Nano-Charged Polypropylene Application: Realistic Perspectives for Enhancing Durability
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Preparation of Samples
2.2. Characterization Methods
2.3. Accelerated Weathering Treatment
2.4. Electrical Properties
3. Results and Discussion
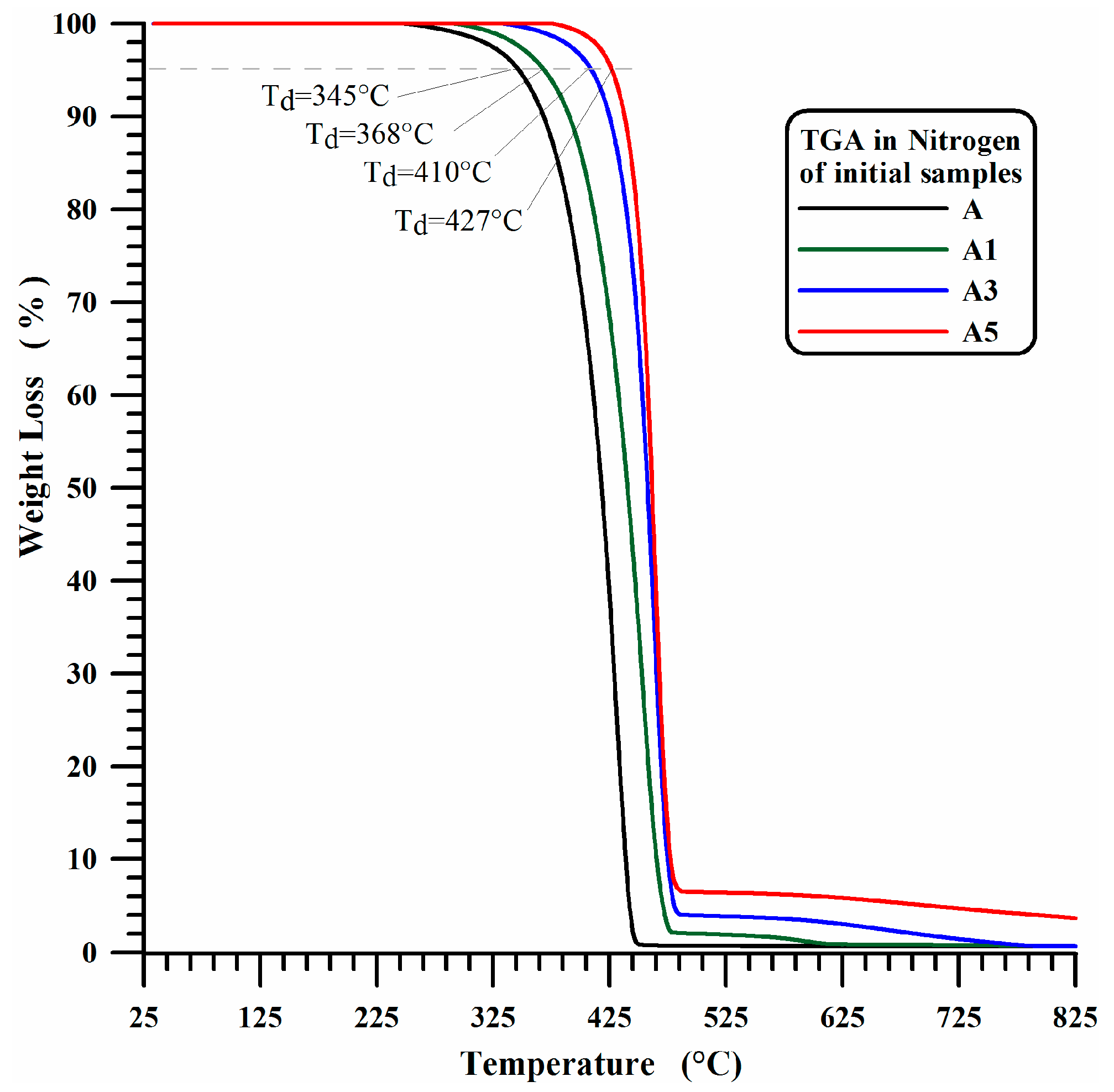
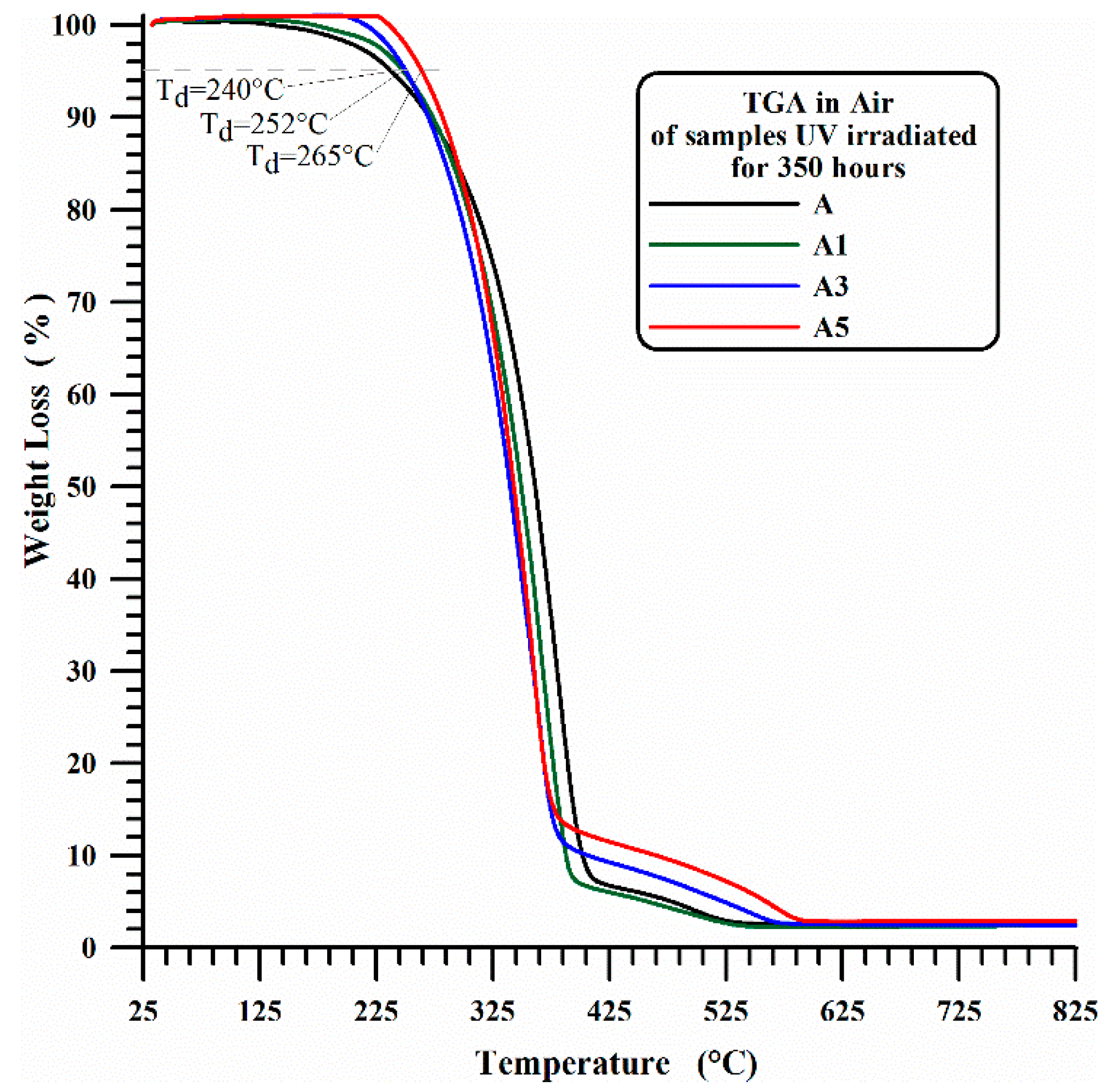
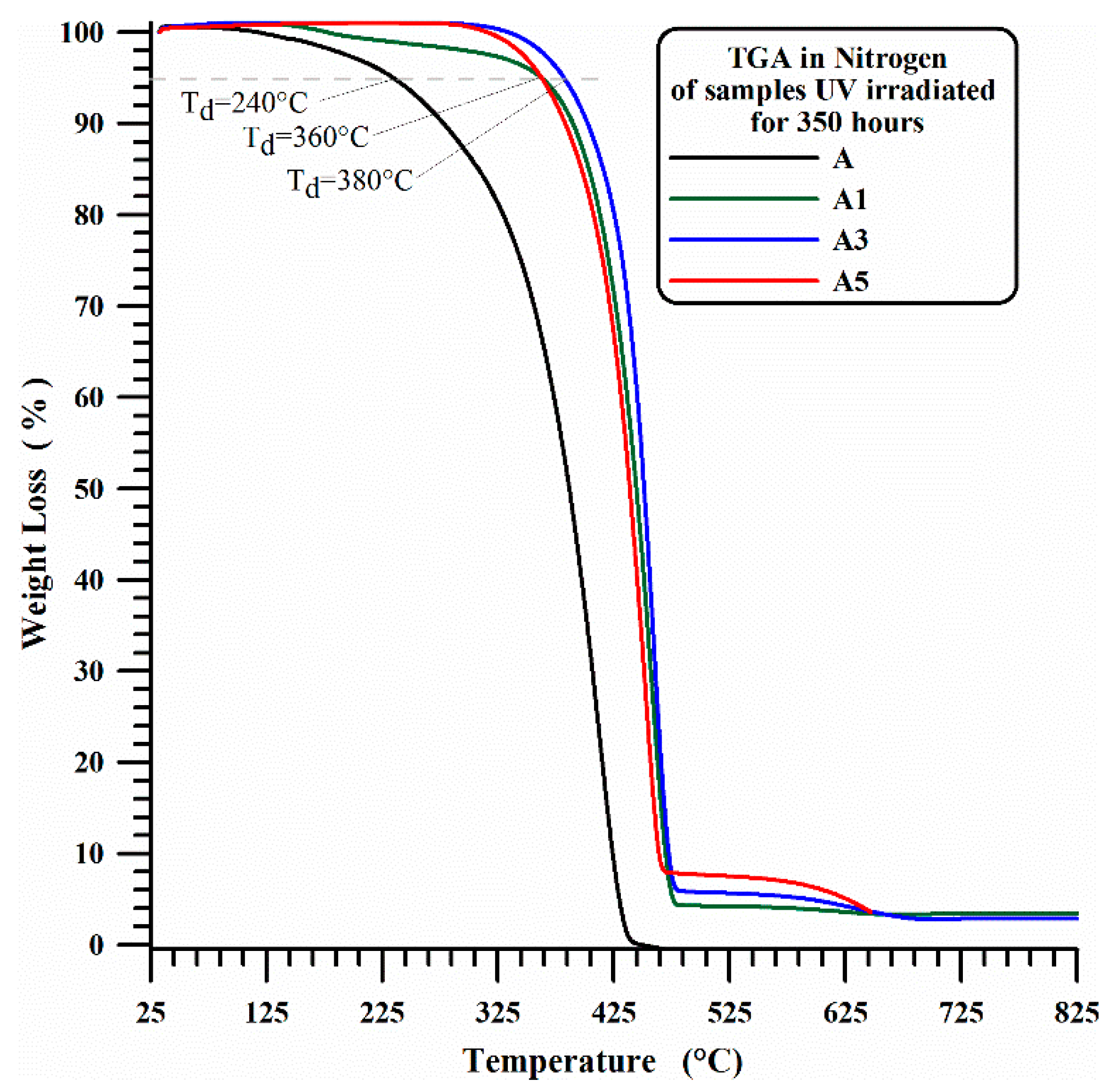
3.1. Thermogravimetric Results
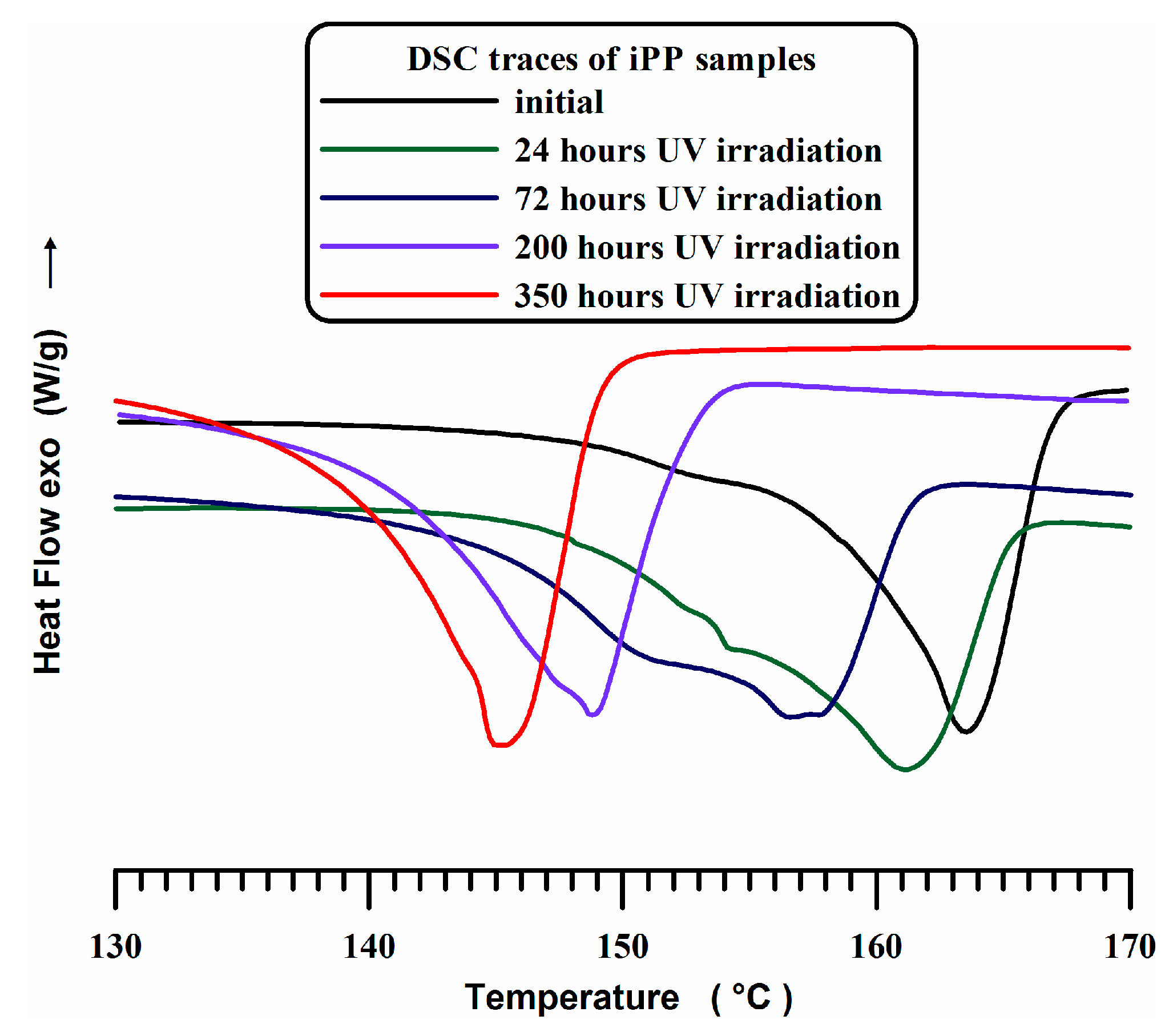
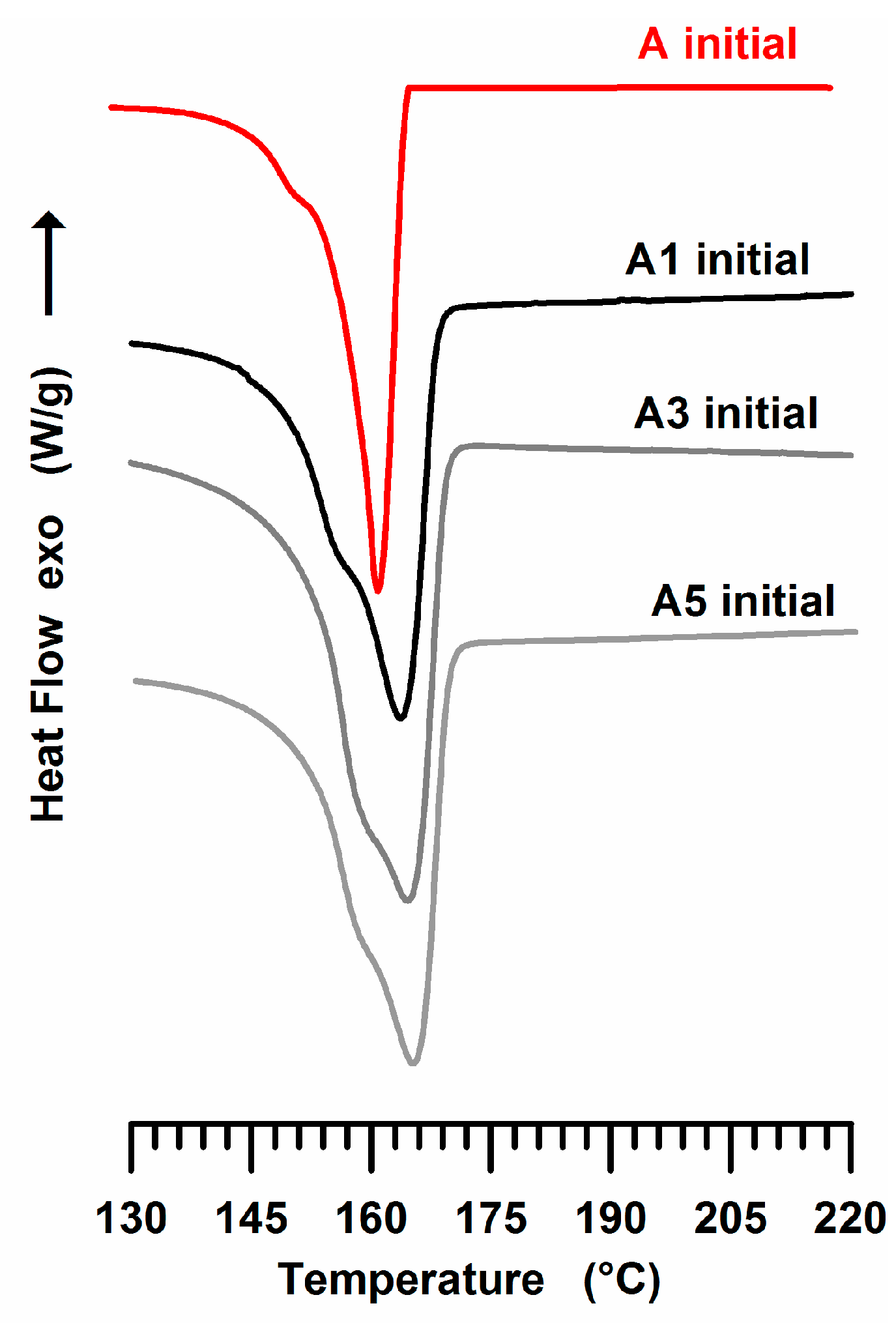
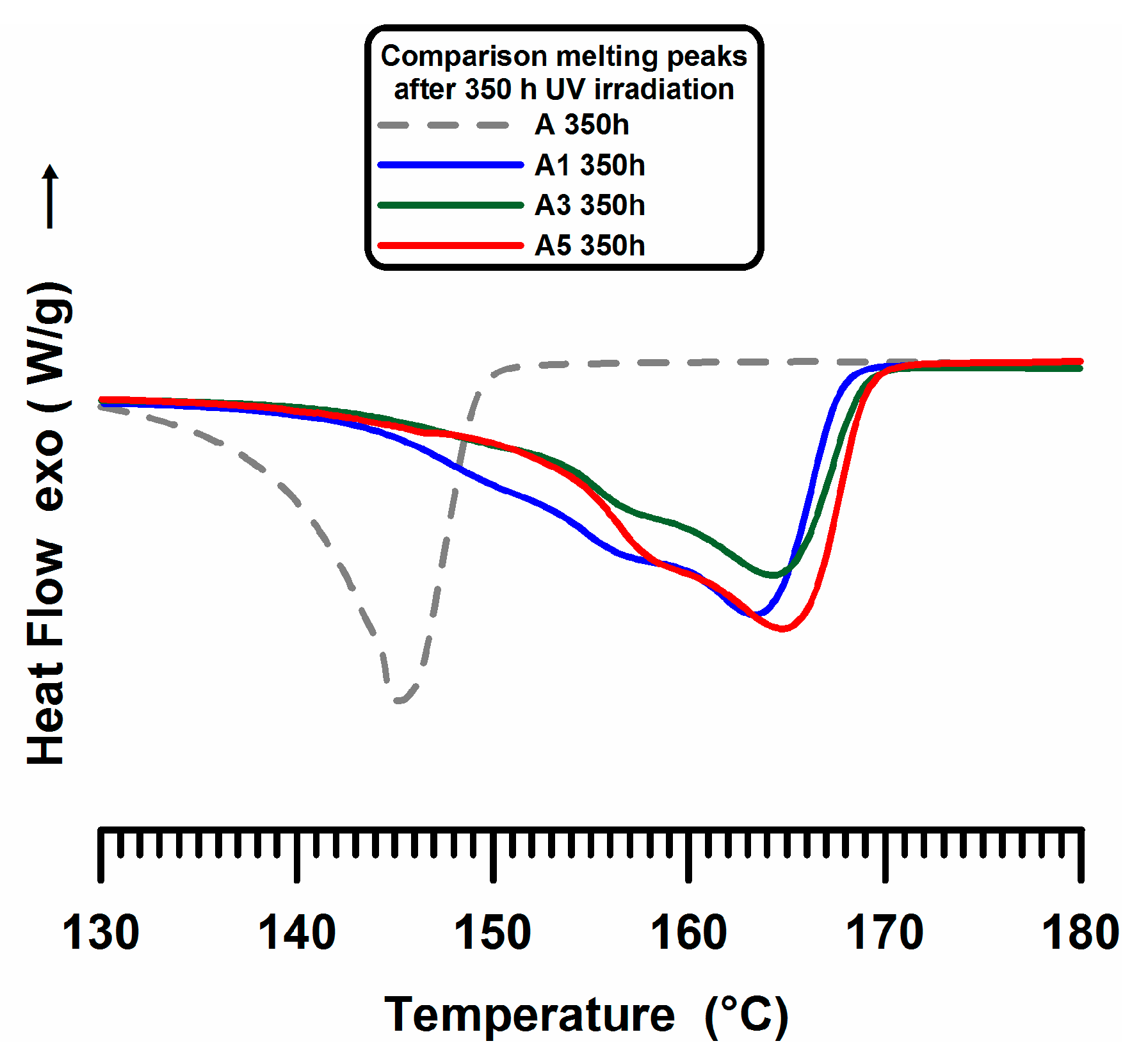
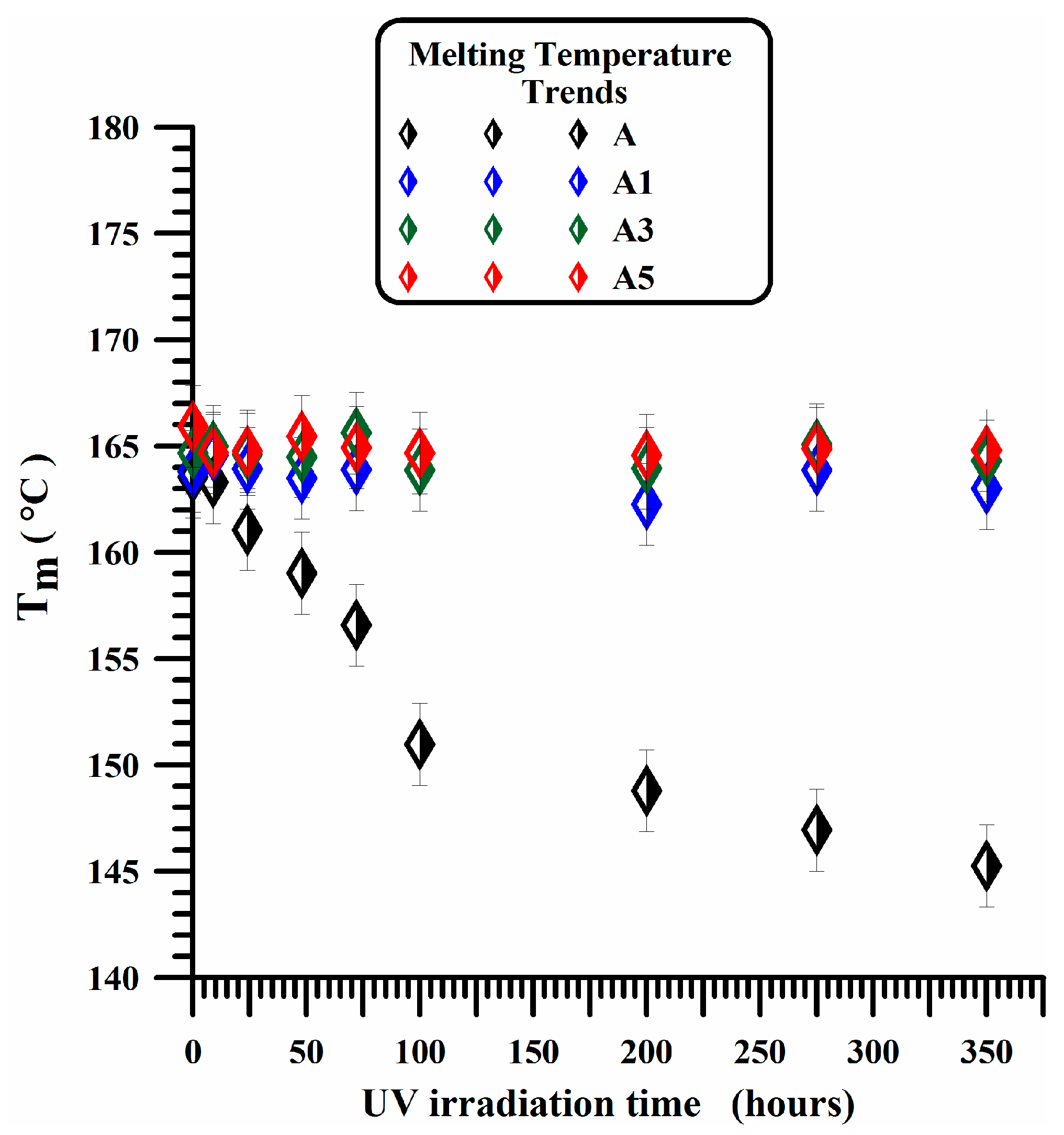
3.2. DSC Experiments
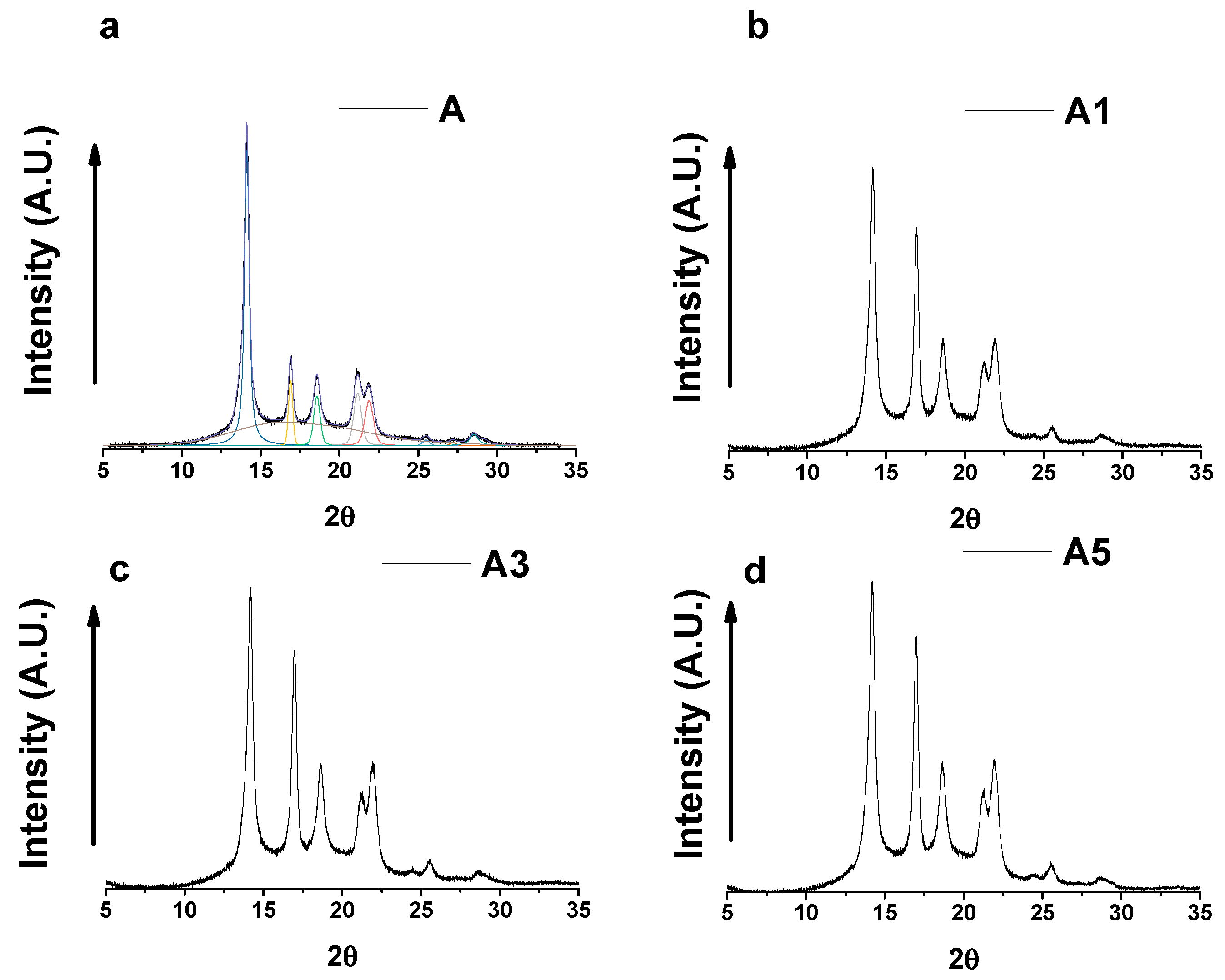
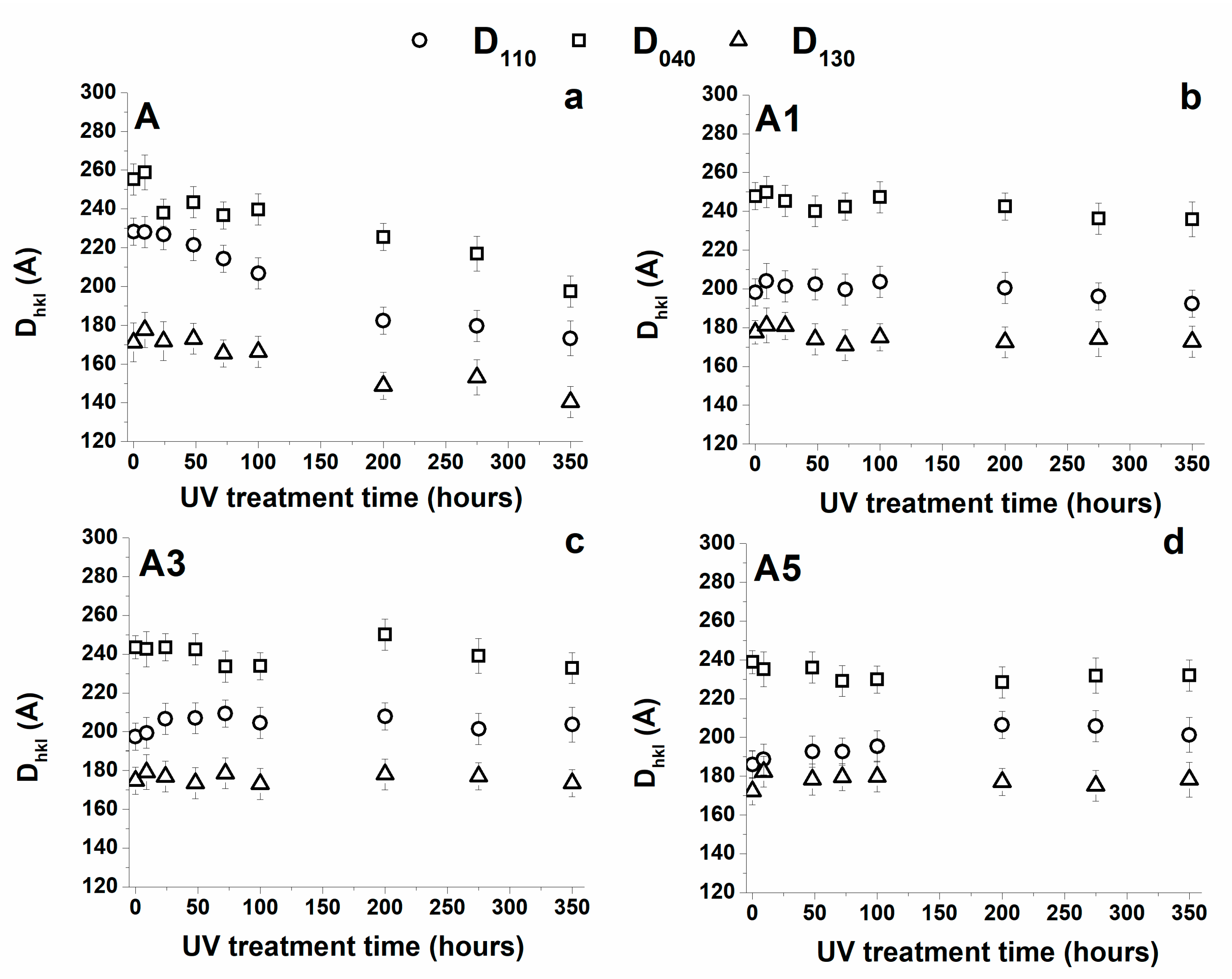
3.3. Wide-Angle X-ray Scattering
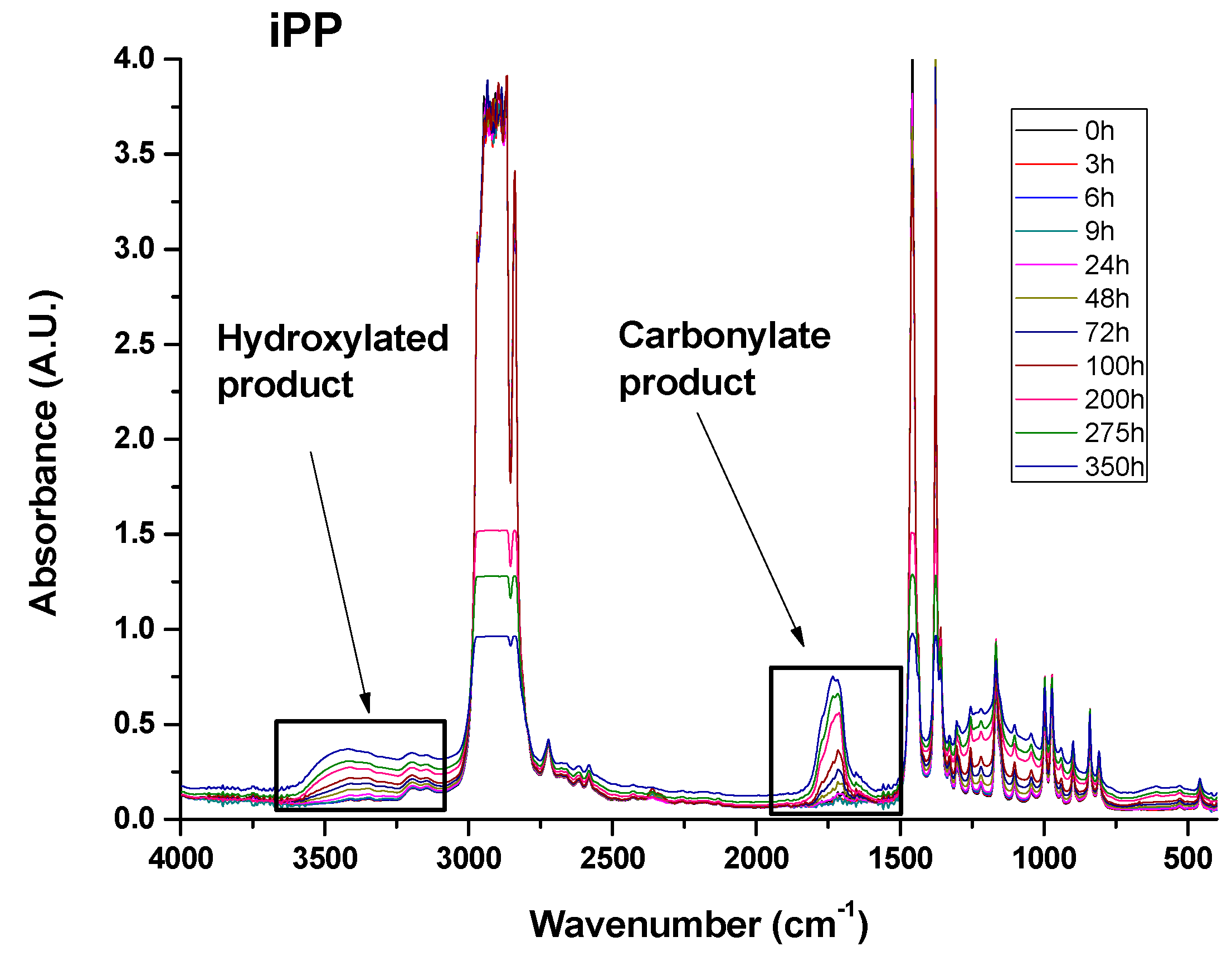
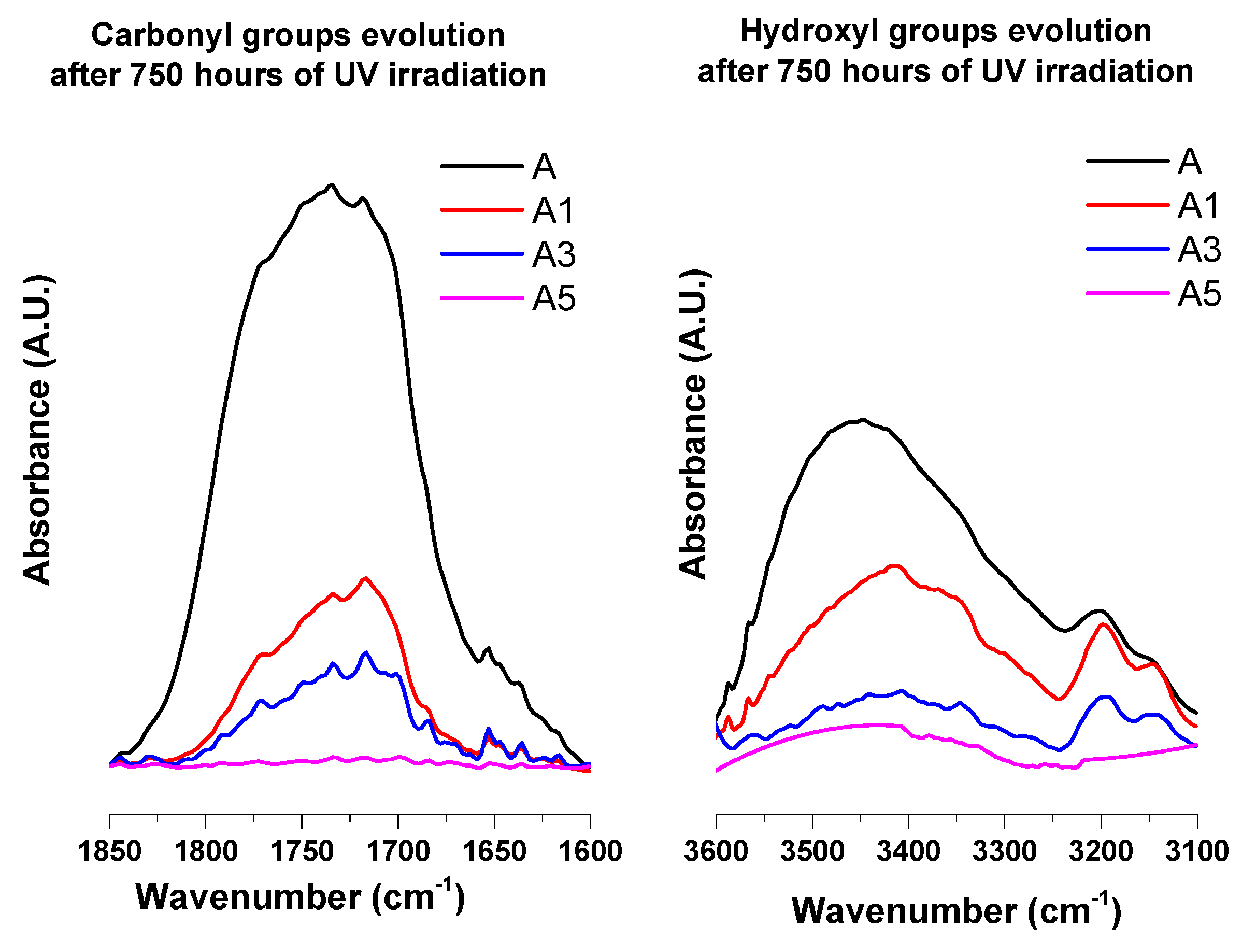
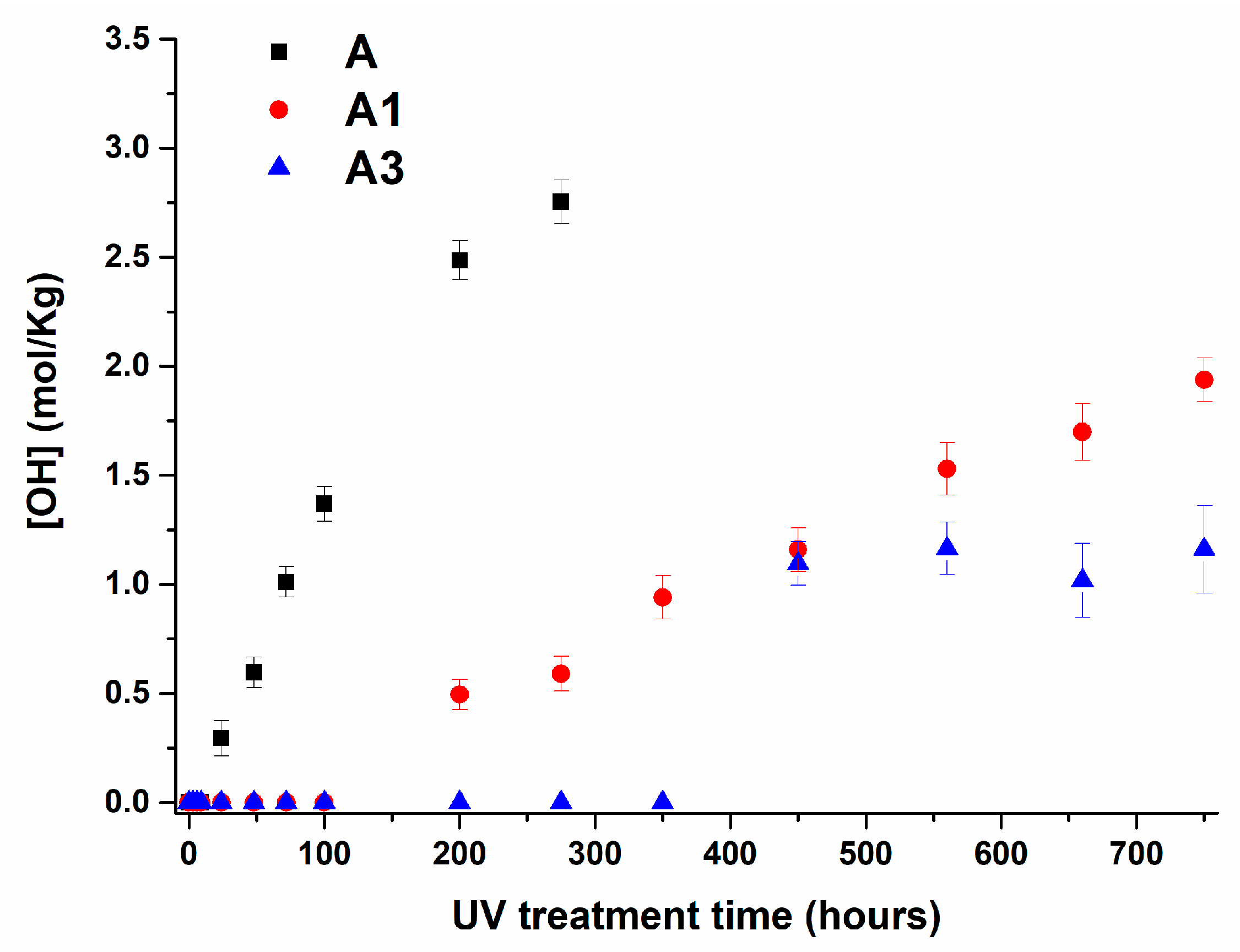
3.4. Infrared Analysis
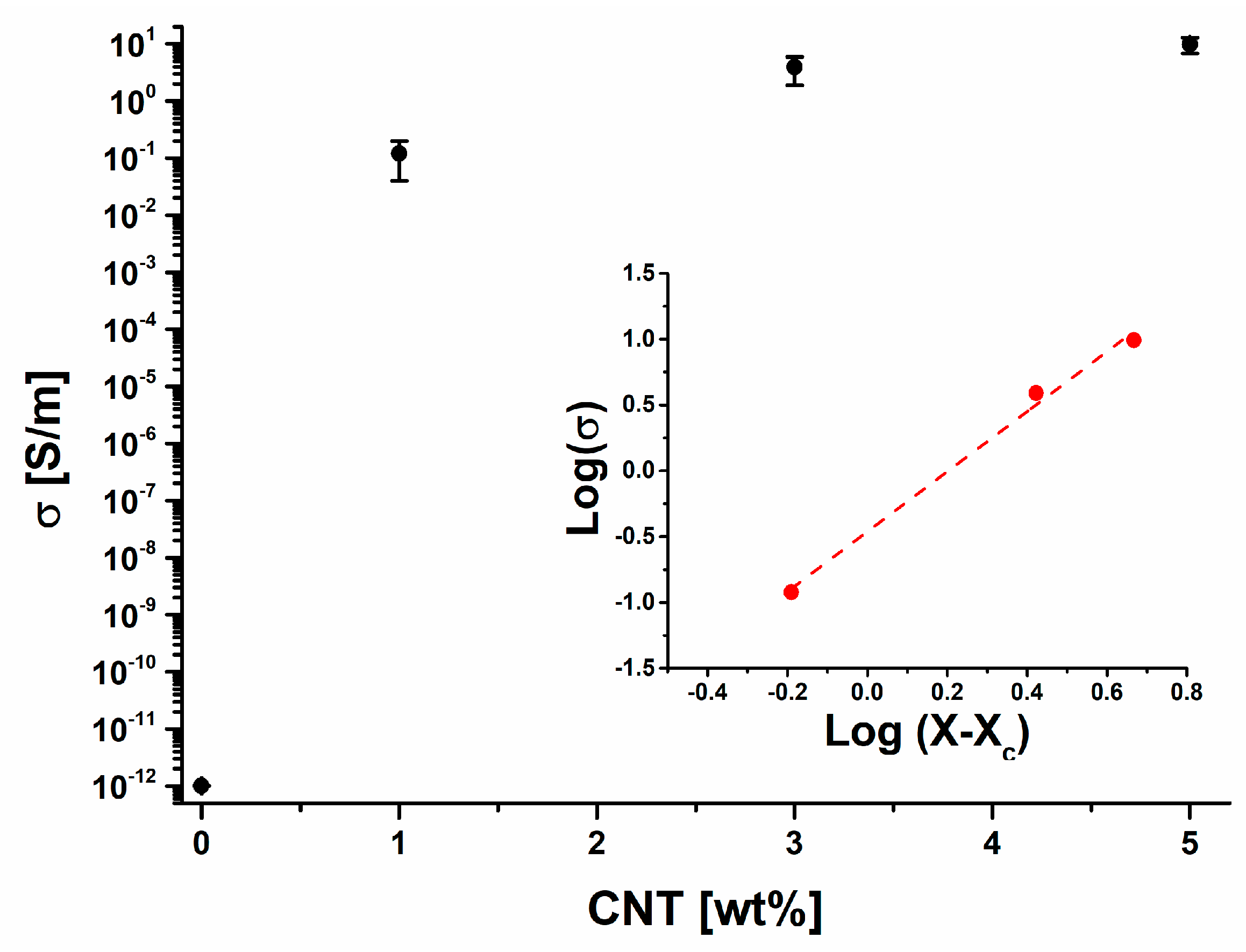
3.5. Electrical Properties
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Faiella, G.; Piscitelli, F.; Lavorgna, M.; Antonucci, V.; Giordano, M. Tuning the insulator to conductor transition in a multiwalled carbon nanotubes/epoxy composite at substatistical percolation threshold. Appl. Phys. Lett. 2009, 95, 153106. [Google Scholar] [CrossRef]
- Bu, Q.; Zhan, Y.; He, F.; Lavorgna, M.; Xia, H. Document Stretchable conductive films based on carbon nanomaterials prepared by spray coating. J. Appl. Polym. Sci. 2016, 133, 43243. [Google Scholar] [CrossRef]
- Esposito Corcione, C.; Manno, R.; Frigione, M. Sunlight-curable boehmite/siloxane-modified methacrylic based nanocomposites as insulating coatings for stone substrates. Prog. Org. Coat. 2016, 95, 107–119. [Google Scholar] [CrossRef]
- Guadagno, L.; Naddeo, C.; Raimondo, M.; Barra, G.; Vertuccio, L.; Russo, S.; Lafdi, K.; Tucci, V.; Spinelli, G.; Lamberti, P. Influence of carbon nanoparticles/epoxy matrix interaction on mechanical, electrical and transport properties of structural advanced materials. Nanotechnology 2017, 28, 094001–094020. [Google Scholar] [CrossRef] [PubMed]
- Esposito Corcione, C.; Manno, R.; Frigione, M. Sunlight curable boehmite/siloxane-modified methacrylic nano-composites: An innovative solution for the protection of carbonate stones. Prog. Org. Coat. 2016, 97, 222–232. [Google Scholar] [CrossRef]
- Romano, V.; Naddeo, C.; Guadagno, L.; Vertuccio, L. Thermal conductivity of epoxy resins filled with MWCNT and hydrotalcite clay: Experimental data and theoretical predictive modeling. Polym. Compos. 2015, 36, 1118–1123. [Google Scholar] [CrossRef]
- Romano, V.; Naddeo, C.; Vertuccio, L.; Lafdi, K.; Guadagno, L. Experimental Evaluation and Modelling of Thermal Conductivity of Tetrafunctional Epoxy Resin Containing Different Carbon Nanostructures. Polym. Eng. Sci. 2017, 57, 779–786. [Google Scholar] [CrossRef]
- Guadagno, L.; Raimondo, M.; Vittoria, V.; Vertuccio, L.; Naddeo, C.; Russo, S.; De Vivo, B.; Lamberti, P.; Spinelli, G.; Tucci, V. Development of epoxy mixtures for application in aeronautics and aerospace. RSC Adv. 2014, 4, 15474–15488. [Google Scholar] [CrossRef]
- Ku-Herrera, J.J.; Aviles, F. Cyclic tension and compression piezoresistivity of carbon nanotube/vinyl ester composites in the elastic and plastic regimes. Carbon 2012, 50, 2592–2598. [Google Scholar] [CrossRef]
- Vertuccio, L.; Guadagno, L.; Spinelli, G.; Lamberti, P.; Tucci, V.; Russo, S. Piezoresistive properties of resin reinforced with carbon nanotubes for health-monitoring of aircraft primary structures. Compos. Part B 2016, 107, 192–202. [Google Scholar] [CrossRef]
- Yan, N.; Capezzuto, F.; Lavorgna, M.; Buonocore, G.G.; Tescione, F.; Xia, H.; Ambrosio, L. Borate cross-linked graphene oxide-chitosan as robust and high gas barrier films. Nanoscale 2016, 8, 10783–10791. [Google Scholar] [CrossRef] [PubMed]
- Guadagno, L.; Raimondo, M.; Vietri, U.; Vertuccio, L.; Barra, G.; De Vivo, B.; Lamberti, P.; Spinelli, G.; Tucci, V.; Volponi, R.; et al. Effective formulation and processing of nanofilled carbon fiber reinforced composites. RSC Adv. 2015, 5, 6033–6042. [Google Scholar] [CrossRef]
- Guadagno, L.; Naddeo, C.; Vittoria, V. Structural and Morphological Changes during UV Irradiation of the Crystalline Helical Form of Syndiotactic Polypropylene. Macromolecules 2004, 37, 9826–9834. [Google Scholar] [CrossRef]
- Guadagno, L.; Naddeo, C.; Raimondo, M.; Vittoria, V. Structural and morphological changes during UV irradiation of the trans-planar form of syndiotactic polypropylene. Polym. Degrad. Stab. 2008, 93, 176–187. [Google Scholar] [CrossRef]
- Naddeo, C.; Guadagno, L.; Vittoria, V. Photooxidation of spherilene linear low-density polyethylene films subjected to environmental weathering 1. Changes in Mechanical properties. Polym. Degrad. Stab. 2004, 85, 1009–1013. [Google Scholar] [CrossRef]
- Guadagno, L.; Naddeo, C.; Vittoria, V.; Camino, G.; Cagnani, C. Chemical and morphological modifications of irradiated linear low density polyethylene (LLDPE). Polym. Degrad. Stab. 2001, 72, 175–186. [Google Scholar] [CrossRef]
- Russo, P.; Acierno, D.; Marinucci, L.; Greco, A.; Frigione, M. Influence of Natural and Accelerated Weathering on Performances of Photoselective Greenhouse Films. J. Appl. Polym. Sci. 2013, 127, 2213–2219. [Google Scholar] [CrossRef]
- Kumar, A.P.; Depan, D.; Torner, N.S.; Singh, R.P. Nanoscale particles for polymer degradation and stabilization–Trends and Future prospective. Prog. Pol. Sci. 2009, 34, 479–515. [Google Scholar] [CrossRef]
- Morlat-Therias, S.; Mailhot, B.; Gonzalez, D.; Gardette, J.L. Photo-oxidation of polypropylene/montmorillonite nanocomposites. 2. Interactions with antioxidants. Chem. Mater. 2005, 17, 1072–1078. [Google Scholar] [CrossRef]
- Mailhot, B.; Morlat, S.; Gardette, J.L.; Boucard, S.; Duchet, J.; Gérard, J.F. Photodegradation of polypropylene nanocomposites. Polym. Degrad. Stab. 2003, 82, 163–167. [Google Scholar] [CrossRef]
- Qin, O.; Zhang, C.; Liu, H.; Xie, S.; Yang, M.; Shen, D. Photooxidative degradation of polypropylene/montmorillonite nanocomposites. Polymer 2005, 46, 3149–3156. [Google Scholar] [CrossRef]
- Morlat, S.; Mailhot, B.; Gonzalez, D.; Gardett, J. Photo-oxidation of Polypropylene/Montmorillonite Nanocomposites. 1. Influence of Nanoclay and Compatibilizing Agent. Chem. Mater. 2004, 16, 377–383. [Google Scholar] [CrossRef]
- Zanetti, M.; Camino, G.; Reichert, P.; Mulhaupt, R. Thermal Behaviour of Poly(propylene) Layered Silicate Nanocomposites. Macromol. Rapid Commun. 2001, 22, 176–180. [Google Scholar] [CrossRef]
- Scarfato, P.; Acierno, D.; Russo, P. Photooxidative Weathering of Biodegradable Nanocomposite Films Containing. Halloysite. Polym. Compos. 2015, 36, 1169–1175. [Google Scholar] [CrossRef]
- Morlat-Therias, S.; Mailhot, B.; Gardette, J.L.; Da Silva, C.; Haidar, B.; Vidal, A. Photooxidation of ethylene–propylene-diene/montmorillonite nanocomposites. Polym. Degrad. Stab. 2005, 90, 78–85. [Google Scholar] [CrossRef]
- Osawa, Z. Role of metals and metal-deactivators in polymer degradation. Polym. Degrad. Stab. 1988, 20, 203–236. [Google Scholar] [CrossRef]
- Schadler, L.S. Polymer-Based and Polymer-Filled Nanocomposites; Ajayan, P.M., Schadler, L.S., Braun, P.V., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003; pp. 55–70. [Google Scholar]
- Tudor, J.; Willington, L.; O’Hare, D.; Royan, B. Intercalation of catalytically active metal complexes in phyllosilicates and their application as propene polymerisation catalysts. Chem. Commun. 1996, 2031–2032. [Google Scholar] [CrossRef]
- Guadagno, L.; Naddeo, C.; Raimondo, M.; Gorrasi, G.; Vittoria, V. Effect of carbon nanotubes on the photo-oxidative durability of syndiotactic polypropylene. Polym. Degrad. Stab. 2010, 95, 1614–1626. [Google Scholar] [CrossRef]
- Guadagno, L.; Raimondo, M.; Naddeo, C.; Di Bartolomeo, A.; Lafdi, K. Influence of multiwall carbon nanotubes on morphological and structural changes during UV irradiation of syndiotactic polypropylene films. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 963–975. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; Arrigo, R.; Catalanotto, F.; Morici, E. Improvement of the photo-stability of polystyrene-block-polybutadiene-block-polystyrene through carbon nanotubes. Polym. Degrad. Stab. 2015, 118, 24–32. [Google Scholar] [CrossRef]
- Morlat-Therias, S.; Fanton, E.; Gardette, J.L.; Peeterbroeck, S.; Alexandre, M.; Dubois, P. Polymer/carbon nanotube nanocomposites: influence of carbon nanotubes on EVA photodegradation. Polym. Degrad. Stab. 2007, 92, 1873–1882. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; La Mantia, F.P.; Malatesta, V. Photo-oxidation behaviour of polyethylene/multi-wall carbon nanotube composite films. Polym. Degrad. Stab. 2009, 94, 162–170. [Google Scholar] [CrossRef]
- Petersen, E.J.; Lam, T.; Gorham, J.M.; Scott, K.C.; Long, C.J.; Stanley, D.; Sharma, R.; Alexander Liddle, J.; Pellegrin, B.; Nguyen, T. Methods to assess the impact of UV irradiation on the surface chemistry and structure of multiwall carbon nanotube epoxy nanocomposites. Carbon 2014, 69, 194–205. [Google Scholar] [CrossRef]
- Asmatulu, R.; Mahmud, G.A.; Hille, C.; Misak, H.E. Effects of UV degradation on surface hydrophobicity, crack, and thickness of MWCNT-based nanocomposite coatings. Prog. Org. Coat. 2011, 72, 553–561. [Google Scholar] [CrossRef]
- Nguyen, T.; Petersen, E.J.; Pellegrin, B.; Gorham, J.M.; Lam, T.; Zhao, M.; Sung, L. Impact of UV irradiation on multiwall carbon nanotubes in nanocomposites: Formation of entangled surface layer and mechanisms of release resistance. Carbon 2017, 116, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Pellegrin, B.; Bernard, C.; Gu, X.; Gorham, J.M.; Stutzman, P.; Stanley, D.; Shapiro, A.; Byrd, E.; Hettenhouser, R.; Chin, J. Fate of nanoparticles during life cycle of polymer nanocomposites. J. Phys. Conf. Ser. 2011, 304, 012061–012072. [Google Scholar] [CrossRef]
- Wohlleben, W.; Meier, M.W.; Vogel, S.; Landsiedel, R.; Cox, G.; Hirth, S.; Tomovic, Z. Elastic CNT-polyurethane nanocomposite: Synthesis, performance and assessment of fragments released during use. Nanoscale 2013, 5, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Vilar, G.; Fernandez-Rosas, E.; Puntes, V.; Jamier, V.; Aubouy, L.; Vazquez-Campos, S. Monitoring migration and transformation of nanomaterials in polymeric composites during accelerated aging. J. Phys. Conf. Ser. 2013, 429, 012044–012050. [Google Scholar] [CrossRef]
- Cerruti, P.; Lavorgna, M.; Carfagna, C.; Nicolais, L. Comparison of photo-oxidative degradation of polyamide 6,6 films stabilized with HALS and CuCl2+KI mixtures. Polymer 2005, 46, 4571–4583. [Google Scholar] [CrossRef]
- Busquets-Fite, M.; Fernandez, E.; Janer, G.; Vilar, G.; Vazquez-Campos, S.; Zanasca, R.; Citterio, C.; Mercante, L.; Puntes, V. Exploring release and recovery of nanomaterials from commercial polymeric nanocomposites. J. Phys. Conf. Ser. 2013, 429, 012048. [Google Scholar] [CrossRef]
- Gorrasi, G.; Sorrentino, A. Photo-oxidative stabilization of carbon nanotubes on polylactic acid. Polym. Degrad. Stab. 2013, 98, 963–971. [Google Scholar] [CrossRef]
- Bocchini, S.; Di Blasio, A.; Frache, A. Influence of MWCNT on polypropylene and polyethylene photooxidation. Macromol. Symp. 2011, 301, 16–22. [Google Scholar] [CrossRef]
- Bikiaris, D. Microstructure and Properties of Polypropylene/Carbon Nanotube Nanocomposites. Materials 2010, 3, 2884–2946. [Google Scholar] [CrossRef]
- Khare, R.; Bose, S. Carbon Nanotube Based Composites-A Review. J. Miner. Mater. Charact. Eng. 2005, 4, 31–46. [Google Scholar] [CrossRef]
- Logakis, E.; Pollatos, E.; Pandis, C.; Peoglos, V.; Zuburtikudis, I.; Delides, C.G.; Vatalis, A.; Gjoka, M.; Syskakis, E.; Viras, K.; et al. Structure–property relationships in isotactic polypropylene/multi-walled carbon nanotubes nanocomposites. Compos. Sci. Technol. 2010, 70, 328–335. [Google Scholar] [CrossRef]
- Sathyanarayana, S.; Hübner, C. Thermoplastic Nanocomposites with Carbon Nanotubes. In Structural Nanocomposites; Springer: Berlin/Heidelberg, Germany, 2013; pp. 19–60. [Google Scholar]
- Li, J. Multiwalled Carbon Nanotubes Reinforced Polypropylene Composite Material. J. Nano Res. 2017. [Google Scholar] [CrossRef]
- Schlagenhauf, L.; Nüesch, F.; Wang, J. Release of carbon nanotubes from polymer nanocomposites. Fibers 2014, 2, 108. [Google Scholar] [CrossRef]
- Hirth, S.; Cena, L.; Cox, G.; Tomović, Ž.; Peters, T.; Wohlleben, W. Scenarios and methods that induce protruding or released cnts after degradation of nanocomposite materials. J. Nano Res. 2013, 15, 1504. [Google Scholar] [CrossRef] [PubMed]
- Guadagno, L.; Vertuccio, L.; Sorrentino, A.; Raimondo, M.; Naddeo, C.; Vittoria, V.; Iannuzzo, G.; Calvi, E.; Russo, S. Mechanical and barrier properties of epoxy resin filled with multi-walled carbon nanotubes. Carbon 2009, 47, 2419–2430. [Google Scholar] [CrossRef]
- Gugumus, F. Thermooxidative degradation of polyolefins in the solid state: part 1 experimental kinetics of functional group formation. Polym. Degrad. Stab. 1996, 52, 131–144. [Google Scholar] [CrossRef]
- Delprat, P.; Deteurtre, X.; Gardette, J.L. Photooxidation of unstabilized and HALS stabilized polyphasic ethylene–polypropylene polymers. Polym. Degrad. Stab. 1995, 50, 1–12. [Google Scholar] [CrossRef]
- Beg, M.D.H.; Pickering, K.L. Accelerated weathering of unbleached and bleached Kraft wood fibre reinforced polypropylene composites. Polym. Degrad. Stab. 2008, 93, 1939–1946. [Google Scholar] [CrossRef]
- Zhao, H.; Li, R.K. A study on the photo-degradation of zinc oxide (ZnO) filled polypropylene nanocomposites. Polymer 2006, 47, 3207–3217. [Google Scholar] [CrossRef]
- Mouffok, S.; Kaci, M. Artificial weathering effect on the structure and properties of polypropylene/polyamide-6 blends compatibilized with PP-g-MA. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Arosfoi, B.B.; Szabo, A.; Marosi, G.; Tabuani, D.; Camino, G.; Pagliari, G. Thermal and spectroscopic characterization of polypropylene–carbon nanotube composites. J. Therm. Anal. Calor. 2006, 86, 669–673. [Google Scholar] [CrossRef]
- Varga, J. Propylene Structure, Blends and Composites; Karger-Kocsis, J., Ed.; Chapman and Hall: Cambridge, UK, 1995; ISBN 978-94-010-4233-8. [Google Scholar]
- Roberts, R.C. The melting behavior of bulk crystallized polymers. J. Polym. Sci Part C 1970, 8, 381–384. [Google Scholar] [CrossRef]
- Morrow, D.R.; Newman, B.A. Crystallization of Low-Molecular-Weight Polypropylene Fractions. J. Appl. Phys. 1968, 39, 4944–4950. [Google Scholar] [CrossRef]
- Mandelkern, L. Kinetics and Mechanisms. In Crystallization of Polymers; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Natale, R.; Russo, R.; Vittoria, V. Crystallinity of isotactic polypropylene films annealed from the quenched state. J. Mater. Sci. 1992, 27, 4350–4354. [Google Scholar] [CrossRef]
- Watts, P.C.P.; Fearon, P.K.; Hsu, W.K.; Billingham, N.C.; Kroto, H.W.; Walton, D.R.M. Carbon nanotubes as polymer antioxidants. J. Mater. Chem. 2003, 13, 491–495. [Google Scholar] [CrossRef]
- Collins, P.G.; Bradley, K.; Ishigami, M.; Zettl, A. Extreme oxygen sensitivity of electronic properties of carbon nanotubes. Science 2000, 287, 1801–1804. [Google Scholar] [CrossRef] [PubMed]
- Sumanasekera, G.U.; Adu, C.; Fang, S.; Eklund, P.C. Effect of gas adsorption and collisions on electrical transport in single-walled carbon nanotubes. Phys. Rev. Lett. 2000, 85, 1096–1099. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Cho, K. Chemical control of nanotubes electronics. Nanotechnology 2000, 11, 57–60. [Google Scholar] [CrossRef]
- Giannozzi, P.; Car, R.; Scoles, G. Oxygen adsorption on graphite and nanotubes. J. Chem. Phys. 2003, 118, 1003–1006. [Google Scholar] [CrossRef]
- Froudakis, G.E.; Schnell, M.; Muhlhauser, M.; Peyerimhoff, S.D.; Andriotis, A.N.; Menon, M.; Sheetz, R.M. Pathways for oxygen adsorption on single-walled carbon nanotubes. Phys. Rev. B 2003, 68, 115435. [Google Scholar] [CrossRef]
- Barone, V.; Heyd, J.; Scuseria, G.E. Effect of oxygen chemisorptions on the energy band gap of a chiral semiconducting single-walled carbon nanotube. Chem. Phys. Lett. 2004, 389, 289–292. [Google Scholar] [CrossRef]
- Walch, S.P. On the reaction of N and O atoms with carbon nanotubes. Chem. Phys. Lett. 2003, 374, 501–505. [Google Scholar] [CrossRef]
- Grujicic, M.; Cao, G.; Rao, A.M.; Tritt, T.M.; Nayak, S. UV-Light enhanced oxidation of carbon nanotubes. Appl. Surf. Sci. 2003, 214, 289–303. [Google Scholar] [CrossRef]
- Savage, T.; Bhattacharya, S.; Sadanadan, B.; Gaillard, J.; Tritt, T.M.; Sun, Y.P.; Ajayan, P.M. Phtoinduced oxidation of carbon nanotubes. J. Phys. Condens. Matter 2003, 15, 5915–5921. [Google Scholar] [CrossRef]
- Guadagno, L.; Naddeo, C.; Vittoria, V.; Sorrentino, A.; Vertuccio, L.; Raimondo, M.; Calvi, E. Cure behavior and physical properties of epoxy Resin-Filled with multiwalled carbon nanotubes. J. Nanosci. Nanotechnol. 2010, 10, 2686–2693. [Google Scholar] [CrossRef] [PubMed]
- Vionnet-Menot, S.; Grimaldi, C.; Maeder, T.; Strässler, S.; Ryser, P. Tunneling percolation origin of non universality: theory and experiments. Phys. Rev. B 2005, 71, 064201–064212. [Google Scholar] [CrossRef]
- Kim, Y.J.; Shin, T.S.; Choi, H.D.; Kwon, J.H.; Chung, Y.C.; Yoon, H.G. Electrical conductivity of chemically modified multiwalled carbon nanotube/epoxy composites. Carbon 2005, 43, 23–30. [Google Scholar] [CrossRef]
- Kymakis, E.; Amaratunga, G.A. Electrical properties of single-wall carbon nanotube-polymer composite films. J. Appl. Phys. 2006, 99, 084302–84307. [Google Scholar] [CrossRef]
- Hernández, J.J.; García-Gutiérrez, M.C.; Nogales, A.; Rueda, D.R.; Kwiatkowska, M.; Szymczyk, A.; Roslaniec, Z.; Concheso, A.; Guinea, I.; Ezquerra, T.A. Influence of preparation procedure on the conductivity and transparency of swcnt-polymer nanocomposites. Compos. Sci. Technol. 2009, 69, 1867–1872. [Google Scholar] [CrossRef]
- Nan, C.W.; Shen, Y.; Ma, J. Physical Properties of Composites near Percolation. Annu. Rev. Mater. Res. 2010, 40, 131–151. [Google Scholar] [CrossRef]
- Bauhofer, W.; Kovacs, J.Z. A review and analysis of electrical percolation in carbon nanotube polymer composites. Compos. Sci. Technol. 2009, 69, 1486–1498. [Google Scholar] [CrossRef]
- Gulrez, S.K.; Ali Mohsin, M.E.; Shaikh, H.; Anis, A.; Pulose, A.M.; Yadav, M.K.; Qua, E.P.; Al-Zahrani, S.M. A review on electrically conductive polypropylene and polyethylene. Polym. Compos. 2014, 35, 900–914. [Google Scholar] [CrossRef]




| Physical Property | Value |
|---|---|
| Melt index | 35 g/10 min (230 °C/2.16 kg) |
| Mol wt | average Mn ~ 50,000 |
| average Mw ~ 190,000 | |
| Hardness | 98 (Rockwell R, ASTM D 785-A) |
| Melting Temperature | Tm 160–165 °C |
| Density | 0.9 g/mL at 25 °C (lit.) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naddeo, C.; Vertuccio, L.; Barra, G.; Guadagno, L. Nano-Charged Polypropylene Application: Realistic Perspectives for Enhancing Durability. Materials 2017, 10, 943. https://doi.org/10.3390/ma10080943
Naddeo C, Vertuccio L, Barra G, Guadagno L. Nano-Charged Polypropylene Application: Realistic Perspectives for Enhancing Durability. Materials. 2017; 10(8):943. https://doi.org/10.3390/ma10080943
Chicago/Turabian StyleNaddeo, Carlo, Luigi Vertuccio, Giuseppina Barra, and Liberata Guadagno. 2017. "Nano-Charged Polypropylene Application: Realistic Perspectives for Enhancing Durability" Materials 10, no. 8: 943. https://doi.org/10.3390/ma10080943
APA StyleNaddeo, C., Vertuccio, L., Barra, G., & Guadagno, L. (2017). Nano-Charged Polypropylene Application: Realistic Perspectives for Enhancing Durability. Materials, 10(8), 943. https://doi.org/10.3390/ma10080943






