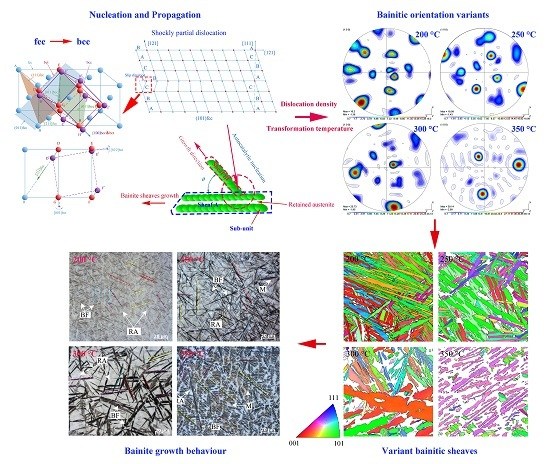
Effect of Isothermal Temperature on Growth Behavior of Nanostructured Bainite in Laser Cladded Coatings
Abstract
:1. Introduction
2. Materials and Experimental Procedures
3. Results and Discussion
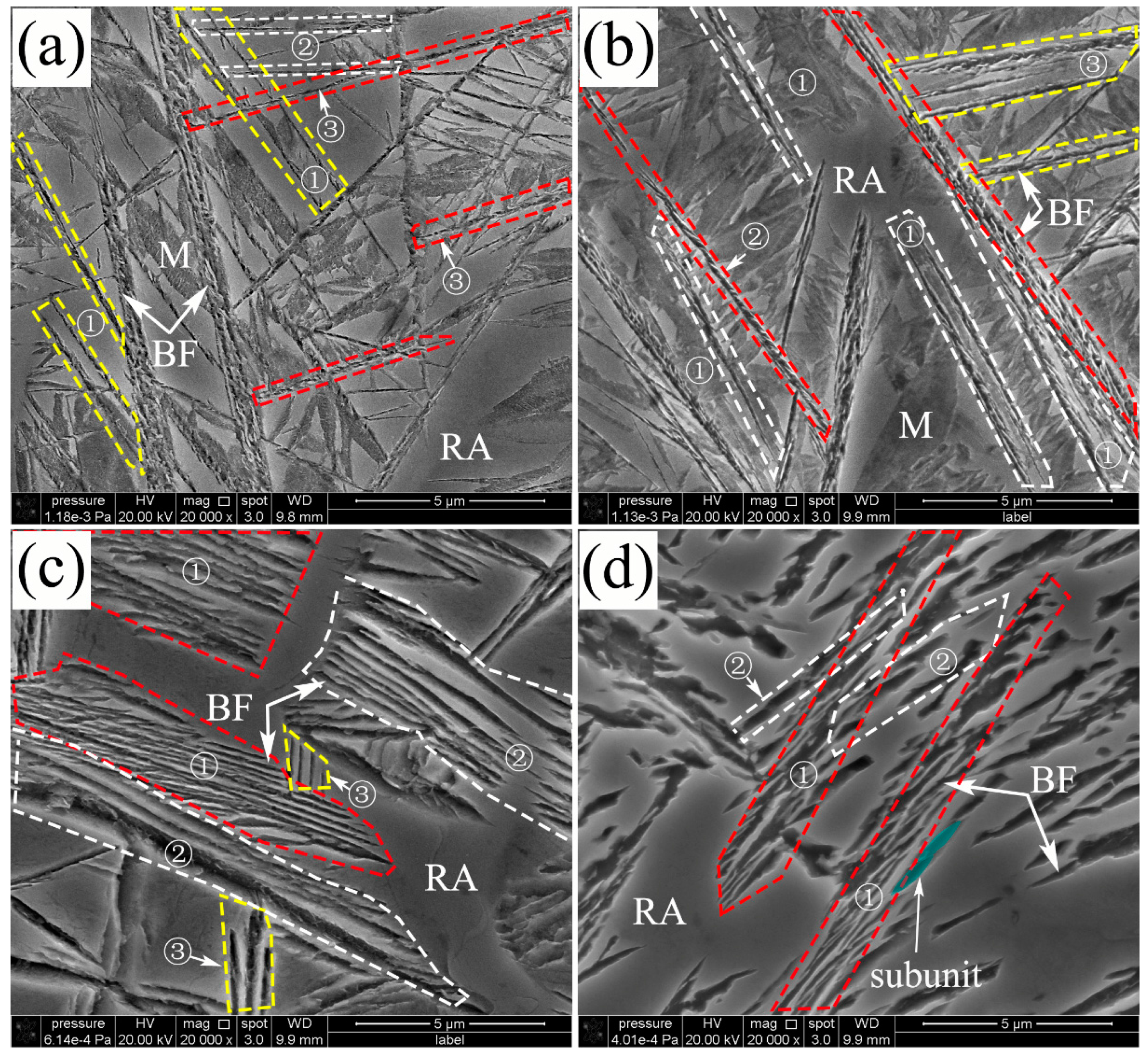
3.1. Characteristics of the Microstructure of Nanobainite
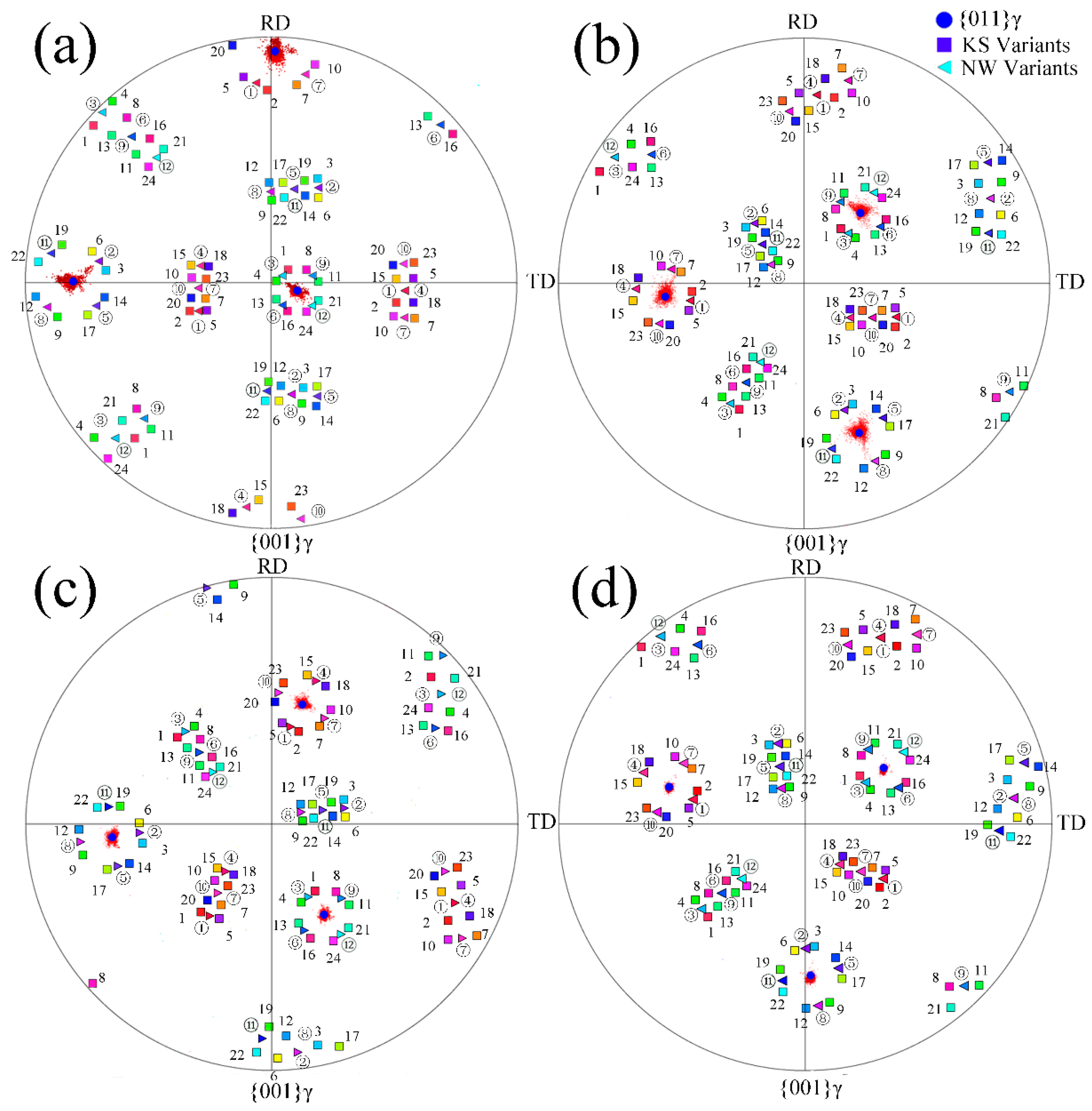
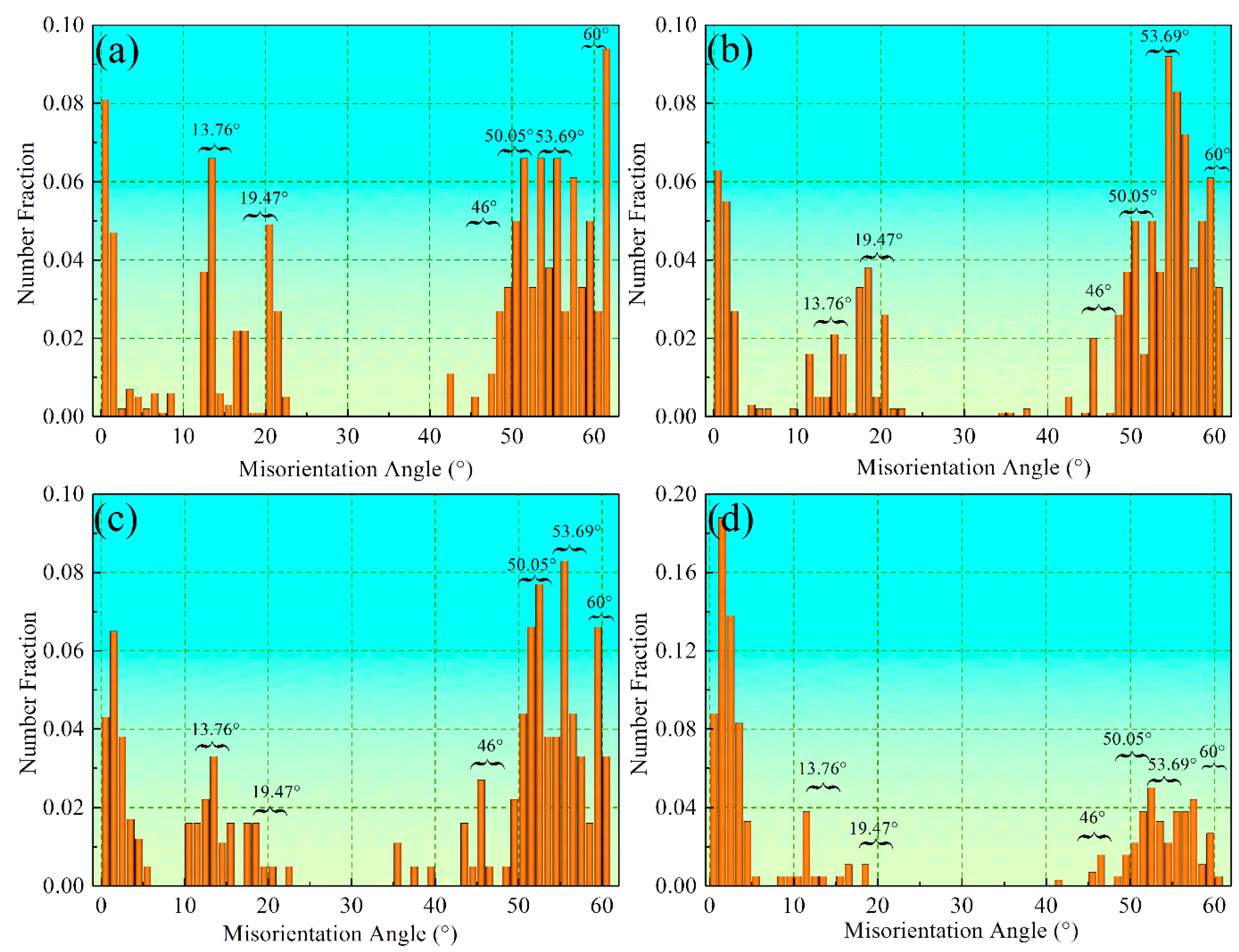
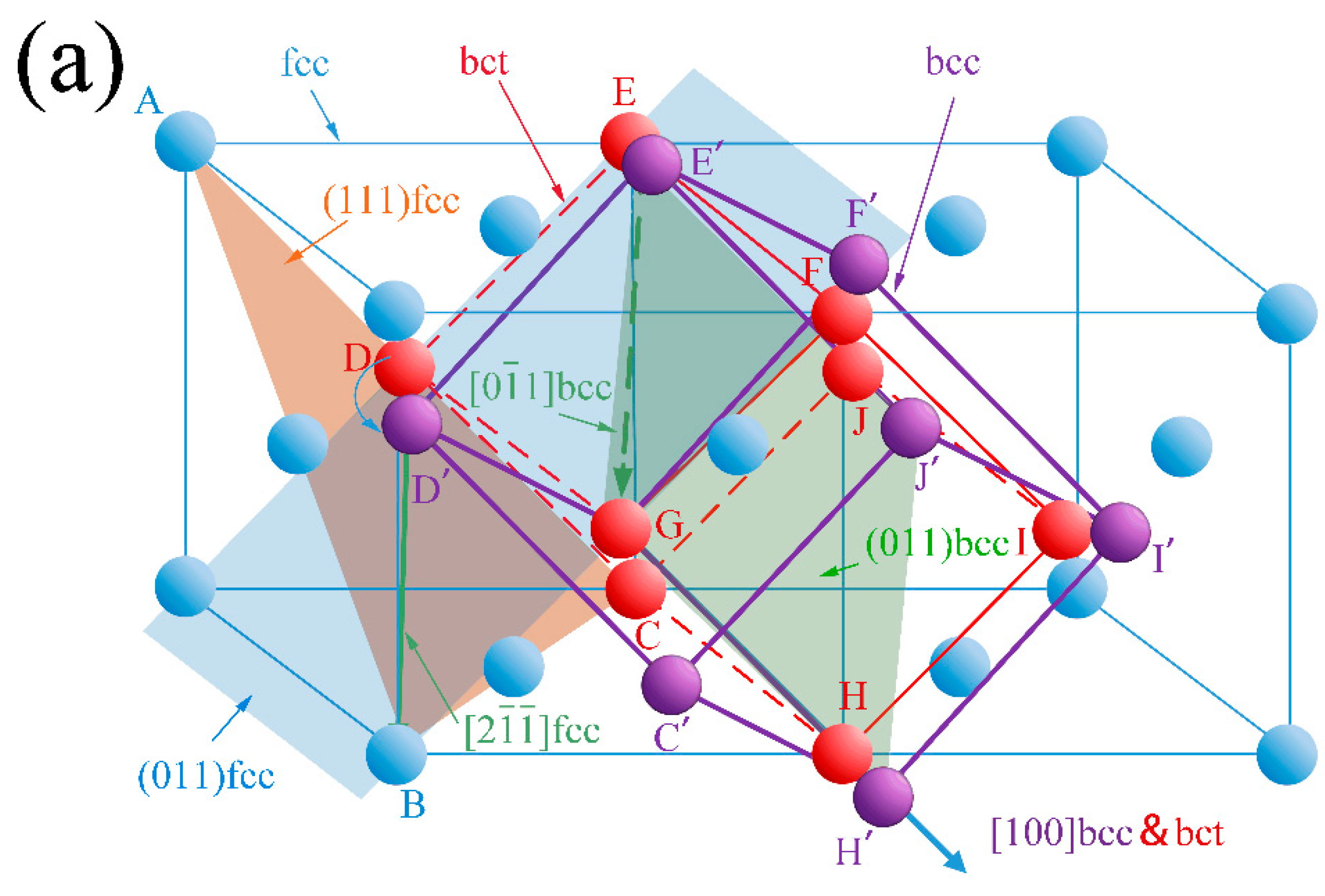
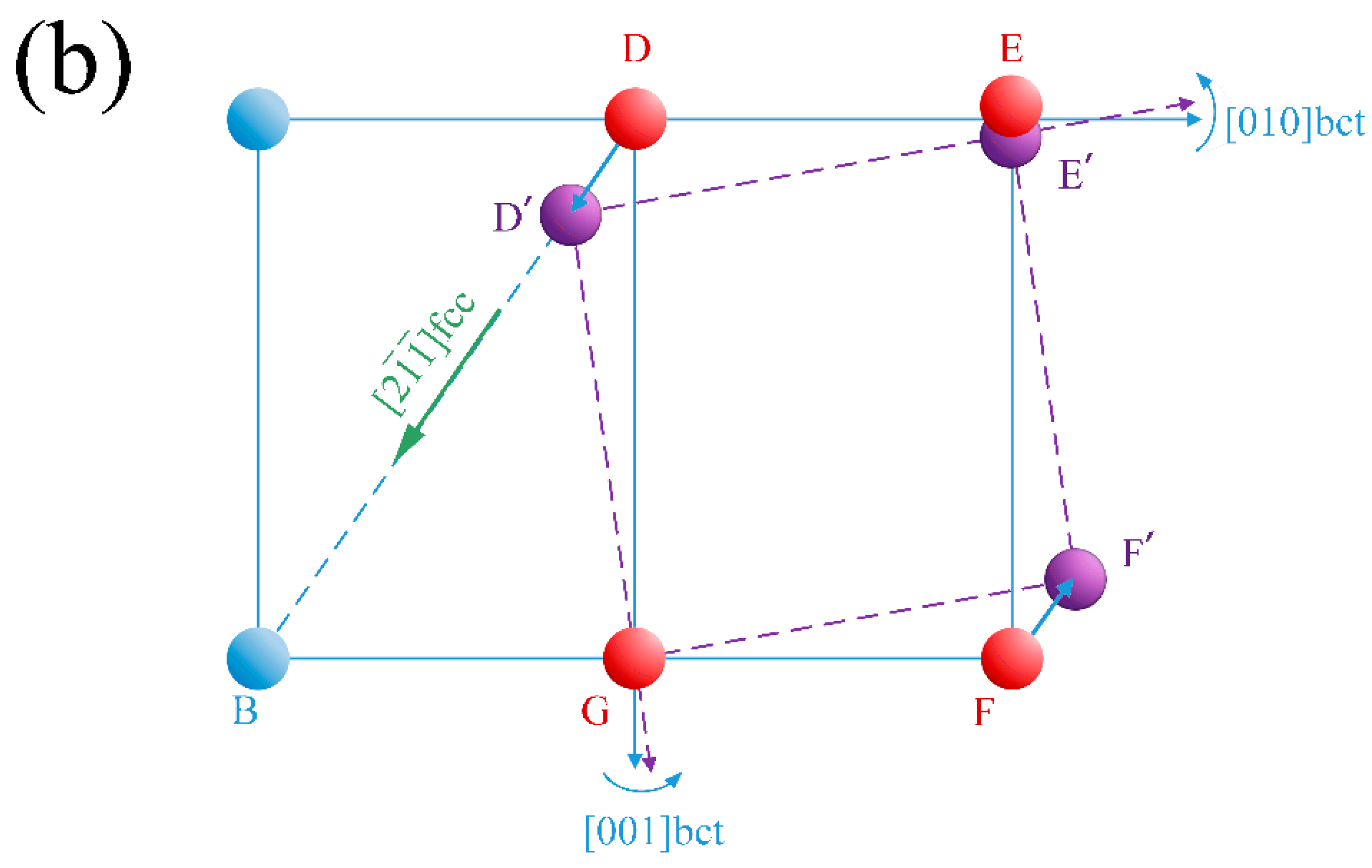
3.2. Crystal Orientation Relationship
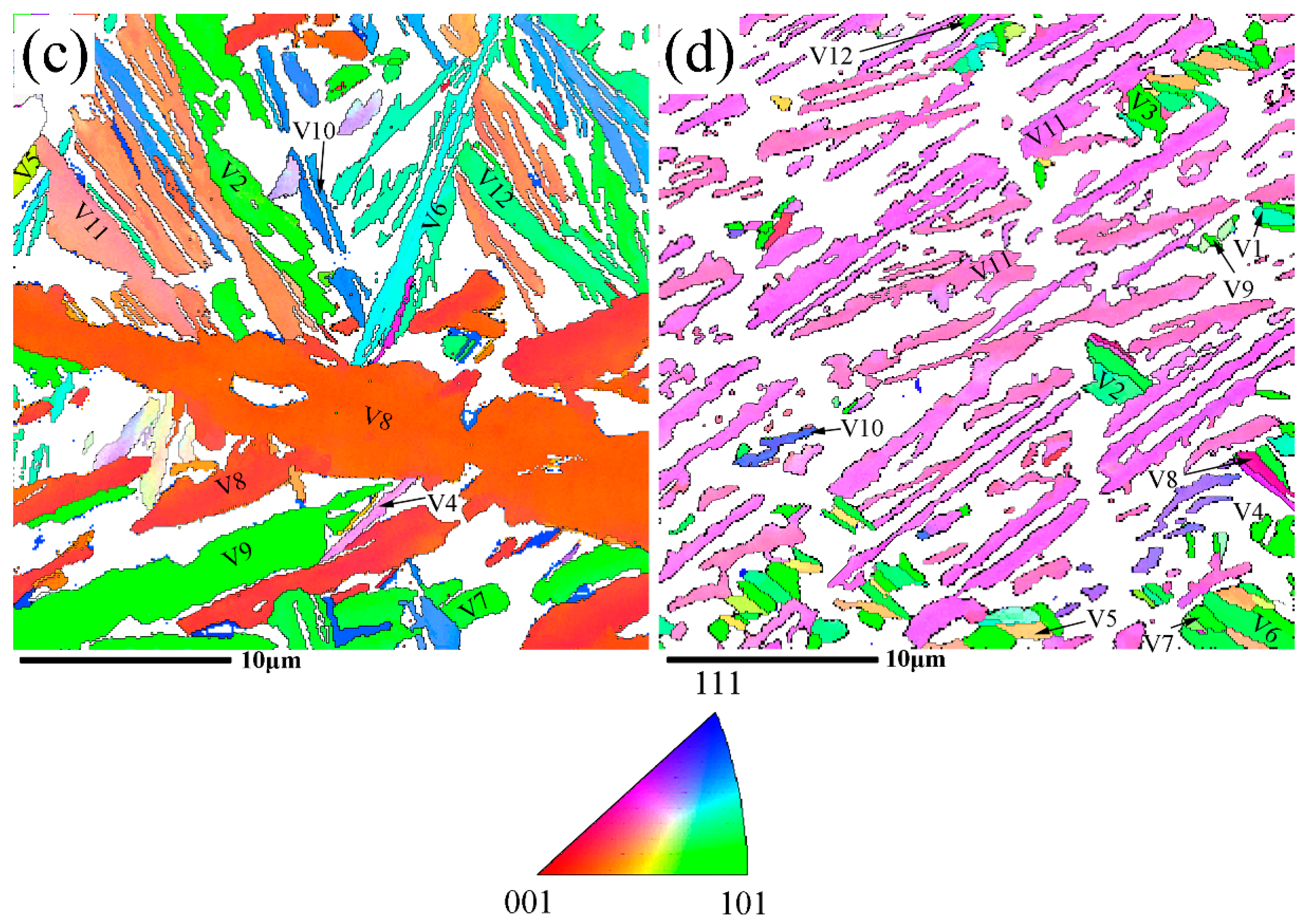
3.3. Variants of Growth Directions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Caballero, F.G.; Bhadeshia, H.K.D.H.; Mawella, K.J.A.; Jones, D.G.; Brown, P. Design of novel high strength bainitic steels: Part 1. Mater. Sci. Technol. 2001, 17, 512–516. [Google Scholar] [CrossRef]
- Caballero, F.G.; Allain, S.; Cornide, J.; Velásquez, J.P.; Garcia-Mateo, C.; Miller, M.K. Design of cold rolled and continuous annealed carbide-free bainitic steels for automotive application. Mater. Des. 2013, 49, 667–680. [Google Scholar] [CrossRef]
- Caballero, F.G.; Garcia-Mateo, C.; Miller, M.K. Design of Novel Bainitic Steels: Moving from UltraFine to Nanoscale Structures. JOM 2014, 66, 747–755. [Google Scholar] [CrossRef]
- Caballero, F.G.; Garcia-Mateo, C.; Miller, M.K. Modern steels at atomic and nanometre scales. Mater. Sci. Technol. 2015, 31, 764–772. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Caballero, F.G.; Sourmail, T.; Smanio, V.; De Andres, C.G. Industrialised nanocrystalline bainitic steels. Design approach. Int. J. Mater. Res. 2014, 105, 725–734. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Bhadeshia, H.K.D.H.; Caballero, F.G. Acceleration of Low-temperature Bainite. ISIJ Int. 2003, 43, 1821–1825. [Google Scholar] [CrossRef]
- Mingxi, L.; Yizhu, H.; Xiaomin, Y. Effect of nano-Y2O3 on microstructure of laser cladding cobalt-based alloy coatings. Appl. Surf. Sci. 2006, 252, 2882–2887. [Google Scholar] [CrossRef]
- Guo, Y.; Li, Z.; Yao, C.; Zhang, K.; Lu, F.; Feng, K.; Huang, J.; Wang, M.; Wu, Y. Microstructure evolution of Fe-based nanostructured bainite coating by laser cladding. Mater. Des. 2014, 63, 100–108. [Google Scholar] [CrossRef]
- Guo, Y.; Feng, K.; Lu, F.; Zhang, K.; Li, Z.; Hosseini, S.R.E.; Wang, M. Effects of isothermal heat treatment on nanostructured bainite morphology and microstructures in laser cladded coatings. Appl. Surf. Sci. 2015, 357, 309–316. [Google Scholar] [CrossRef]
- Morito, S.; Yoshida, H.; Maki, T.; Huang, X. Effect of block size on the strength of lath martensite in low carbon steels. Mater. Sci. Eng. A 2006, 438, 237–240. [Google Scholar] [CrossRef]
- Gourgues, A.F.; Flower, H.M.; Lindley, T.C. Electron backscattering diffraction study of acicular ferrite, bainite, and martensite steel microstructures. Mater. Sci. Technol. 2000, 16, 26–40. [Google Scholar] [CrossRef]
- Bakhtiari, R.; Ekrami, A. The effect of bainite morphology on the mechanical properties of a high bainite dual phase (HBDP) steel. Mater. Sci. Eng. A 2009, 525, 159–165. [Google Scholar] [CrossRef]
- Abbaszadeh, K.; Saghafian, H.; Kheirandish, S. Effect of Bainite Morphology on Mechanical Properties of the Mixed Bainite-martensite Microstructure in D6AC Steel. J. Mater. Sci. Technol. 2012, 28, 336–342. [Google Scholar] [CrossRef]
- Beladi, H.; Adachi, Y.; Timokhina, I.; Hodgson, P.D. Crystallographic analysis of nanobainitic steels. Scr. Mater. 2009, 60, 455–458. [Google Scholar] [CrossRef]
- Gong, W.; Tomota, Y.; Adachi, Y.; Paradowska, A.M.; Kelleher, J.F.; Zhang, S.Y. Effects of ausforming temperature on bainite transformation, microstructure and variant selection in nanobainite steel. Acta Mater. 2013, 61, 4142–4154. [Google Scholar] [CrossRef]
- Beladi, H.; Timokhina, I.B.; Hodgson, P.D.; Adachi, Y. Characterization of nanostructured bainitic steel. Int. J. Mod. Phys. 2012, 5, 1–8. [Google Scholar]
- Furuhara, T.; Kawata, H.; Morito, S.; Miyamoto, G.; Maki, T. Variant Selection in Grain Boundary Nucleation of Upper Bainite. Metall. Mater. Trans. A 2008, 39, 1003–1013. [Google Scholar] [CrossRef]
- Guo, Z.; Lee, C.S.; Morris, W., Jr. On coherent transformations in steel. Acta Mater. 2004, 52, 5511–5518. [Google Scholar] [CrossRef]
- Gaude-Fugarolas, D.; Jacques, P.J. A New Physical Model for the Kinetics of the Bainite Transformation. ISIJ Int. 2006, 46, 712–717. [Google Scholar] [CrossRef]
- Tszeng, T.C. Autocatalysis in bainite transformations. Mater. Sci. Eng. A 2000, 293, 185–190. [Google Scholar] [CrossRef]
- Matsuzaki, A.; Bhadeshia, H.K.D.H. Effect of austenite grain size and bainite morphology on overall kinetics of bainite transformation in steels. Mater. Sci. Technol. 1999, 15, 518–522. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H.; Christian, J.W. Bainite in steels. MTA 1990, 21, 767–797. [Google Scholar] [CrossRef]
- Rees, G.I.; Bhadeshia, H.K.D.H. Bainite transformation kinetics Part 1 Modified model. Mater. Sci. Technol. 1992, 8, 985–993. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Bainite in Steels, 3rd ed.; Taylor & Francis Ltd.: Oxford, UK, 2015; pp. 19–60. [Google Scholar]
- Gu, X.F.; Furuhara, T.; Zhang, W.Z. PTCLab: A python program to calculate phase transformation crystallography. J. Appl. Cryst. 2014, 49, 1099–1106. [Google Scholar] [CrossRef]
- Kundu, S.; Bhadeshia, H.K.D.H. Crystallographic texture and intervening transformations. Scr. Mater. 2007, 57, 869–872. [Google Scholar] [CrossRef]
- Takayama, N.; Miyamoto, G.; Furuhara, T. Effects of transformation temperature on variant pairing of bainitic ferrite in low carbon steel. Acta Mater. 2012, 60, 2387–2396. [Google Scholar] [CrossRef]
- Kitahara, H.; Ueji, R.; Ueda, M.; Tsuji, N.; Minamino, Y. Crystallographic analysis of plate martensite in Fe–28.5 at.% Ni by FE-SEM/EBSD. Mater. Charact. 2005, 54, 378–386. [Google Scholar] [CrossRef]
- Van Bohemen, S.M.; Sietsma, J. Modeling of isothermal bainite formation based on the nucleation kinetics. Int. J. Mater. Res. 2008, 99, 739–747. [Google Scholar] [CrossRef]
- Abbasi-Bani, A.; Zarei-Hanzaki, A.; Pishbin, M.H.; Haghdadi, N. A comparative study on the capability of Johnson-Cook and Arrhenius-type constitutive equations to describe the flow behavior of Mg-6Al-1Zn alloy. Mech. Mater. 2014, 71, 52–61. [Google Scholar] [CrossRef]







| Laser Cladding | Isothermal Heat Treatment | ||||
|---|---|---|---|---|---|
| Laser Power (W) | Preheating Temperature (°C) | Scanning Velocity (mm/s) | Powder Feed Rate (g/min) | Isothermal Temperature (°C) | Isothermal Times (h) |
| 2500 | 220 | 10 | 30 | 200 | 1, 2, 4, 8, 12, 24, 48, |
| 2400 | 270 | 10 | 30 | 250 | 1/2, 1, 2, 3, 4, 8, 12, 16 |
| 2300 | 320 | 10 | 30 | 300 | 1/4, 1/2, 3/4, 1, 3/2, 2, 3, 4, 6 |
| 2200 | 370 | 10 | 30 | 350 | 1/4, 1/3, 1/2, 3/4, 1, 1.5, 2, 3, 4 |
| Isothermal Temperature (°C) | Orientation of the Retained Austenite | |
|---|---|---|
| Plane | Direction | |
| 200 | (0.0898 0.2007 0.9755) | [0.9955 0.0114 −0.094] |
| 250 | (0.5175 0.4221 0.7443) | [−0.0624 −0.8489 0.5248] |
| 300 | (0.6015 0.4011 0.6909) | [0.7738 −0.0776 −0.6286] |
| 350 | (0.5122 0.4406 0.7373) | [0.2266 −0.8973 0.3788] |
| Variant | Parallel Plane | Parallel Direction | Rotation Axis in V1 | Rotation Angle (deg) |
|---|---|---|---|---|
| V1 | (111)γ//(011)α | [01]γ//[1]α | ||
| V2 | (111)γ//(011)α | [01]γ//[1]α | [0.58 −0.58 0.58] | 60.00 |
| V3 | (111)γ//(011)α | [01]γ//[1]α | [0.00 −0.71 −0.71] | 60.00 |
| V4 | (111)γ//(011)α | [01]γ//[1]α | [0.00 0.71 0.71] | 10.53 |
| V5 | (111)γ//(011)α | [10]γ//[1]α | [0.00 0.71 0.71] | 60.00 |
| V6 | (111)γ//(011)α | [10]γ//[1]α | [0.00 −0.71 −0.71] | 49.47 |
| V7 | (11)γ//(011)α | [10]γ//[1]α | [−0.58 −0.58 0.58] | 49.47 |
| V8 | (11)γ//(011)α | [10]γ//[1]α | [0.58 −0.58 0.58] | 10.53 |
| V9 | (11)γ//(011)α | [0]γ//[1]α | [−0.19 0.77 0.61] | 50.51 |
| V10 | (11)γ//(011)α | [0]γ//[1]α | [−0.49 −0.46 0.74] | 50.51 |
| V11 | (11)γ//(011)α | [011]γ//[1]α | [0.35 −0.93 −0.07] | 14.88 |
| V12 | (11)γ//(011)α | [011]γ//[1]α | [0.36 −0.71 0.60] | 57.21 |
| V13 | (11)γ//(011)α | [01]γ//[1]α | [0.93 0.35 0.07] | 14.88 |
| V14 | (11)γ//(011)α | [01]γ//[1]α | [0.74 0.46 −0.49] | 50.51 |
| V15 | (11)γ//(011)α | [0]γ//[1]α | [−0.25 −0.63 −0.74] | 57.21 |
| V16 | (11)γ//(011)α | [0]γ//[1]α | [0.66 0.66 0.36] | 20.61 |
| V17 | (11)γ//(011)α | [110]γ//[1]α | [−0.66 0.36 −0.66] | 51.73 |
| V18 | (11)γ//(011)α | [110]γ//[1]α | [−0.30 −0.63 −0.72] | 47.11 |
| V19 | (11)γ//(011)α | [10]γ//[1]α | [−0.61 0.19 −0.77] | 50.51 |
| V20 | (11)γ//(011)α | [10]γ//[1]α | [−0.36 −0.60 −0.71] | 57.21 |
| V21 | (11)γ//(011)α | [0]γ//[1]α | [0.96 0.00 −0.30] | 20.61 |
| V22 | (11)γ//(011)α | [0]γ//[1]α | [−0.72 0.30 −0.63] | 47.11 |
| V23 | (11)γ//(011)α | [101]γ//[1]α | [−0.74 −0.25 0.63] | 57.21 |
| V24 | (11)γ//(011)α | [101]γ//[1]α | [0.91 −0.41 0.00] | 21.06 |
| Variant | Parallel Plane | Parallel Direction | Rotation Axis in V1 | Rotation Angle (deg) |
|---|---|---|---|---|
| V1 | (111)γ//(011)α | [2]γ//[01]α | ||
| V2 | (111)γ//(011)α | [21]γ//[01]α | [0.000 −0.707 −0.707] | 60.00 |
| V3 | (111)γ//(011)α | [2]γ//[01]α | [0.00 0.707 0.707] | 60.00 |
| V4 | (11)γ//(011)α | []γ//[01]α | [1.000 0.000 0.000] | 49.47 |
| V5 | (11)γ//(011)α | [12]γ//[01]α | [−0.223 −0.697 −0.681] | 10.53 |
| V6 | (11)γ//(011)α | [02]γ//[01]α | [−0.223 0.697 0.681] | 50.51 |
| V7 | (11)γ//(011)α | [21]γ//[01]α | [0.706 −0.706 −0.060] | 14.88 |
| V8 | (11)γ//(011)α | []γ//[01]α | [−0.681 −0.223 0.697] | 50.51 |
| V9 | (11)γ//(011)α | [12]γ//[01]α | [−0.624 0.471 −0.624] | 57.21 |
| V10 | (1)γ//(011)α | [21]γ//[01]α | [0.706 0.706 0.060] | 50.51 |
| V11 | (1)γ//(011)α | [21]γ//[01]α | [−0.624 −0.471 0.624] | 57.21 |
| V12 | (1)γ//(011)α | [2]γ//[01]α | [−0.681 0.223 −0.697] | 20.61 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Yao, C.; Feng, K.; Li, Z.; Chu, P.K.; Wu, Y. Effect of Isothermal Temperature on Growth Behavior of Nanostructured Bainite in Laser Cladded Coatings. Materials 2017, 10, 800. https://doi.org/10.3390/ma10070800
Guo Y, Yao C, Feng K, Li Z, Chu PK, Wu Y. Effect of Isothermal Temperature on Growth Behavior of Nanostructured Bainite in Laser Cladded Coatings. Materials. 2017; 10(7):800. https://doi.org/10.3390/ma10070800
Chicago/Turabian StyleGuo, Yanbing, Chengwu Yao, Kai Feng, Zhuguo Li, Paul K. Chu, and Yixiong Wu. 2017. "Effect of Isothermal Temperature on Growth Behavior of Nanostructured Bainite in Laser Cladded Coatings" Materials 10, no. 7: 800. https://doi.org/10.3390/ma10070800
APA StyleGuo, Y., Yao, C., Feng, K., Li, Z., Chu, P. K., & Wu, Y. (2017). Effect of Isothermal Temperature on Growth Behavior of Nanostructured Bainite in Laser Cladded Coatings. Materials, 10(7), 800. https://doi.org/10.3390/ma10070800