Laser Surface Microstructuring of Biocompatible Materials Using a Microlens Array and the Talbot Effect: Evaluation of the Cell Adhesion
Abstract
:1. Introduction
2. Materials and Methods
3. Results
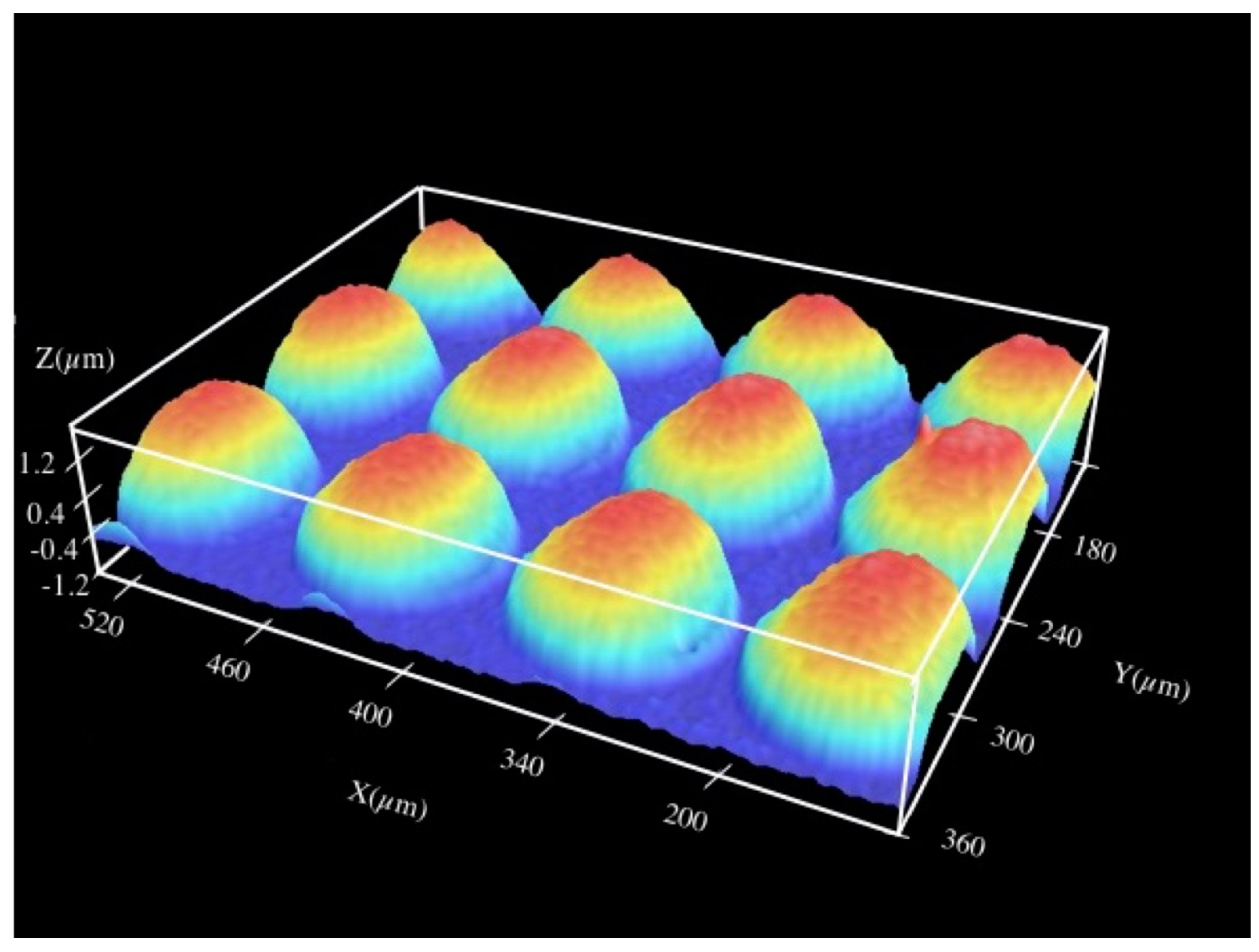
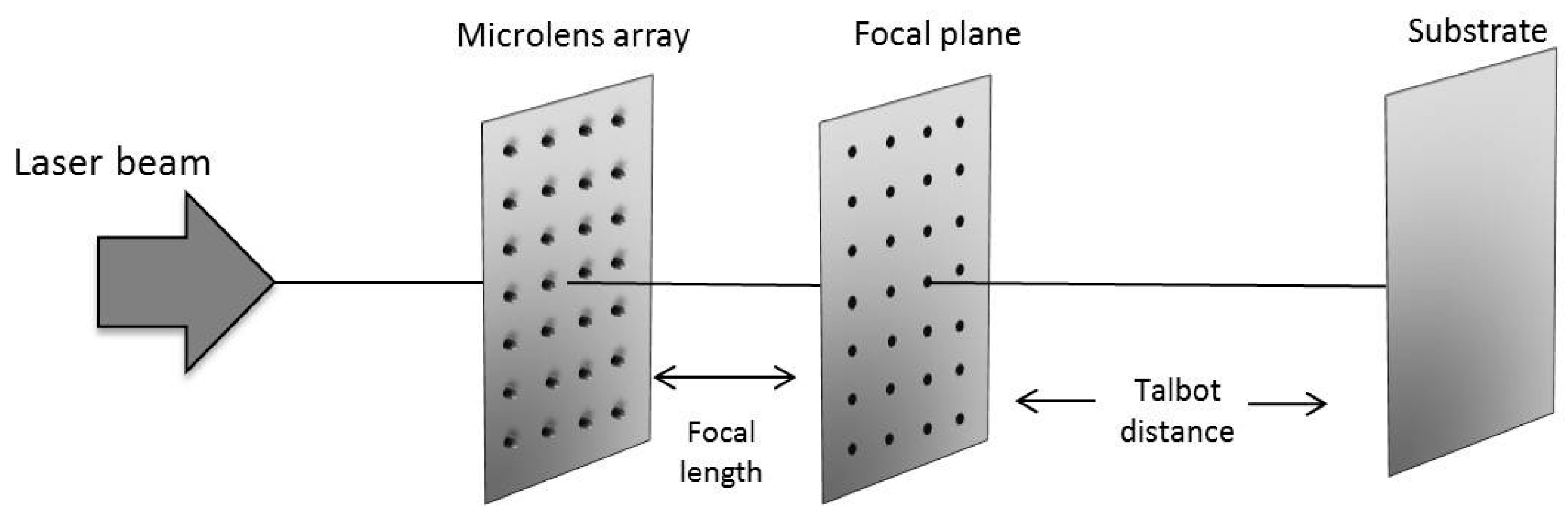
3.1. Surface Multistructuring
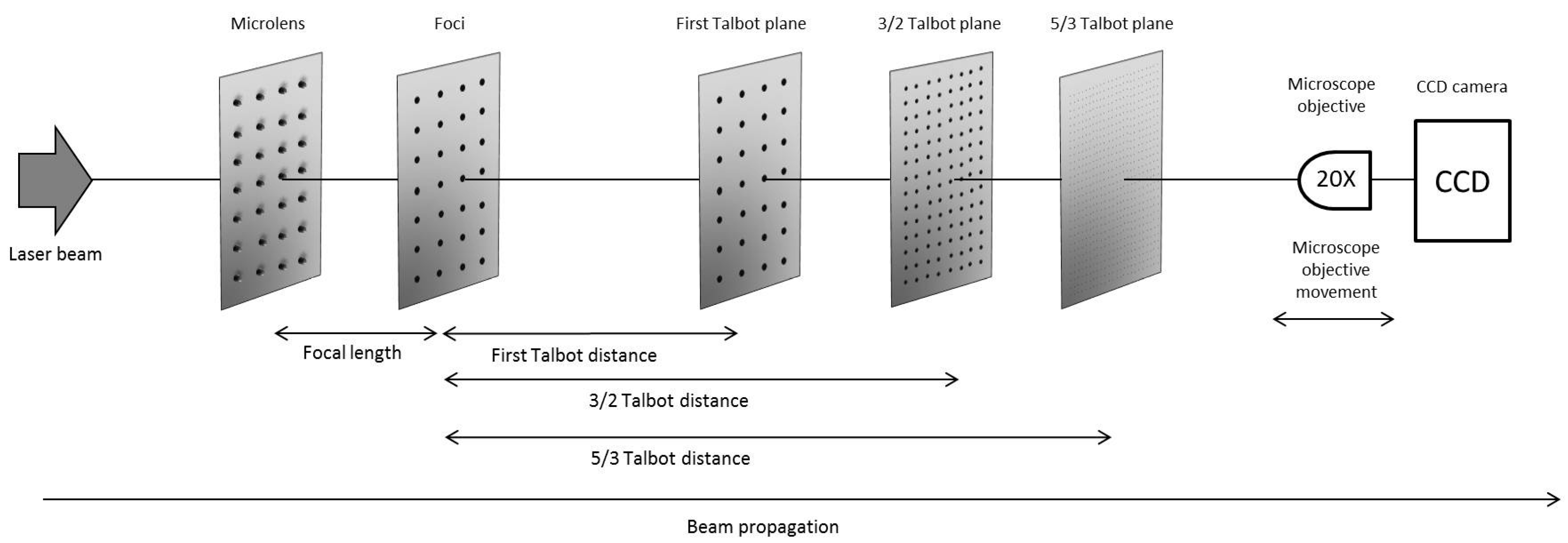
3.1.1. Integer and Fractional Talbot Effect
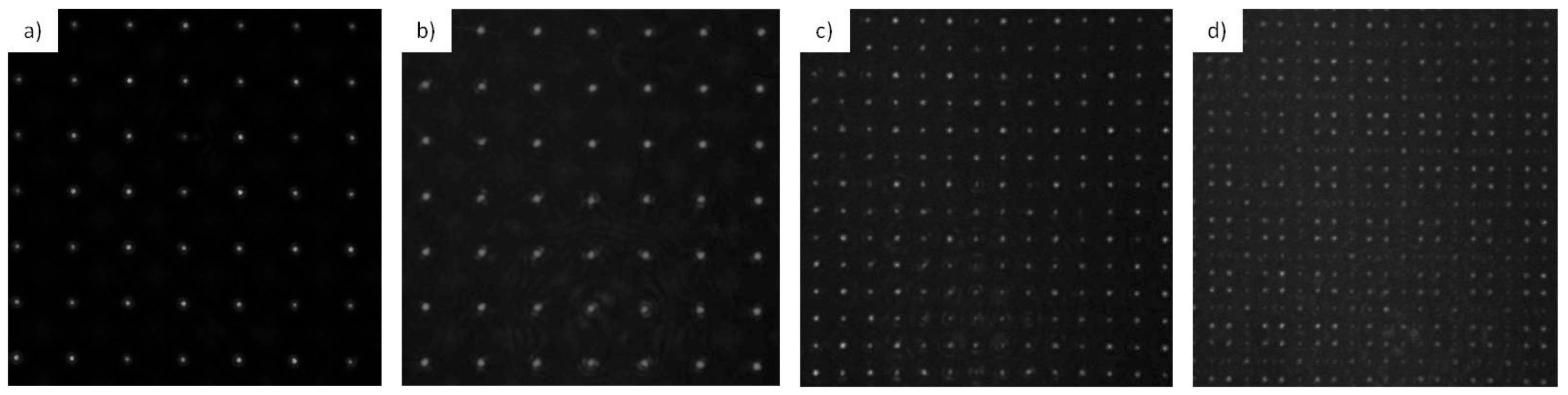
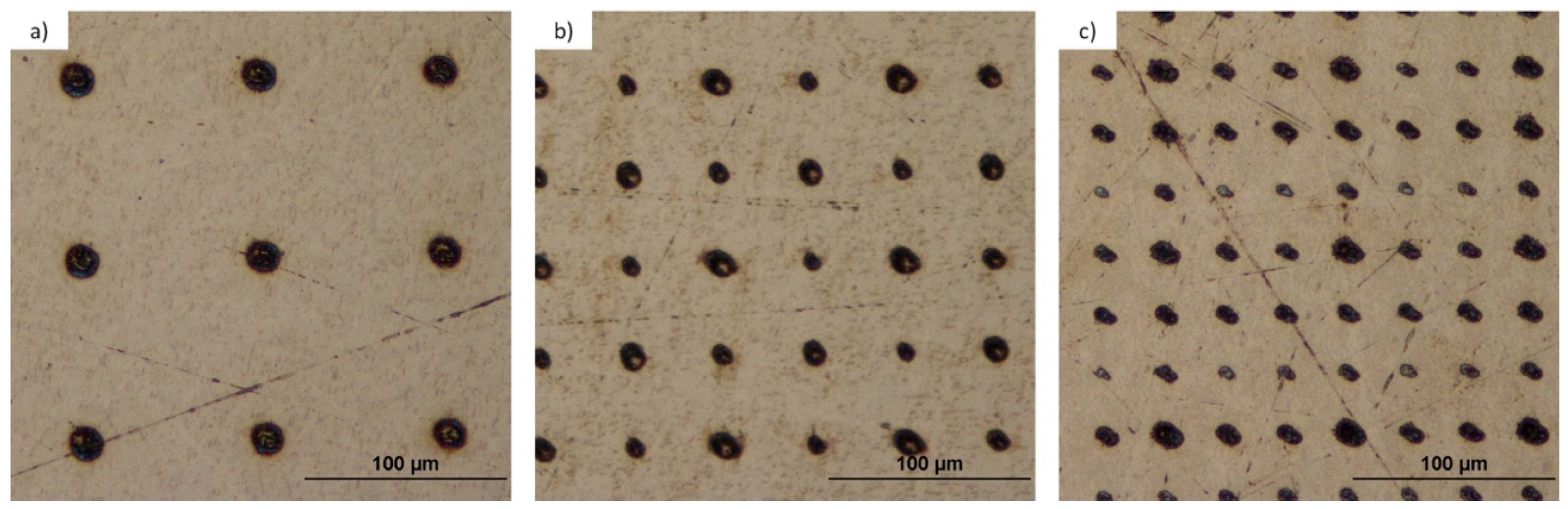
3.1.2. Identification of the Talbot Planes
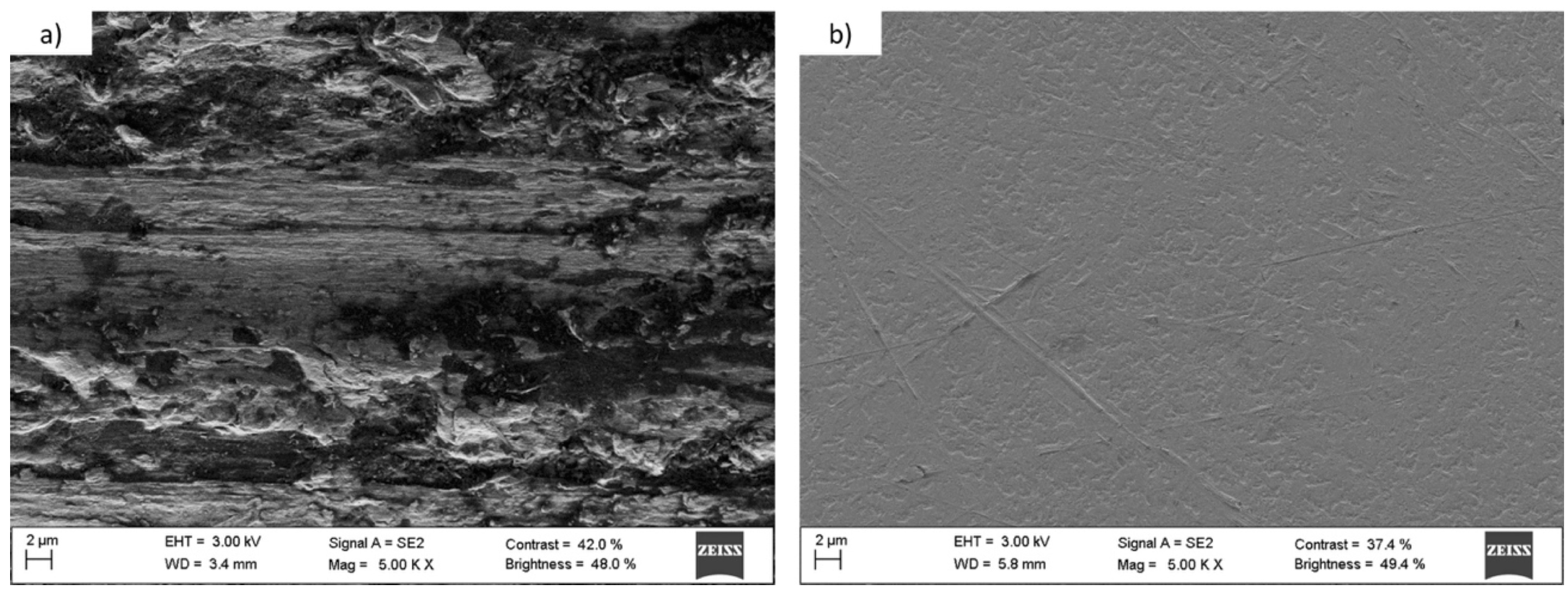
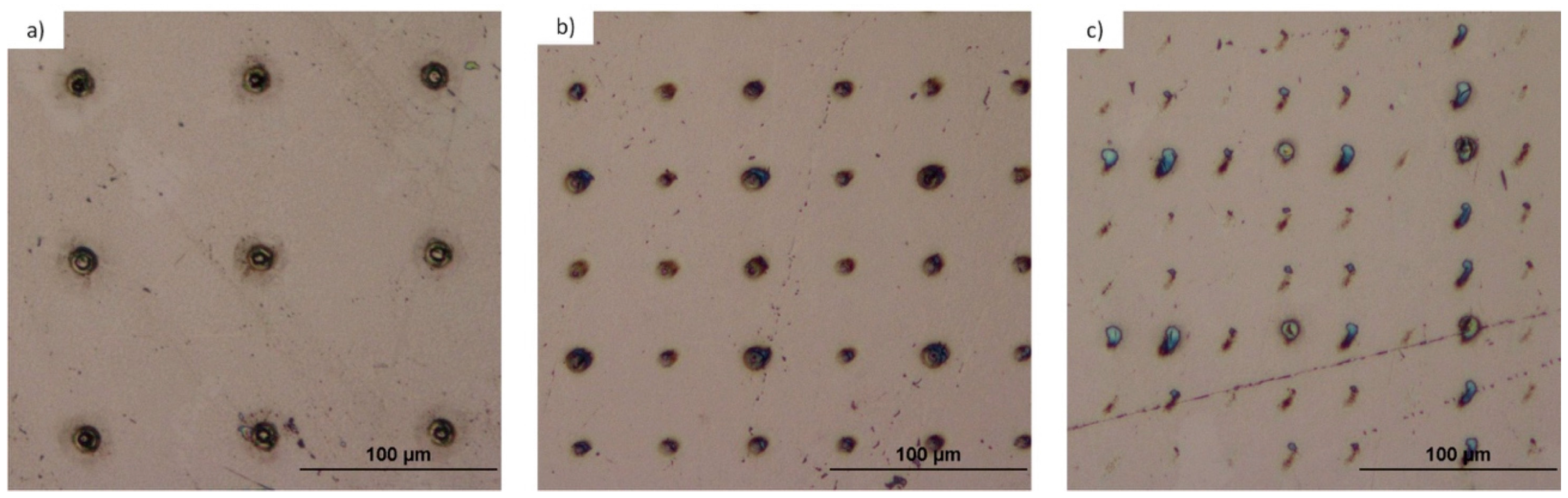
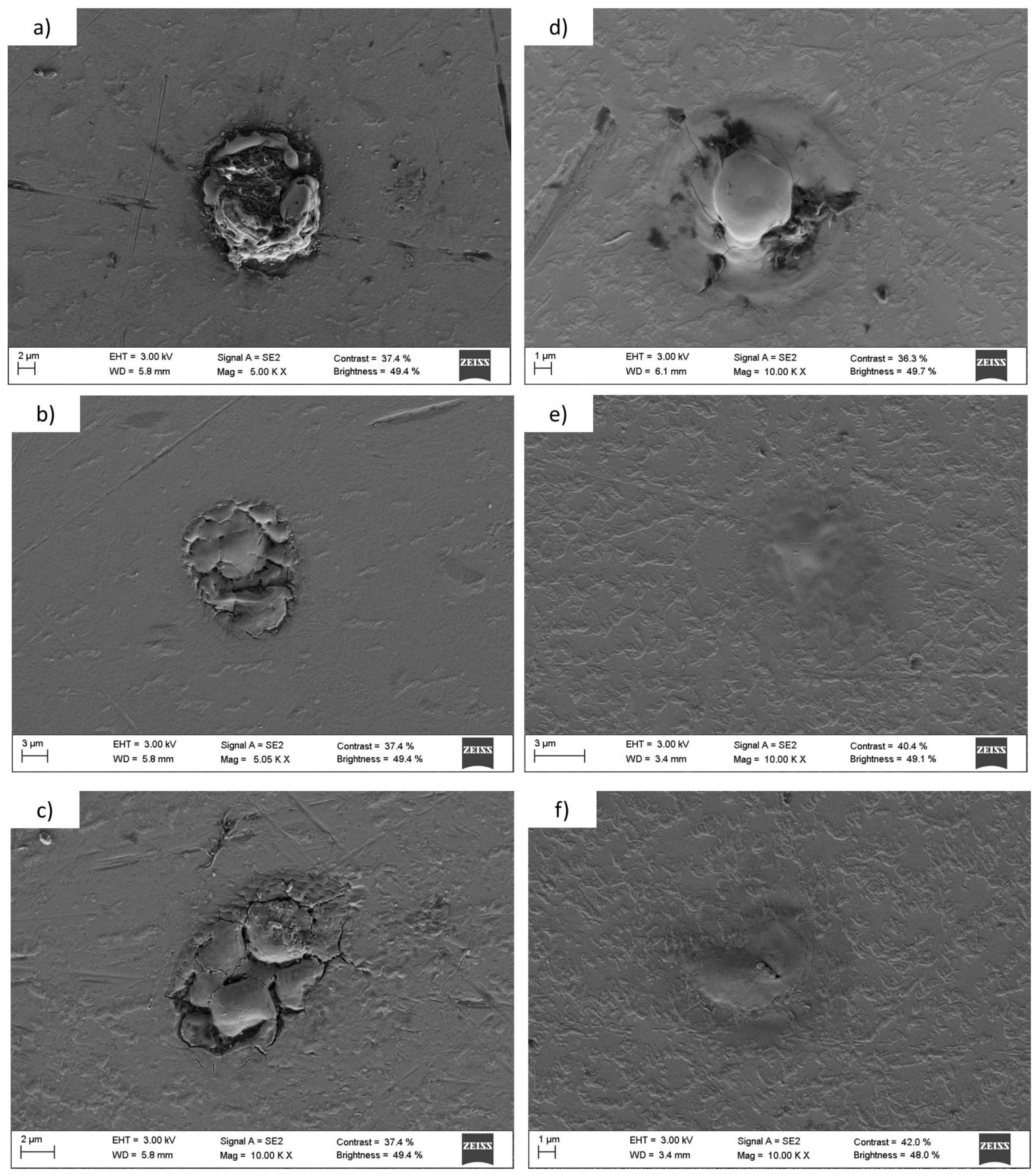
3.1.3. Titanium and Tantalum Ablation
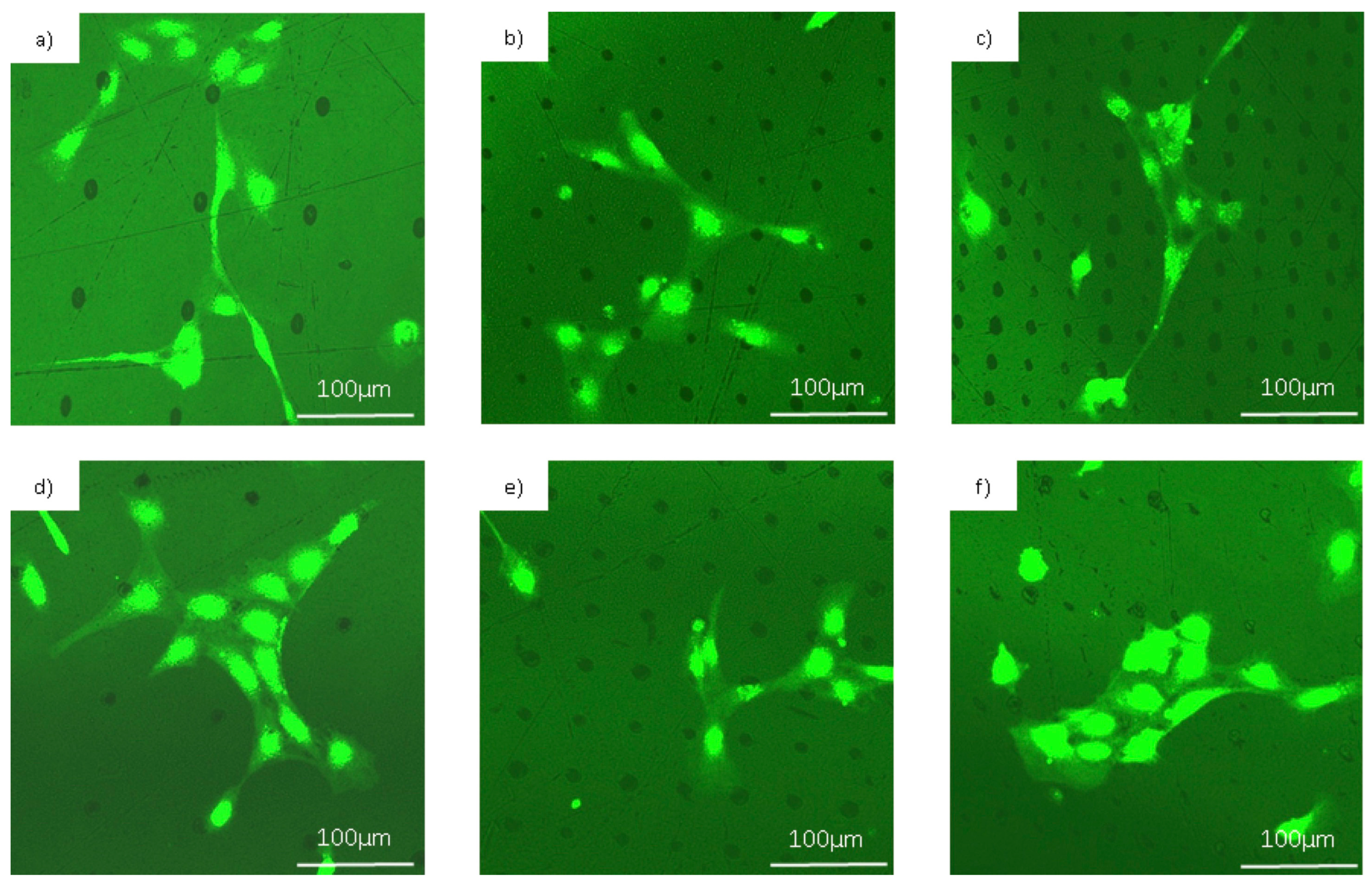
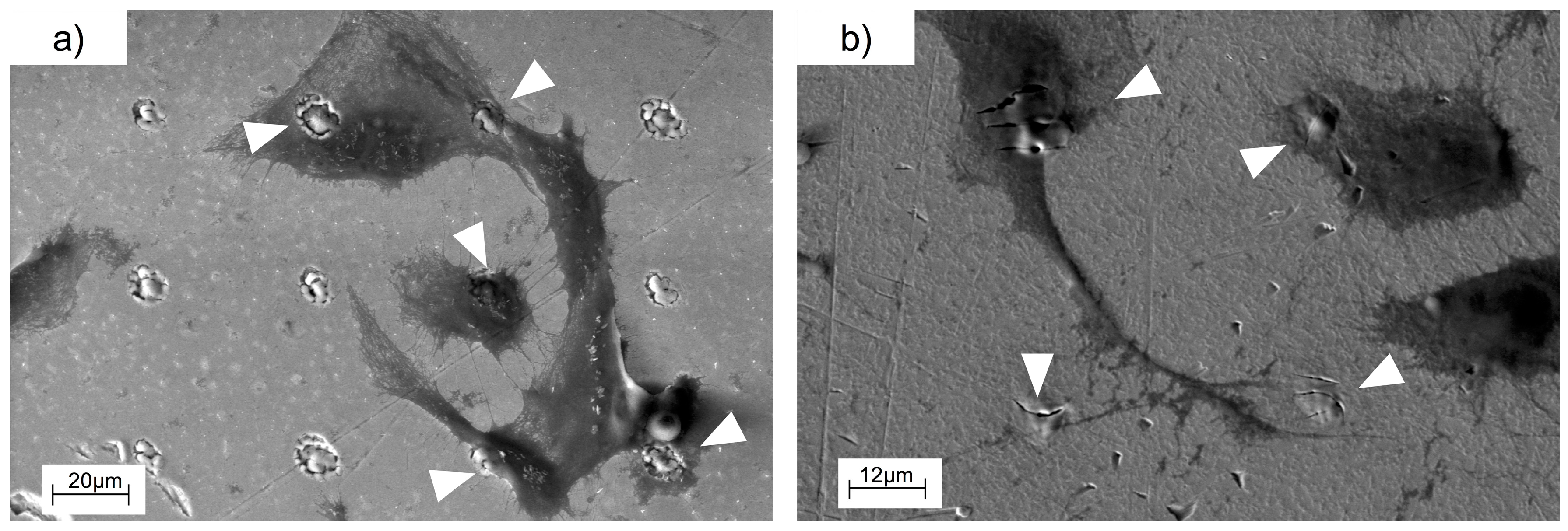
3.2. Cell Behavior
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Romano, V.; Weber, H.P.; Dumitru, G.; Pimenov, S.M.; Kononenko, T.V.; Konov, V.I.; Haefke, H.; Gerbig, Y.; Sentis, M.L.; Hermann, J.; et al. Laser surface microstructuring to improve tribological systems. In Laser Processing of Advanced Materials and Laser Microtechnologies; Dausinger, F.H., Ed.; The International Society for Optics and Photonics (SPIE): Bellingham, WA, USA, 2003; Volume 5121, pp. 199–211. [Google Scholar]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Weibel, D.E.; Michels, A.F.; Feil, A.F.; Amaral, L.; Teixeira, S.R.; Horowitz, F. Adjustable hydrophobicity of Al substrates by chemical surface functionalization of nano/microstructures. J. Phys. Chem. C 2010, 114, 13219–13225. [Google Scholar] [CrossRef]
- Tien, N.C.; Jeong, S.; Phinney, L.M.; Fushinobu, K.; Bokor, J. Surface adhesion reduction in silicon microstructures using femtosecond laser pulses. Appl. Phys. Lett. 1996, 68, 197–199. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Mirhosseini, N.; Michael, A.; Liu, Z.; Wang, T. Enhancement of endothelialisation of coronary stents by laser surface engineering. Lasers Surg. Med. 2013, 45, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.G.; Pishko, M.V. Fabrication of cell-containing hydrogel microstructures inside microfluidic devices that can be used as cell-based biosensors. Anal. Bioanal. Chem. 2006, 385, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.H.; Ho, C.T.; Lee, T.H.; Lee, T.L.; Liao, K.K.; Chen, F.L. Effect of surface roughness of ground titanium on initial cell adhesion. Biomol. Eng. 2004, 21, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Pelham, R.J.; Wang, Y.L. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA 1997, 94, 13661–13665. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.M.; Jiang, Z.; Bastmeyer, M.; Lahann, J. Physical aspects of cell culture substrates: Topography, roughness, and elasticity. Small 2012, 8, 336–355. [Google Scholar] [CrossRef] [PubMed]
- Craighead, H.G.; Turner, S.W.; Davis, R.C.; James, C.; Perez, A.M.; John, P.S.; Isaacson, M.S.; Kam, L.; Shaim, W.; Turner, J.N.; et al. Chemical and topographical surface modification for control of central nervous system cell adhesion. Biomed. Microdevices 1998, 1, 49–64. [Google Scholar] [CrossRef]
- Turner, A.M.P.; Dowell, N.; Turner, S.W.P.; Kam, L.; Isaacson, M.; Turner, J.N.; Craighead, H.G.; Shain, W. Attachment of astroglial cells to microfabricated pillar arrays of different geometries. J. Biomed. Mater. Res. 2000, 51, 430–441. [Google Scholar] [CrossRef]
- Altomare, L.; Gadegaard, N.; Visai, L.; Tanzi, M.C.; Fare, S. Biodegradable microgrooved polymeric surfaces obtained by photolithography for skeletal muscle cell orientation and myotube development. Acta Biomater. 2010, 6, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Dolatshahi-Pirouz, A.; Jensen, T.; Kraft, D.C.; Foss, M.; Kingshott, P.; Hansen, J.L.; Larsen, A.N.; Chevalier, J.; Besenbacher, F. Fibronectin adsorption, cell adhesion, and proliferation on nanostructured tantalum surfaces. ACS Nano 2010, 4, 2874–2882. [Google Scholar] [CrossRef] [PubMed]
- Ziebart, T.; Schnell, A.; Walter, C.; Kämmerer, P.W.; Pabst, A.; Lehmann, K.M.; Ziebart, J.; Klein, M.O.; Al-Nawas, B. Interactions between endothelial progenitor cells (EPC) and titanium implant surfaces. Clin. Oral Investig. 2013, 17, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Cristea, D.; Ghiuta, I.; Munteanu, D. Tantalum based materials for implants and prostheses applications. Bull. Transilv. Univ. Brasov. Eng. Sci. Ser. I 2015, 8, 151. [Google Scholar]
- Khang, D.; Lu, J.; Yao, C.; Haberstroh, K.M.; Webster, T.J. The role of nanometer and sub-micron surface features on vascular and bone cell adhesion on titanium. Biomaterials 2008, 29, 970–983. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Rao, M.P.; MacDonald, N.C.; Khang, D.; Webster, T.J. Improved endothelial cell adhesion and proliferation on patterned titanium surfaces with rationally designed, micrometer to nanometer features. Acta Biomater. 2008, 4, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Vandrangi, P.; Gott, S.C.; Kozaka, R.; Rodgers, V.G.; Rao, M.P. Comparative endothelial cell response on topographically patterned titanium and silicon substrates with micrometer to sub-micrometer feature sizes. PLoS ONE 2014, 9, e111465. [Google Scholar] [CrossRef] [PubMed]
- Justesen, J.; Lorentzen, M.; Andersen, L.K.; Hansen, O.; Chevallier, J.; Modin, C.; Füchtbauer, A.; Foss, M.; Besenbacher, F.; Duch, M.; et al. Spatial and temporal changes in the morphology of preosteoblastic cells seeded on microstructured tantalum surfaces. J. Biomed. Mater. Res. A 2009, 89, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.H.; Park, C.; Whitesides, G.M. Generation of submicrometer structures by photolithography using arrays of spherical microlenses. J. Colloid Interface Sci. 2003, 265, 304–309. [Google Scholar] [CrossRef]
- Akashi, T.; Yoshimura, Y. Deep reactive ion etching of borosilicate glass using an anodically bonded silicon wafer as an etching mask. J. Micromech. Microeng. 2006, 16, 1051. [Google Scholar] [CrossRef]
- Etsion, I. State of the art in laser surface texturing. J. Tribol. 2005, 127, 248–253. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, J.; Liang, C.; Wang, H.; Zhu, X.; Zhang, N. Surface microstructuring of Ti plates by femtosecond lasers in liquid ambiences: A new approach to improving biocompatibility. Opt. Express 2009, 17, 21124–21133. [Google Scholar] [CrossRef] [PubMed]
- Nieto, D.; Vara, G.; Diez, J.A.; O’Connor, G.M.; Arines, J.; Gómez-Reino, C.; Flores-Arias, M.T. Laser-based microstructuring of surfaces using low-cost microlens arrays. J. Micro/Nanolithogr. MEMS MOEMS 2012, 11, 023014. [Google Scholar] [CrossRef]
- He, M.; Yuan, X.C.; Ngo, N.; Bu, J.; Tao, S. Low-cost and efficient coupling technique using reflowed sol-gel microlens. Opt. Express 2003, 11, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Aymerich, M.; Nieto, D.; Flores-Arias, M.T. Laser-based surface multistructuring using optical elements and the Talbot effect. Opt. Express 2015, 23, 24369–24382. [Google Scholar] [CrossRef] [PubMed]
- Patorski, K. The Self-Imaging Phenomenon and its Applications. Prog. Opt. 1989, 27, 1–108. [Google Scholar]
- Berry, M.V.; Klein, S. Integer, fractional and fractal Talbot effects. J. Mod. Opt. 1996, 43, 2139–2164. [Google Scholar] [CrossRef]
- Lin, Y.; Hong, M.H.; Chen, G.X.; Lim, C.S.; Wang, Z.B.; Tan, L.S.; Shi, L.P.; Chong, T.C. Patterning of phase change films with microlens arrays. J. Alloys Compd. 2008, 449, 253–257. [Google Scholar] [CrossRef]
- Lim, C.S.; Hong, M.H.; Lin, Y.; Chen, G.X.; Kumar, A.S.; Rahman, M.; Tan, L.S.; Fuh, J.Y.H.; Lim, G.C. Sub-micron surface patterning by laser irradiation through microlens arrays. J. Mater. Process. Technol. 2007, 192, 328–333. [Google Scholar] [CrossRef]
- Rodiño-Janeiro, B.K.; González-Peteiro, M.; Ucieda-Somoza, R.; González-Juanatey, J.R.; Álvarez, E. Glycated albumin, a precursor of advanced glycation end-products, up-regulates NADPH oxidase and enhances oxidative stress in human endothelial cells: Molecular correlate of diabetic vasculopathy. Diabetes Metab. Res. 2010, 26, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, A.W.; Mendlovic, D.; Shabtay, G. Talbot (1836), Montgomery (1967), Lau (1948) and Wolf (1955) on periodicity in optics. Pure Appl. Opt. 1998, 7, 1121. [Google Scholar] [CrossRef]
- Montgomery, W.D. Self-imaging objects of infinite aperture. J. Opt. Soc. Am. 1967, 57, 772–775. [Google Scholar] [CrossRef]
- Reino, C.G.; Pérez, M.V.; Bao, C. Gradient-Index Optics: Fundamentals and Applicatioins; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Besold, B.; Lindlein, N. Fractional Talbot effect for periodic microlens arrays. Opt. Eng. 1997, 36, 1099–1105. [Google Scholar] [CrossRef]
- Deligianni, D.D.; Katsala, N.; Ladas, S.; Sotiropoulou, D.; Amedee, J.; Missirlis, Y.F. Effect of surface roughness of the titanium alloy Ti–6Al–4V on human bone marrow cell response and on protein adsorption. Biomaterials 2001, 22, 1241–1251. [Google Scholar] [CrossRef]
- Klein, M.O.; Bijelic, A.; Ziebart, T.; Koch, F.; Kämmerer, P.W.; Wieland, M.; Konerding, M.A.; Al-Nawas, B. Submicron scale-structured hydrophilic titanium surfaces promote early osteogenic gene response for cell adhesion and cell differentiation. Clin. Implant Dent. Relat. Res. 2013, 15, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K.; Bigerelle, M. On the relation between surface roughness of metallic substrates and adhesion of human primary bone cells. Scanning 2014, 36, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Donahue, H.J. Cell sensing and response to micro-and nanostructured surfaces produced by chemical and topographic patterning. Tissue Eng. 2007, 13, 1879–1891. [Google Scholar] [CrossRef] [PubMed]

| TITANIUM | TANTALUM | |||||
|---|---|---|---|---|---|---|
| First Talbot Plane | 3/2 Talbot Plane | 5/3 Talbot Plane | First Talbot Plane | 3/2 Talbot Plane | 5/3 Talbot Plane | |
| Microlens-substrate distance (mm) | 16.21 ± 0.01 | 23.85 ± 0.01 | 26.42 ± 0.01 | 16.21 ± 0.01 | 23.85 ± 0.01 | 26.42 ± 0.01 |
| Period (μm) | 90.28 ± 0.10 | 45.14 ± 0.10 | 29.62 ± 0.10 | 89.84 ± 0.10 | 45.61 ± 0.10 | 29.92 ± 0.10 |
| Spot diameter (μm) | 16.15 ± 0.10 | 12.20 ± 0.10 | 5.52 ± 0.10 | 13.26 ± 0.10 | 10.77 ± 0.10 | 5.44 ± 0.10 |
| Spot depth (μm) | 3.1 ± 0.3 | 2.2 ± 0.3 | 0.8 ± 0.3 | 2.3 ± 0.3 | 1.7 ± 0.3 | 0.6 ± 0.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aymerich, M.; Nieto, D.; Álvarez, E.; Flores-Arias, M.T. Laser Surface Microstructuring of Biocompatible Materials Using a Microlens Array and the Talbot Effect: Evaluation of the Cell Adhesion. Materials 2017, 10, 214. https://doi.org/10.3390/ma10020214
Aymerich M, Nieto D, Álvarez E, Flores-Arias MT. Laser Surface Microstructuring of Biocompatible Materials Using a Microlens Array and the Talbot Effect: Evaluation of the Cell Adhesion. Materials. 2017; 10(2):214. https://doi.org/10.3390/ma10020214
Chicago/Turabian StyleAymerich, María, Daniel Nieto, Ezequiel Álvarez, and María T. Flores-Arias. 2017. "Laser Surface Microstructuring of Biocompatible Materials Using a Microlens Array and the Talbot Effect: Evaluation of the Cell Adhesion" Materials 10, no. 2: 214. https://doi.org/10.3390/ma10020214
APA StyleAymerich, M., Nieto, D., Álvarez, E., & Flores-Arias, M. T. (2017). Laser Surface Microstructuring of Biocompatible Materials Using a Microlens Array and the Talbot Effect: Evaluation of the Cell Adhesion. Materials, 10(2), 214. https://doi.org/10.3390/ma10020214







