Anti-Inflammatory, Immunomodulatory, and Tissue Repair Activity on Human Keratinocytes by Green Innovative Nanocomposites
Abstract
:1. Introduction
2. Results
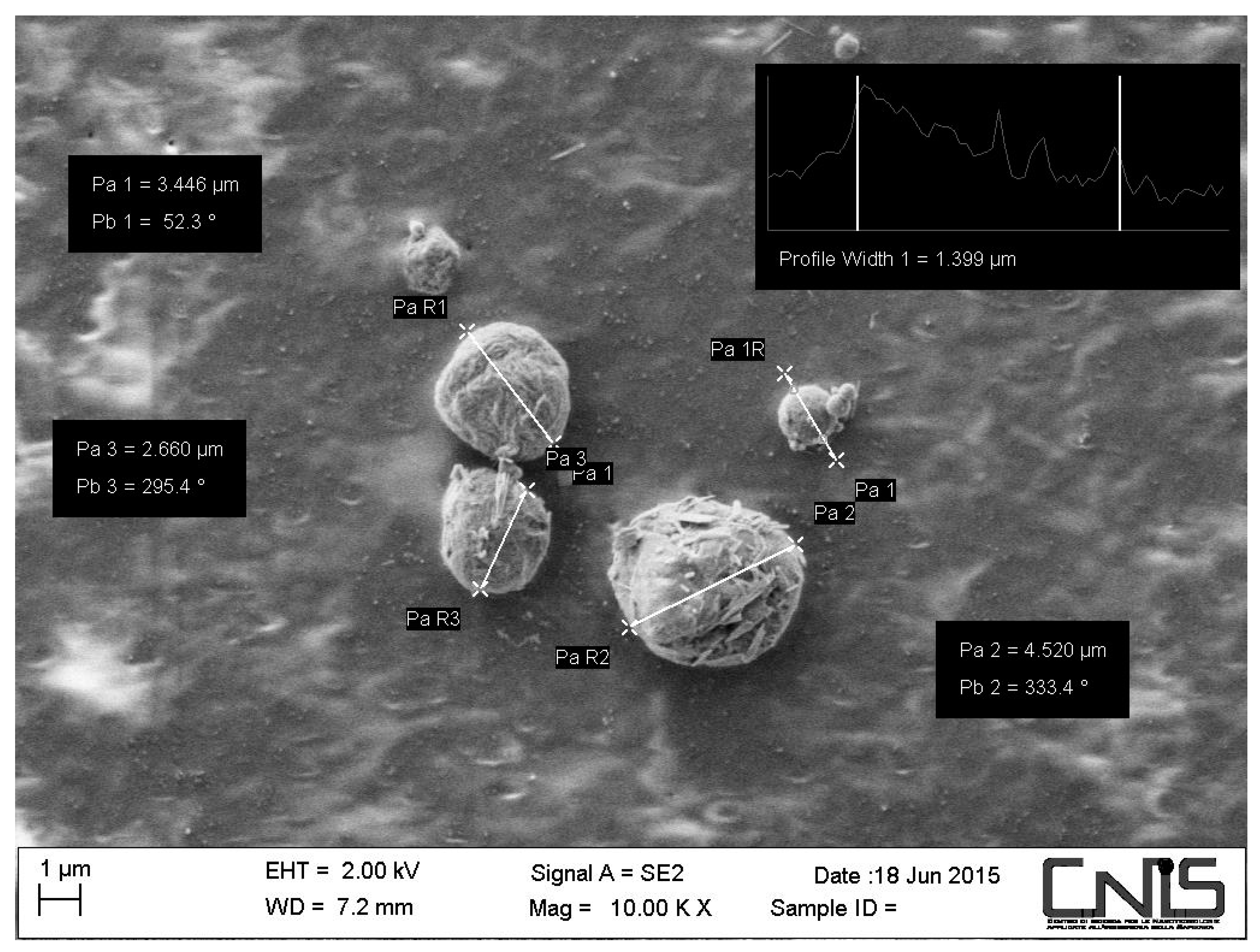
2.1. Morphology of the CN-Bio-Lignin Nanoparticles and the Bio-Nano-Composite Non-Woven Tissues
2.2. In Vitro Effectiveness of CN-Bio-Lignin Non-Woven Tissues on Cell Viability
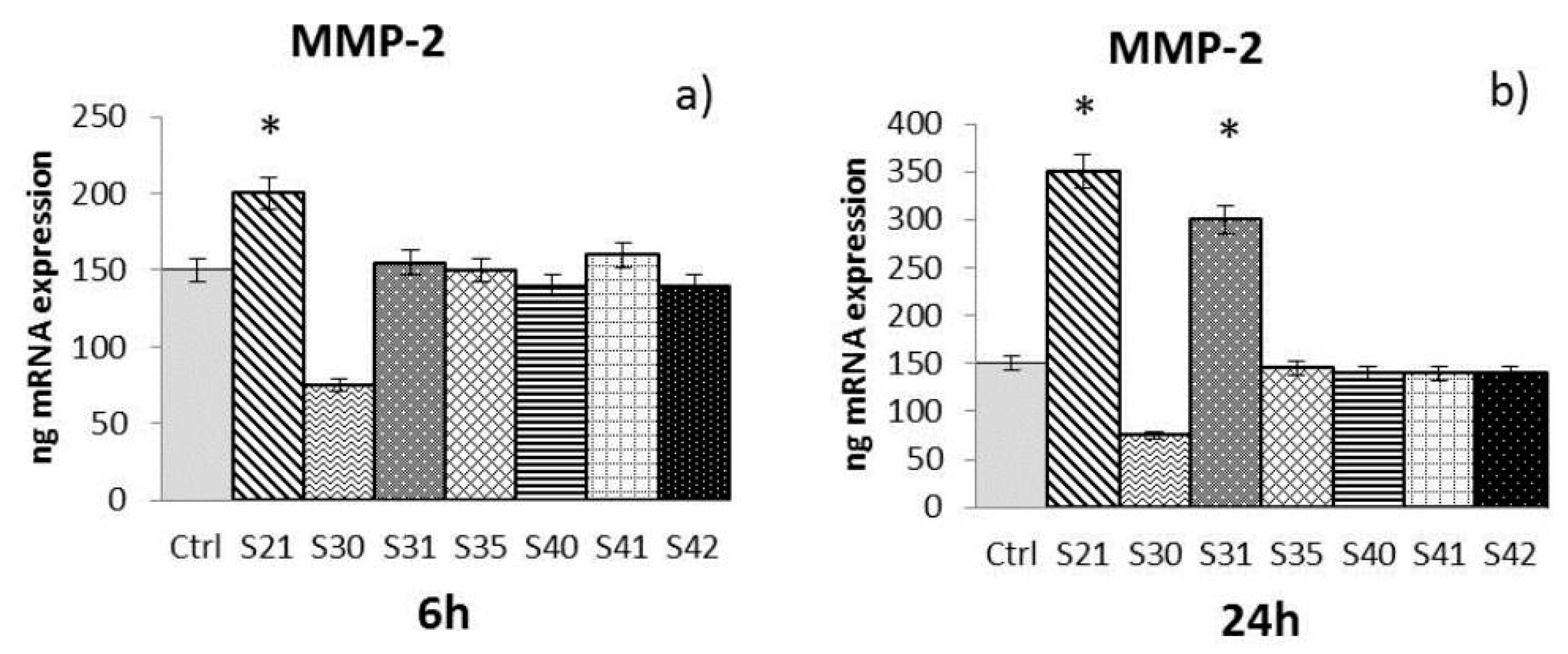
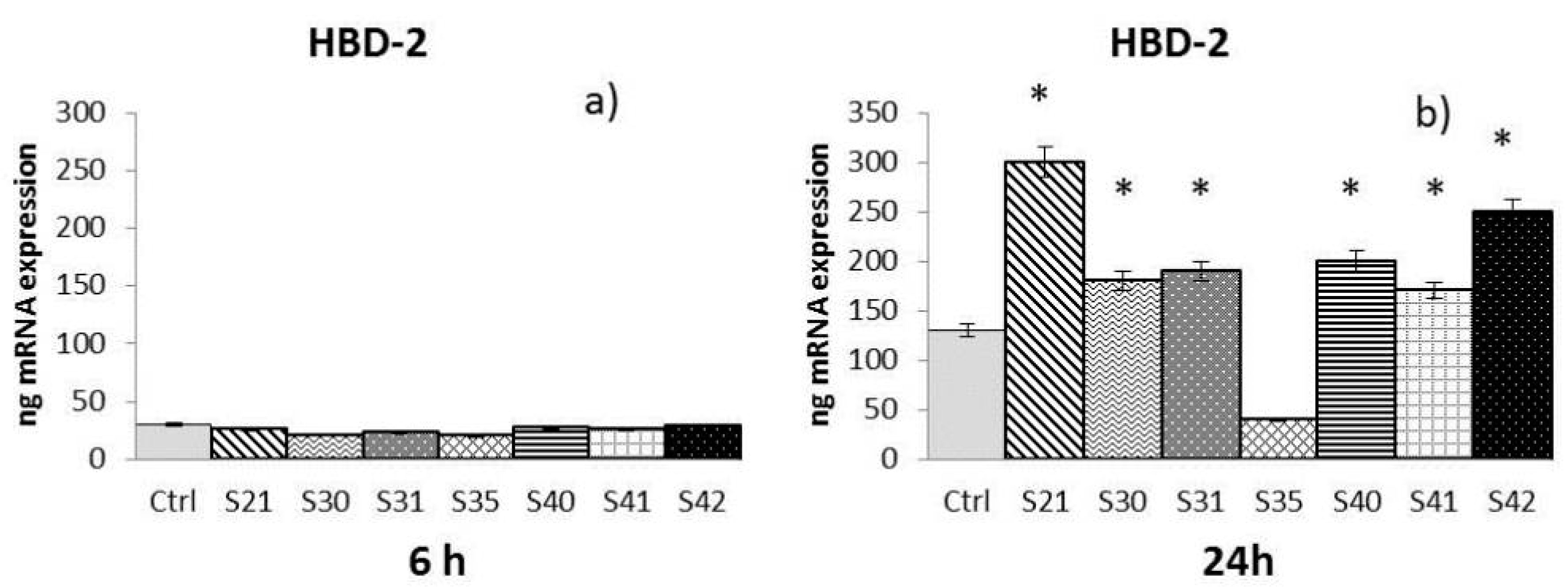
2.3. In Vitro Effectiveness of CN-Bio-Lignin Non-Woven Tissues on HBD-2 and MMPs Release from HaCat Cells
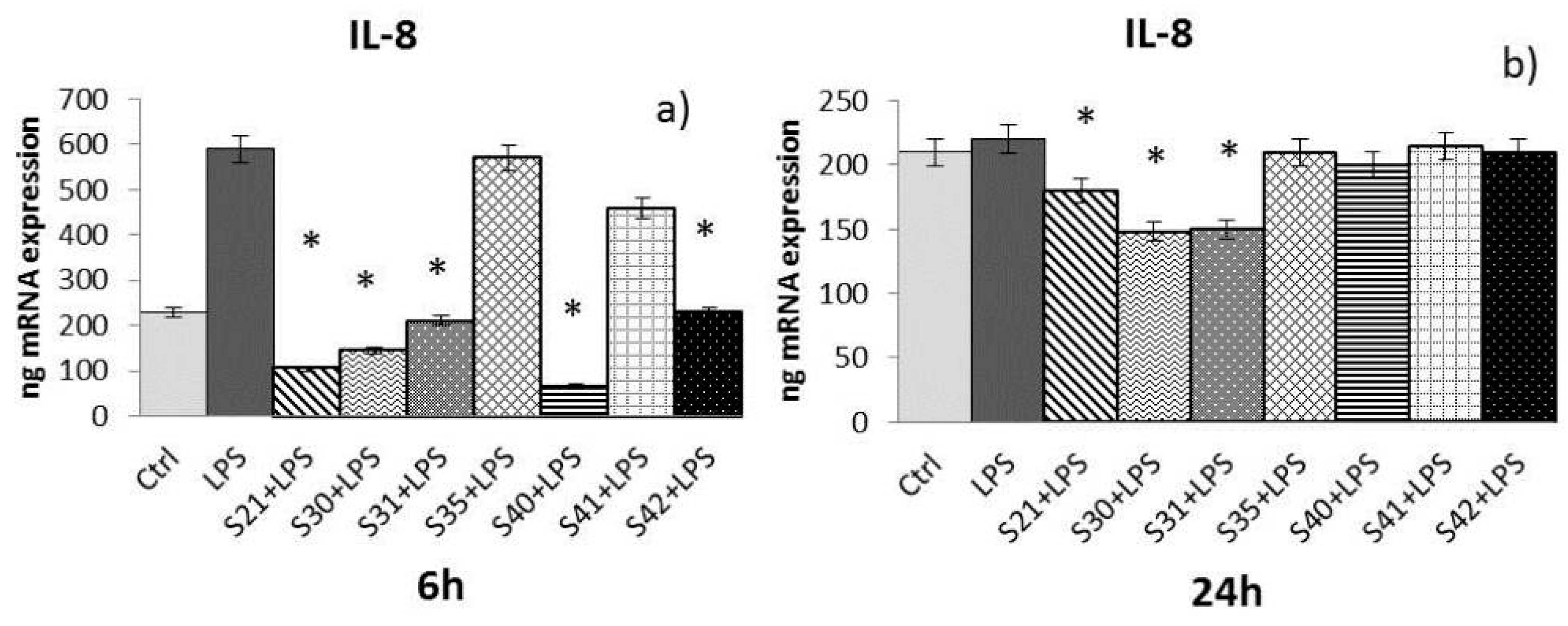
- -
- -
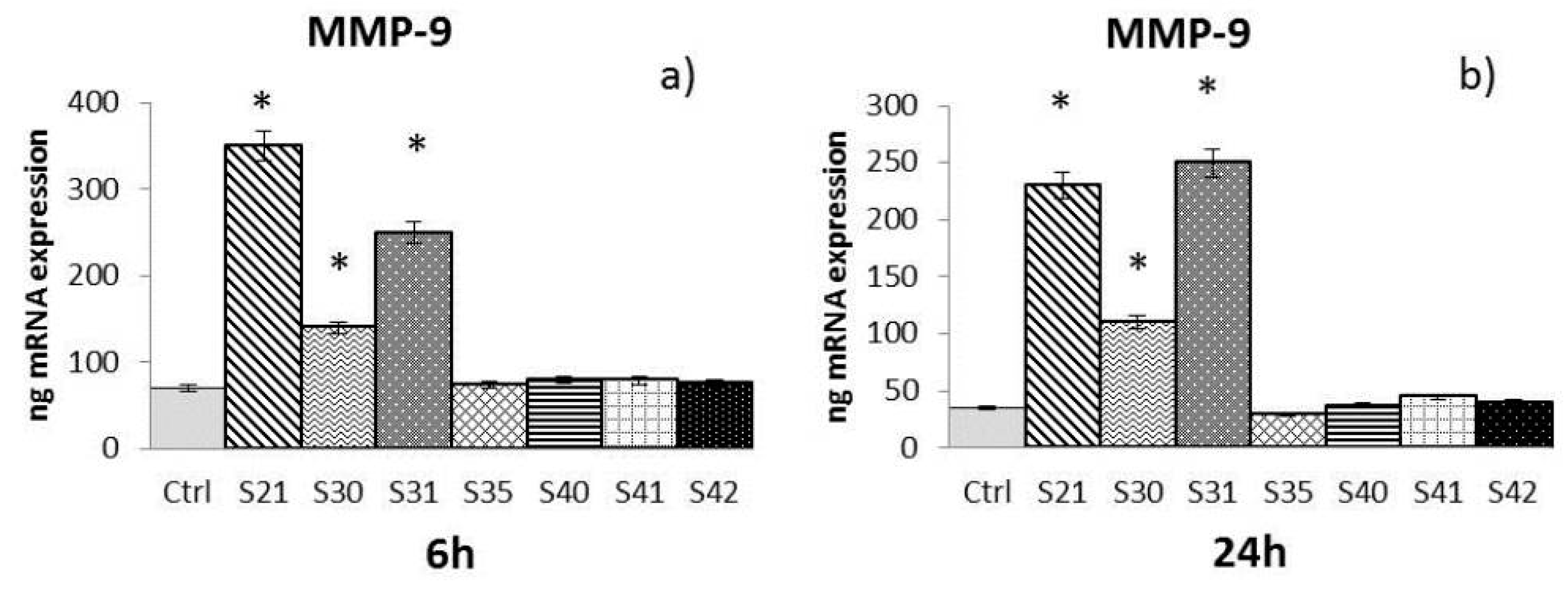
- MMP-9: After both 6 h and 24 h (Figure 4a,b), only S21, S30 and S31 among the bio-composites tested showed a significant increase of MMP-9 compared to control.
- -
2.4. In Vitro Effectiveness of CN-Bio-Lignin Non-Woven Tissues on Inflammatory Cytokines Released from HaCat Cells Treated with Lipopolysaccharide (LPS)
- -
- -
- -
- TNF-α: After both 6 and 24 h (Figure 8a,b) of treatment, only S21, S30, and S31 showed a significant reduction of TNF-α induced by LPS.
2.5. Statistical Analysis
3. Discussion
4. Materials and Methods
4.1. Materials and Non-Woven Tissue Preparation
4.2. Characterization of Nanoparticles and Non-Woven Tissues
4.3. Cell Culture
4.4. Cell Treatments
4.5. MTT Proliferation Assay
4.6. Real-Time PCR
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Iavicoli, I.; Leso, V.; Ricciardi, W.; Hodson, L.L.; Hoover, M.D. Opportunities and challenges of nanotechnology in the green economy. Environ. Health 2014, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wysokowski, M.; Petrenko, I.; Stelling, A.L.; Stawski, D.; Jesionowski, T.; Ehrlich, H. Poriferan Chitin as a Versatile Template for Extreme Biomimetics. Polymers 2015, 7, 235–265. [Google Scholar] [CrossRef]
- Salaberria, A.M.; Diaz, R.H.; Labidi, J.; Fernandez, S.C.M. Preparing valuable, renewable nanocomposite films based exclusively on ocean biomass-chitin nano fillers and chitosan. React. Funct. Polym. 2015, 89, 31–39. [Google Scholar] [CrossRef]
- Zdarta, J.; Klapiszewski, L.; Wysokowski, M.; Norman, M.; Kołodziejczak-Radzimska, A.; Moszyński, D.; Ehrlich, H.; Maciejewski, H.; Stelling, A.L.; Jesionowski, T. Chitin-Lignin Material as a Novel Matrix for Enzyme Immobilization. Mar. Drugs 2015, 13, 2424–2446. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Yamauchi, M.; Kim, S.K.; Kusaoke, H. Biomaterials: Chitosan and Collagen for Regenerative Medicine. BioMed Res. Intern. 2014. [Google Scholar] [CrossRef] [PubMed]
- Morganti, P. Bionanotechnology & Bioeconomy for a Greener Development. J. Appl. Cosmetol. 2015, 33, 51–65. [Google Scholar]
- Klapiszewski, L.; Wysokowski, M.; Majchrzak, I.; Szatkowski, T.; Nowacka, M.; Siwińska-Stefańska, K.; Szwarc-Rzepka, K.; Bartczak, P.; Ehrlich, H.; Jesionowski, T. Preparation and characterization of multifunctional chitin/lignin materials. J. Nanomater. 2013, 2013, 1–13. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and Chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 12, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Morganti, P. Green economy and Bionanotechnology to transform waste materials in useful goods: Results of EU research projects. Eurocosmetics 2015, 22, 12–16. [Google Scholar]
- Morganti, P.; Carezzi, F.; Del Ciotto, P.; Tischenko, G.; Chianese, A. A green multifunctional polymer from discarded material: Chitin Nanofibrils. Br. J. Appl. Sci. Technol. 2014, 4, 4175–4190. [Google Scholar] [CrossRef]
- Wysokowski, M.; Klapiszewski, L.; Moszyński, D.; Bartczak, P.; Szatkowski, T.; Majchrzak, I.; Siwińska-Stefańska, K.; Bazhenov, V.V.; Jesionowski, T. Modification of Chitin with Kraft Lignin and Development of New Biosorbents for Removal of Cadmium(II) and Nickel(II) Ions. Mar. Drugs 2014, 12, 2245–2268. [Google Scholar] [CrossRef] [PubMed]
- Harmita, H.; Karthikeyan, K.G.; Pan, X. Copper and cadmium sorption onto kraft and organosolv lignins. Biosource Technol. 2009, 100, 6183–6191. [Google Scholar] [CrossRef] [PubMed]
- Klapiszewski, L.; Bartczak, P.; Wysokowski, M.; Jesionowski, T. Silica conjugated with kraft lignin and its use as a novel ‘green’ sorbent for hazardous metal ions removal. Chem. Eng. J. 2015, 260, 684–693. [Google Scholar] [CrossRef]
- Morganti, P.; Palombo, M.; Carezzi, F.; Nunziata, M.L.; Morganti, G.; Cardillo, M.; Chianese, A. Green Nanotechnology Serving the Bioeconomy: Natural Beauty Masks to Save the Environment. Cosmetics 2016, 3, 41. [Google Scholar] [CrossRef]
- Morganti, P.; Del Ciotto, P.; Stoller, M.; Chianese, A. Antibacterial and Anti-inflammatory Green Nanocomposites. Chem. Eng. Trans. 2016, 47, 61–66. [Google Scholar]
- Buommino, E.; De Filippis, A.; Gaudiello, F.; Balato, A.; Balato, N.; Tufano, M.A.; Ayala, F. Modification of osteopontin and MMP-9 levels in patients with psoriasis on anti-TNF-α therapy. Arch. Dermatol. Res. 2012, 304, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Liotta, L.A.; Kohn, E.C. The microenvironment of the tumour-host interface. Nature 2001, 411, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Streuli, C. Extracellular matrix remodelling and cellular differentiation. Curr. Opin. Cell Biol. 1999, 11, 634–640. [Google Scholar] [CrossRef]
- Filippov, S.; Koenig, G.C.; Chun, T.H.; Hotary, K.B.; Ota, I.; Bugge, T.H.; Roberts, J.D.; Fay, W.P.; Birkedal-Hansen, H.; Holmbeck, K.; et al. MT1 matrix metalloproteinase directs arterial wall invasion and neointima formation by vascular smooth muscle cells. J. Exp. Med. 2005, 202, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.L.; Caley, M.; O’Toole, E.A. Matrix metalloproteinases and epidermal wound repair. Cell Tissue Res. 2013, 351, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Donnarumma, G.; Paoletti, I.; Fusco, A.; Perfetto, B.; Buommino, E.; de Gregorio, V.; Baroni, A. β-Defensins: Work in Progress. Adv. Exp. Med. Biol. 2016, 901, 59–76. [Google Scholar] [PubMed]
- Morganti, P.; Febo, P.; Cardillo, M.; Donnarumma, G.; Baroni, A. Chitin Nanofibril and Nanolignin: Natural Polymers of Biomedical Interest. J. Clin. Cosmet. Dermatol. 2017, 1. [Google Scholar] [CrossRef]
- Eide, K.B.; Norberg, A.N.; Heggset, E.B.; Lindbom, A.R.; Varum, K.M.; Eijsink, V.G.H.; Sorlie, M. Human Chitotriosidase-Catalyzed Hydrolysis of Chitosan. Biochemistry 2012, 51, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Ugartondo, V.; Mitjans, M.; Vinardell, M.P. Comparative antioxidant and cytotoxic effects of lignins from different sources. Bioresour. Technol. 2008, 99, 6683–6687. [Google Scholar] [CrossRef] [PubMed]
- Ugartondo, V.; Mitjans, M.; Vinardell, M.P. Applicability of lignins from different sources as antioxidant based on the protective effects on lipid peroxidation induced by oxygen radicals. Ind. Crops Prod. 2009, 30, 184–187. [Google Scholar] [CrossRef]
- Garcia, A.; Alrios, M.G.; Spigno, G.; Labidi, J. Lignin as natural radical scavenger. Effect of the obtaining and purification processes on the antioxidant behaviour of lignin. Biochem. Eng. J. 2012, 67, 173–185. [Google Scholar] [CrossRef]
- Yang, Y.; Rosenberg, G.A. Matrix metalloproteinases as therapeutic targets for stroke. Brain Res. 2015, 1623, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Angrisano, T.; Pero, R.; Paoletti, I.; Keller, S.; Lembo, L.; Baroni, A.; Chiariotti, L.; Lembo, F.; Donnarumma, G. Epigenetic regulation of IL-8 and β-defensin genes in human keratinocytes in response to Malassezia furfur. J. Investig. Dermatol. 2013, 133, 2101–2104. [Google Scholar] [CrossRef] [PubMed]
- Donnarumma, G.; De Gregorio, V.; Fusco, A.; Farina, E.; Baroni, A.; Esposito, V.; Contaldo, M.; Petruzzi, M.; Pannone, G.; Serpico, R. Inhibition of HSV-1 replication by laser diode-irradiation: Possible mechanism of action. Int. J. Immunopathol. Pharmacol. 2010, 23, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Donnarumma, G.; Paoletti, I.; Buommino, E.; Iovene, M.R.; Tudisco, L.; Cozza, V.; Tufano, M.A. Anti-inflammatory effects of moxifloxacin and human beta-defensin 2 association in human lung epithelial cell line (A549) stimulated with lipopolysaccharide. Peptides 2007, 28, 2286–2292. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, I.; Fusco, A.; Grimaldi, E.; Perillo, L.; Coretti, L.; Di Domenico, M.; Cozza, V.; Cataldo, M.; Serpico, R.; Guida, A.; et al. Assessment of host defence mechanisms induced by Candida species. Int. J. Immunopathol. Pharmacol. 2013, 26, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Baroni, A.; Donnarumma, G.; Paoletti, I.; Longanesi-Cattani, I.; Bifulco, K.; Tufano, M.A.; Carriero, M.V. Antimicrobial human beta-defensin-2 stimulates migration, proliferation and tube formation of human umbilical vein endothelial cells. Peptides 2009, 30, 267–272. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, F.; Brongo, S.; Ferraro, G.; Baroni, A. Prevention and treatment of keloids with intralesional verapamil. Dermatology 2002, 204, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Petrik, S.; Maly, M. Production Nozzle-Less Electrospinning Nanofiber Technology. MRS Online Proc. Libr. Arch. 2009. [Google Scholar] [CrossRef]



| Sample | Concentration (10 µg/mL) | O.D. |
|---|---|---|
| Ctr | 1.56 | |
| S35 | 10 | 1.7 |
| 50 | 1.5 | |
| 100 | 1.7 | |
| S21 | 10 | 1.4 |
| 50 | 1.3 | |
| 100 | 1.35 | |
| S40 | 10 | 1.6 |
| 50 | 1.56 | |
| 100 | 1.61 | |
| S42 | 10 | 1.35 |
| 50 | 1.36 | |
| 100 | 1.38 | |
| S41 | 10 | 1.71 |
| 50 | 1.69 | |
| 100 | 1.5 | |
| S30 | 10 | 1.3 |
| 50 | 1.2 | |
| 100 | 1.26 | |
| S31 | 10 | 1.3 |
| 50 | 1.36 | |
| 100 | 1.4 |
| Samples | Main Composites | ||
|---|---|---|---|
| S35 | PEO 7% | CN 30% (w/w) | ///// |
| S21 | PEO 7% | CN 30% (w/w) | Lignin 0.1% |
| S40 | PEO 7% | CN 30% (w/w) | Lignin 1% |
| S42 | PEO 7% | CN 30% (w/w) | Lignin 2.5% |
| S41 | PEO 7% | CN 30% (w/w) | Lignin 5% |
| S30 | PEO 7% | CN 30% (w/w) | Lignin I 0.1% |
| S31 | PEO 7% | CN 30% (w/w) | Lignin II 0.1% |
| Gene | Primers Sequence | Conditions | Product Size (bp) |
|---|---|---|---|
| IL-8 | 5-ATGACTTCCAAGCTGGCCGTG-3′ | 5″ at 94 °C, 6″ at 55 °C, | 297 |
| 5-TGAATTCTCAGCCCTCTTCAAAAACTTCTC-3 | 12″ at 72 °C for 40 cycles | ||
| IL-1 α | 5′-CATGTCAAATTTCACTGCTTCATCC-3′ | 5″ at 95 °C, 8″ at 55 °C, | 421 |
| 5′-GTCTCTGAATCAGAAATCCTTCTATC-3′ | 17″ at 72 °C for 45 cycles | ||
| TNF- α | 5′-CAGAGGGAAGAGTTCCCCAG-3′ | 5″ at 95 °C, 6″ at 57 °C, | 324 |
| 5′-CCTTGGTCTGGTAGGAGACG-3′ | 13″ at 72 °C for 40 cycles | ||
| MMP-2 | 5′-TGACGGTAAGGACGGACTC-3′ | 5″ at 94 °C, 7″ at 57 °C, | 342 |
| 5′-TGGAAGCGGATTGGAAACT-3′ | 14″ at 72 °C for 40 cycles | ||
| MMP-9 | 5′-GCCAACTACGACACCGACGA-3′ | 5″ at 95 °C, 6″ at 56 °C, | 310 |
| 5′-CGCTGGTACAGGTCGAGTAC-3′ | 12″ at 72 °C for 40 cycles | ||
| HBD-2 | 5′-GGATCCATGGGTATAGGCGATCCTGTTA-3′ | 5″ at 94 °C, 6″ at 63 °C, | 198 |
| 5′-AAGCTTCTCTGATGAGGGAGCCCTTTCT-3′ | 10″ at 72 °C for 50 cycles |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morganti, P.; Fusco, A.; Paoletti, I.; Perfetto, B.; Del Ciotto, P.; Palombo, M.; Chianese, A.; Baroni, A.; Donnarumma, G. Anti-Inflammatory, Immunomodulatory, and Tissue Repair Activity on Human Keratinocytes by Green Innovative Nanocomposites. Materials 2017, 10, 843. https://doi.org/10.3390/ma10070843
Morganti P, Fusco A, Paoletti I, Perfetto B, Del Ciotto P, Palombo M, Chianese A, Baroni A, Donnarumma G. Anti-Inflammatory, Immunomodulatory, and Tissue Repair Activity on Human Keratinocytes by Green Innovative Nanocomposites. Materials. 2017; 10(7):843. https://doi.org/10.3390/ma10070843
Chicago/Turabian StyleMorganti, Pierfrancesco, Alessandra Fusco, Iole Paoletti, Brunella Perfetto, Paola Del Ciotto, Marco Palombo, Angelo Chianese, Adone Baroni, and Giovanna Donnarumma. 2017. "Anti-Inflammatory, Immunomodulatory, and Tissue Repair Activity on Human Keratinocytes by Green Innovative Nanocomposites" Materials 10, no. 7: 843. https://doi.org/10.3390/ma10070843
APA StyleMorganti, P., Fusco, A., Paoletti, I., Perfetto, B., Del Ciotto, P., Palombo, M., Chianese, A., Baroni, A., & Donnarumma, G. (2017). Anti-Inflammatory, Immunomodulatory, and Tissue Repair Activity on Human Keratinocytes by Green Innovative Nanocomposites. Materials, 10(7), 843. https://doi.org/10.3390/ma10070843







