Antibacterial Activity of Commercial Dentine Bonding Systems against E. faecalis–Flow Cytometry Study
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Eluate Preparation
4.2. Microbank System
4.3. Bacteria Suspension Preparation
4.4. Bacteria Incubation
4.5. Flow Cytometry Staining Procedure
4.6. Statistical Analysis
5. Conclusions
- (1)
- Flow cytometry seemed to be a very useful evaluation method of antibacterial activity of dentine bonding systems.
- (2)
- Self-etching bonding systems exhibit significantly higher antibacterial activity against E. faecalis in comparison to total-etching DBS.
- (3)
- The highest percentage of dead bacteria cells was found for G-ænial Bond, while the lowest–for Te-Econom Bond.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cocco, A.R.; de Oliveira da Rosa, W.L.; da Silva, A.F.; Lund, R.G.; Piva, E. A systematic review about antibacterial monomers used in dental adhesive systems: Current status and further prospects. Dent. Mater. 2015, 31, 1345–1362. [Google Scholar] [CrossRef] [PubMed]
- Penmetsa, R.K.R.; Sri Rekha, A.; Poppuri, K.C.; Sai Prashanth, P.; Garapati, S. An invitro evaluation of antibacterial properties of self etching dental adhesive systems. J. Clin. Diagn. Res. 2014. [Google Scholar] [CrossRef]
- Amin, S.; Shetty, H.K.; Varma, R.K.; Amin, V.; Nair, P.M.S. Comparative evaluation of antibacterial activity of total-etch and self-etch adhesive systems: An ex vitro study. JCD 2014, 17, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Ozel, E.; Kolayli, F.; Tuna, E.B.; Er, D. In vitro antibacterial activity of various adhesive materials against oral streptococci. Biotechnol. Biotechnol. Equip. 2016, 30, 121–126. [Google Scholar] [CrossRef]
- Łukomska, M.; Olbert-Sieroszewska, V.; Żurawska-Olszewska, J.; Szczerba, I.; Krzemiński, Z.; Sokołowski, J. Antibacterial Properties of Total-Etch Bonding Systems. Pol. J. Environ. Stud. 2009, 18, 267–273. [Google Scholar]
- Esteves, C.M.; Ota-Tsuzuki, C.; Reis, A.F.; Rodrigues, J.A. Antibacterial activity of various self-etching adhesive systems against oral streptococci. Oper. Dent. 2010, 35, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, O.; Matalon, S.; Slutzky, H.; Weiss, E.I. Antibacterial properties of self-etching dental adhesive systems. J. Am. Dent. Assoc. 2007, 138, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Paradella, T.C.; Koga-Ito, C.Y.; Jorge, A.O.C. In vitro antibacterial activity of adhesive systems on Streptococcus mutans. J. Adhes. Dent. 2009, 11, 95–99. [Google Scholar] [PubMed]
- Kasacka, I.; Łapińska, J. Salivary cells in patients with dental amalgam and composite resin material restorations—A morphological investigation. Pol. J. Environ. Stud. 2010, 19, 1223–1227. [Google Scholar]
- Trubiani, O.; Cataldi, A.; De Angelis, F.; D’Arcangelo, C.; Caputi, S. Overexpression of interleukin-6 and -8, cell growth inhibition and morphological changes in 2-hydroxyethyl methacrylate-treated human dental pulp mesenchymal stem cells. Int. Endod. J. 2012, 45, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Trubiani, O.; Caputi, S.; Di Iorio, D.; D’Amario, M.; Paludi, M.; Giancola, R.; Di Nardo Di Maio, F.; de Angelis, F.; D’Arcangelo, C. The cytotoxic effects of resin-based sealers on dental pulp stem cells. Int. Endod. J. 2010, 43, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S.; Russell, R.R.; McCabe, J.F. Antibacterial activity of MDPB polymer incorporated in dental resin. J. Dent. 1995, 23, 177–181. [Google Scholar] [CrossRef]
- Imazato, S.; Kinomoto, Y.; Tarumi, H.; Torii, M.; Russell, R.R.B.; McCabe, J.F. Incorporation of Antibacterial Monomer MDPB into Dentin Primer. J. Dent. Res. 1997, 76, 768–772. [Google Scholar] [CrossRef]
- Imazato, S.; Ehara, A.; Torii, M.; Ebisu, S. Antibacterial activity of dentine primer containing MDPB after curing. J. Dent. 1998, 26, 267–271. [Google Scholar] [CrossRef]
- Imazato, S.; Imai, T.; Ebisu, S. Antibacterial activity of proprietary self-etching primers. Am. J. Dent. 1998, 11, 106–108. [Google Scholar] [PubMed]
- Imazato, S.; Imai, T.; Russell, R.R.B.; Torii, M.; Ebisu, S. Antibacterial activity of cured dental resin incorporating the antibacterial monomer MDPB and an adhesion-promoting monomer. J. Biomed. Mater. Res. 1998, 39, 511–515. [Google Scholar] [CrossRef]
- Imazato, S.; Ebi, N.; Tarumi, H.; Russell, R.R.B.; Kaneko, T.; Ebisu, S. Bactericidal activity and cytotoxicity of antibacterial monomer MDPB. Biomaterials 1999, 20, 899–903. [Google Scholar] [CrossRef]
- Imazato, S.; Torii, Y.; Takatsuka, T.; Inoue, K.; Ebi, N.; Ebisu, S. Bactericidal effect of dentin primer containing antibacterial monomer methacryloyloxydodecylpyridinium bromide (MDPB) against bacteria in human carious dentin. J. Oral Rehabil. 2001, 28, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S.; Kuramoto, A.; Kaneko, T.; Ebisu, S.; Russell, R.R.B. Comparison of antibacterial activity of simplified adhesive systems. Am. J. Dent. 2002, 15, 356–360. [Google Scholar] [PubMed]
- Imazato, S. Antibacterial properties of resin composites and dentin bonding systems. Dent. Mater. 2003, 19, 449–457. [Google Scholar] [CrossRef]
- Imazato, S.; Kinomoto, Y.; Tarumi, H.; Ebisu, S.; Tay, F.R. Antibacterial activity and bonding characteristics of an adhesive resin containing antibacterial monomer MDPB. Dent. Mater. 2003, 19, 313–319. [Google Scholar] [CrossRef]
- Ozer, F.; Karakaya, S.; Unlü, N.; Erganiş, O.; Kav, K.; Imazato, S. Comparison of antibacterial activity of two dentin bonding systems using agar well technique and tooth cavity model. J. Dent. 2003, 31, 111–116. [Google Scholar] [CrossRef]
- Imazato, S.; Kaneko, T.; Takahashi, Y.; Noiri, Y.; Ebisu, S. In vivo antibacterial effects of dentin primer incorporating MDPB. Oper. Dent. 2004, 29, 369–375. [Google Scholar] [PubMed]
- Lobo, M.M.; Gonçalves, R.B.; Pimenta, L.A.F.; Bedran-Russo, A.K.B.; Pereira, P.N.R. In vitro evaluation of caries inhibition promoted by self-etching adhesive systems containing antibacterial agents. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 75B, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S.; Kuramoto, A.; Takahashi, Y.; Ebisu, S.; Peters, M.C. In vitro antibacterial effects of the dentin primer of Clearfil Protect Bond. Dent. Mater. 2006, 22, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Marczuk-Kolada, G.; Jakoniuk, P.; Mystkowska, J.; Łuczaj-Cepowicz, E.; Waszkiel, D.; Dąbrowski, J.; Leszczyńska, K. Fluoride release and antibacterial activity of selected dental materials. Postępy Higieny I Medycyny Doświadczalnej 2006, 60, 416–420. [Google Scholar] [PubMed]
- Imazato, S.; Tay, F.R.; Kaneshiro, A.V.; Takahashi, Y.; Ebisu, S. An in vivo evaluation of bonding ability of comprehensive antibacterial adhesive system incorporating MDPB. Dent. Mater. 2007, 23, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Walter, R.; Duarte, W.R.; Pereira, P.N.R.; Heymann, H.O.; Swift, E.J.; Arnold, R.R. In Vitro Inhibition of Bacterial Growth Using Different Dental Adhesive Systems. Oper. Dent. 2007, 32, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S.; Ohmori, K.; Russell, R.R.B.; McCabe, J.F.; Momoi, Y.; Maeda, N. Determination of bactericidal activity of antibacterial monomer MDPB by a viability staining method. Dent. Mater. J. 2008, 27, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Luczaj-Cepowicz, E.; Pawińska, M.; Marczuk-Kolada, G.; Leszczyńska, K.; Waszkiel, D. Antibacterial activity of two Mineral Trioxide Aggregate materials in vitro evaluation. Ann. Acad. Med. Stetin. 2008, 54, 147–150. [Google Scholar] [PubMed]
- Li, F.; Chen, J.; Chai, Z.; Zhang, L.; Xiao, Y.; Fang, M.; Ma, S. Effects of a dental adhesive incorporating antibacterial monomer on the growth, adherence and membrane integrity of Streptococcus mutans. J. Dent. 2009, 37, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, M.; Sheehy, E.C.; Gilbert, S.C.; Beighton, D. Antimicrobial properties of dentine bonding agents determined using in vitro and ex vivo methods. J. Dent. 2009, 37, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Izutani, N.; Imazato, S.; Noiri, Y.; Ebisu, S. Antibacterial effects of MDPB against anaerobes associated with endodontic infections. Int. Endod. J. 2010, 43, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Izutani, N.; Imazato, S.; Nakajo, K.; Takahashi, N.; Takahashi, Y.; Ebisu, S.; Russell, R.R.B. Effects of the antibacterial monomer 12-methacryloyloxydodecylpyridinium bromide (MDPB) on bacterial viability and metabolism. Eur. J. Oral Sci. 2011, 119, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Łukomska-Szymańska, M.; Olbert-Sieroszewska, V.; Żurawska-Olszewska, J.; Szczerba, I.; Krzemiński, Z.; Sokołowski, J. Właściwości przeciwbakteryjne wybranych systemów wiążących VI generacji Antibacterial Properties of 6th Generation Bonding Systems. Dent. Med. Probl. 2010, 47, 304–308. [Google Scholar]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Cadenaro, M.; Antoniolli, F.; Sauro, S.; Tay, F.R.; Di Lenarda, R.; Prati, C.; Biasotto, M.; Contardo, L.; Breschi, L. Degree of conversion and permeability of dental adhesives. Eur. J. Oral Sci. 2005, 113, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.I.; Shalhav, M.; Fuss, Z. Assessment of antibacterial activity of endodontic sealers by a direct contact test. Endod. Dent. Traumatol. 1996, 12, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Cobankara, F.; Altinoz, H.; Erganis, O.; Kav, K.; Belli, S. In Vitro Antibacterial Activities of Root-Canal Sealers By Using Two Different Methods. J. Endod. 2004, 30, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Matalon, S.; Slutzky, H.; Weiss, E.I. Antibacterial properties of 4 orthodontic cements. Am. J. Orthod. Dentofac. Orthop. 2005, 127, 56–63. [Google Scholar] [CrossRef]
- Lewinstein, I.; Matalon, S.; Slutzkey, S.; Weiss, E.I. Antibacterial properties of aged dental cements evaluated by direct-contact and agar diffusion tests. J. Prosthet. Dent. 2005, 93, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Giammanco, G.M.; Cumbo, E.M.G.; Luciani, A.; Gallina, G.; Mammina, C.; Pizzo, G. In vitro evaluation of the antibacterial activity of cured dentin/enamel adhesive incorporating the antimicrobial agent MDPB. New Microbiol. 2009, 32, 385–390. [Google Scholar] [PubMed]
- Tomás, I.; García-Caballero, L.; Cousido, M.C.; Limeres, J.; Álvarez, M.; Diz, P. Evaluation of chlorhexidine substantivity on salivary flora by epifluorescence microscopy. Oral Dis. 2009, 15, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Weiger, R.; Netuschil, L.; Wester-Ebbinghaus, T.; Brecx, M. An approach to differentiate between antibacterial and antiadhesive effects of mouthrinses in vivo. Arch. Oral Biol. 1998, 43, 559–565. [Google Scholar] [CrossRef]
- Weiger, R.; von Ohle, C.; Decker, E.; Axmann-Krcmar, D.; Netuschil, L. Vital microorganisms in early supragingival dental plaque and in stimulated human saliva. J. Periodontal Res. 1997, 32, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Ihalin, R.; Nuutila, J.; Loimaranta, V.; Lenander, M.; Tenovuo, J.; Lilius, E.M. Susceptibility of Fusobacterium nucleatum to killing by peroxidase-iodide-hydrogen peroxide combination in buffer solution and in human whole saliva. Anaerobe 2003, 9, 23–30. [Google Scholar] [CrossRef]
- Berney, M.; Vital, M.; Hülshoff, I.; Weilenmann, H.-U.; Egli, T.; Hammes, F. Rapid, cultivation-independent assessment of microbial viability in drinking water. Water Res. 2008, 42, 4010–4018. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, C.J.; Nebe-von-Caron, G. The Application of multi-parameter flow cytometry to monitor individual microbial cell physiological state. Adv. Biochem. Eng. Biotechnol. 2004, 89, 197–223. [Google Scholar] [PubMed]
- Brunzel, S.; Yang, B.; Wolfart, S.; Kern, M. Tensile bond strength of a so-called self-adhesive luting resin cement to dentin. J. Adhes. Dent. 2010, 12, 143–150. [Google Scholar] [PubMed]
- Shapiro, H.M. Practical Flow Cytometry. Cytometry 1995, 19, 376. [Google Scholar]
- Lee, J.A.; Spidlen, J.; Boyce, K.; Cai, J.; Crosbie, N.; Dalphin, M.; Furlong, J.; Gasparetto, M.; Goldberg, M.; Goralczyk, E.M.; et al. MIFlowCyt: The minimum information about a flow cytometry experiment. Cytom. Part A 2008, 73, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Lehtinen, P.O.; Foster, A.S.; Ma, Y.; Krasheninnikov, A.V.; Nieminen, R.M. Irradiation-induced magnetism in graphite: A density functional study. Phys. Rev. Lett. 2004, 93, 187202. [Google Scholar] [CrossRef] [PubMed]
- Berney, M.; Hammes, F.; Bosshard, F.; Weilenmann, H.-U.; Egli, T. Assessment and Interpretation of Bacterial Viability by Using the LIVE/DEAD BacLight Kit in Combination with Flow Cytometry. Appl. Environ. Microbiol. 2007, 73, 3283–3290. [Google Scholar] [CrossRef] [PubMed]
- Joux, F.; Lebaron, P. Use of fluorescent probes to assess physiological functions of bacteria at single-cell level. Microbes Infect. 2000, 2, 1523–1535. [Google Scholar] [CrossRef]
- Łukomska‑Szymańska, M.; Zarzycka, B.; Grzegorczyk, J.; Półtorak, K.; Sokołowski, J.; Łapińska, B. Streptococcus mutans and Enterococcus faecalis as crucial pathogens of oral cavity. Dent. Forum 2016, 44, 47–52. [Google Scholar]
- Pinheiro, E.; Mayer, M. Enterococcus faecalis in Oral Infections. JBR J. Interdiscip. Med. Dent. Sci. 2014, 3, 160. [Google Scholar]
- Chávez de Paz, L.E.; Bergenholtz, G.; Dahlén, G.; Svensäter, G. Response to alkaline stress by root canal bacteria in biofilms. Int. Endod. J. 2007, 40, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Hollenbeck, B.L.; Rice, L.B. Intrinsic and acquired resistance mechanisms in enterococcus. Virulence 2012, 3, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Arias-Moliz, M.T.; Ferrer-Luque, C.M.; Espigares-Rodríguez, E.; Liébana-Ureña, J.; Espigares-García, M. Bactericidal activity of phosphoric acid, citric acid, and EDTA solutions against Enterococcus faecalis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 106, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Radcliffe, C.E.; Potouridou, L.; Qureshi, R.; Habahbeh, N.; Qualtrough, A.; Worthington, H.; Drucker, D.B. Antimicrobial activity of varying concentrations of sodium hypochlorite on the endodontic microorganisms Actinomyces israelii, A. naeslundii, Candida albicans and Enterococcus faecalis. Int. Endod. J. 2004, 37, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.P.F.A.; Ferraz, C.C.R.; Vianna, M.E.; Berber, V.B.; Teixeira, F.B.; Souza-Filho, F.J. In vitro antimicrobial activity of several concentrations of sodium hypochlorite and chlorhexidine gluconate in the elimination of Enterococcus faecalis. Int. Endod. J. 2001, 34, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Vahdaty, A.; Pitt Ford, T.R.; Wilson, R.F. Efficacy of chlorhexidine in disinfecting dentinal tubules in vitro. Endod. Dent. Traumatol. 1993, 9, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Noites, R.; Pina-Vaz, C.; Rocha, R.; Carvalho, M.F.; Gonçalves, A.; Pina-Vaz, I. Synergistic antimicrobial action of chlorhexidine and ozone in endodontic treatment. BioMed Res. Int. 2014, 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Haapasalo, M.; Orstavik, D. In vitro infection and disinfection of dentinal tubules. J. Dent. Res. 1987, 66, 1375–1379. [Google Scholar] [CrossRef] [PubMed]
- Sukawat, C.; Srisuwan, T. A comparison of the antimicrobial efficacy of three calcium hydroxide formulations on human dentin infected with Enterococcus faecalis. J. Endod. 2002, 28, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F.; de Uzeda, M. Disinfection by calcium hydroxide pastes of dentinal tubules infected with two obligate and one facultative anaerobic bacteria. J. Endod. 1996, 22, 674–676. [Google Scholar] [CrossRef]
- Poggio, C.; Arciola, C.R.; Cepurnykh, S.; Chiesa, M.; Scribante, A.; Selan, L.; Imbriani, M.; Visai, L. In vitro antibacterial activity of different self-etch adhesives. Int. J. Artif. Organs 2012, 35, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chai, Z.G.; Sun, M.N.; Wang, F.; Ma, S.; Zhang, L.; Fang, M.; Chen, J.H. Anti-biofilm effect of dental adhesive with cationic monomer. J. Dent. Res. 2009, 88, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Weir, M.D.; Chen, J.; Xu, H.H.K. Effect of charge density of bonding agent containing a new quaternary ammonium methacrylate on antibacterial and bonding properties. Dent. Mater. 2014, 30, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S.; Ma, S.; Chen, J.H.; Xu, H.H.K. Therapeutic polymers for dental adhesives: Loading resins with bio-active components. Dent. Mater. 2014, 30, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.G.d; Puppin-Rontani, R.M.; Fúcio, S.B.P.d; Negrini, T.d.C.; Carlo, H.L.; Garcia-Godoy, F. Analysis by confocal laser scanning microscopy of the MDPB bactericidal effect on S. mutans biofilm CLSM analysis of MDPB bactericidal effect on biofilm. J. Appl. Oral Sci. 2012, 20, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, Y.; Ozalp, M.; Attar, N. Comparison of the antibacterial activity of different self-etching primers and adhesives. J. Contemp. Dent. Pract. 2008, 9, 57–64. [Google Scholar] [PubMed]
- Imazato, S. Bio-active restorative materials with antibacterial effects: New dimension of innovation in restorative dentistry. Dent. Mater. J. 2009, 28, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, E.; Ionescu, A.; Fadini, L.; Mazzoni, A.; Imazato, S.; Pashley, D.; Breschi, L.; Gagliani, M. Influence of MDPB-containing primer on Streptococcus mutans biofilm formation in simulated Class I restorations. J. Adhes. Dent. 2013, 15, 431–438. [Google Scholar] [PubMed]
- Chai, Z.; Li, F.; Fang, M.; Wang, Y.; Ma, S.; Xiao, Y.; Huang, L.; Chen, J. The bonding property and cytotoxicity of a dental adhesive incorporating a new antibacterial monomer. J. Oral Rehabil. 2011, 38, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, C.; Camilleri, J. Antimicrobial properties of conventional restorative filling materials and advances in antimicrobial properties of composite resins and glass ionomer cements—A literature review. Dent. Mater. 2015, 31, e89–e99. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Weir, M.D.; Fouad, A.F.; Xu, H.H.K. Time-kill behaviour against eight bacterial species and cytotoxicity of antibacterial monomers. J. Dent. 2013, 41, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Adan, A.; Alizada, G.; Kiraz, Y.; Baran, Y.; Nalbant, A.; Nel Alizada, G.; Mur Kiraz, Y. Critical Reviews in Biotechnology Flow cytometry: Basic principles and applications. Yusuf Baran Ayten Nalbant Crit. Rev. Biotechnol. 2016, 37, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Bridier, A.; Hammes, F.; Canette, A.; Bouchez, T.; Briandet, R. Fluorescence-based tools for single-cell approaches in food microbiology. Int. J. Food Microbiol. 2015, 213, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Buysschaert, B.; Byloos, B.; Leys, N.; van Houdt, R.; Boon, N. Reevaluating multicolor flow cytometry to assess microbial viability. Appl. Microbiol. Biotechnol. 2016, 100, 9037–9051. [Google Scholar] [CrossRef] [PubMed]
- Grimwade, L.F.; Fuller, K.A.; Erber, W.N. Applications of imaging flow cytometry in the diagnostic assessment of acute leukaemia. Methods 2016, 112, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Fleisher, T.A.; Madkaikar, M.; Rosenzweig, S.D. Application of Flow Cytometry in the Evaluation of Primary Immunodeficiencies. Indian J. Pediatr. 2016, 83, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Schmidlin, P.R.; Zehnder, M.; Göhring, T.N.; Waltimo, T.M. Glutaraldehyde in bonding systems disinfects dentin in vitro. J. Adhes. Dent. 2004, 6, 61–64. [Google Scholar] [PubMed]
- Atac, A.S.; Cehreli, Z.C.; Sener, B. Antibacterial activity of fifth-generation dentin bonding systems. J. Endod. 2001, 27, 730–733. [Google Scholar] [PubMed]
- Cehreli, Z.C.; Stephan, A.; Sener, B. Antimicrobial properties of self-etching primer-bonding systems. Oper. Dent. 2003, 28, 143–148. [Google Scholar] [PubMed]
- Hamouda, I.M.; Al-Khodary, A.M.; El Shami, F.M. Degree of conversion and antimicrobial activity of etch-and-rinse versus self-etching adhesives. J. Adhes. Dent. 2010, 12, 33–38. [Google Scholar] [PubMed]
- Baca, P.; de Freitas, M.F.A.; Ferrer-Luque, C.M.; González-Rodríguez, M.P.; Arias-Moliz, M.T. In vitro enterococcus faecalis biofilm formation on five adhesive systems. Med. Oral Patol. Oral Cir. Bucal 2012, 17, 501–505. [Google Scholar] [CrossRef]
- Van Meerbeek, B.; De Munck, J.; Yoshida, Y.; Inoue, S.; Vargas, M.; Vijay, P.; van Landuyt, K.; Lambrechts, P.; Vanherle, G. Buonocore memorial lecture. Adhesion to enamel and dentin: Current status and future challenges. Oper. Dent. 2003, 28, 215–235. [Google Scholar] [PubMed]
- Arora, R.; Rao, M.H. Comparative evaluation of the antibacterial effects of four dentine bonding systems: An in vitro study. J. Conserv. Dent. JCD 2013, 16, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Asar, N.V.; Korkmaz, T.; Gül, E.B. The effect of wollastonite incorporation on the linear firing shrinkage and flexural strength of dental aluminous core ceramics: A preliminary study. Mater. Des. 2010, 31, 2540–2545. [Google Scholar] [CrossRef]
- Türkün, L.S.; Ateş, M.; Türkün, M.; Uzer, E. Antibacterial activity of two adhesive systems using various microbiological methods. J. Adhes. Dent. 2005, 7, 315–320. [Google Scholar] [PubMed]
- Poggio, C.; Beltrami, R.; Scribante, A.; Colombo, M.; Chiesa, M. Shear bond strength of one-step self-etch adhesives: pH influence. Dent. Res. J. 2015, 12, 209–214. [Google Scholar]
- Priyadharshini, S.S.; Ahmed, A.S.; Savadamoorthi, K.S. In vitro antibacterial effectiveness of three different dentin bonding systems against Streptococcus mutans and Enterococcus faecalis. J. Int. Oral Heal. 2017, 9, 33–37. [Google Scholar] [CrossRef]
- Tagami, A.; Takahashi, R.; Nikaido, T.; Tagami, J. The effect of curing conditions on the dentin bond strength of two dual-cure resin cements. J. Prosthodont. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Beltrami, R.; Chiesa, M.; Scribante, A.; Allegretti, J.; Poggio, C. Comparison of shear bond strength of universal adhesives on etched and nonetched enamel. J. Appl. Biomater. Funct. Mater. 2016, 14, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Łukomska-Szymańska, M.; Zarzycka, B.; Grzegorczyk, J.; Sokołowski, K.; Półtorak, K.; Sokołowski, J.; Łapińska, B. Antibacterial Properties of Calcium Fluoride-Based Composite Materials: In Vitro Study. BioMed Res. Int. 2016, 2016, 1–7. [Google Scholar] [CrossRef] [PubMed]
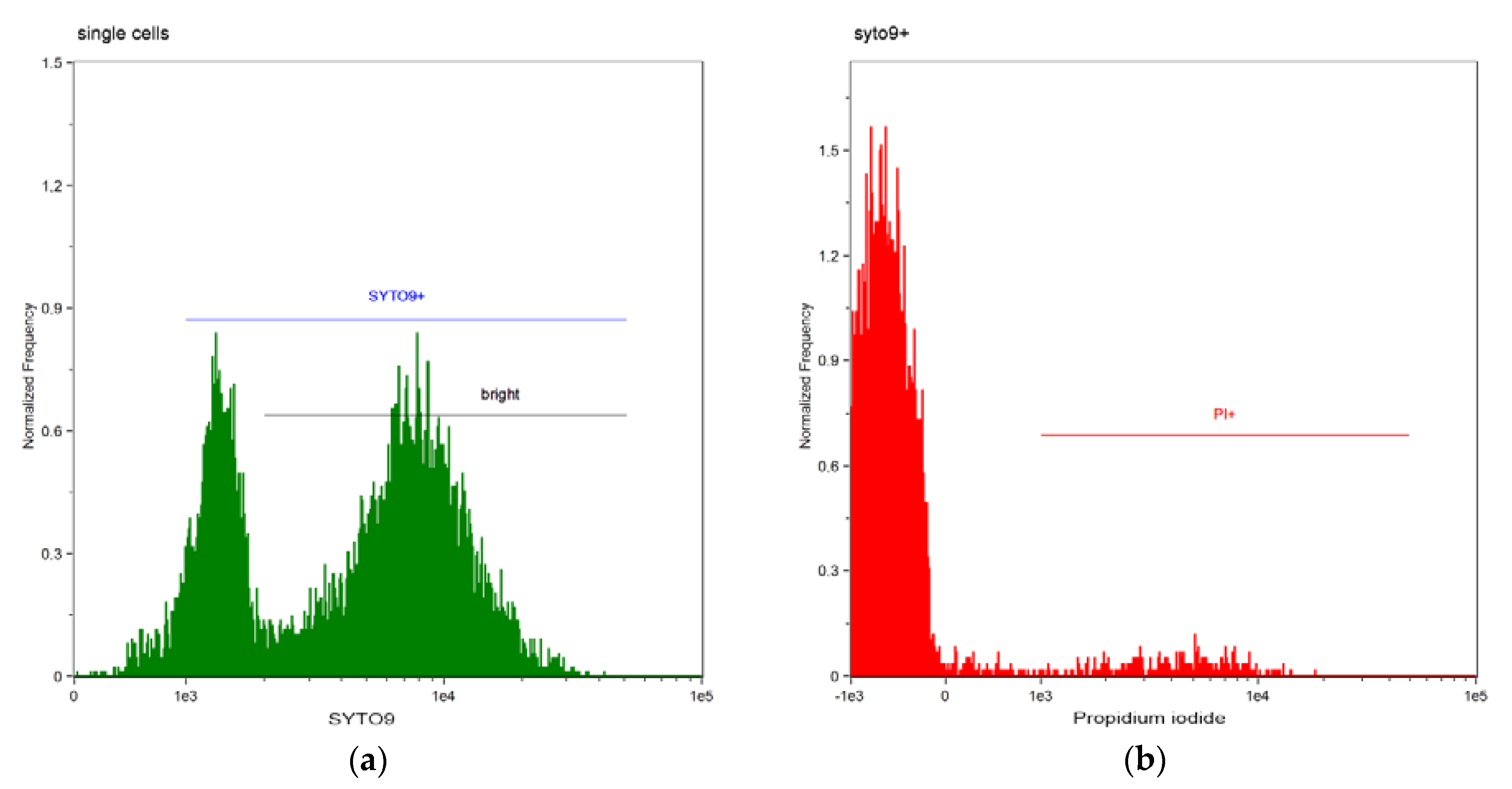
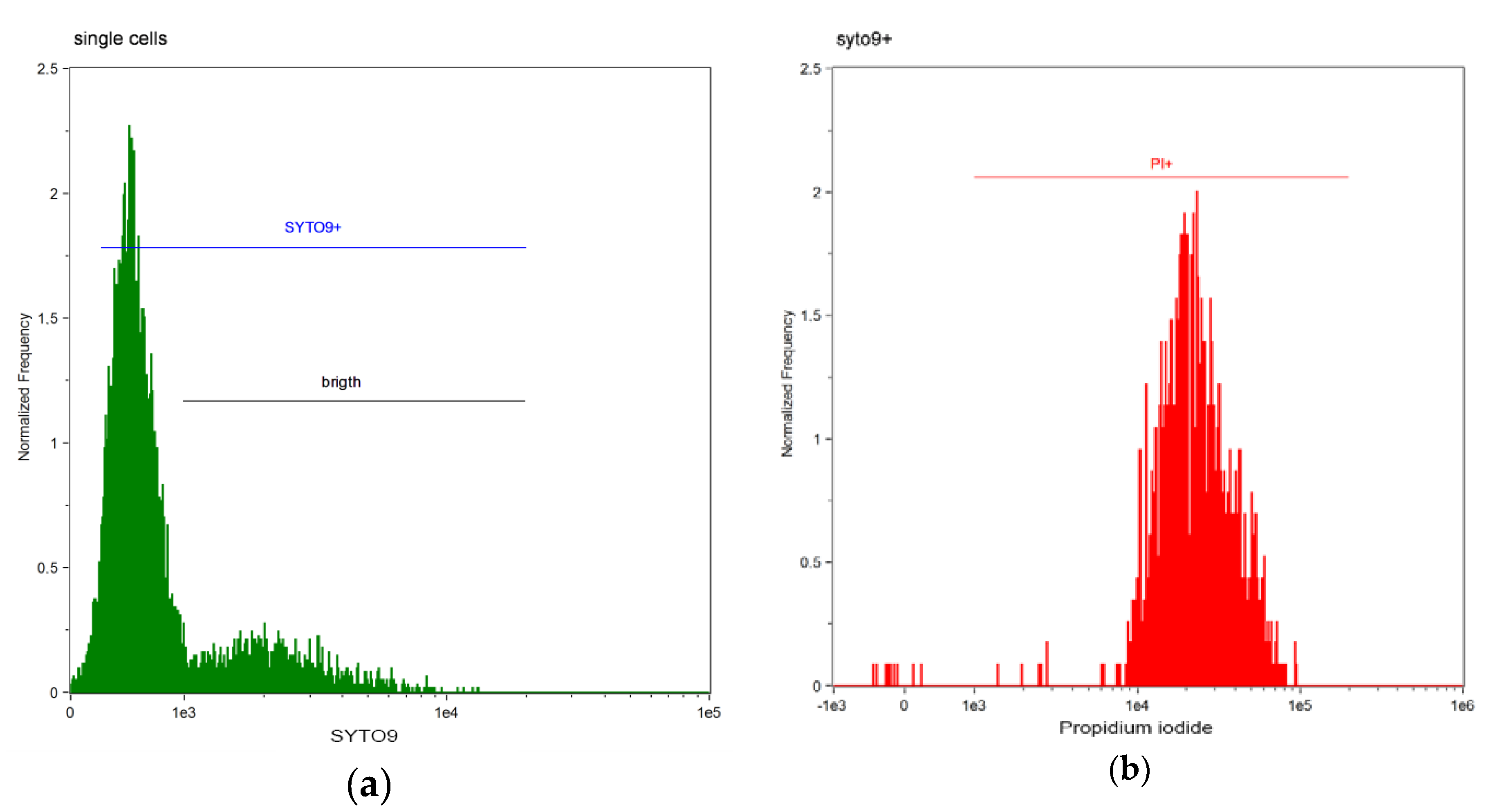
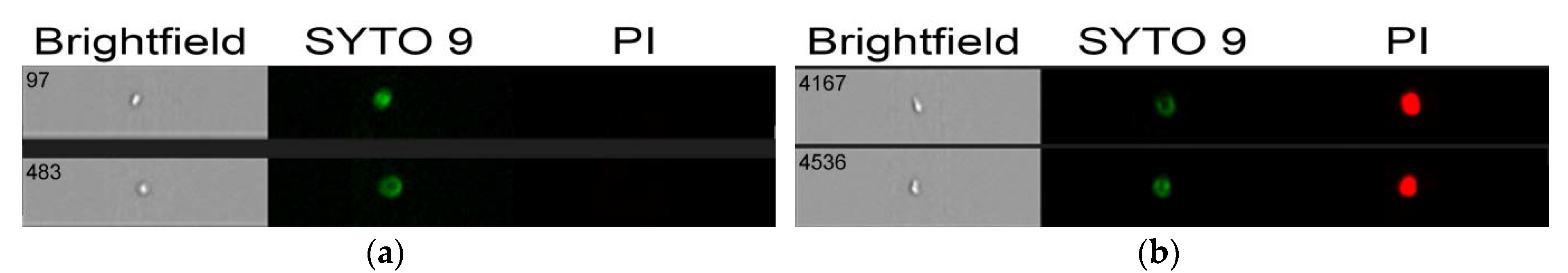

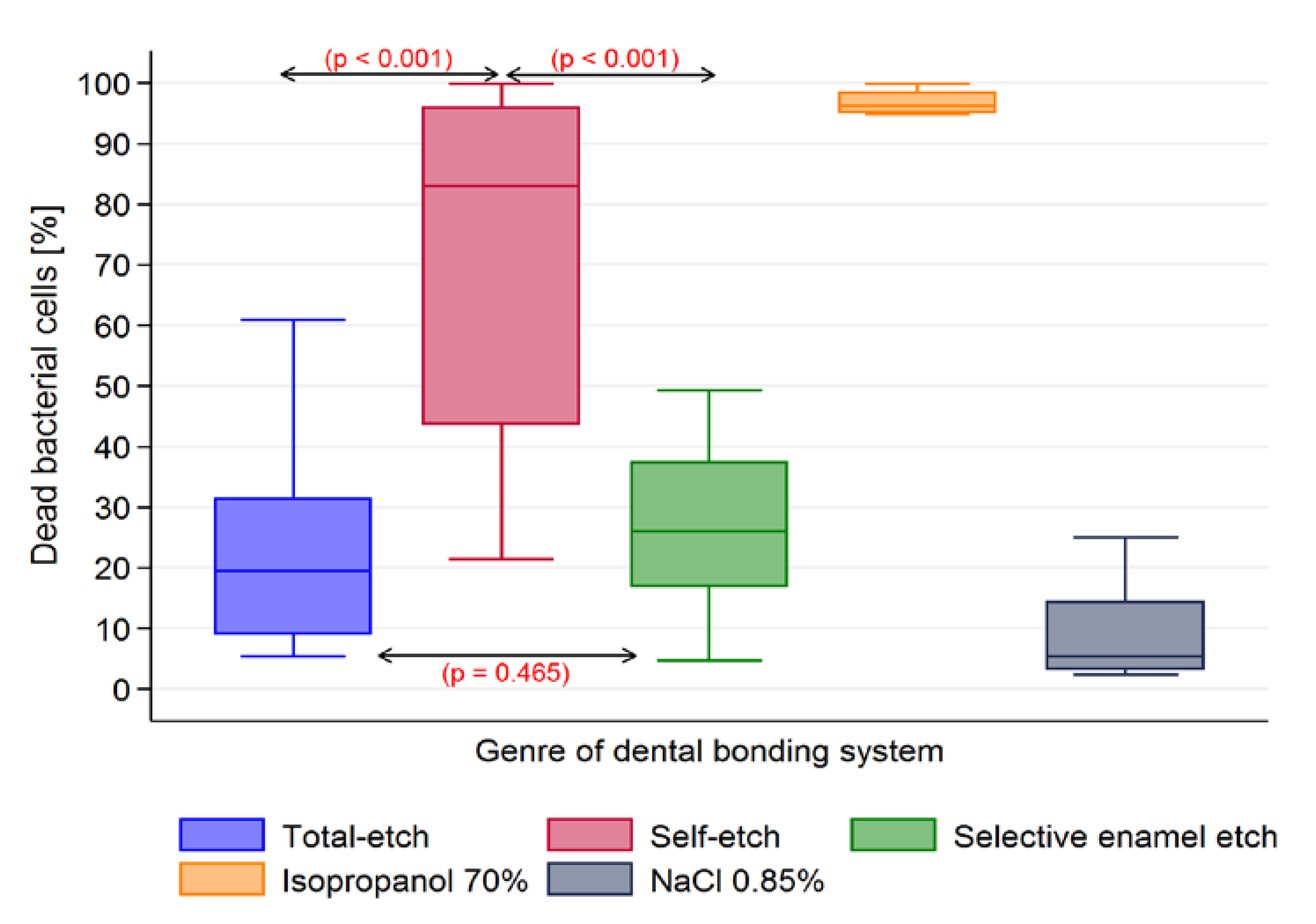
| DBS | Dead Bacterial Cells (%) | ||||
|---|---|---|---|---|---|
| M | SD | CV | Min.–Max. | ||
| TE | Prime&Bond one Etch&Rinse | 72.04 | 27.47 | 38.13% | 31.80–98.20 |
| Te-Econom Bond | 9.19 | 7.31 | 79.53% | 3.43–21.71 | |
| ExciTE F | 13.76 | 12.26 | 89.11% | 2.29–30.98 | |
| OptiBond Solo Plus | 13.13 | 8.39 | 63.91% | 5.88–28.45 | |
| SE | G-ænial Bond | 92.24 | 3.64 | 3.78% | 90.00–99.92 |
| G-Bond | 60.46 | 35.16 | 58.16% | 21.38–98.74 | |
| Clearfil S3 Bond Plus | 88.02 | 13.70 | 15.57% | 60.20–99.82 | |
| Panavia F 2.0 ED Primer II | 86.67 | 14.20 | 16.39% | 63.48–99.73 | |
| SEE | Prime&Bond® One Select | 30.53 | 18.86 | 61.78% | 4.63–60.23 |
| Futurabond M+ | 28.87 | 15.58 | 53.96% | 7.57–49.23 | |
| Control | Isopropranol 70% | 95.41 | 5.96 | 6.24% | 76.75–99.81 |
| NaCl 0.85% | 6.56 | 7.25 | 110.44% | 1.49–25.00 | |
| DBS | Prime&Bond one Etch&Rinse | Te-Econom Bond | ExciTE F | OptiBond Solo Plus | G-ænial Bond | G-Bond | Clearfil S3 Bond Plus | Panavia F 2.0 ED Primer II | Prime&Bond® One Select | Futura Bond M+ | Isopropanol 70% | NaCl 0.85% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prime&Bond one Etch&Rinse | - | =0.006 | =0.023 | =0.010 | =0.002 | =0.010 | =0.138 | =0.112 | <0.001 | =0.016 | =0.001 | <0.001 |
| Te-Econom Bond | <0.001 | - | =0.575 | =0.844 | <0.001 | =0.056 | <0.001 | <0.001 | =0.617 | =0.038 | <0.001 | =0.657 |
| ExciTE F | <0.001 | =0.575 | - | =0.715 | <0.001 | =0.170 | <0.001 | <0.001 | =0.952 | =0.122 | <0.001 | =0.278 |
| OptiBond Solo Plus | <0.001 | =0.844 | =0.715 | - | <0.001 | 0.085 | <0.001 | <0.001 | =0.761 | =0.058 | <0.001 | =0.503 |
| G-ænial Bond | =0.002 | <0.001 | =0.002 | <0.001 | - | =0.020 | =0.077 | =0.096 | =0.001 | =0.023 | =0.921 | <0.001 |
| G-Bond | =0.010 | =0.056 | =0.170 | =0.085 | <0.001 | - | <0.001 | <0.001 | =0.153 | =0.857 | <0.001 | =0.010 |
| Clearfil S3 Bond Plus | =0.138 | =0.001 | =0.006 | =0.002 | =0.077 | <0.001 | - | =0.913 | =0.004 | <0.001 | =0.052 | <0.001 |
| Panavia F 2.0 ED Primer II | =0.112 | <0.001 | =0.008 | =0.003 | =0.096 | <0.001 | =0.913 | - | <0.001 | <0.001 | =0.068 | <0.001 |
| Prime&Bond® One Select | <0.001 | =0.617 | =0.952 | =0.761 | <0.001 | =0.153 | <0.001 | <0.001 | - | =0.109 | <0.001 | =0.309 |
| Futura bond M+ | =0.016 | =0.038 | =0.122 | =0.058 | <0.001 | =0.857 | <0.001 | <0.001 | =0.109 | - | <0.001 | =0.006 |
| Isopropanol 70% | =0.001 | <0.001 | <0.001 | <0.001 | =0.921 | <0.001 | =0.052 | =0.068 | <0.001 | <0.001 | - | <0.001 |
| NaCl 0.85% | <0.001 | =0.657 | =0.278 | =0.503 | <0.001 | =0.010 | <0.001 | <0.001 | =0.309 | =0.006 | <0.001 | - |
| Name | Manufacturer | Number of Components | Type | Resin/Monomer | pH | Mode of Etching | ||
|---|---|---|---|---|---|---|---|---|
| Total-Etching | Self-Etching | Selective Enamel Etching | ||||||
| Prime&Bond One Etch&Rinse | Dentsply, UK | 1 | 2-step | TCB resin, phosphoric acid modified acrylate resin (PENTA), UDMA, TEGDMA, HEMA | 2.5 * | + | ||
| Te-Econom Bond | Ivoclar Vivadent, Germany | 1 | 2-step | HEMA, di- and mono-methacrylates | 2.6 * | + | ||
| ExciTE® F | Ivoclar Vivadent, Germany | 1 | 2-step | Bis-GMA, HEMA, phosphoric acid acrylate, dimethacrylates | 2.5 * | + | ||
| OptiBond™ Solo Plus | Kerr/USA | 1 | 2-step | Bis-GMA, GPDM, HEMA | 2.2 * | + | ||
| G-ænial® Bond | GC, Japan | 1 | 1-step | 4-MET, phosphoric acid ester monomer | 1.5 [92] | + | ||
| G-Bond® | GC, Japan | 1 | 1-step | UDMA | 2.0 [93] | + | ||
| Clearfil S3 Bond Plus | Kuraray America, USA | 1 | 1-step | MDP, Bis-GMA, HEMA | 2.3 * | + | ||
| Panavia F 2.0 ED Primer II | Kuraray America, USA | 2 (A + B) | 2-step | 2-Hydroxyethyl methacrylate, 10-methacryloyloxydecyl dihydrogen phosphate N-Methacryloyl-5-aminosalicylic acid | 2.4 [94] | + | ||
| Prime&Bond® One Select | Dentsply, UK | 1 | 1- or 2-step | bifunctional acrylate, acidic acrylate, phosphoric acid ester | 1.6 * | + | + | + |
| Futurabond M+ | VOCO, Germany | 1 | 1- or 2-step | Bis-GMA, HEMA | 2.0 [95] | + | + | + |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukomska-Szymanska, M.; Konieczka, M.; Zarzycka, B.; Lapinska, B.; Grzegorczyk, J.; Sokolowski, J. Antibacterial Activity of Commercial Dentine Bonding Systems against E. faecalis–Flow Cytometry Study. Materials 2017, 10, 481. https://doi.org/10.3390/ma10050481
Lukomska-Szymanska M, Konieczka M, Zarzycka B, Lapinska B, Grzegorczyk J, Sokolowski J. Antibacterial Activity of Commercial Dentine Bonding Systems against E. faecalis–Flow Cytometry Study. Materials. 2017; 10(5):481. https://doi.org/10.3390/ma10050481
Chicago/Turabian StyleLukomska-Szymanska, Monika, Magdalena Konieczka, Beata Zarzycka, Barbara Lapinska, Janina Grzegorczyk, and Jerzy Sokolowski. 2017. "Antibacterial Activity of Commercial Dentine Bonding Systems against E. faecalis–Flow Cytometry Study" Materials 10, no. 5: 481. https://doi.org/10.3390/ma10050481
APA StyleLukomska-Szymanska, M., Konieczka, M., Zarzycka, B., Lapinska, B., Grzegorczyk, J., & Sokolowski, J. (2017). Antibacterial Activity of Commercial Dentine Bonding Systems against E. faecalis–Flow Cytometry Study. Materials, 10(5), 481. https://doi.org/10.3390/ma10050481







