A Novel Synthesis Routine for Woodwardite and Its Affinity towards Light (La, Ce, Nd) and Heavy (Gd and Y) Rare Earth Elements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Woodwardite and REEs Addition
2.2. Characterisation Methods
3. Results and Discussion
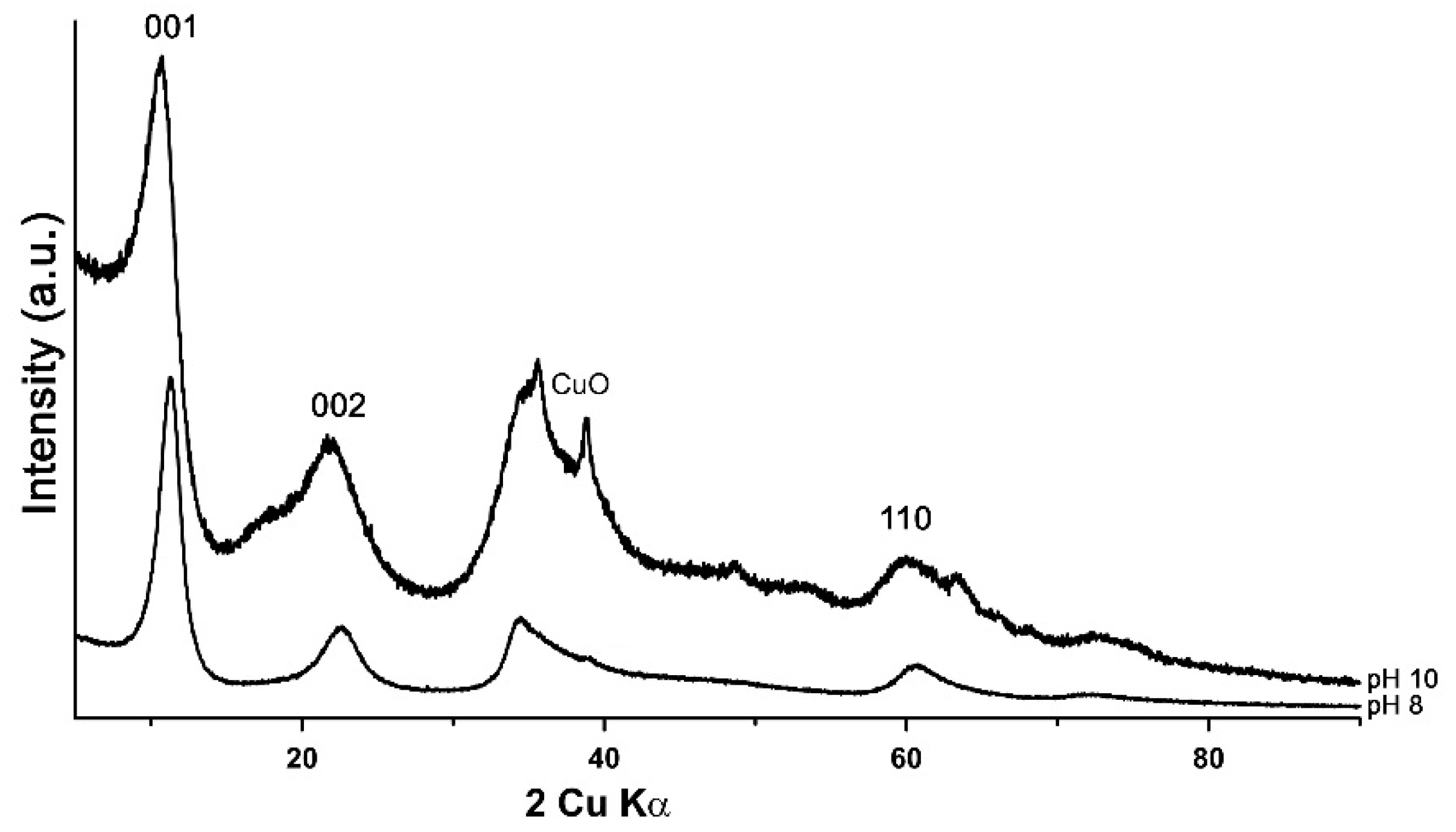
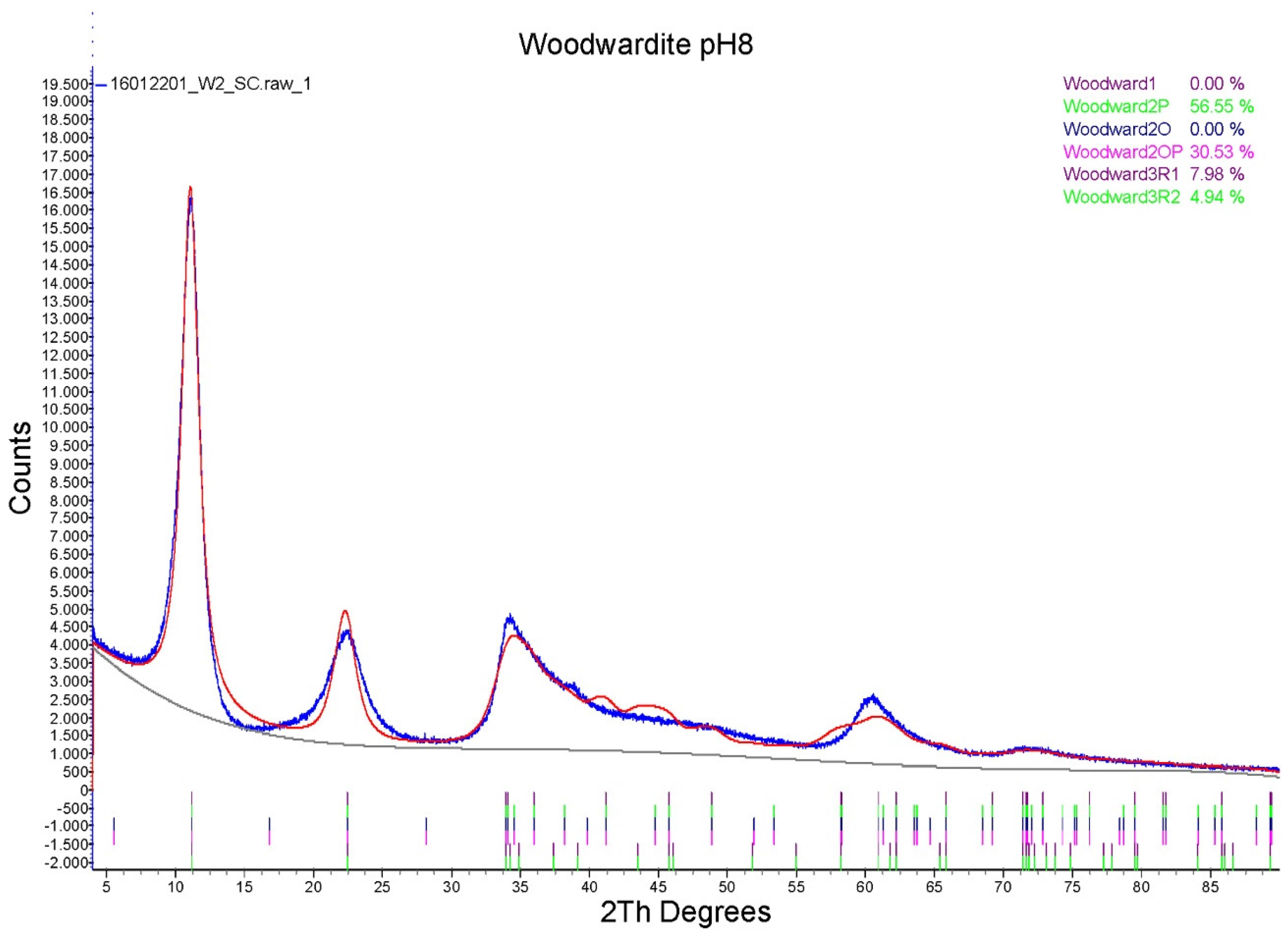
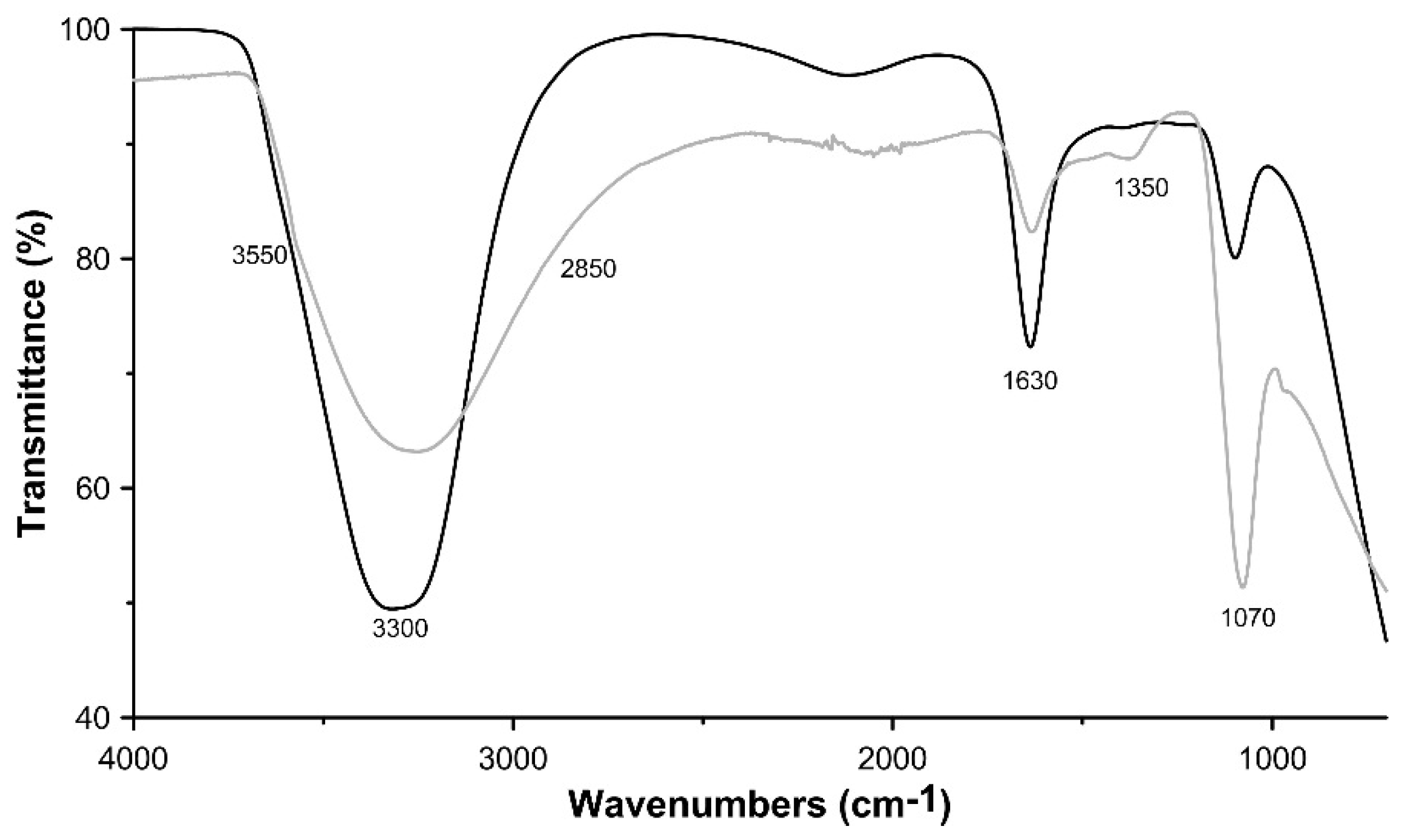
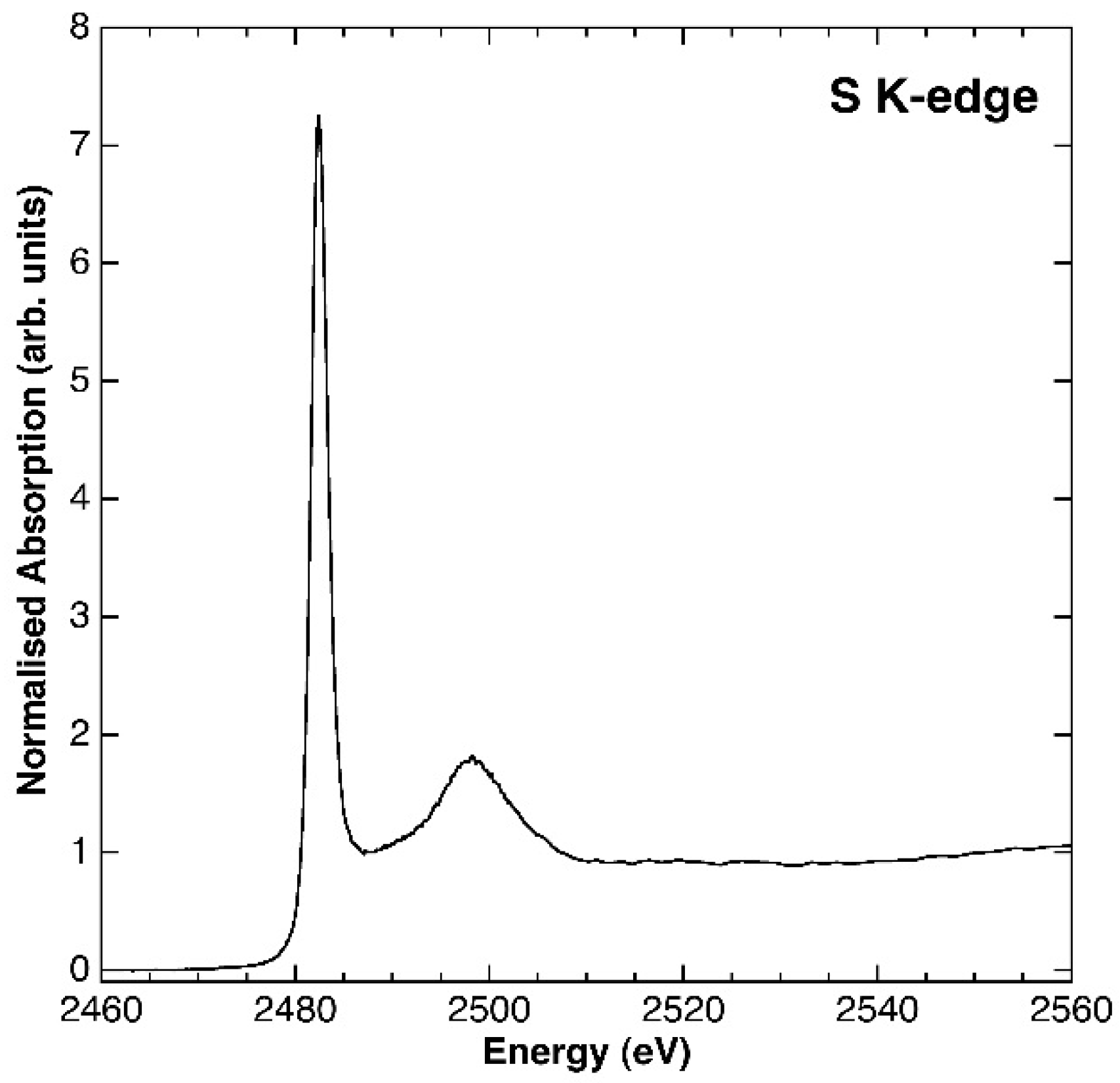
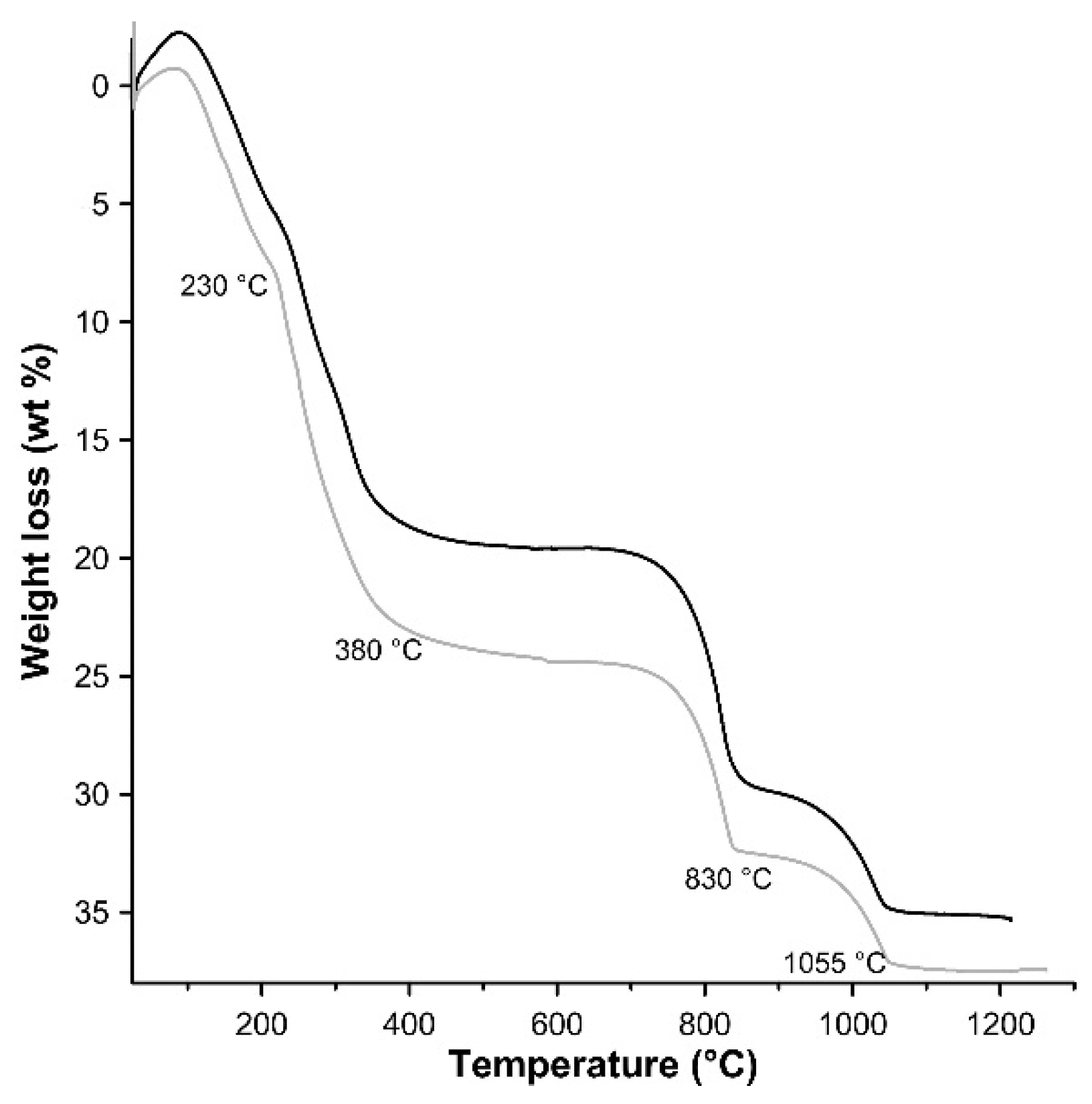
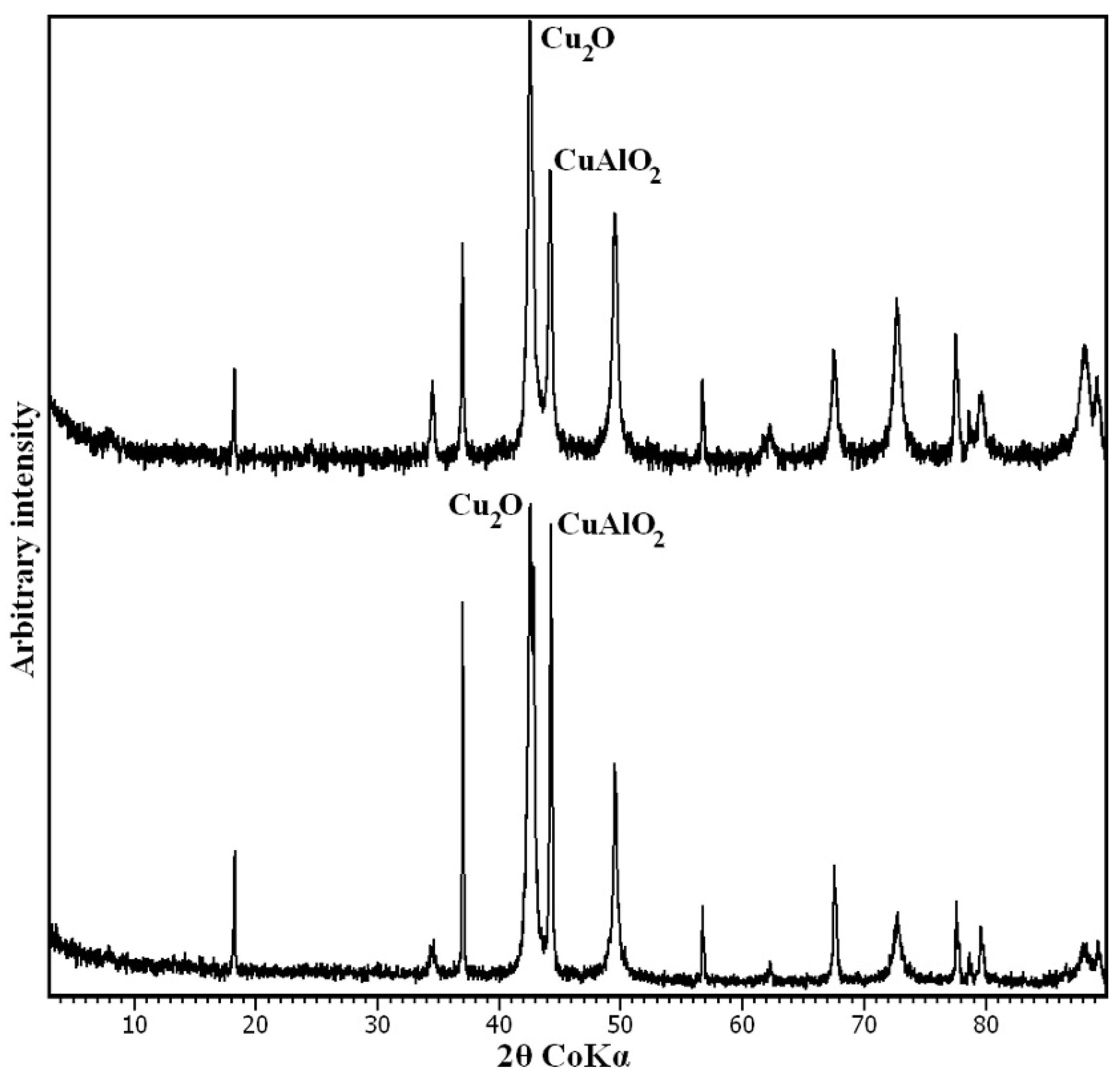
3.1. Characterisation of the Synthesis Product
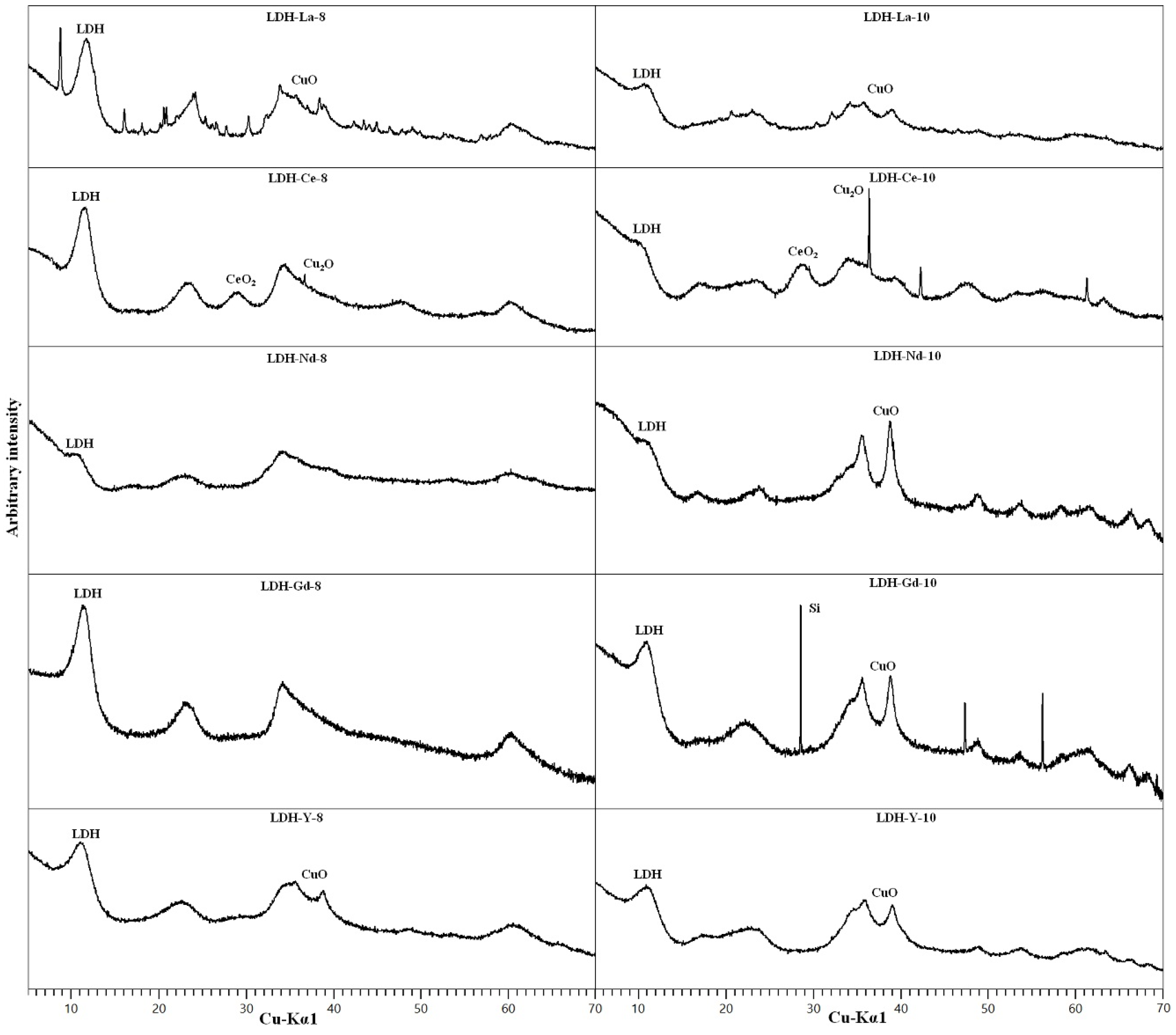
3.2. REE-Doped Samples
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Castor, S.B.; Hedrick, J.B. Rare Earth Elements. In Industrial Minerals and Rocks: Commodities, Markets, and Uses, 7th ed.; Kogel, J.E., Trivedi, N.C., Barker, J.M., Krukowski, S.T., Eds.; Society for Mining, Metallurgy, and Exploration: Littleton, CO, USA, 2006; pp. 769–792. ISBN 978-0873352499. [Google Scholar]
- Ayora, C.; Macías, F.; Torres, E.; Lozano, A.; Carrero, S.; Nieto, J.-M.; Pérez-López, R.; Fernández-Martínez, A.; Castillo-Michel, H. Recovery of Rare Earth Elements and Yttrium from Passive-Remediation Systems of Acid Mine Drainage. Environ. Sci. Technol. 2016, 50, 8255–8262. [Google Scholar] [CrossRef] [PubMed]
- Brookins, D.G. Aqueous geochemistry of Rare Earth Elements. In Geochemistry and Mineralogy of Rare Earth Elements; Lipin, B.R., McKay, G.A., Eds.; Mineralogical Society of America: Washington, DC, USA, 1989; Review in Mineralogy 21; pp. 201–225. ISBN 0-939950-25-1. [Google Scholar]
- Mills, S.J.; Christy, A.G.; Génin, J.-M.R.; Kameda, T.; Colombo, F. Nomenclature of the hydrotalcite supergroup: Natural layered double hydroxides. Miner. Mag. 2012, 76, 1289–1336. [Google Scholar] [CrossRef]
- Witzke, T. Untersuchung Natürlicher Sulfathaltiger Hybrider Schichtstrukturen: Charaktereisierung, Systematik, Strukturmodellierung und Rietveld-Verfeinerung. Ph.D. Thesis, Martin-Luther-Universität Halle, Halle, Germany, 1995. [Google Scholar]
- Dinelli, E.; Tateo, F. Different types of fine-grained sediments associated with acid mine drainage in the Libiola Fe–Cu mine area (Ligurian Apennines, Italy). Appl. Geochem. 2002, 17, 1081–1092. [Google Scholar] [CrossRef]
- Tumiati, S.; Godard, G.; Masciocchi, N.; Martin, S.; Monticelli, D. Environmental factors controlling the precipitation of Cu-bearing hydrotalcite-like compounds from mine waters. The case of the “Eve verda” spring (Aosta Valley, Italy). Eur. J. Miner. 2008, 20, 73–94. [Google Scholar] [CrossRef]
- Carbone, C.; Dinelli, E.; Marescotti, P.; Gasparotto, G.; Lucchetti, G. The role of AMD secondary minerals in controlling environmental pollution: Indications from bulk leaching tests. J. Geochem. Explor. 2013, 132, 188–200. [Google Scholar] [CrossRef]
- Woo, E.S.; Kim, J.J.; Kim, Y.H.; Jeong, G.C.; Jang, Y.D.; Dick, W.A. Mineralogical and geochemical characterization of precipitates on stream receiving acid mine water, Korea. Environ. Earth Sci. 2013, 69, 2199–2209. [Google Scholar] [CrossRef]
- Bookin, A.S.; Drits, V.A. Polytype diversity of the hydrotalcite-like minerals. I. Possible polytypes and their diffraction features. Clays Clay Miner. 1993, 41, 551–557. [Google Scholar] [CrossRef]
- Kolitsch, U.; Giester, G.; Pippinger, T. The crystal structure of cualstibite-1M (formerly cyanophyllite), its revised chemical formula and its relation to cualstibite-1T. Miner. Petrol. 2013, 107, 171–178. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Yakovenchuk, V.N.; Zhitova, E.S.; Zolotarev, A.A.; Pakhomovsky, Y.A.; Ivanyuk, G.Y.U. Crystal chemistry of natural layered double hydroxides. 1. Quintinite-2H-3c from the Kovdor alkaline massif, Kola peninsula, Russia. Miner. Mag. 2013, 74, 821–832. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Yakovenchuk, V.N.; Zhitova, E.S.; Zolotarev, A.A.; Pakhomovsky, Y.A.; Ivanyuk, G.Y.U. Crystal chemistry of natural layered double hydroxides. 2. Quintinite-1M: First evidence of a monoclinic polytype in M2+-M3+ layered double hydroxides. Miner. Mag. 2010, 74, 833–840. [Google Scholar] [CrossRef]
- Mills, S.J.; Christy, A.G.; Kampf, A.R.; Housley, R.M.; Favreau, G.; Boulliard, J.-C.; Bourgoin, V. Zincalstibite-9R: The first nine-layer polytype with the layered double hydroxide structure-type. Miner. Mag. 2012, 76, 1337–1345. [Google Scholar] [CrossRef]
- Mills, S.J.; Kampf, A.R.; Housley, R.M.; Favreau, G.; Pasero, M.; Biagioni, C.; Merlino, S.; Berbain, C.; Orlandi, P. Omsite, (Ni,Cu)2Fe3+(OH)6[Sb(OH)6], a new member of the cualstibite group from Oms, France. Miner. Mag. 2012, 76, 1347–1354. [Google Scholar] [CrossRef]
- Zhitova, E.S.; Yakovenchuk, V.N.; Krivovichev, S.V.; Zolotarev, A.A.; Pakhomovsky, Y.A.; Ivanyuk, G.Y.U. Crystal chemistry of natural layered double hydroxides. 3. The crystal structure of Mg,Al-disordered quintinite-2H. Miner. Mag. 2010, 74, 841–848. [Google Scholar] [CrossRef]
- Miyata, S. Anion-exchange properties of hydrotalcite-like compounds. Clays Clay Miner. 1983, 31, 305–311. [Google Scholar] [CrossRef]
- Ardau, C.; Frau, F.; Lattanzi, P. Antimony removal from aqueous solutions by the use of Zn-Al sulphate Layered Double Hydroxide. Water Air Soil Pollut. 2016, 227, 227–344. [Google Scholar] [CrossRef]
- Duan, X.; Lu, J.; Evans, D.G. Assembly Chemistry of Anion-intercalated Layered Materials. In Modern Inorganic Synthetic Chemistry; Xu, R., Pang, W., Huo, Q., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 375–404. [Google Scholar] [CrossRef]
- Li, F.; Duan, X. Applications of Layered Double Hydroxides. In Layered Double Hydroxides; Duan, X., Evans, D.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Structure and Bonding 119; pp. 193–223. ISBN 978-3-540-28279-2. [Google Scholar]
- Chang, Z.; Evans, D.; Duan, X.; Boutinaud, P.; de Roy, M.; Forano, C. Preparation and characterization of rare earth-containing layered double hydroxides. J. Phys. Chem. Solids 2006, 67, 1054–1057. [Google Scholar] [CrossRef]
- Gasser, M.S.; Aly, M.I. Separation and recovery of rare earth elements from spent nickel–metal-hydride batteries using synthetic adsorbent. Int. J. Miner Process. 2013, 121, 31–38. [Google Scholar] [CrossRef]
- Iftekhar, S.; Srivastava, V.; Sillanpää, M. Synthesis and application of LDH intercalated cellulose nanocomposite for separation of rare earth elements (REEs). Chem. Eng. J. 2017, 309, 130–139. [Google Scholar] [CrossRef]
- Kameda, T.; Hoshi, K.; Yoshioka, T. Preparation of Cu-Al layered double hydroxide intercalated with ethylenediaminetetraacetate by coprecipitation and its uptake of rare earth ions from aqueous solution. Solid State Sci. 2013, 17, 28–34. [Google Scholar] [CrossRef]
- Reichle, W.T. Catalytic Reactions by Thermally Activated, Synthetic, Anionic Clay Minerals. J. Catal. 1985, 94, 547–557. [Google Scholar] [CrossRef]
- Ardau, C.; Frau, F.; Lattanzi, P. New data on arsenic sorption properties of Zn–Al sulphate layered double hydroxides: Influence of competition with other anions. Appl. Clay Sci. 2013, 80–81, 1–9. [Google Scholar] [CrossRef]
- Bîrjega, R.; Pavel, O.D.; Costentin, G.; Che, M.; Angelescu, E. Rare-earth elements modified hydrotalcites and corresponding mesoporous mixed oxides as basic solid catalysts. Appl. Catal. A-Gen. 2005, 288, 185–193. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiró, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Biscoe, J.; Warren, B.E. An X-ray Study of Carbon Black. J. Appl. Phys. 1942, 13, 364–371. [Google Scholar] [CrossRef]
- Evans, D.G.; Slade, R.C.T. Structural Aspects of Layered Double Hydroxides. In Layered Double Hydroxides; Duan, X., Evans, D.G., Eds.; Springer: Berlin/Heidelberg, Germnay, 2006; Structure and Bonding 119; pp. 1–87. ISBN 978-3-540-28279-2. [Google Scholar]
- Witzke, T.; Raade, G. Zincowoodwardite, [Zn1-xAlx(OH)2][(SO4)x/2(H2O)n], a new mineral of the hydrotalcite group. N. Jb. Miner. Mh. 2000, 10, 455–465. [Google Scholar]
- Witzke, T. Hydrowoodwardite, a new mineral of the hydrotalcite group from Königswalde near Annaberg, Saxony/Germany and other localities. N. Jb. Miner. Mh. 1999, 2, 75–86. [Google Scholar]
- Raade, G.; Elliott, C.J.; Din, V.K. New data in glaucocerinite. Miner. Mag. 1985, 49, 583–590. [Google Scholar] [CrossRef]
- Paris, E.; Giuli, G.; Carroll, M.; Davoli, I. Sulfur valence and speciation in glasses by X-ray Absorption Spectroscopy. Can. Miner. 2001, 39, 331–339. [Google Scholar] [CrossRef]
- Mori, R.A.; Paris, E.; Giuli, G.; Eeckhout, S.G.; Kavcic, M.; Zitnik, M.; Bukar, K.; Pettersson, L.G.M.; Glatzel, P. Sulfur-Metal Orbital Hybridization in Sulfur-Bearing Compounds Studied by X-ray Emission Spectroscopy. Inorg. Chem. 2010, 49, 6468–6473. [Google Scholar] [CrossRef] [PubMed]
- Kovanda, F.; Rojka, T.; Bezdička, P.; Jirátová, K.; Obalová, L.; Pacultová, K.; Bastl, Z.; Grygar, T. Effect of hydrothermal treatment on properties of Ni–Al layered double hydroxides and related mixed oxides. J. Solid State Chem. 2009, 182, 27–36. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Das, J.; Das, D.; Parida, K.M. Preparation and characterization of Mg–Al hydrotalcite-like compounds containing cerium. J. Colloid Interface Sci. 2006, 301, 569–574. [Google Scholar] [CrossRef] [PubMed]
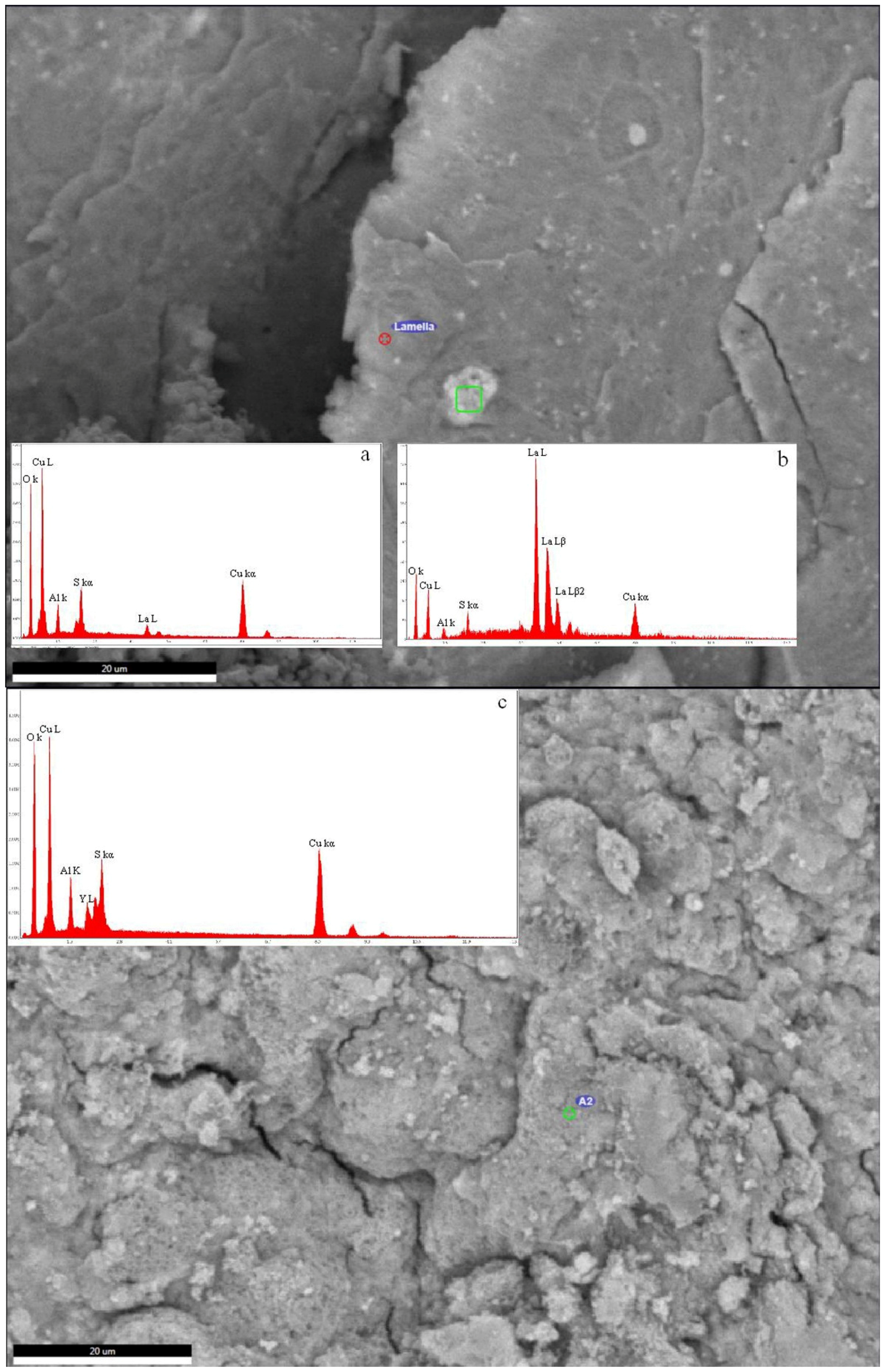
| Sample | Cu | Al | S | Y | La | Ce | Nd | Gd |
|---|---|---|---|---|---|---|---|---|
| LDH 3CuAl·SO4-pH8 | 39.83 | 5.78 | 4.79 | - | - | - | - | - |
| LDH 3CuAl·SO4-pH10 | 44.7 | 3.59 | 4.50 | - | - | - | - | - |
| LDH-Y-8 | 40.60 | 4.50 | 4.40 | 3.50 | - | - | - | - |
| LDH-Y-10 | 41.50 | 4.60 | 3.40 | 3.50 | - | - | - | - |
| LDH-La-8 | 37.30 | 3.70 | 4.90 | - | 4.90 | - | - | - |
| LDH-La-10 | 32.70 | 4.10 | 6.30 | - | 5.20 | - | - | - |
| LDH-Ce-8 | 33.8 | 4.20 | 6.40 | - | - | 3.8 | - | - |
| LDH-Ce-10 | 42.10 | 4.90 | 5.00 | - | - | 5.10 | - | - |
| LDH-Nd-8 | 32.9 | 4.05 | 6.20 | - | - | - | 4.4 | - |
| LDH-Nd-10 | 35.8 | 2.32 | 5.60 | - | - | - | 8.0 | - |
| LDH-Gd-8 | 36.76 | 4.34 | 4.12 | - | - | - | - | 4.04 |
| LDH-Gd-10 | 36.30 | 4.33 | 5.15 | - | - | - | - | 4.26 |
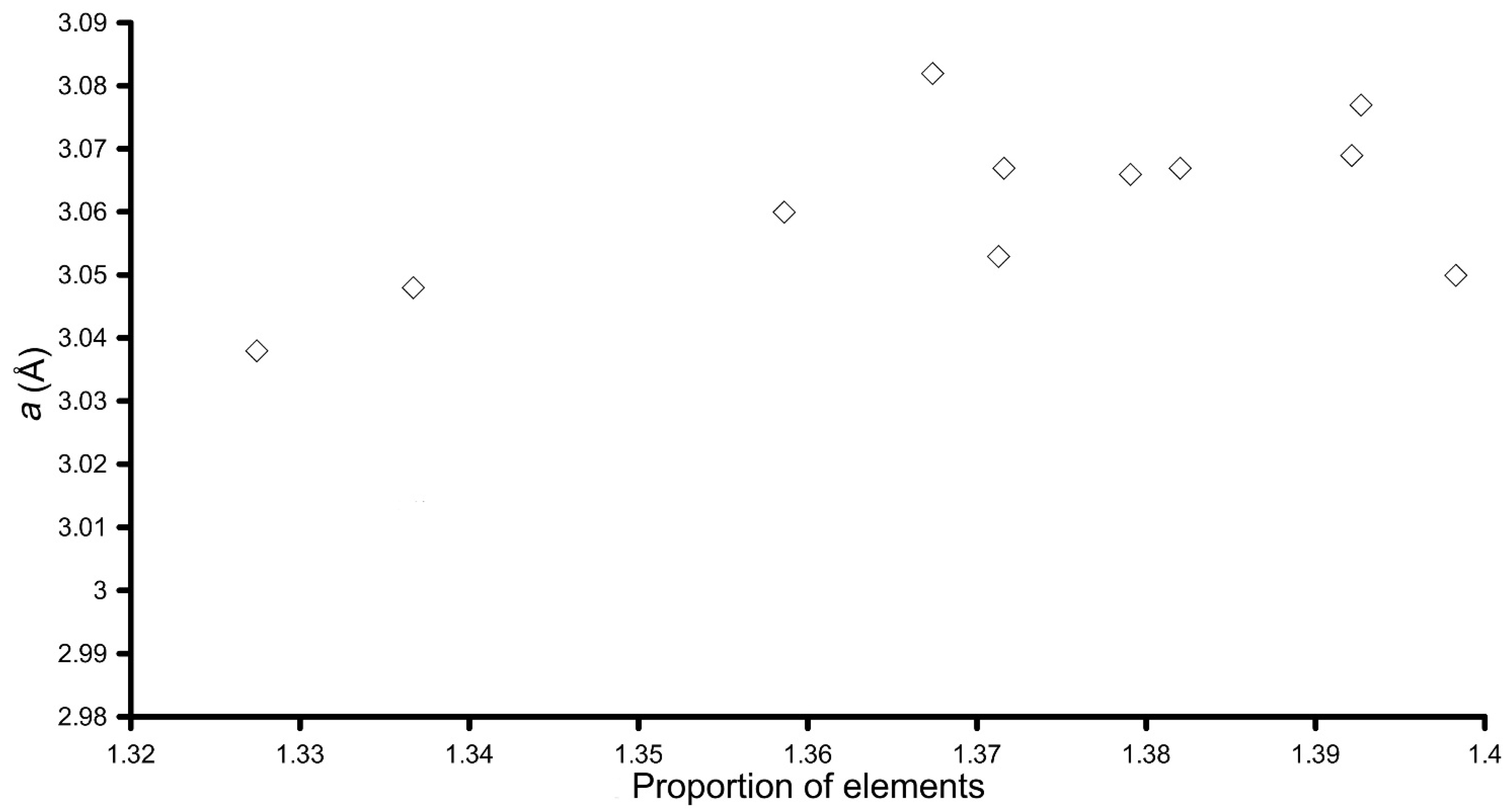
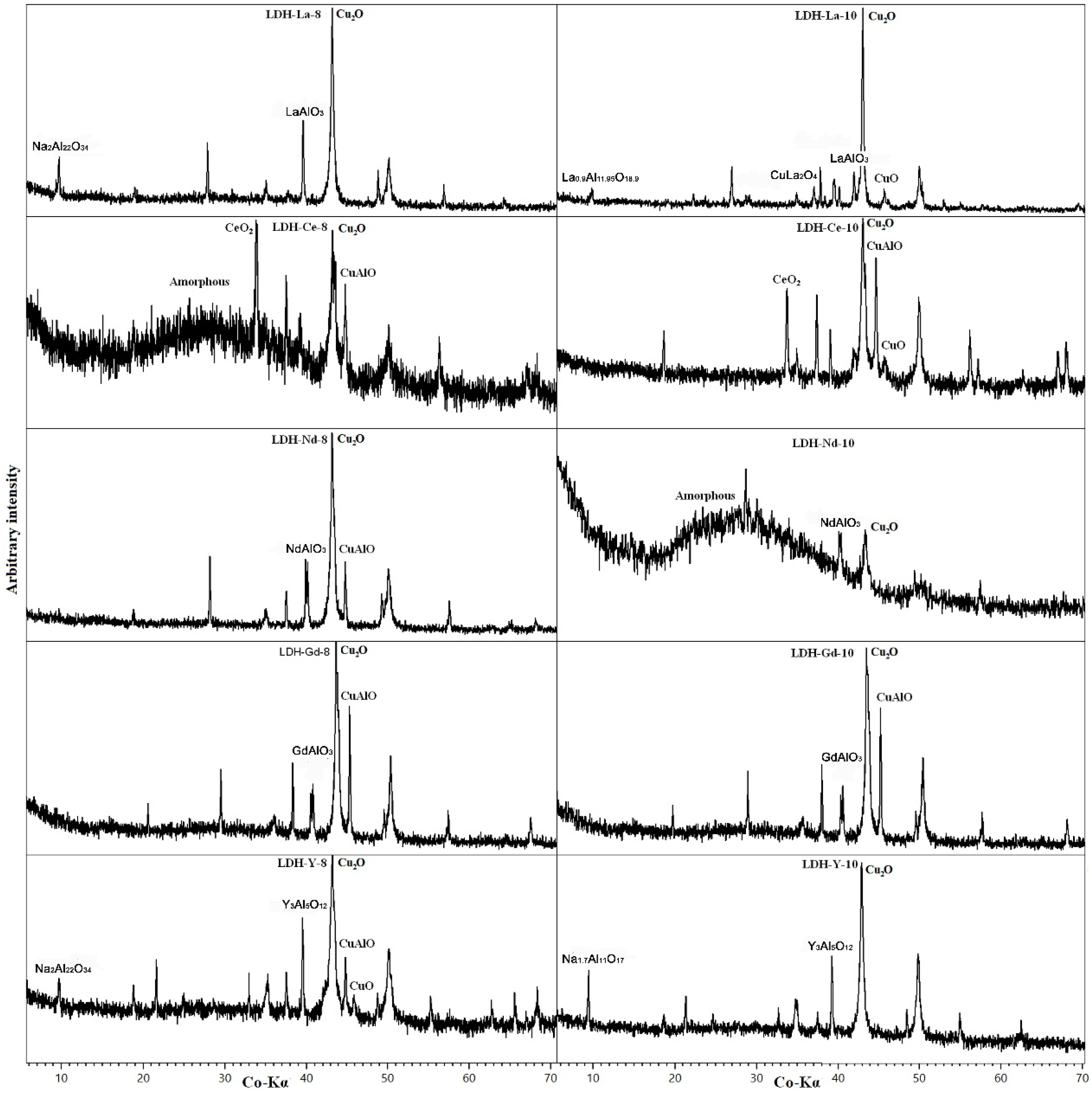
| Sample | Molar Ratio (in Solution) | LDH Formula | x (Al/Cu + Al) | d001 (Å) | 2x d002 (Å) | a = 2x d110 (Å) |
|---|---|---|---|---|---|---|
| LDH 3CuAl·SO4-pH8 | 3Cu:Al | [Cu5.92Al2.08(OH)16]S1.04 | 0.25 | 7.90 | 7.92 | 3.060 |
| LDH 3CuAl·SO4-pH10 | 3Cu:Al | [Cu6.40Al1.60(OH)16]S0.80 | 0.20 | 8.17 | 8.03 | 3.067 |
| LDH-Y-8 | 3Cu:0.8Al:0.2Y | [Cu5.68(Al1.84Y0.48)Σ2.32(OH)16]S1.16 | 0.24 | 7.88 | 7.83 | 3.048 |
| LDH-Y-10 | 3Cu:0.8Al:0.2Y | [Cu5.49(Al2.03Y0.48)Σ2.51(OH)16]S1.255 | 0.23 | 8.03 | 8.20 | 3.038 |
| LDH-La-8 | 3Cu:0.8Al:0.2La | [Cu6.34Al1.66(OH)16]S0.83 | 0.21 | 7.70 | 7.61 | 3.066 |
| LDH-La-10 | 3Cu:0.8Al:0.2La | [Cu6.18Al1.82(OH)16]S0.91 | 0.23 | 8.25 | 8.02 | 3.053 |
| LDH-Ce-8 | 3Cu:0.8Al:0.2Ce | [Cu6.13(Al1.79Ce0.08)Σ1.87(OH)16]S0.935 | 0.23 | 7.74 | 7.64 | 3.067 |
| LDH-Ce-10 | 3Cu:0.8Al:0.2Ce | [Cu6.10Al1.90(OH)16]S0.95 | 0.24 | 8.39 | 8.22 | 3.082 |
| LDH-Nd-8 | 3Cu:0.8Al:0.2Nd | [Cu5.93(Al1.72Nd0.35)Σ2.07(OH)16]S1.035 | 0.22 | 8.05 | 7.74 | 3.050 |
| LDH-Nd-10 | 3Cu:0.8Al:0.2Nd | [Cu6.39(Al0.98Nd0.63)Σ1.61(OH)16]S0.805 | 0.13 | 7.92 | - | - |
| LDH-Gd-8 | 3Cu:0.8Al:0.2Gd | [Cu6.05(Al1.68Gd0.27)Σ1.95(OH)16]S0.975 | 0.22 | 7.78 | 7.68 | 3.069 |
| LDH-Gd-10 | 3Cu:0.8Al:0.2Gd | [Cu6.02(Al1.69Gd0.29)Σ1.98(OH)16]S0.99 | 0.22 | 8.06 | 7.93 | 3.077 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Consani, S.; Balić-Žunić, T.; Cardinale, A.M.; Sgroi, W.; Giuli, G.; Carbone, C. A Novel Synthesis Routine for Woodwardite and Its Affinity towards Light (La, Ce, Nd) and Heavy (Gd and Y) Rare Earth Elements. Materials 2018, 11, 130. https://doi.org/10.3390/ma11010130
Consani S, Balić-Žunić T, Cardinale AM, Sgroi W, Giuli G, Carbone C. A Novel Synthesis Routine for Woodwardite and Its Affinity towards Light (La, Ce, Nd) and Heavy (Gd and Y) Rare Earth Elements. Materials. 2018; 11(1):130. https://doi.org/10.3390/ma11010130
Chicago/Turabian StyleConsani, Sirio, Tonci Balić-Žunić, Anna Maria Cardinale, Walter Sgroi, Gabriele Giuli, and Cristina Carbone. 2018. "A Novel Synthesis Routine for Woodwardite and Its Affinity towards Light (La, Ce, Nd) and Heavy (Gd and Y) Rare Earth Elements" Materials 11, no. 1: 130. https://doi.org/10.3390/ma11010130
APA StyleConsani, S., Balić-Žunić, T., Cardinale, A. M., Sgroi, W., Giuli, G., & Carbone, C. (2018). A Novel Synthesis Routine for Woodwardite and Its Affinity towards Light (La, Ce, Nd) and Heavy (Gd and Y) Rare Earth Elements. Materials, 11(1), 130. https://doi.org/10.3390/ma11010130





