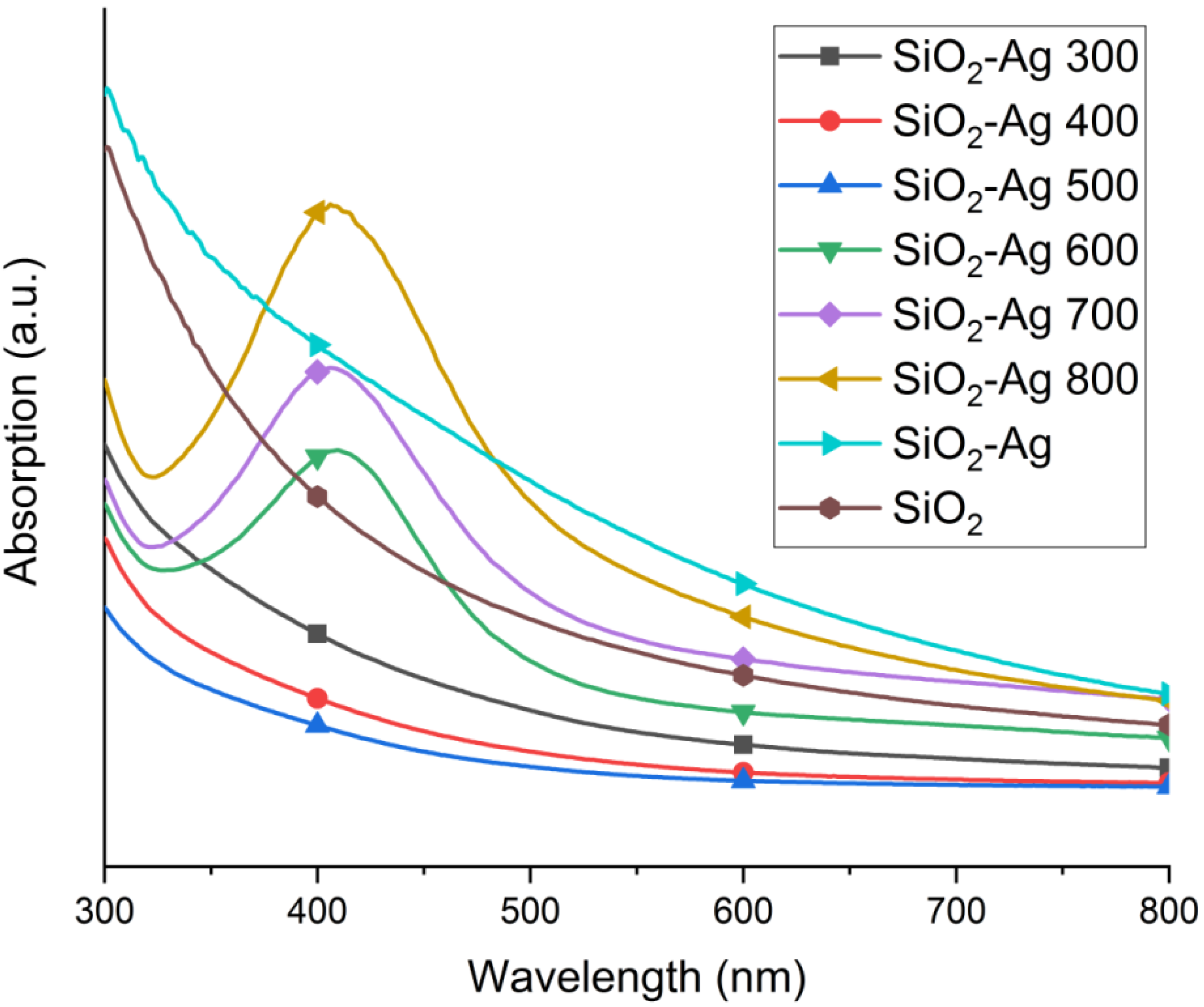
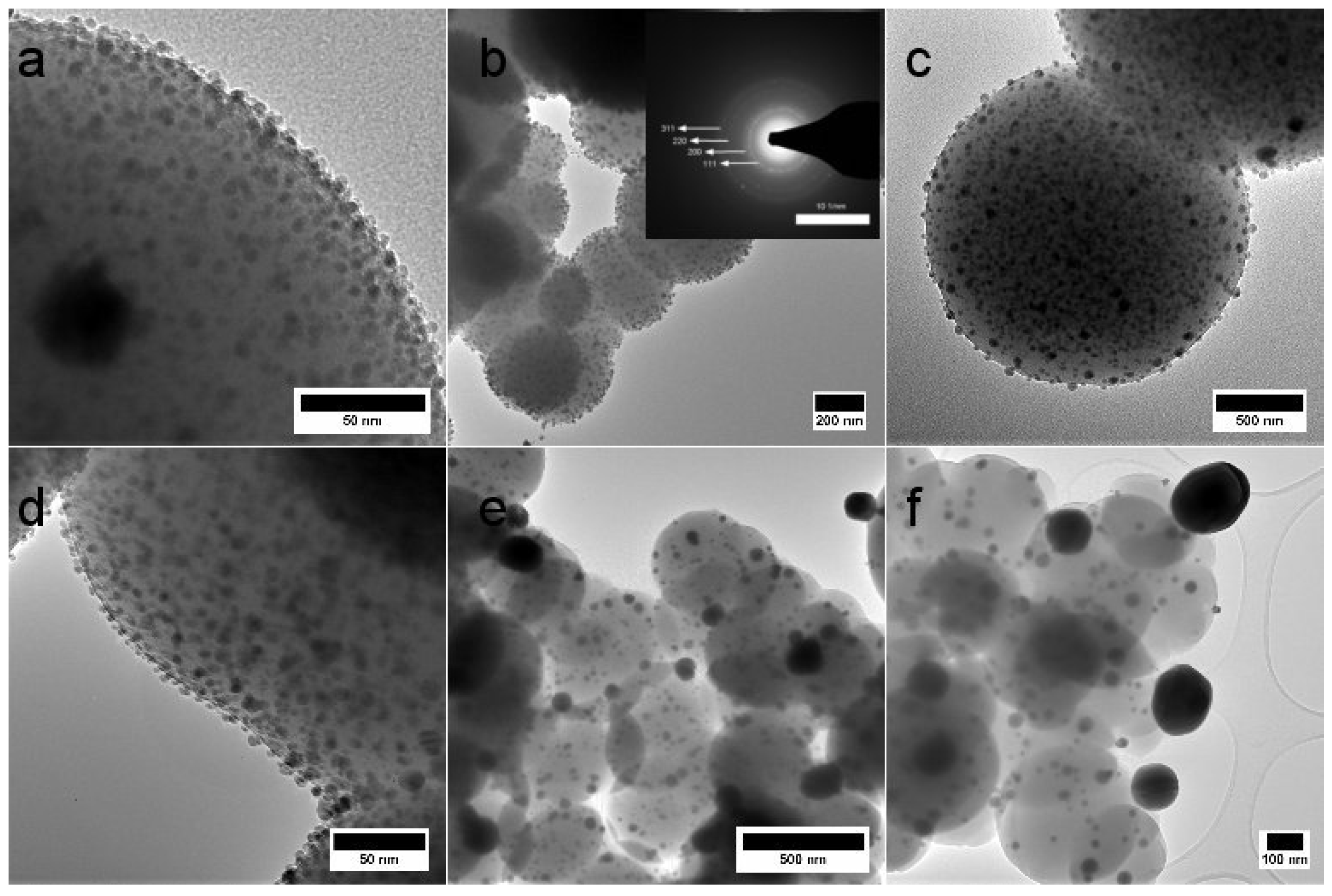
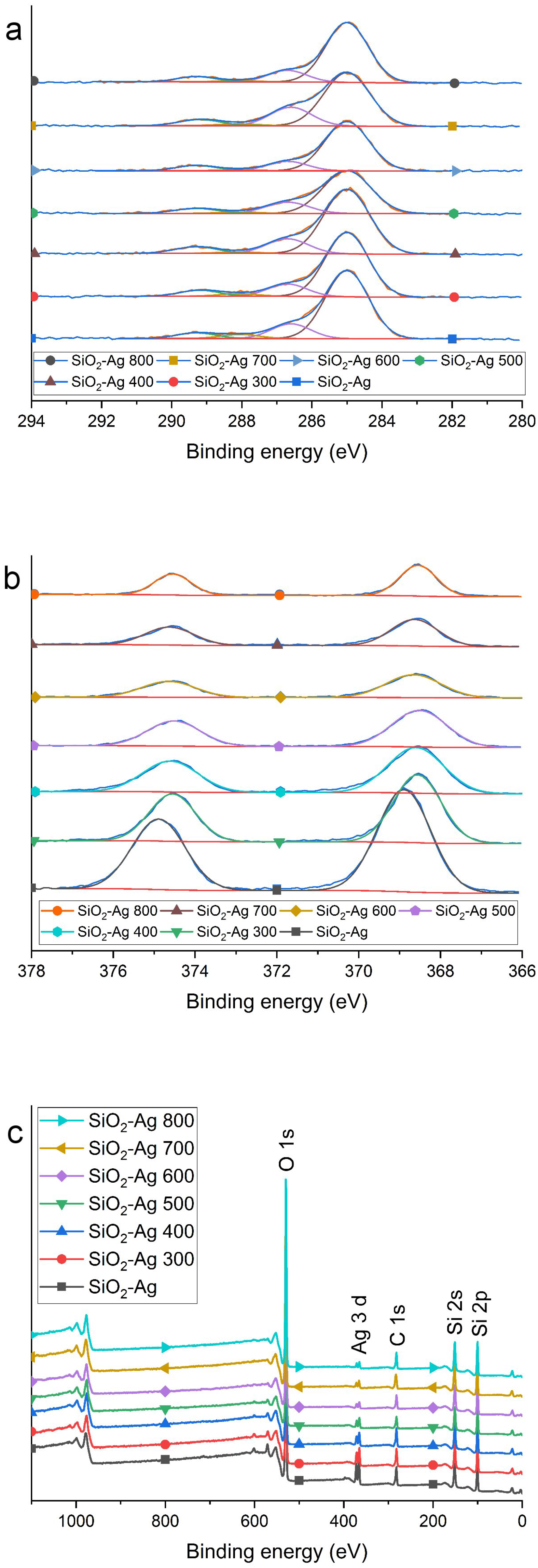
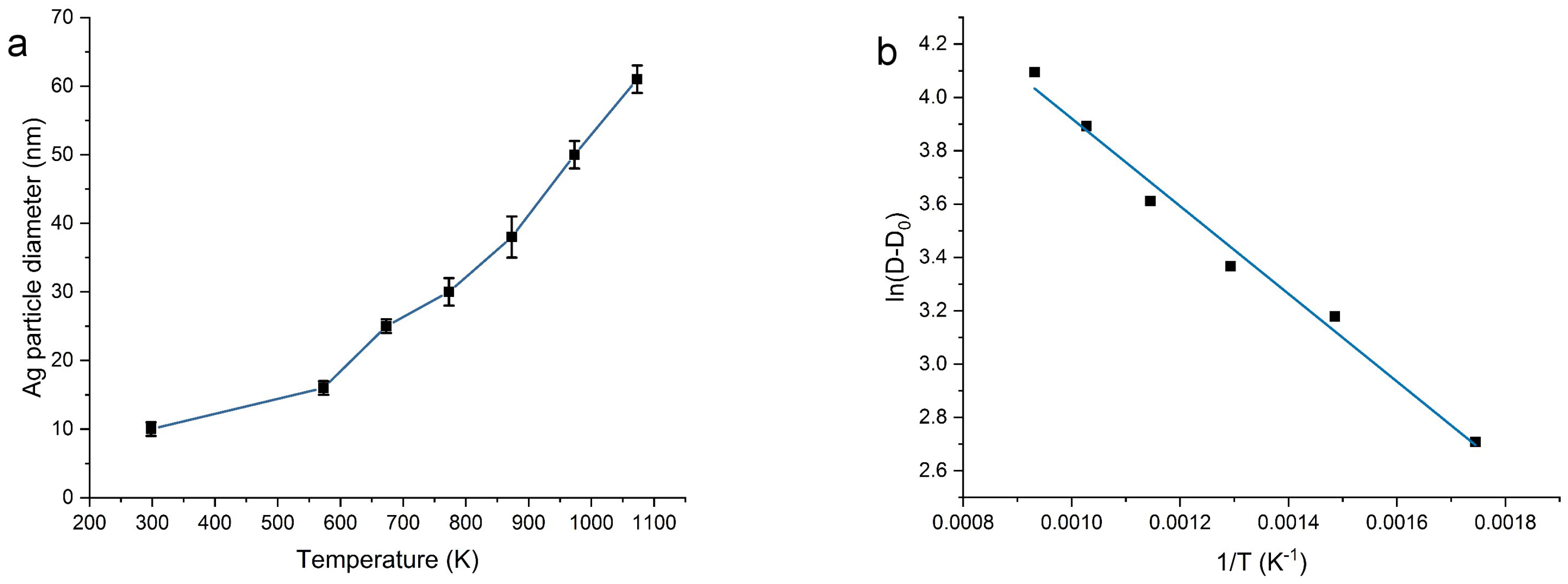
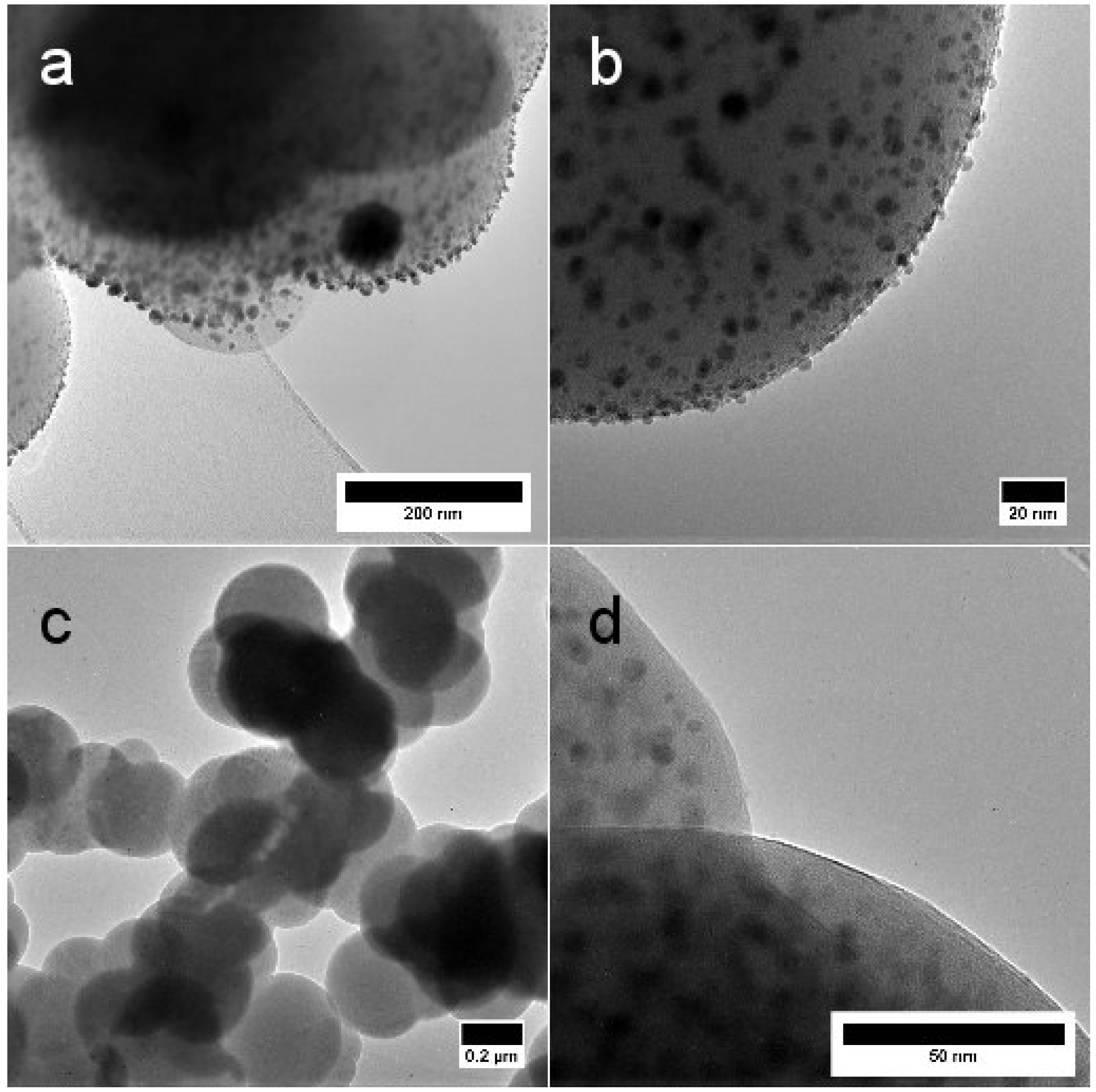
| T (°C) | N Total Particles | Mean ± SE (nm) | Min (nm) | Max (nm) | Surface Area () | Surface Area-Dis () |
|---|---|---|---|---|---|---|
| RT | 60 | 10 ± 1 | 4 | 48 | 4.2 | 4.5 |
| 300 | 175 | 16 ± 1 | 4 | 65 | 5.2 | 4.2 |
| 400 | 186 | 25 ± 1 | 4 | 122 | 4.9 | 4.3 |
| 500 | 247 | 30 ± 2 | 4 | 199 | 4.9 | 4.6 |
| 600 | 188 | 28 ± 3 | 4 | 210 | 4.7 | 4.3 |
| 700 | 298 | 50 ± 2 | 7 | 202 | 4.6 | 4.8 |
| 800 | 495 | 61 ± 2 | 4 | 283 | 5.2 | 4.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granbohm, H.; Larismaa, J.; Ali, S.; Johansson, L.-S.; Hannula, S.-P. Control of the Size of Silver Nanoparticles and Release of Silver in Heat Treated SiO2-Ag Composite Powders. Materials 2018, 11, 80. https://doi.org/10.3390/ma11010080
Granbohm H, Larismaa J, Ali S, Johansson L-S, Hannula S-P. Control of the Size of Silver Nanoparticles and Release of Silver in Heat Treated SiO2-Ag Composite Powders. Materials. 2018; 11(1):80. https://doi.org/10.3390/ma11010080
Chicago/Turabian StyleGranbohm, Henrika, Juha Larismaa, Saima Ali, Leena-Sisko Johansson, and Simo-Pekka Hannula. 2018. "Control of the Size of Silver Nanoparticles and Release of Silver in Heat Treated SiO2-Ag Composite Powders" Materials 11, no. 1: 80. https://doi.org/10.3390/ma11010080
APA StyleGranbohm, H., Larismaa, J., Ali, S., Johansson, L.-S., & Hannula, S.-P. (2018). Control of the Size of Silver Nanoparticles and Release of Silver in Heat Treated SiO2-Ag Composite Powders. Materials, 11(1), 80. https://doi.org/10.3390/ma11010080






