The Effect of Coatings and Nerve Growth Factor on Attachment and Differentiation of Pheochromocytoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. PC12 Cell Line
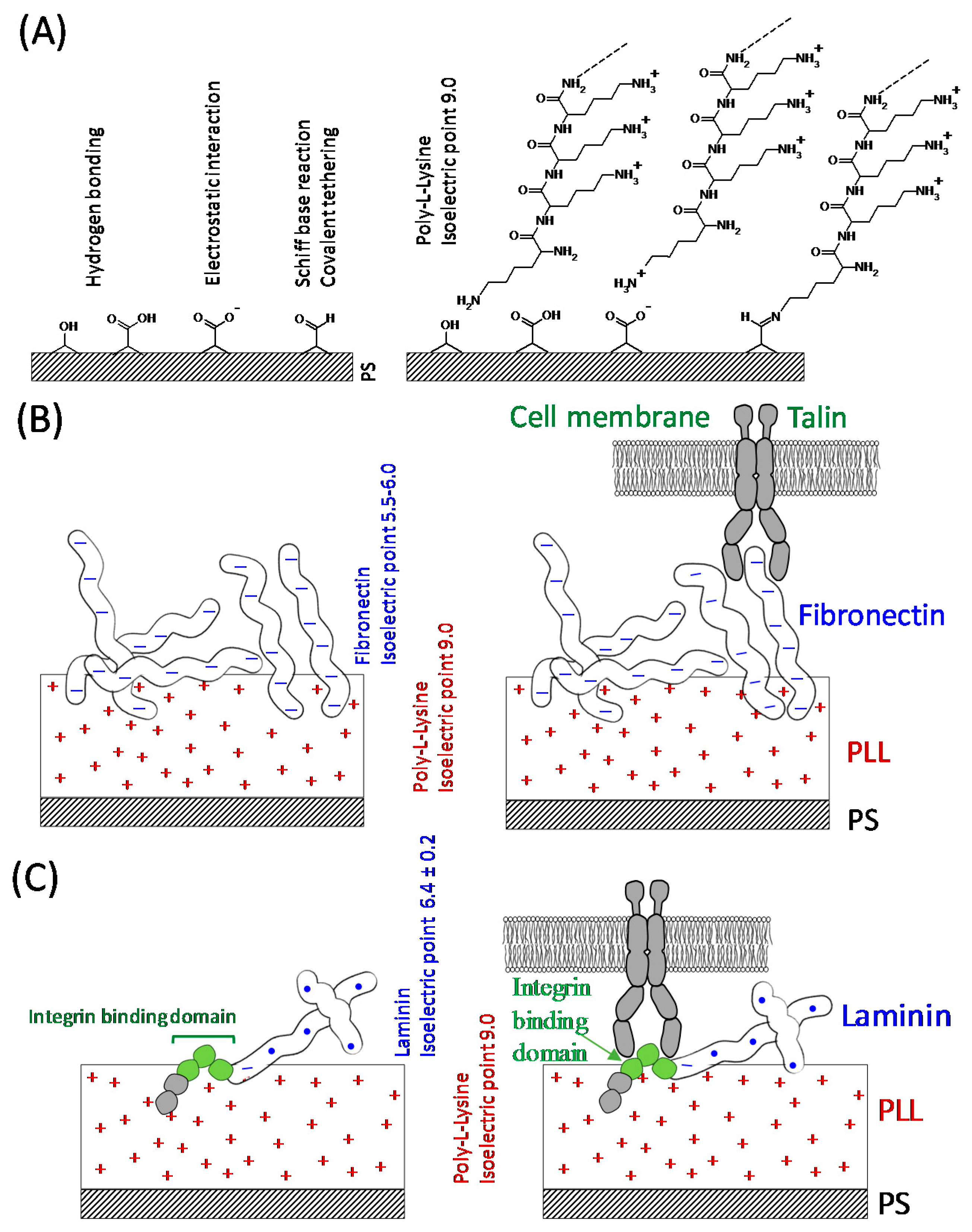
2.2. Coatings
2.2.1. Poly-L-lysine
2.2.2. Fibronectin
2.2.3. Laminin
2.3. Atomic Force Microscopy
2.4. Differentiation of PC12 Cells
2.5. Metabolic Activity
2.6. Assessment of Neurite Outgrowth
2.7. Widefield Fluorescence Microscopy
2.8. Statistical Analysis
3. Results
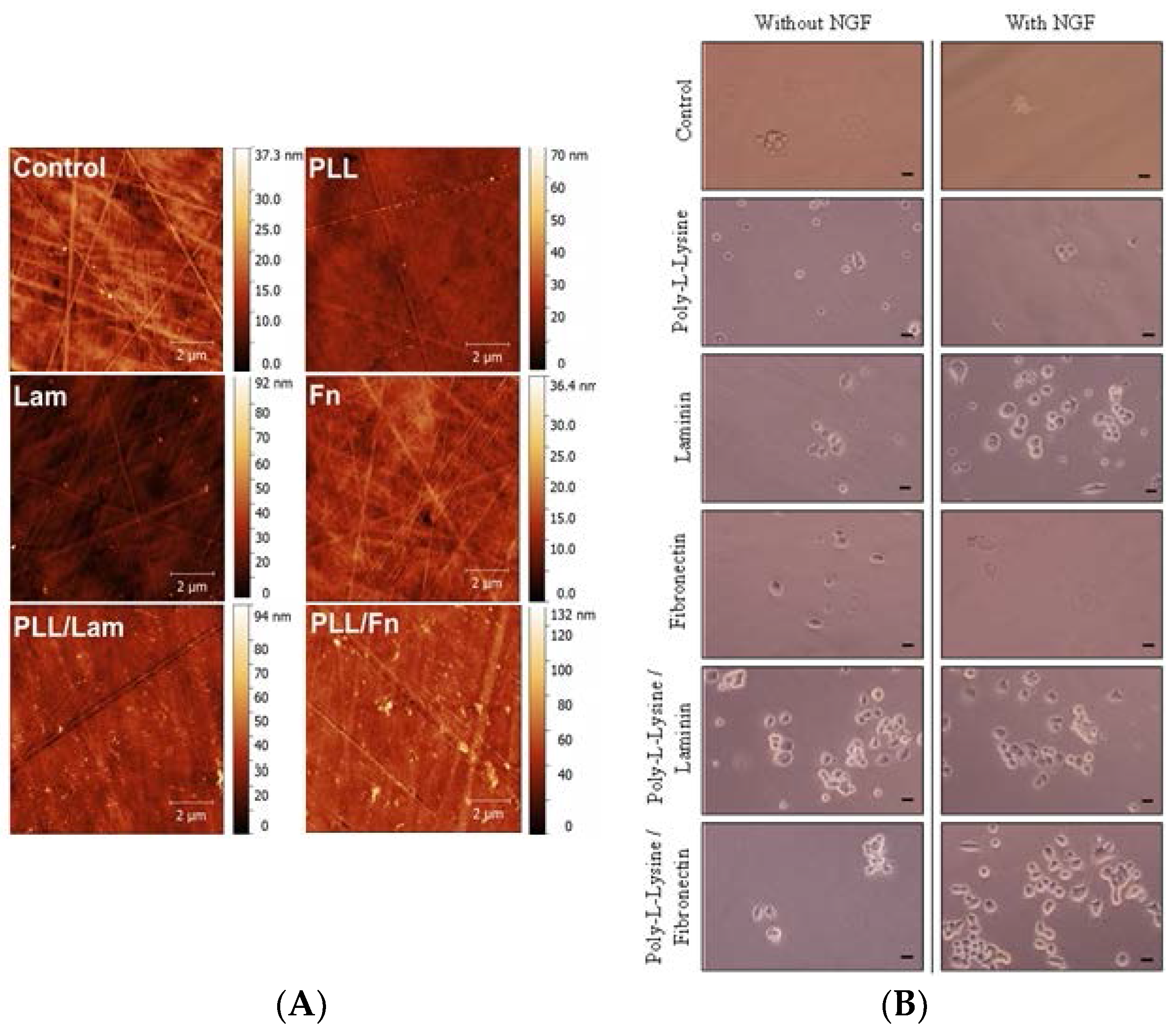
3.1. Protein Distribution on the Substratum
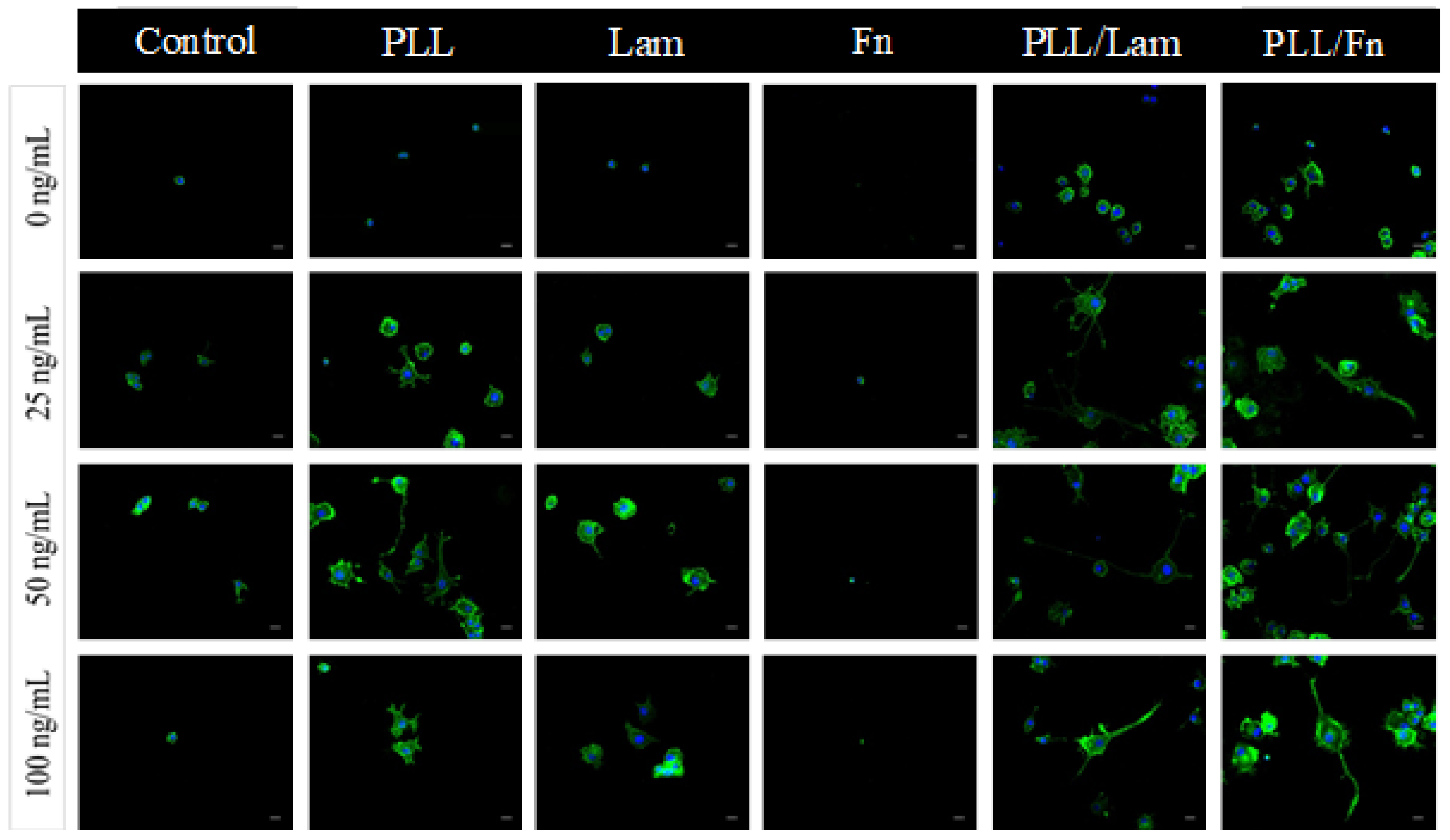
3.2. PC12 Cell Attachment and Initial Differentiation in the Presence of NGF
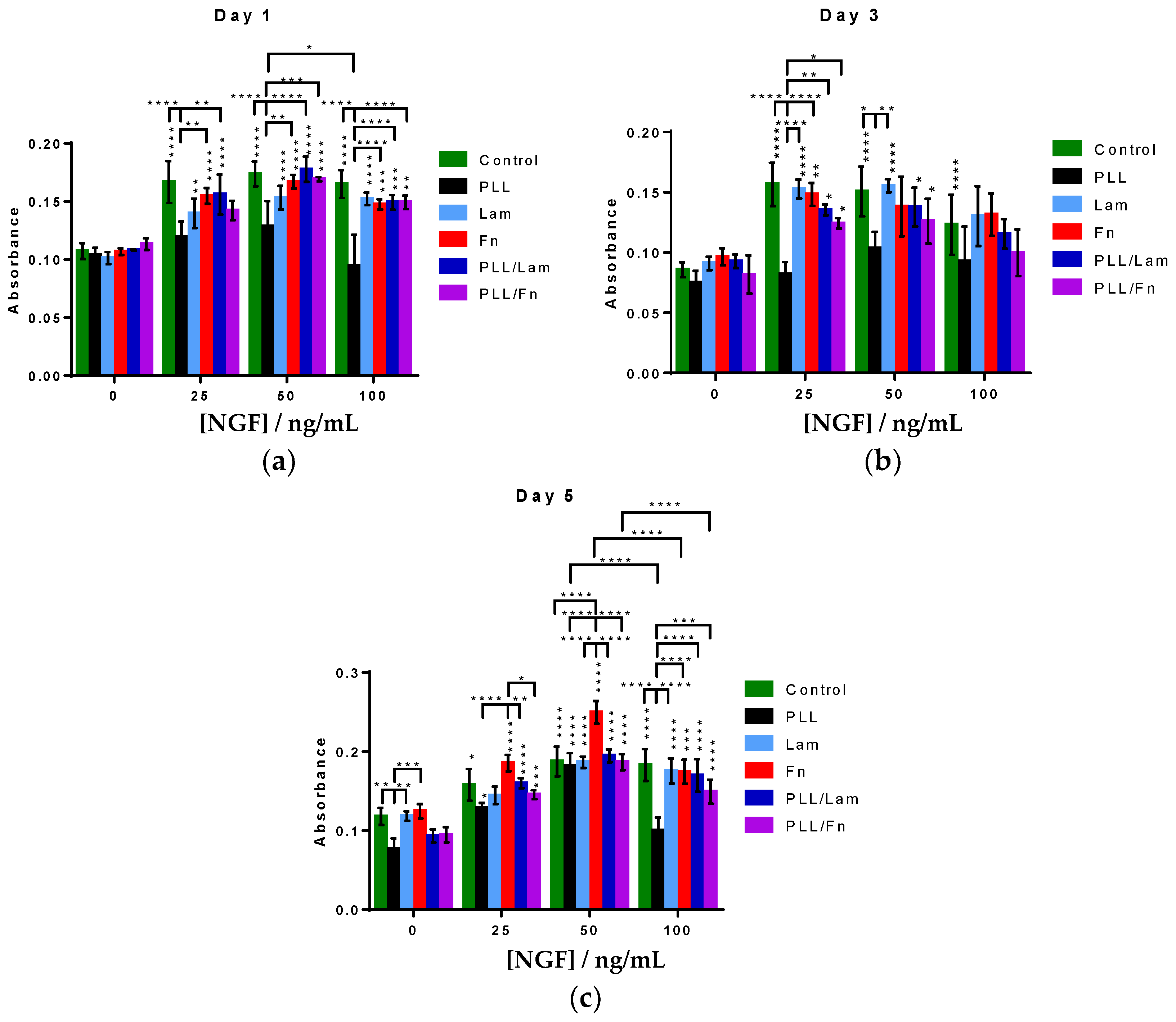
3.3. PC12 Cells Metabolic Activity and Proliferation
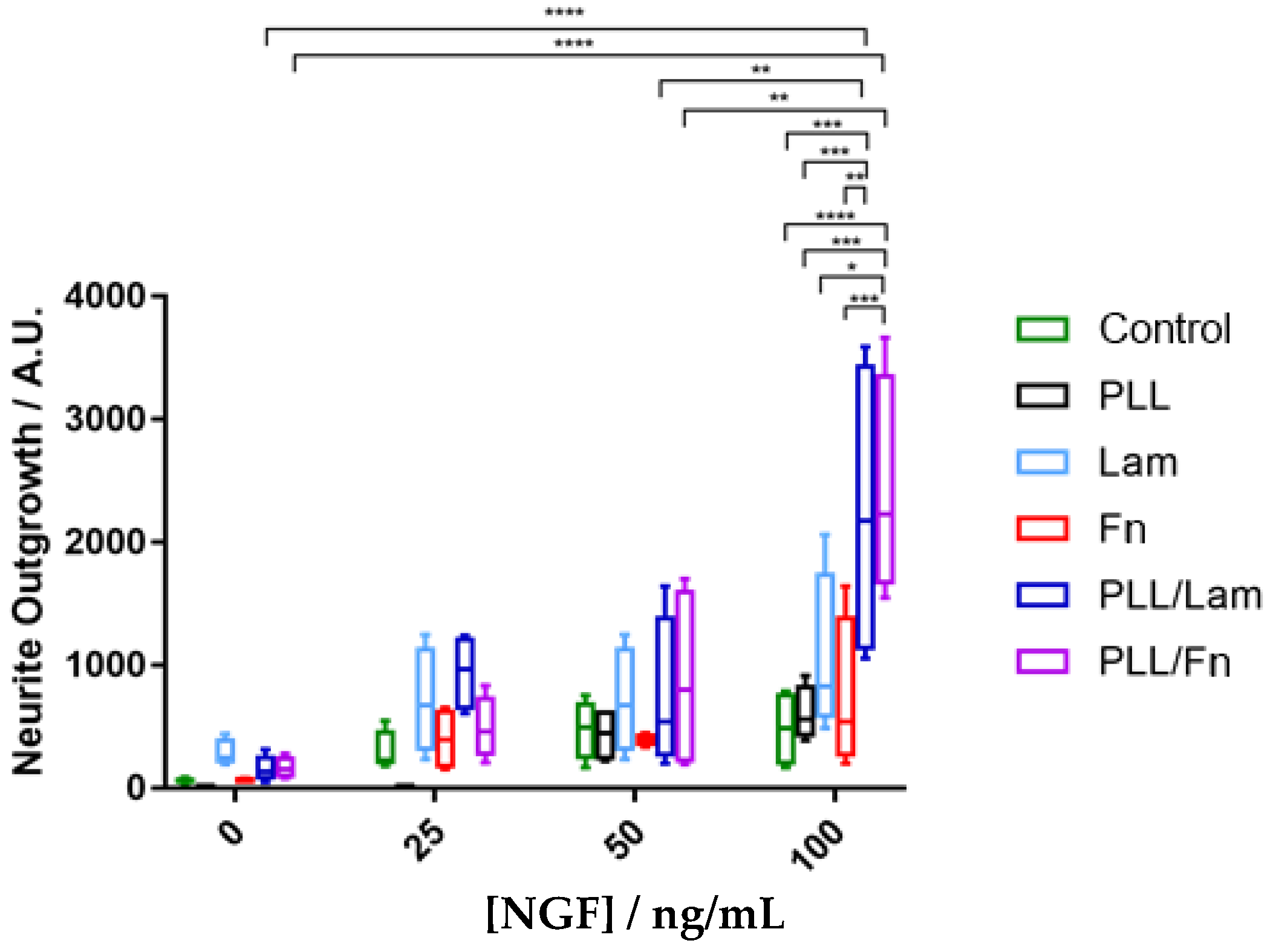
3.4. Neurite Outgrowth
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jesky, R.; Chen, H. The neuritogenic and neuroprotective potential of senegenin against A b-induced neurotoxicity in PC12 cells. BMC Complement. Altern. Med. 2016, 16, 26. [Google Scholar] [CrossRef]
- Westerink, H.R.; Ewing, A.G. The PC12 cell as model for neurosecretion. Acta Physiol. 2008, 192, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.; Amini, S.; White, M.K. General overview of neuronal cell culture. Methods Mol. Biol. 2013, 1078, 1–8. [Google Scholar] [PubMed]
- Fuji, D.K.; Massoglia, S.L.; Savion, N.; Gospodarowicz, D. Neurite outgrowth and protein synthesis by PC12 cells as a function of substratum and nerve growth factor. J. Neurosci. 1982, 2, 1157–1175. [Google Scholar]
- Akeson, R.; Warren, S.L. PC12 adhesion and neurite formation on selected substratums are inhibited by some glycosaminoglycans and a fibronetion-derived tetrapeptide. Exp. Cell Res. 1986, 162, 347–362. [Google Scholar] [CrossRef]
- Attiah, G.D.; Kopher, R.A.; Desai, T.A. Characterization of PC12 cell proliferation and differentiation-stimulated by ECM adhesion proteins and neurotrophic factors. J. Mater. Sci. Mater. Med. 2003, 14, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Kleinman, H.K.; Luckenbill-Edds, L.; Cannon, F.W.; Sephel, G.C. Use of extracellular matrix components for cell culture. Anal. Biochem. 1987, 166, 1–13. [Google Scholar] [CrossRef]
- Ergin, V.; Erdogan, M.; Menevse, A. Regulation of shootin1 gene expression involves ngf-induced alternative splicing during neuronal differentiation of PC12 cells. Sci. Rep. 2015, 5, 17931. [Google Scholar] [CrossRef] [PubMed]
- Ogra, Y.; Tejima, A.; Hatakeyama, N.; Shiraiwa, M.; Wu, S.; Ishikawa, T.; Yawata, A.; Anan, Y.; Suzuki, N. Changes in intracellular copper concentration and copper-regulating gene expression after PC12 differentiation into neurons. Sci. Rep. 2016, 6, 33007. [Google Scholar] [CrossRef] [PubMed]
- Boczek, T.; Kozaczuk, A.; Ferenc, B.; Kosiorek, M.; Pikula, S.; Zylinska, L. Gene expression pattern in PC12 cells with reduced PMCA2 or PMCA3 isoform: Selective up-regulation of calmodulin and neuromodulin. Mol. Cell. Biochem. 2012, 360, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.; Ciofani, G.; Filippeschi, C.; Pellegrino, M.; Pellegrini, M.; Orsini, P.; Pasqualetti, M.; Mattoli, V.; Mazzolai, B. Two-photon polymerization of sub-micrometric patterned surfaces: Investigation of cell-substratum interactions and improved differentiation of neuron-like cells. ACS Appl. Mater. Interfaces 2013, 5, 13012–13021. [Google Scholar] [CrossRef] [PubMed]
- Cowley, S.; Paterson, H.; Kemp, P.; Marshall, C.J. Activation of MAP kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell 1994, 77, 841–852. [Google Scholar] [CrossRef]
- Shafer, J.T.; Atchison, W.D. Transmitter, ion channel and receptor properties of pheochromocytoma (PC12) cells: A model for neurotoxicological studies. Neurotoxicology 1991, 12, 473–492. [Google Scholar] [PubMed]
- Chen, T.-I.; Chiu, H.-W.; Pan, Y.-C.; Hsu, S.-T.; Lin, J.-H.; Yang, K.-T. Intermittent hypoxia-induced protein phosphatase 2A activation reduces PC12 cell proliferation and differentiation. J. Biomed. Sci. 2014, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Singh, P.; Jauhari, A.; Singh, T.; Khan, F.; Pant, A.B.; Parmar, D.; Yadav, S. Critical role of the miR-200 family in regulating differentiation and proliferation of neurons. J. Neurochem. 2015, 133, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Connolly, L.J.; Green, S.A.; Greene, L.A. Comparison of rapid changes in surface morphology and coated pit formation of PC12 cells in response to nerve growth factor epidermal growth factor and dibutyryl cyclicl AMP. J. Cell Biol. 1984, 98, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Tomaselli, J.K.; Damsky, C.H.; Reichardt, L.F. Purification and characterization of mammalian integrins expressed by a rat neuronal cell line (PC12) evidence that they function as α/β heterodimeric receptors for laminin and type IV collagen. J. Cell Biol. 1988, 107, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Parra, P.; Cavaliere, F.; Maroto, M.; Bilbao, L.; Obieta, I.; de Munain, A.L.; Álava, J.I.; Izeta, A. Modeling neural differentiation on micropatterned substratums coated with neural matrix components. Front. Cell. Neurosci. 2012, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Open Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Gadelmawla, E.S.; Koura, M.M.; Maksoud, T.M.A.; Elewa, I.M.; Soliman, H.H. Roughness parameters. J. Mater. Process. Technol. 2002, 123, 133–145. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biologicical-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Freire, E.; Gomes, F.C.; Linden, R.; Neto, V.M.; Coelho-Sampaio, T. Structure of laminin substratum modulates cellular signalling for neuritogenesis. J. Cell Sci. 2002, 115, 4867–4876. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rogers, S.L.; Letourneau, P.C.; Palm, S.L.; McCarthy, J.; Furcht, L.T. Neurite extension by peripheral and central nervous system neurons in response to substratum-bound fibronectin and laminin. Dev. Biol. 1983, 98, 212–220. [Google Scholar] [CrossRef]
- Mazia, D.; Schatten, G.; Sale, W. Adhesion of cells to surfaces coated with polylysine. J. Cell Biol. 1975, 66, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Ajioka, I. Coordination of proliferation and neuronal differentiation by the retinoblastoma protein family. Dev. Growth Differ. 2014, 56, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.V.; Tan, A.S. Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT):subcellular localization, substratum dependance, and involvement of mitochondrial electron transport in MTT reduction. Arch. Biochem. Biophys. 1993, 303, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Vartio, T. Characterization of the binding domains in the fragments cleaved by cathepsin g from human plasma fibronectin. FEBS J. 1982, 123, 223–233. [Google Scholar] [CrossRef]
- Paulsson, M.; Deutzmann, R.; Timpl, R.; Dalzoppo, D.; Odermatt, E.; Engel, J. Evidence for coiled-coil α-helical regions in the long arm of laminin. EMBO J. 1985, 4, 309–316. [Google Scholar] [PubMed]
- Pierschbacher, D.M.; Ruoslahti, E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 1984, 309, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: Versatility, modulation and signalling in cell adhesion. Cell 1992, 69, 11–25. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlowska, A.; Perera, P.T.; Al Kobaisi, M.; Dias, A.; Nguyen, H.K.D.; Ghanaati, S.; Baulin, V.; Crawford, R.J.; Ivanova, E.P. The Effect of Coatings and Nerve Growth Factor on Attachment and Differentiation of Pheochromocytoma Cells. Materials 2018, 11, 60. https://doi.org/10.3390/ma11010060
Orlowska A, Perera PT, Al Kobaisi M, Dias A, Nguyen HKD, Ghanaati S, Baulin V, Crawford RJ, Ivanova EP. The Effect of Coatings and Nerve Growth Factor on Attachment and Differentiation of Pheochromocytoma Cells. Materials. 2018; 11(1):60. https://doi.org/10.3390/ma11010060
Chicago/Turabian StyleOrlowska, Anna, Pallale Tharushi Perera, Mohammad Al Kobaisi, Andre Dias, Huu Khuong Duy Nguyen, Shahram Ghanaati, Vladimir Baulin, Russell J. Crawford, and Elena P. Ivanova. 2018. "The Effect of Coatings and Nerve Growth Factor on Attachment and Differentiation of Pheochromocytoma Cells" Materials 11, no. 1: 60. https://doi.org/10.3390/ma11010060
APA StyleOrlowska, A., Perera, P. T., Al Kobaisi, M., Dias, A., Nguyen, H. K. D., Ghanaati, S., Baulin, V., Crawford, R. J., & Ivanova, E. P. (2018). The Effect of Coatings and Nerve Growth Factor on Attachment and Differentiation of Pheochromocytoma Cells. Materials, 11(1), 60. https://doi.org/10.3390/ma11010060





