Fabrication of Cellulose Nanofiber/AlOOH Aerogel for Flame Retardant and Thermal Insulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CNF Colloid Solution
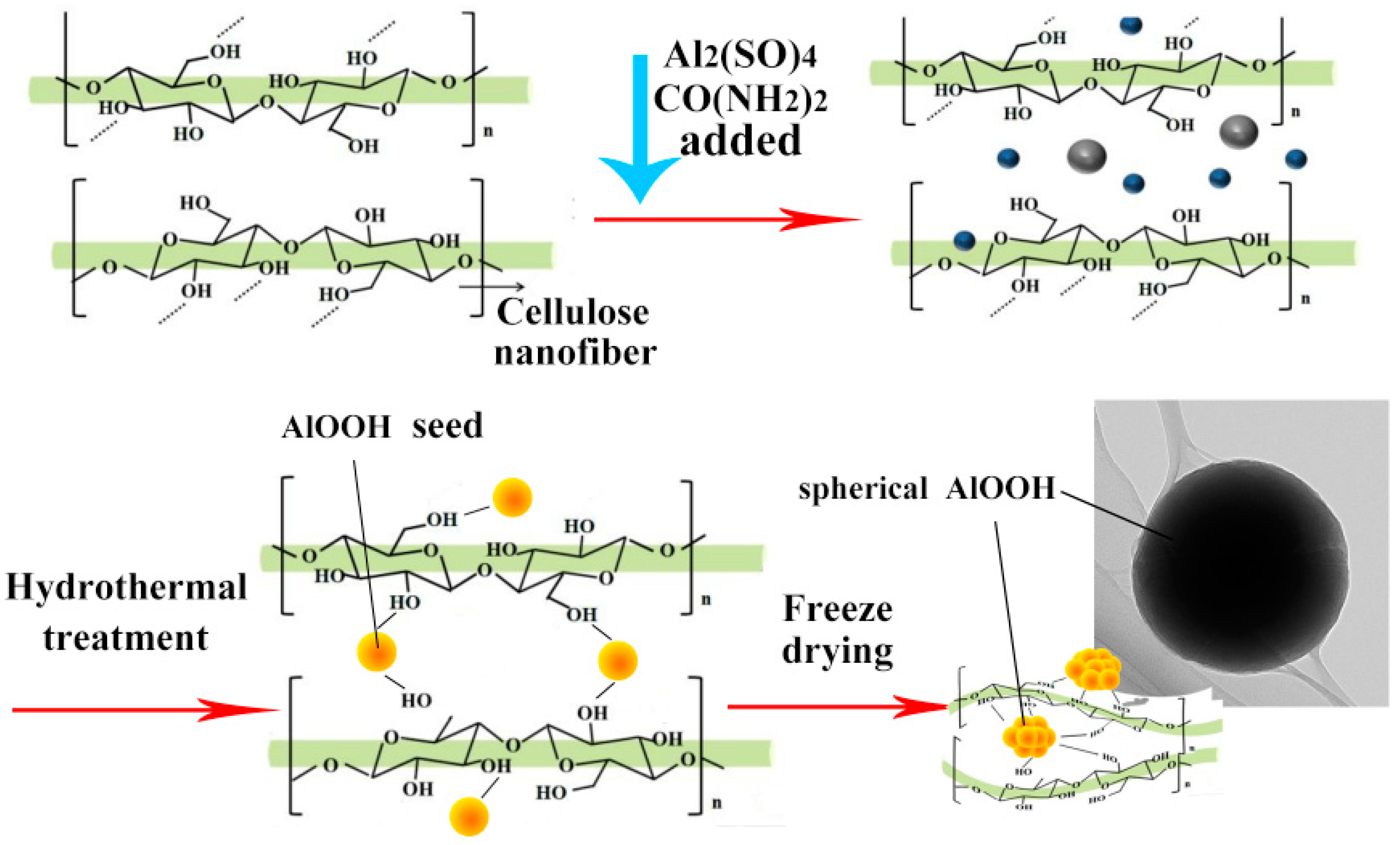
2.3. Preparation of CNF and CNFA Aerogels
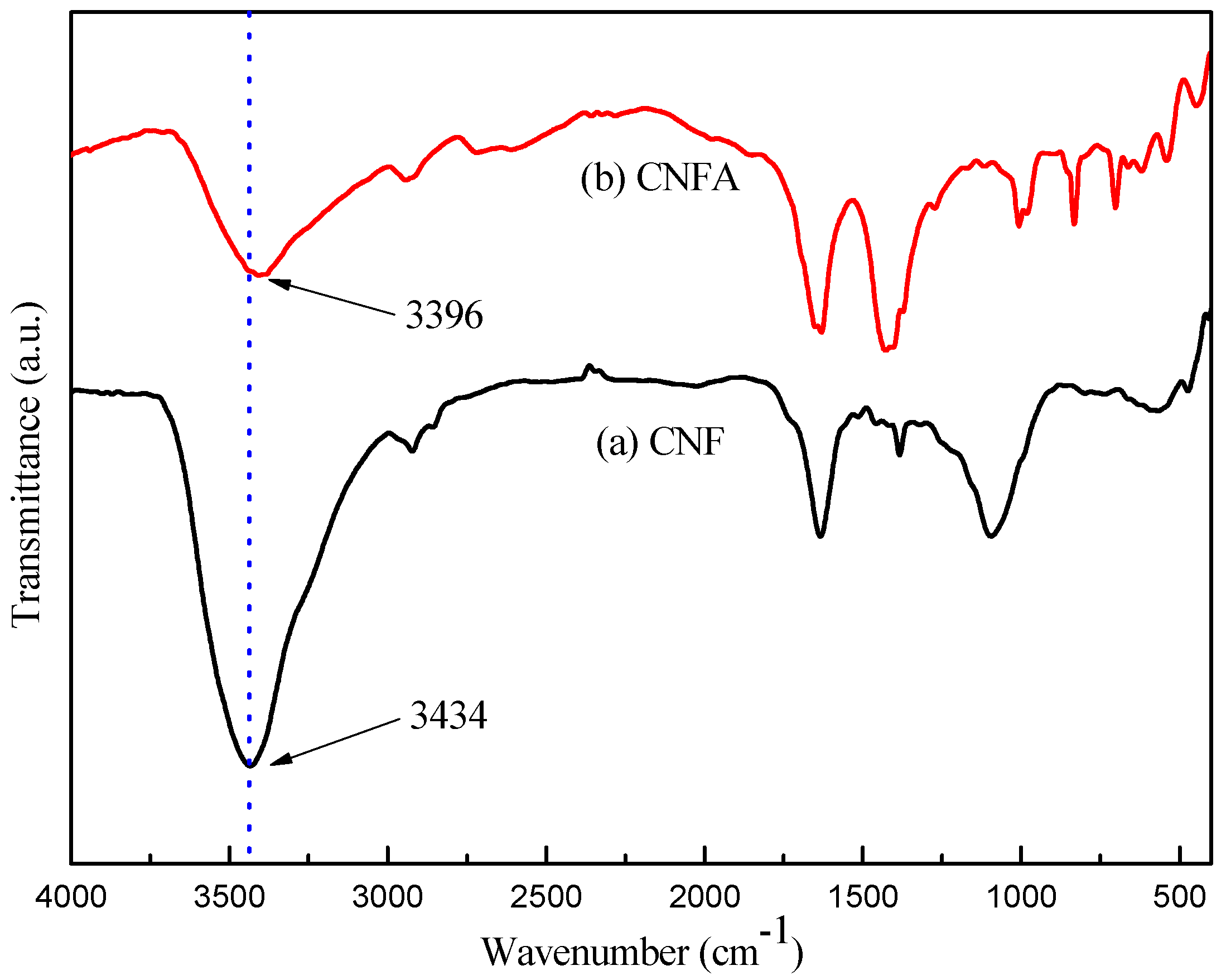
2.4. Characterization
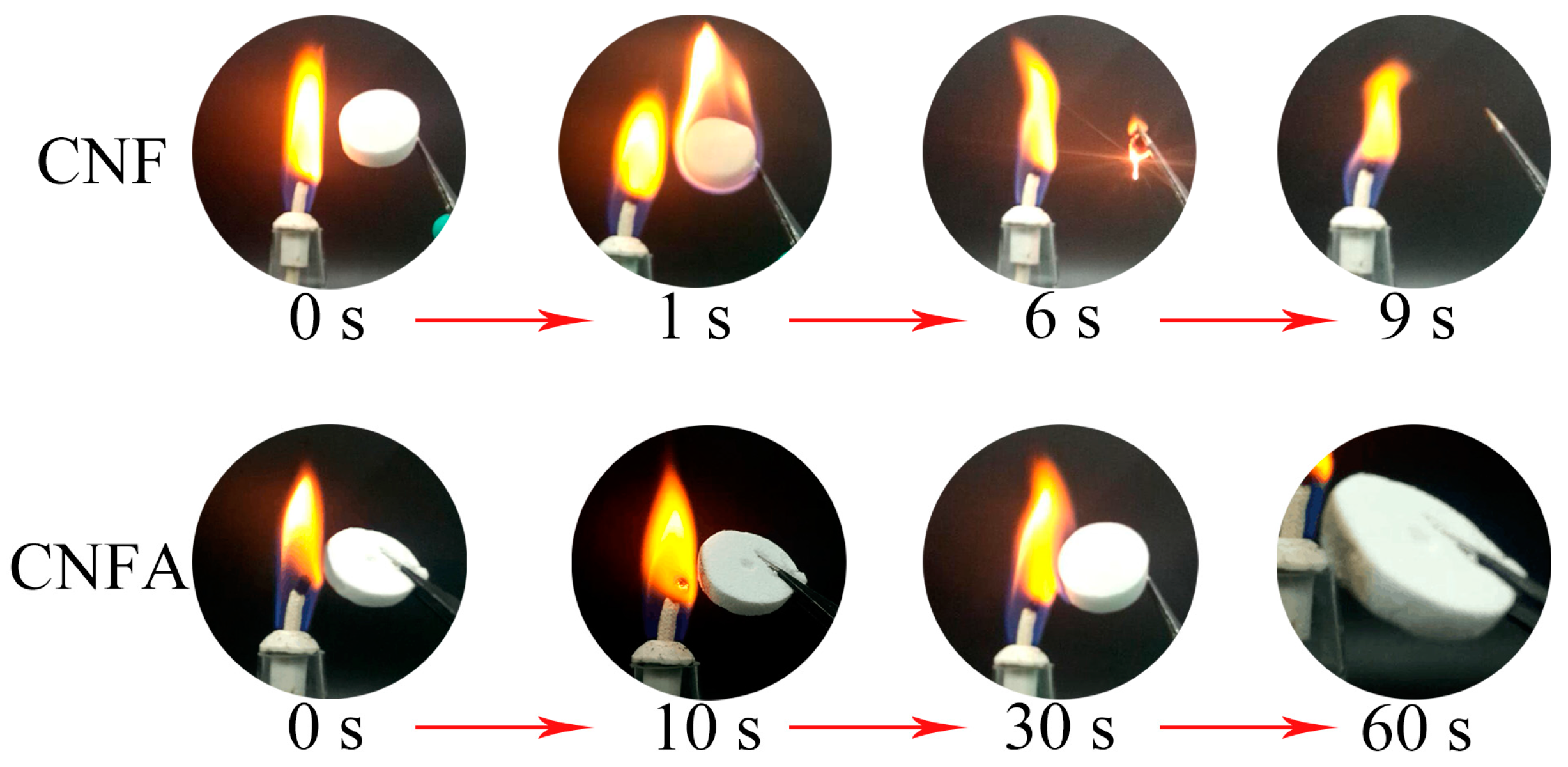
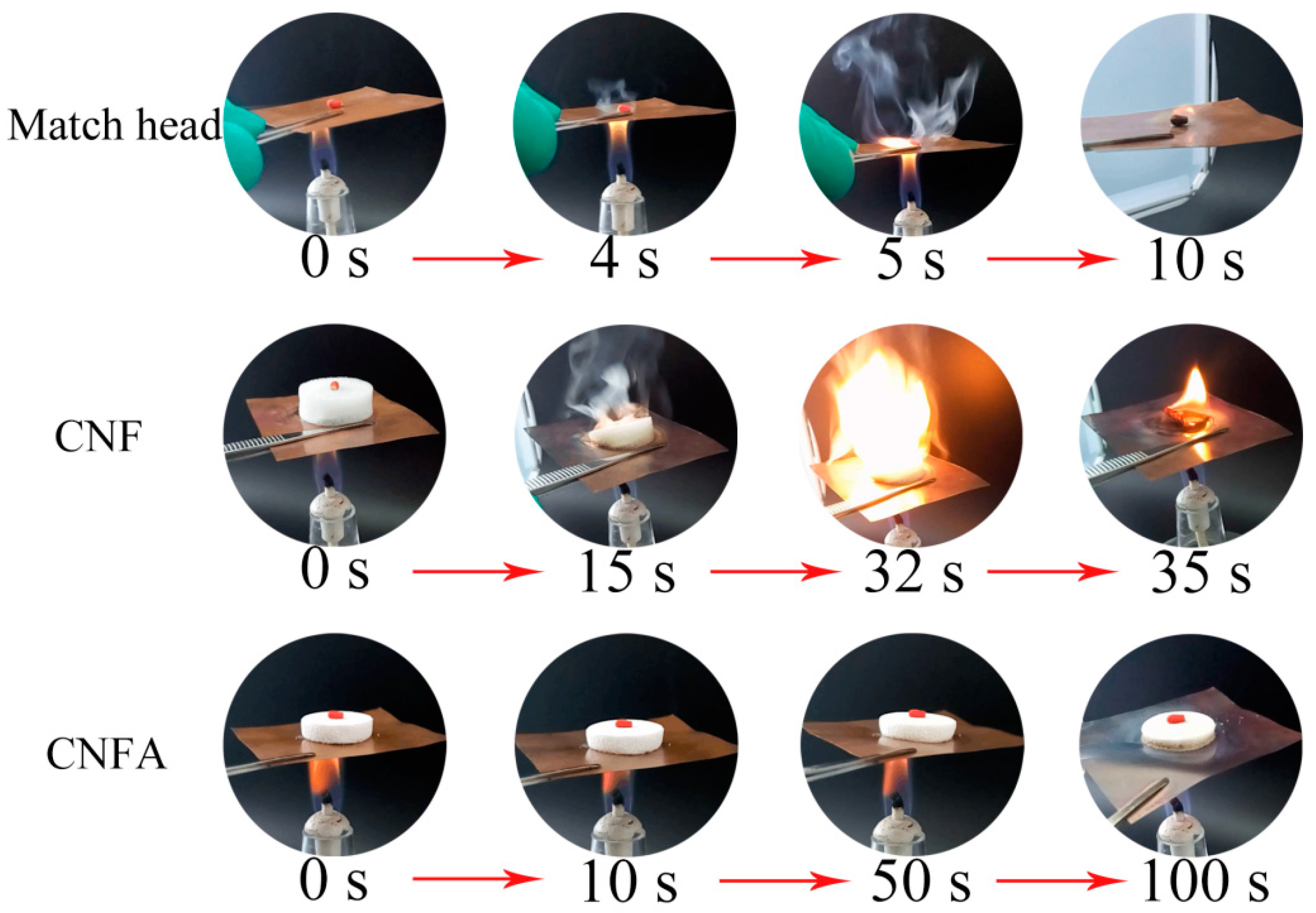
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jeencham, R.; Suppakarn, N.; Jarukumjorn, K. Effect of flame retardants on flame retardant, mechanical, and thermal properties of sisal fiber/polypropylene composites. Compos. Part B-Eng. 2014, 56, 249–253. [Google Scholar] [CrossRef]
- Wicklein, B.; Kocjan, A.; Salazar-Alvarez, G.; Carosio, F.; Camino, G.; Antonietti, M.; Bergström, L. Thermally insulating and fire-retardant lightweight anisotropic foams based on nanocellulose and graphene oxide. Nat. Nanotechnol. 2015, 10, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Coz, F.L.; Arurault, L.; Fontorbes, S.; Vilar, V.; Datas, L.; Winterton, P. Chemical composition and structural changes of porous templates obtained by anodising aluminium in phosphoric acid electrolyte. Surf. Interface Anal. 2010, 42, 227–233. [Google Scholar] [CrossRef]
- Fedorov, V.A.; Kostomarov, D.V.; Antonov, E.V. Chemical Processes in Al2O3-Mo and Al2O3-W systems in a weakly reducing atmosphere. Crystallogr. Rep. 2014, 59, 132–136. [Google Scholar] [CrossRef]
- Xiao, S.; Wang, F.; Yang, Y.; Chang, Z.; Wu, Y. An environmentally friendly and economic membrane based on cellulose as a gel polymer electrolyte for lithium ion batteries. RSC Adv. 2013, 4, 76. [Google Scholar] [CrossRef]
- Peng, N.; Wang, Y.; Ye, Q.; Liang, L.; An, Y.; Li, Q.; Chang, C. Biocompatible cellulose-based superabsorbent hydrogels with antimicrobial activity. Carbohyd. Polym. 2016, 137, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, R.; Silva, M.; Morais, A.; Osajima, J.; Santos, M.; Airoldi, C.; Filho, E. Phosphated cellulose as an efficient biomaterial for aqueous drug ranitidine removal. Materials 2014, 7, 7907–7924. [Google Scholar] [CrossRef]
- Machado, M.; Souza, S.G.U.; Morgado, A.F.; Caldas, P.; Ptak, F.; Prioli, R. Influence of cellulose fibers and fibrils on nanoscale friction in kraft paper. Cellulose 2016, 23, 2653–2661. [Google Scholar] [CrossRef]
- Brinkmann, A.; Chen, M.; Couillard, M.; Jakubek, Z.J.; Leng, T.; Johnston, L.J. Correlating cellulose nanocrystal particle size and surface area. Langmuir 2016, 32, 6105–6114. [Google Scholar] [CrossRef] [PubMed]
- Li, M.C.; Wu, Q.; Song, K.; Lee, S.; Yan, Q.; Wu, Y. Cellulose nanoparticles: Structure morphology rheology relationships. ACS Sustain. Chem. Eng. 2015, 3, 821–832. [Google Scholar] [CrossRef]
- Varanasi, S.; Batchelor, W. Superior non-woven sheet forming characteristics of low-density cationic polymer-cellulose nanofibre colloids. Cellulose 2014, 21, 3541–3550. [Google Scholar] [CrossRef]
- Numata, Y.; Sakata, T.; Furukawa, H.; Tajima, K. Bacterial cellulose gels with high mechanical strength. Mater. Sci. Eng. C-Mater. 2015, 47, 57. [Google Scholar] [CrossRef] [PubMed]
- Masmoudi, F.; Bessadok, A.; Dammak, M.; Jaziri, M.; Ammar, E. Biodegradable packaging materials conception based on starch and polylactic acid (PLA) reinforced with cellulose. Environ. Sci. Pollut. R. 2016, 23, 1–11. [Google Scholar] [CrossRef] [PubMed]
- McKee, J.R.; Appel, E.A.; Seitsonen, J.; Kontturi, E.; Scherman, O.A.; Ikkala, O. Healable, stable and stiff hydrogels: Combining conflicting properties using dynamic and selective three-component recognition with reinforcing cellulose nanorods. Adv. Funct. Mater. 2014, 24, 2706–2713. [Google Scholar] [CrossRef]
- Mølgaard, S.L.; Henriksson, M.; Cárdenas, M.; Svagan, A.J. Cellulose-nanofiber/polygalacturonic acid coatings with high oxygen barrier and targeted release properties. Carbohyd. Polym. 2014, 114, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.; Mun, S.; Min, S.K.; Kim, G.W.; Kim, J. Fabrication of cellulose ZnO hybrid nanocomposite and its strain sensing behavior. Materials 2014, 7, 7000–7009. [Google Scholar] [CrossRef]
- Mahadeva, S.K.; Kim, J. Hybrid nanocomposite based on cellulose and tin oxide: Growth, structure, tensile and electrical characteristics. Sci. Technol. Adv. Mat. 2011, 12, 055006. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhou, L.; Li, M.C.; Wu, Q.; Kojima, Y.; Zhou, D. Preparation and properties of electrospun poly (vinyl pyrrolidone)/cellulose nanocrystal/silver nanoparticle composite fibers. Materials 2016, 9, 523. [Google Scholar] [CrossRef]
- Xiong, R.; Wang, Y.; Zhang, X.; Lu, C.; Lan, L. In situ growth of gold nanoparticles on magnetic g-Fe2O3@cellulose nanocomposites: A highly active and recyclable catalyst for reduction of 4-nitrophenol. RSC Adv. 2014, 4, 6454–6462. [Google Scholar] [CrossRef]
- Mahmoud, K.H.; Abdel-Rahim, F.M. Investigation of optical properties of 40 wt % nickel chloride doped carboxymethyl cellulose film treated with nitrogen plasma. Polym. Compos. 2016. [Google Scholar] [CrossRef]
- Gray, D.; Mu, X. Chiral nematic structure of cellulose nanocrystal suspensions and films: Polarized light and atomic force microscopy. Materials 2015, 8, 7873–7888. [Google Scholar] [CrossRef]
- Xie, J.; Hse, C.Y.; Hoop, C.F.D.; Hu, T.; Qi, J.; Shupe, T.F. Isolation and characterization of cellulose nanofibers from bamboo using microwave liquefaction combined with chemical treatment and ultrasonication. Carbohydr. Polym. 2016, 151, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, X.; Wu, X.; Lu, C. Flame Retardant, Heat Insulating Cellulose Aerogels from Waste Cotton Fabrics by in Situ Formation of Magnesium Hydroxide Nanoparticles in Cellulose Gel Nanostructures. ACS Sustain. Chem. Eng. 2015, 3, 1853–1859. [Google Scholar] [CrossRef]
- Ovalle-Serrano, S.A.; Carrillo, V.S.; Blanco-Tirado, C.; Hinestroza, J.P.; Combariza, M.Y. Controlled synthesis of ZnO particles on the surface of natural cellulosic fibers: Effect of concentration, heating and sonication. Cellulose 2015, 22, 1841–1852. [Google Scholar] [CrossRef]
- Asadi, A.A.; Bazmi, M.; Alavi, S.M.; Royaee, S.J. Neutralization of NaAlO2 solution with CO2 for synthesis of γ-Al2O3 nanoparticles, Part 1: Effects of synthesis parameters in semi-batch membrane dispersion microstructured reactor. RSC Adv. 2016, 6, 109681–109691. [Google Scholar] [CrossRef]
- Yuan, B.; Zhang, J.; Yu, J.; Song, R.; Mi, Q.; He, J.; Zhang, J. Transparent and flame retardant cellulose/aluminum hydroxide nanocomposite aerogels. Sci. China Chem. 2016, 59, 1–7. [Google Scholar] [CrossRef]
- Bendahou, D.; Bendahou, A.; Seantier, B.; Grohens, Y.; Kaddami, H. Nano-fibrillated cellulose-zeolites based new hybrid composites aerogels with super thermal insulating properties. Ind. Crop Prod. 2015, 65, 374–382. [Google Scholar] [CrossRef]
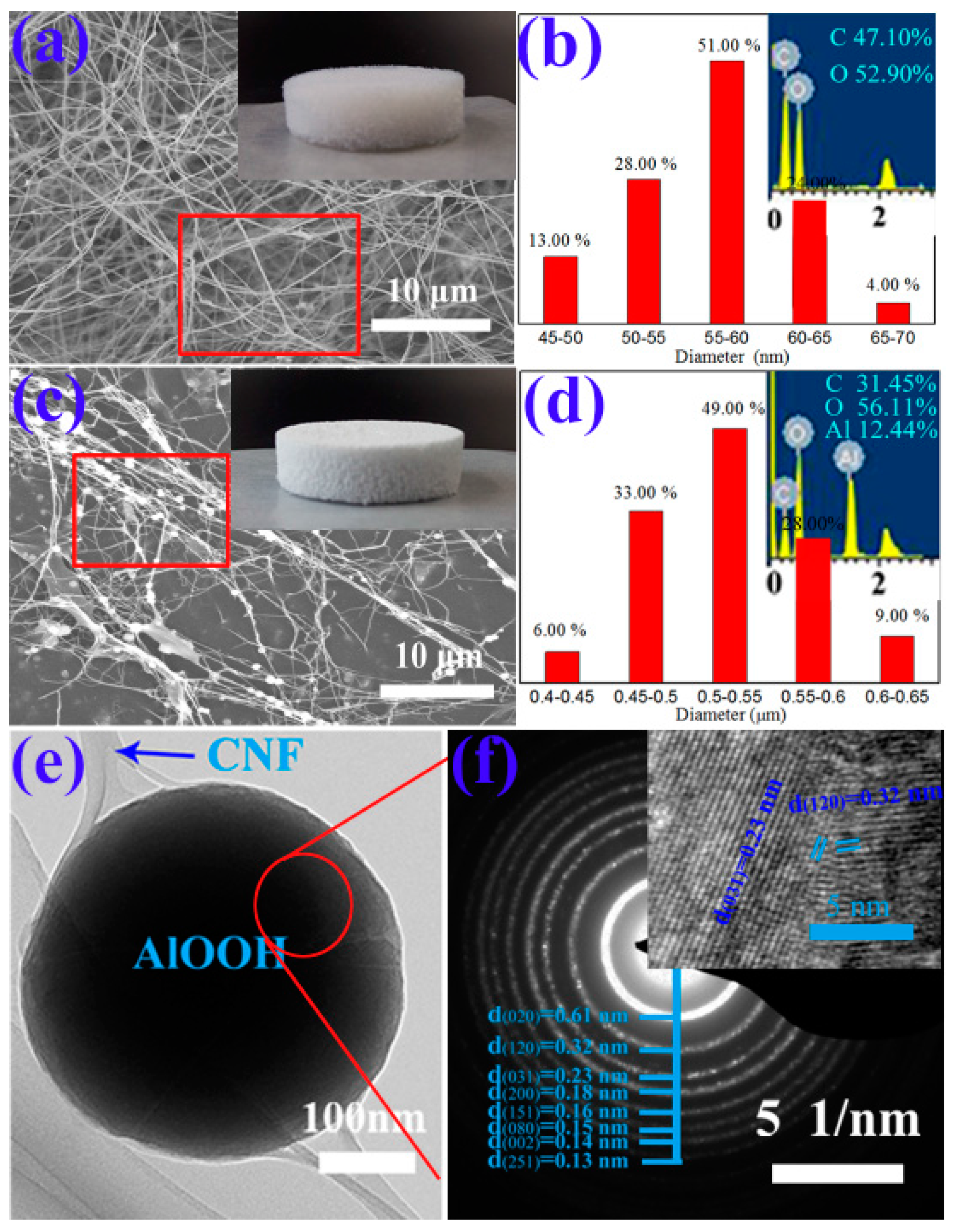
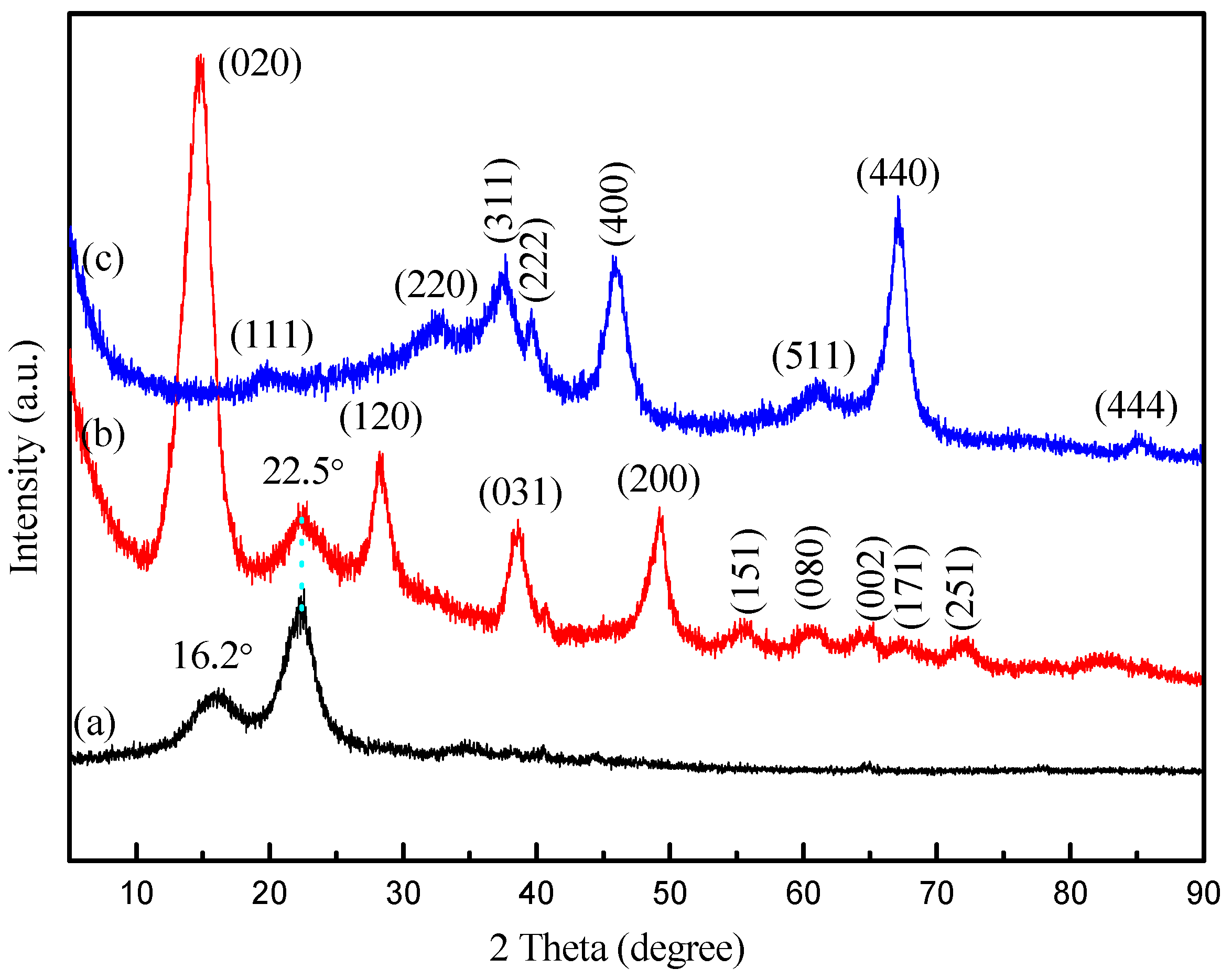
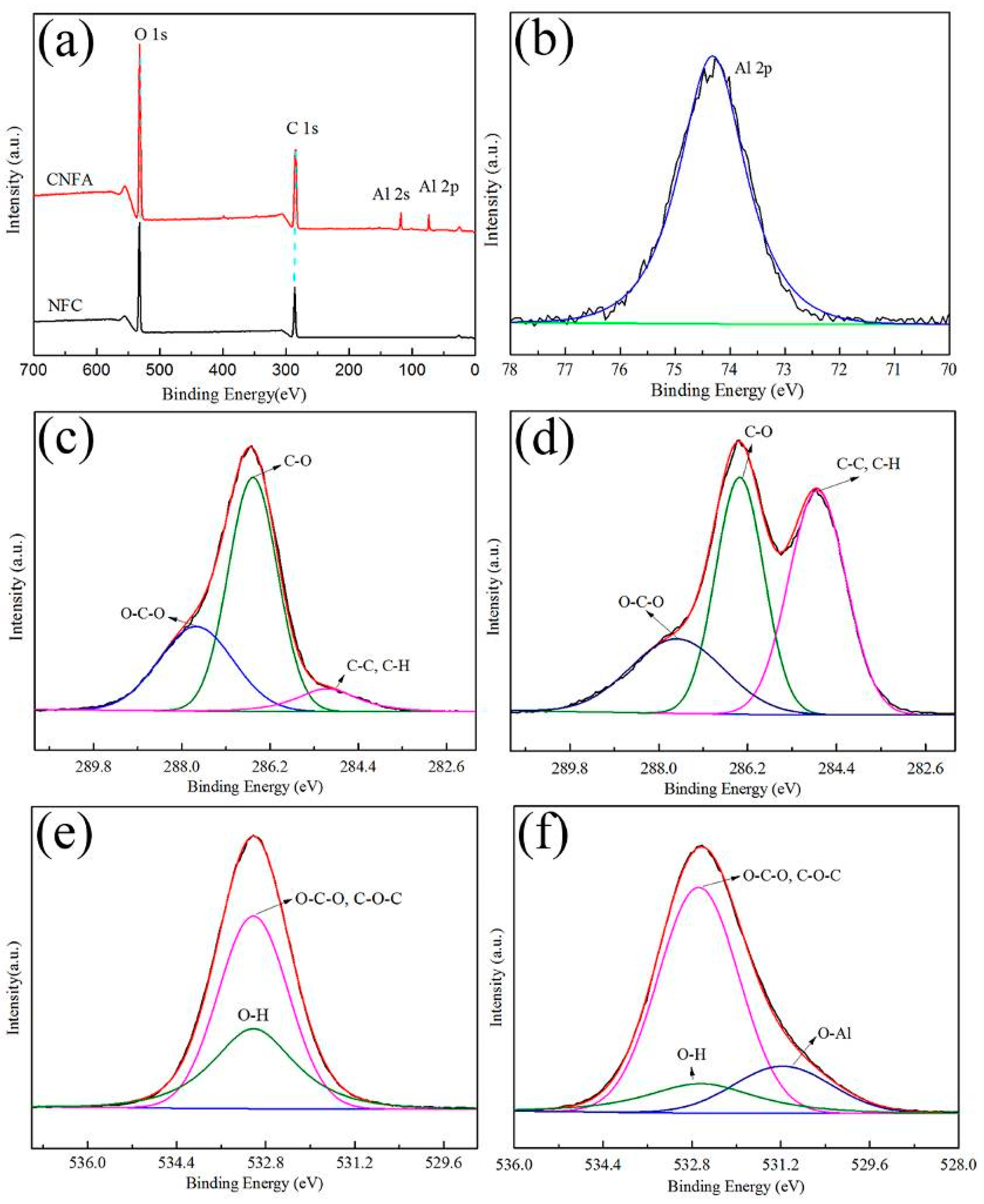
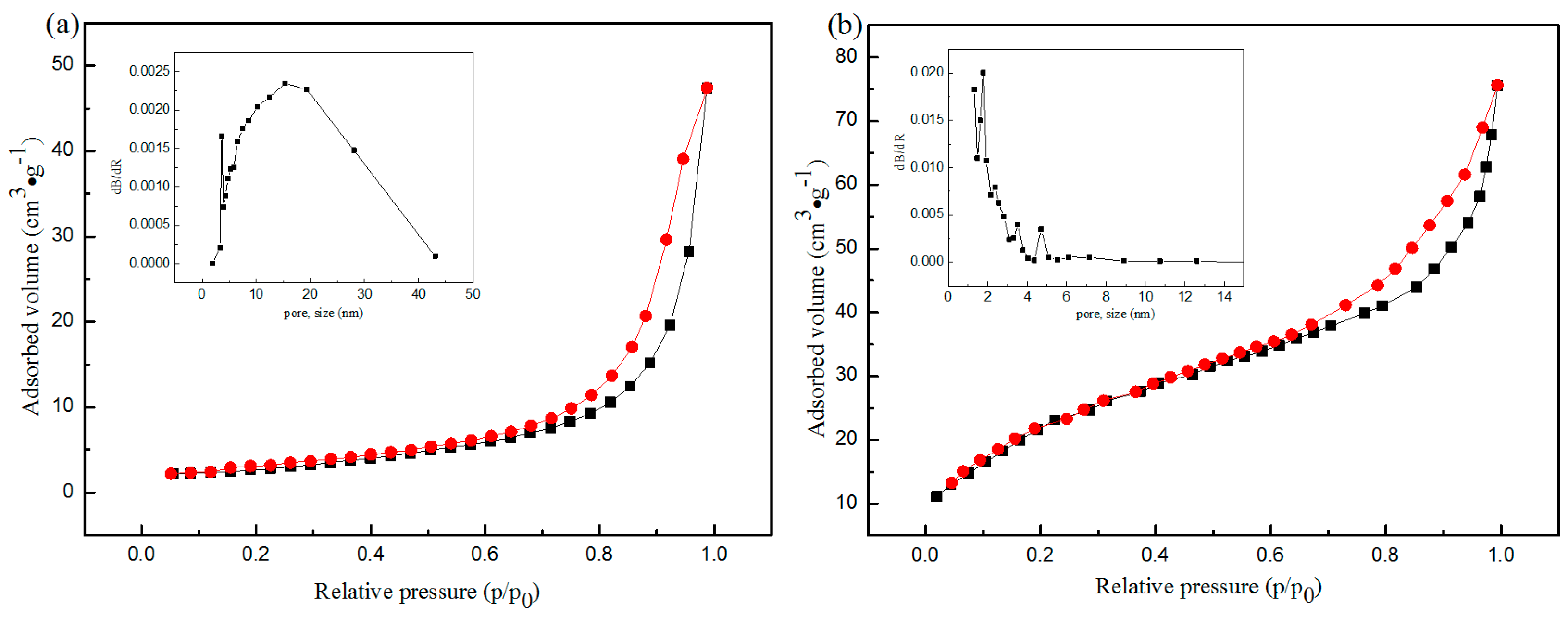
| Sample | SBET (m2·g−1) | Average Pore Diameter (nm) | Vtotal (cm3·g−1) |
|---|---|---|---|
| CNF | 10.1 | 29.3 | 0.073 |
| CNFA | 66.9 | 5.57 | 0.093 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, B.; Chen, S.; Yao, Q.; Sun, Q.; Jin, C. Fabrication of Cellulose Nanofiber/AlOOH Aerogel for Flame Retardant and Thermal Insulation. Materials 2017, 10, 311. https://doi.org/10.3390/ma10030311
Fan B, Chen S, Yao Q, Sun Q, Jin C. Fabrication of Cellulose Nanofiber/AlOOH Aerogel for Flame Retardant and Thermal Insulation. Materials. 2017; 10(3):311. https://doi.org/10.3390/ma10030311
Chicago/Turabian StyleFan, Bitao, Shujun Chen, Qiufang Yao, Qingfeng Sun, and Chunde Jin. 2017. "Fabrication of Cellulose Nanofiber/AlOOH Aerogel for Flame Retardant and Thermal Insulation" Materials 10, no. 3: 311. https://doi.org/10.3390/ma10030311
APA StyleFan, B., Chen, S., Yao, Q., Sun, Q., & Jin, C. (2017). Fabrication of Cellulose Nanofiber/AlOOH Aerogel for Flame Retardant and Thermal Insulation. Materials, 10(3), 311. https://doi.org/10.3390/ma10030311





