3D Printing of Cytocompatible Water-Based Light-Cured Polyurethane with Hyaluronic Acid for Cartilage Tissue Engineering Applications
Abstract
:1. Introduction
2. Results and Discussion
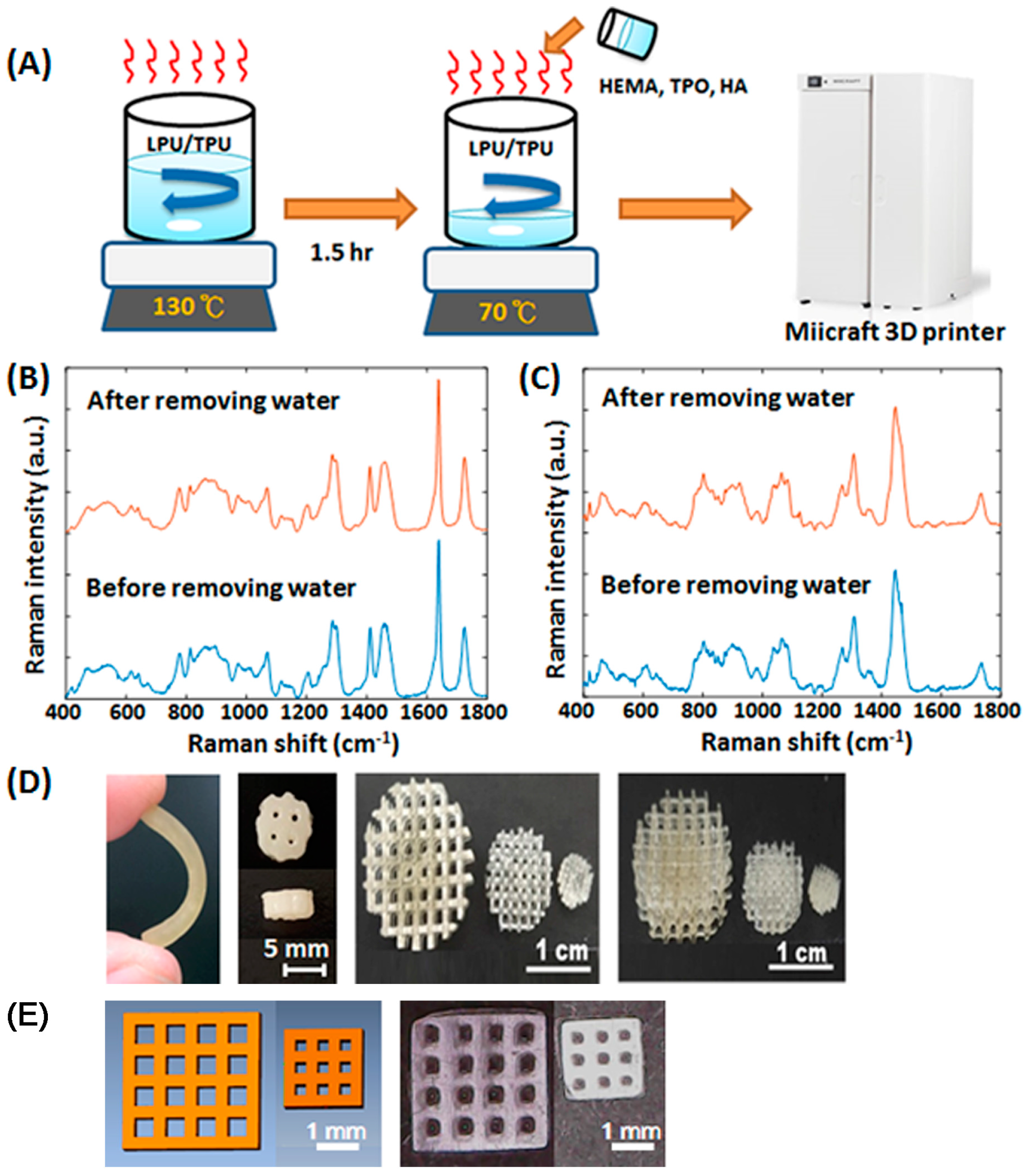
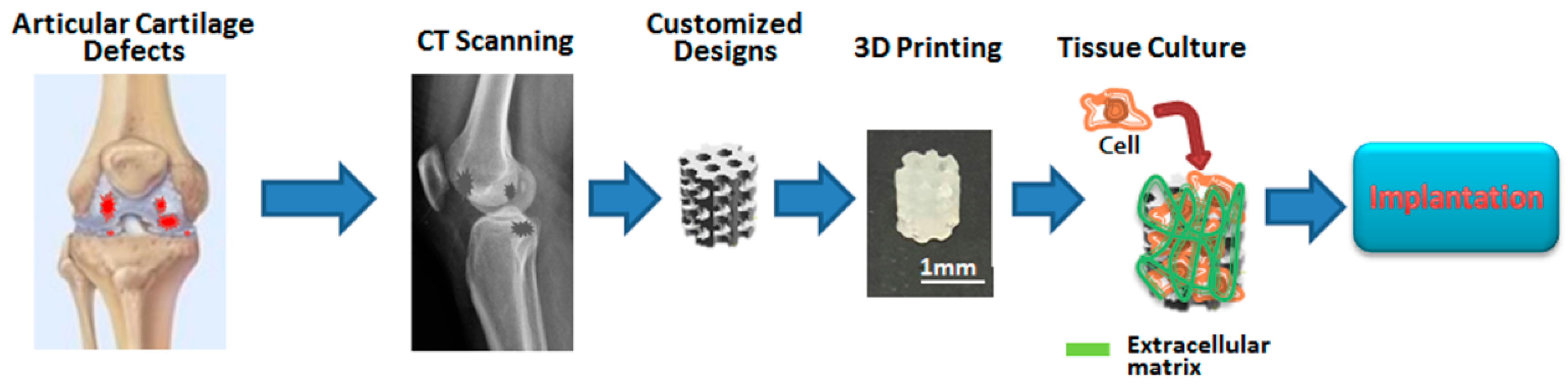
2.1. Fabrication of Customized Scaffolds
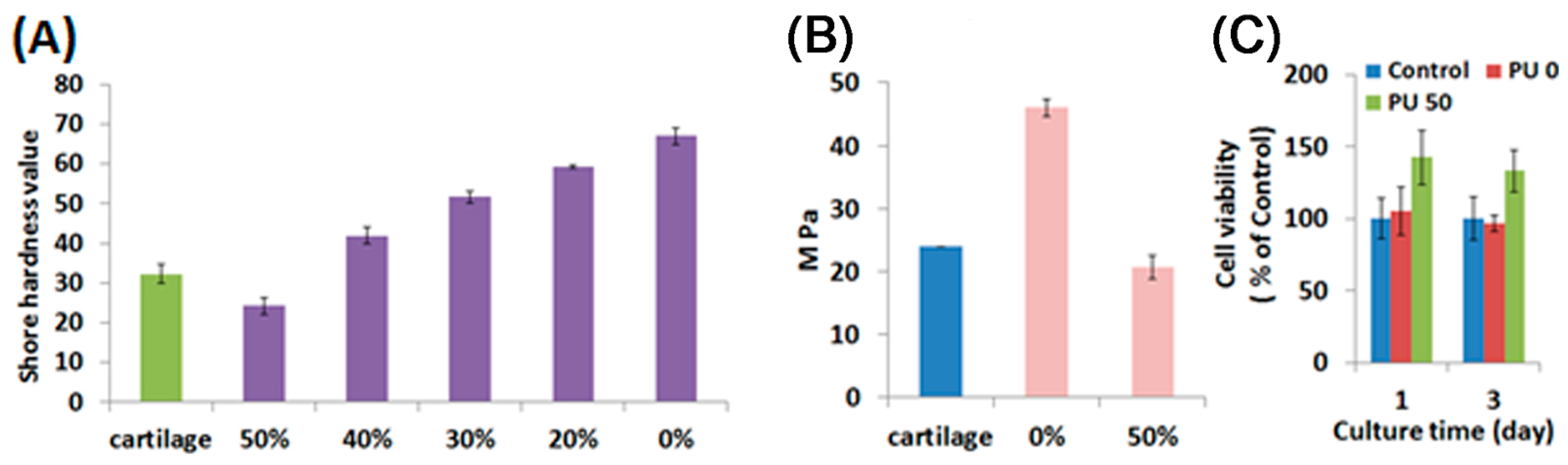
2.2. Characterization of Water-Based Light-Cured PU/TPU Specimens
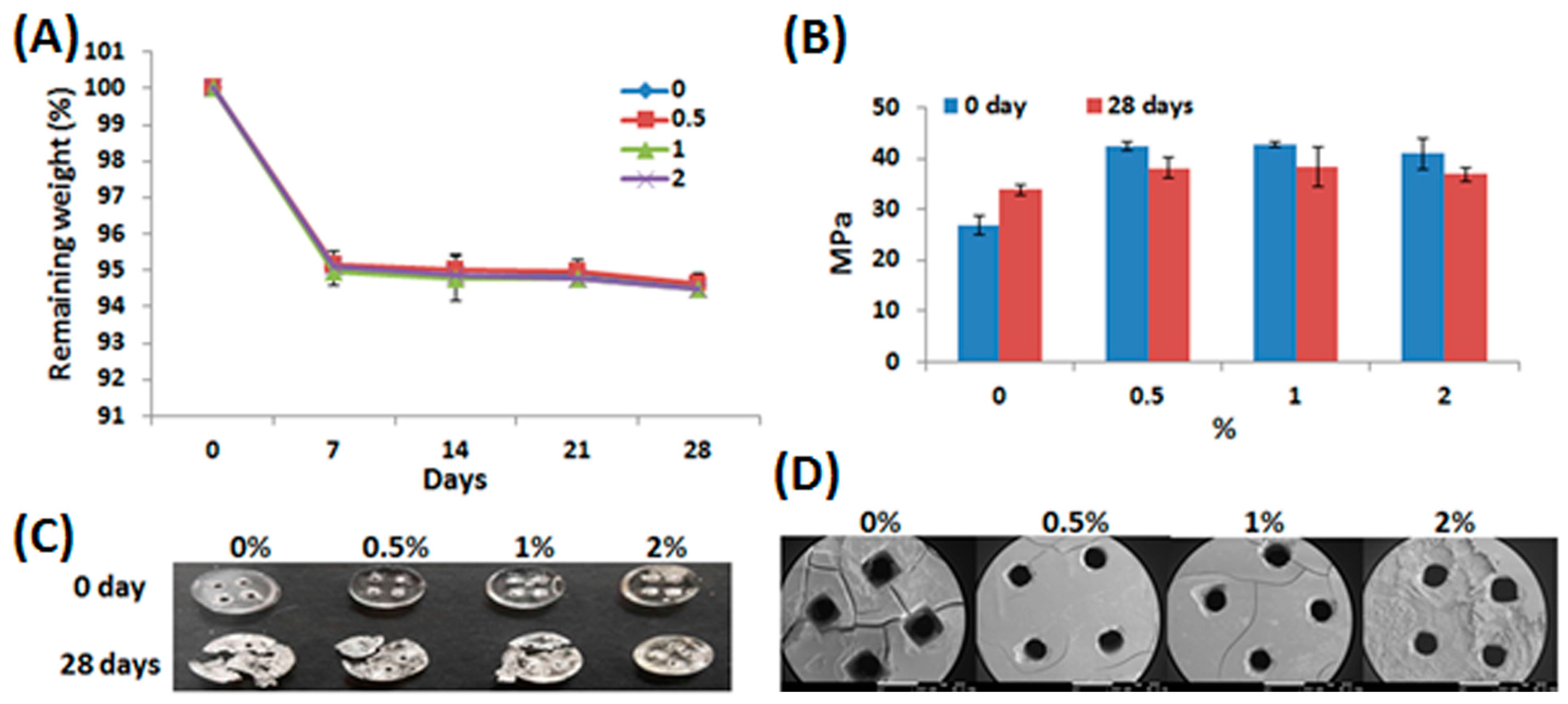
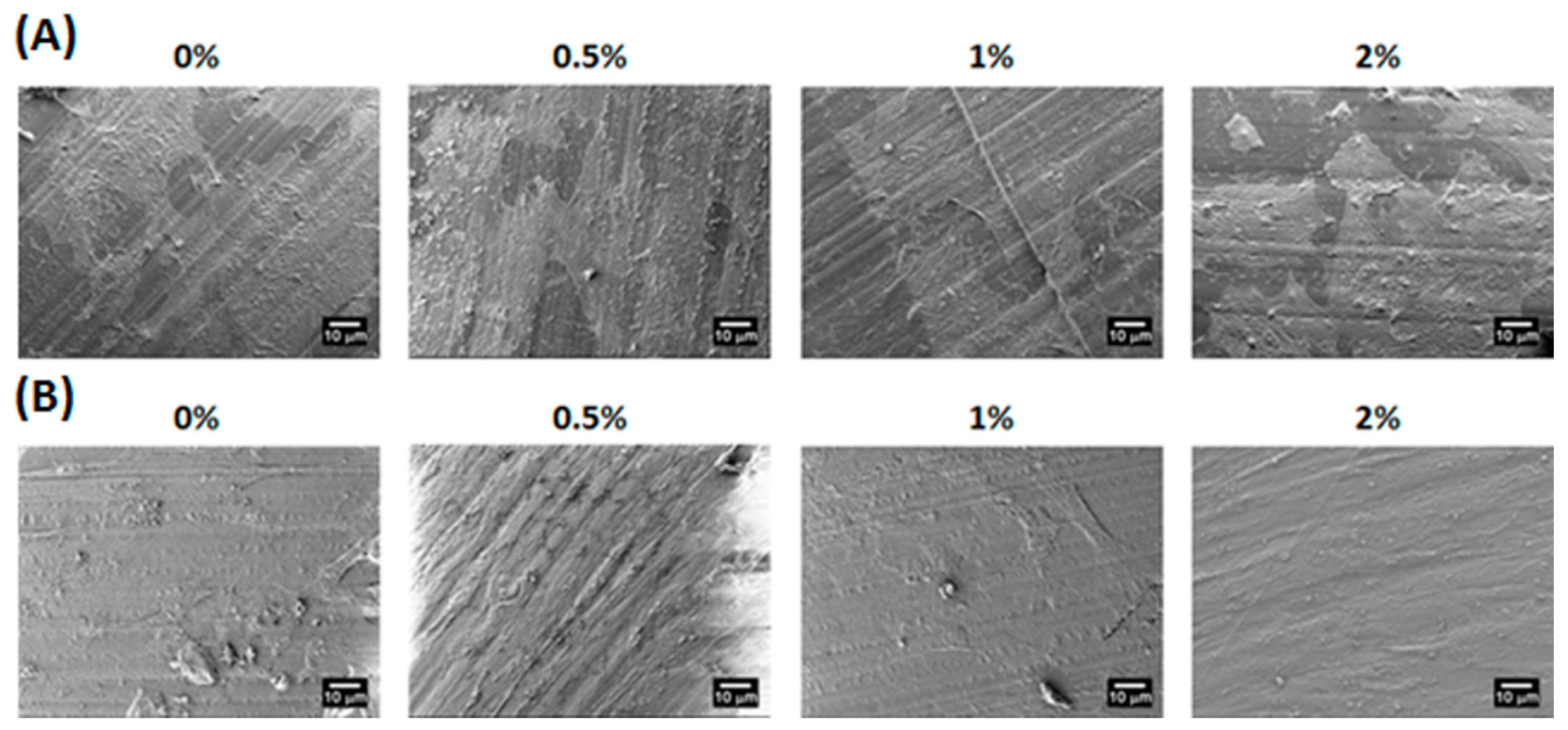
2.3. Characterization of Water-Based Light-Cured PU/HA Scaffolds
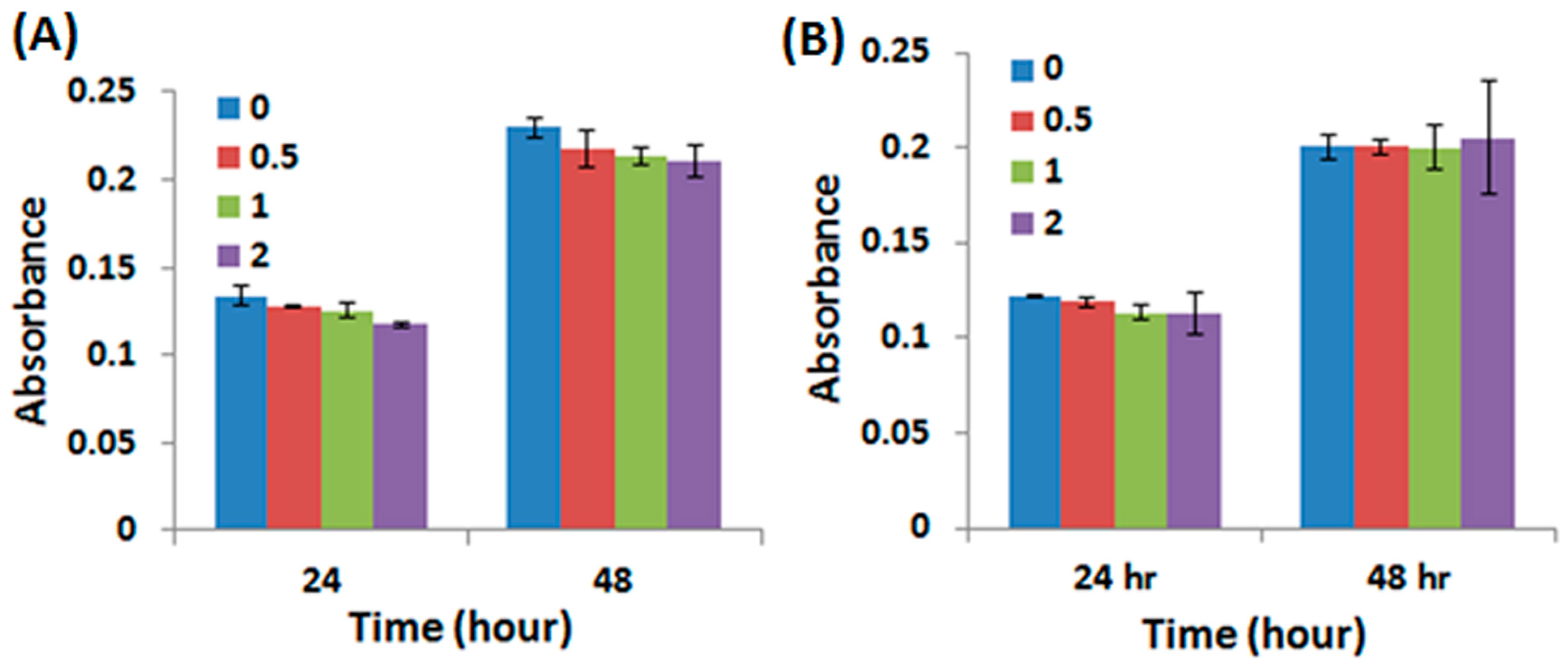
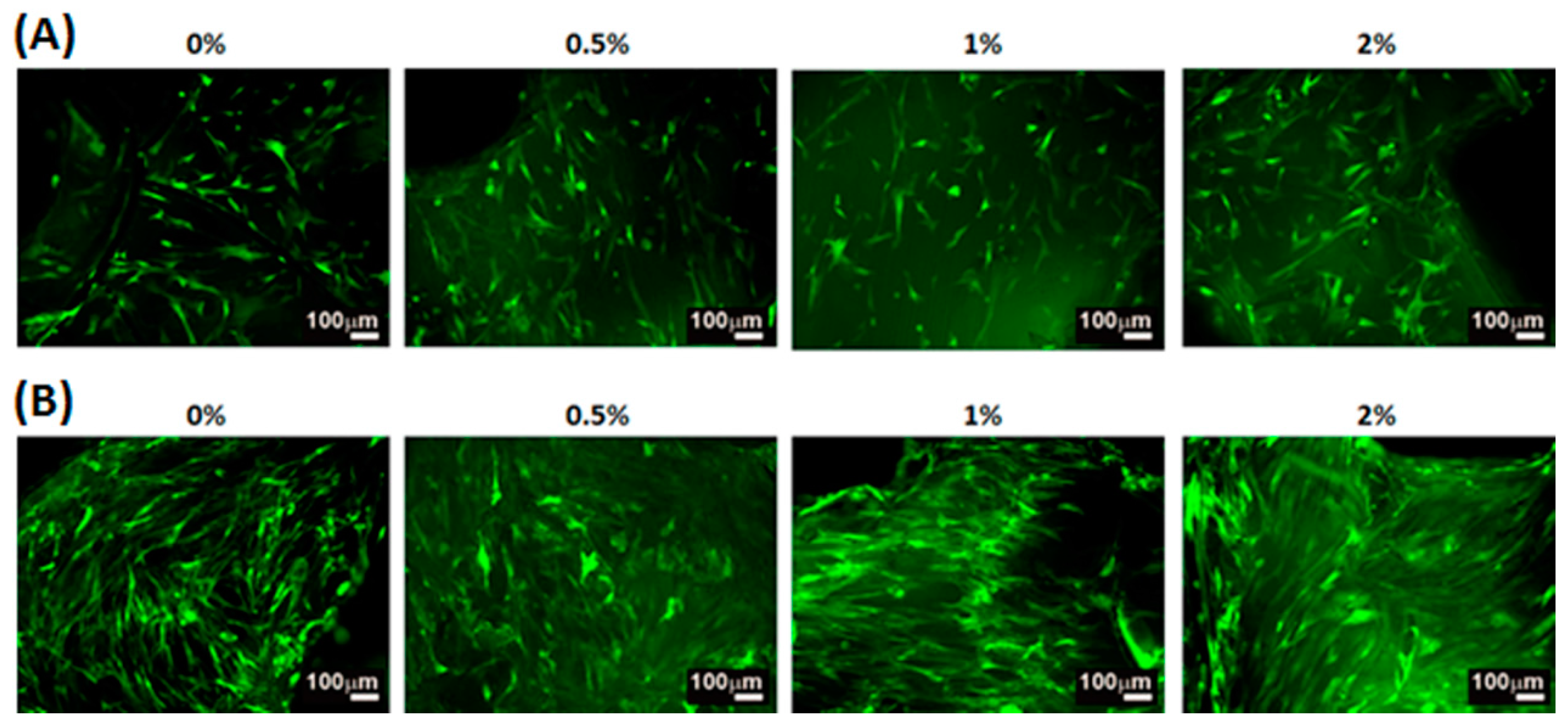
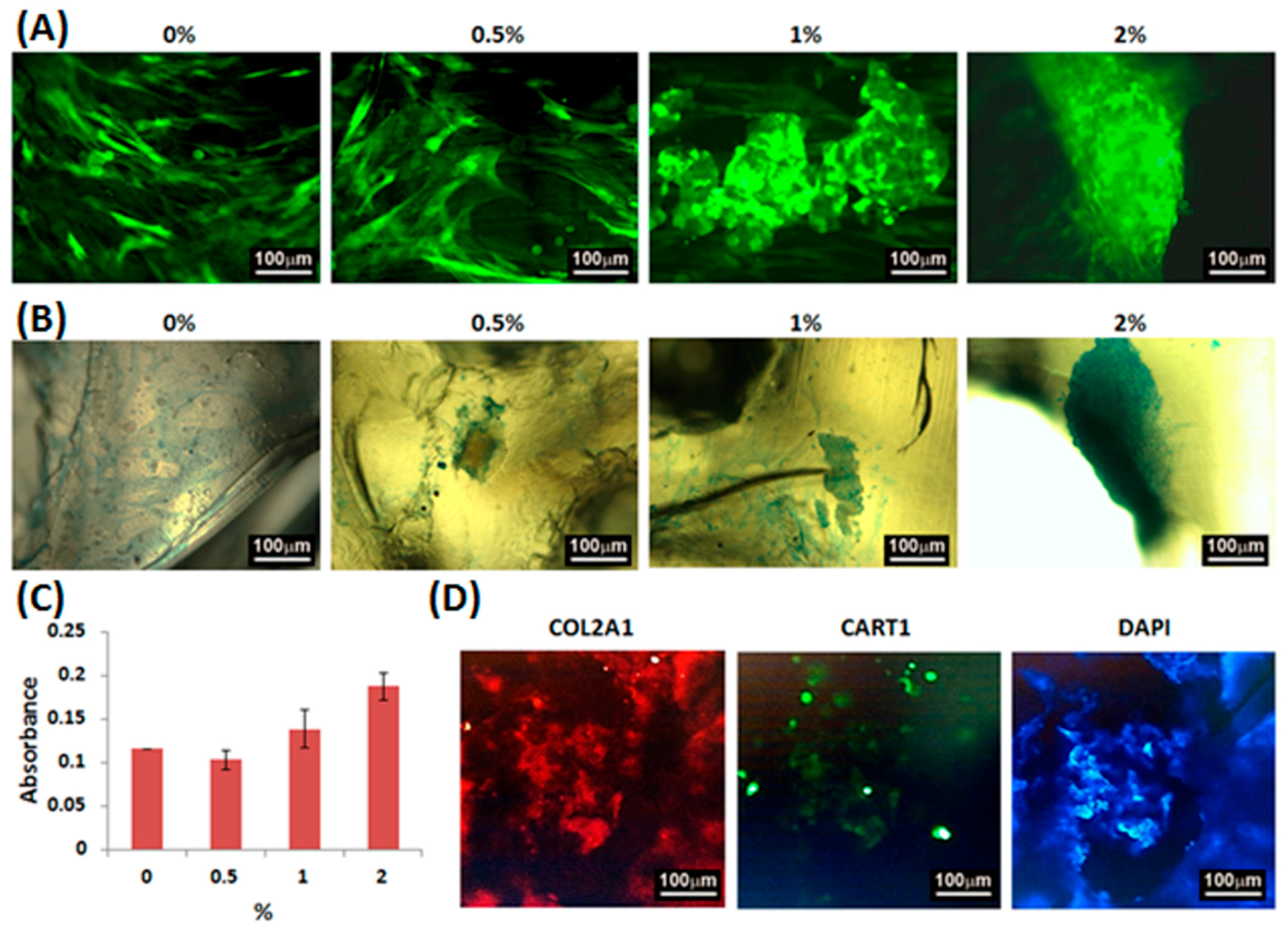
2.4. Adhesion, Proliferation and Chondrogenic Differentiation of Cells Cultured on Water-Based Light-Cured PU/HA Scaffolds
3. Materials and Methods
3.1. The Preparation of Water-Based Polyurethane Based Composites
3.2. Graft Fabrication
3.3. Printing Accuracy Analysis
3.4. Mechanical Properties
3.5. Raman Spectroscopy
3.6. Degradation In Vitro
3.7. Cell Viability
3.8. Cell Morphology
3.9. Fluorescent Images
3.10. Micromass Culture
3.11. Alcian Blue Staining and Analysis
3.12. Immunofluorescence Staining
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, J.; Wang, C.; Lu, S.; Wu, J.; Guo, X.; Duan, C.; Dong, L.; Song, Y.; Zhang, J.; Jing, D.; et al. In vivo chondrogenesis of adult bone-marrow-derived autologous mesenchymal stem cells. Cell Tissue Res. 2005, 319, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.P. Articular cartilage repair. Am. J. Sports Med. 1998, 26, 309–324. [Google Scholar] [PubMed]
- Malafaya, P.B.; Reis, R.L. Bilayered chitosan-based scaffolds for osteochondral tissue engineering: Influence of hydroxyapatite on in vitro cytotoxicity and dynamic bioactivity studies in a specific double-chamber bioreactor. Acta Biomater. 2009, 5, 644–660. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Li, C.; Heng, B.C.; Cao, G.; Yang, Z. Functional biomaterials for cartilage regeneration. J. Biomed. Mater. Res. A 2012, 100, 2526–2536. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Zhang, L.; Li, K.; Fan, H.; Fan, Y.; Liang, J.; Zhang, X. Collagen hydrogel as an immunomodulatory scaffold in cartilage tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Lau, T.T.; Leong, W.; Gong, Y.; Wang, D.-A. Creating a living hyaline cartilage graft free from non-cartilaginous constituents: An intermediate role of a biomaterial scaffold. Adv. Funct. Mater. 2012, 22, 972–978. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Li, Y.; Moran, S.; Khang, G.; Ge, Z. Poly(l-lactide-co-caprolactone) scaffolds enhanced with poly(beta-hydroxybutyrate-co-beta-hydroxyvalerate) microspheres for cartilage regeneration. Biomed. Mater. 2013, 8, 25005. [Google Scholar] [CrossRef] [PubMed]
- Kalson, N.S.; Gikas, P.D.; Briggs, T.W.R. Current strategies for knee cartilage repair. Int. J. Clin. Pract. 2010, 64, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Binder, K.W.; Albanna, M.Z.; Dice, D.; Zhao, W.; Yoo, J.J.; Atala, A. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication 2013, 5, 15001. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.-C.; Tseng, C.-S.; Hsu, S.-H. Synthesis and 3D printing of biodegradable polyurethane elastomer by a water-based process for cartilage tissue engineering applications. Adv. Healthc. Mater. 2014, 3, 1578–1587. [Google Scholar] [CrossRef] [PubMed]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martinez Avila, H.; Hagg, D.; Gatenholm, P. 3D Bioprinting human chondrocytes with nanocellulose-alginate bioink for cartilage tissue engineering applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Qiao, Z.; Jiang, W.; Li, H.; Wei, J.; Zhou, G.; Dai, K. Regeneration of a goat femoral head using a tissue-specific, biphasic scaffold fabricated with CAD/CAM technology. Biomaterials 2013, 34, 6706–6716. [Google Scholar] [CrossRef] [PubMed]
- Chien, K.B.; Makridakis, E.; Shah, R.N. Three-dimensional printing of soy protein scaffolds for tissue regeneration. Tissue Eng. Part C Methods 2013, 19, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Yeong, W.Y.; Sudarmadji, N.; Yu, H.Y.; Chua, C.K.; Leong, K.F.; Venkatraman, S.S.; Boey, Y.C.F.; Tan, L.P. Porous polycaprolactone scaffold for cardiac tissue engineering fabricated by selective laser sintering. Acta Biomater. 2010, 6, 2028–2034. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.-Y.; Lin, H.-Y.; Chen, Y.-W.; Lin, C.-Y.; Hsu, T.-T.; Kao, C.-T. Laser Sintered Magnesium-Calcium Silicate/Poly-ε-Caprolactone Scaffold for Bone Tissue Engineering. Materials 2017, 10. [Google Scholar] [CrossRef]
- Sherwood, J.K.; Riley, S.L.; Palazzolo, R.; Brown, S.C.; Monkhouse, D.C.; Coates, M.; Griffith, L.G.; Landeen, L.K.; Ratcliffe, A. A three-dimensional osteochondral composite scaffold for articular cartilage repair. Biomaterials 2002, 23, 4739–4751. [Google Scholar] [CrossRef]
- Gauvin, R.; Chen, Y.-C.; Lee, J.W.; Soman, P.; Zorlutuna, P.; Nichol, J.W.; Bae, H.; Chen, S.; Khademhosseini, A. Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography. Biomaterials 2012, 33, 3824–3834. [Google Scholar] [CrossRef] [PubMed]
- Dean, D.; Wallace, J.; Siblani, A.; Wang, M.O.; Kim, K.; Mikos, A.G.; Fisher, J.P. Continuous digital light processing (cDLP): Highly accurate additive manufacturing of tissue engineered bone scaffolds. Virtual Phys. Prototyp. 2012, 7, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-W.; Wicker, R.; Lee, S.-H.; Choi, K.-H.; Ha, C.-S.; Chung, I. Fabrication of 3D biocompatible/biodegradable micro-scaffolds using dynamic mask projection microstereolithography. J. Mater. Process. Technol. 2009, 209, 5494–5503. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Elomaa, L.; Teixeira, S.; Hakala, R.; Korhonen, H.; Grijpma, D.W.; Seppälä, J.V. Preparation of poly(ε-caprolactone)-based tissue engineering scaffolds by stereolithography. Acta Biomater. 2011, 7, 3850–3856. [Google Scholar] [CrossRef] [PubMed]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A poly(d,l-lactide) resin for the preparation of tissue engineering scaffolds by stereolithography. Biomaterials 2009, 30, 3801–3809. [Google Scholar] [CrossRef] [PubMed]
- Seck, T.M.; Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. Designed biodegradable hydrogel structures prepared by stereolithography using poly(ethylene glycol)/poly(d,l-lactide)-based resins. J. Control. Release 2010, 148, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Eglin, D.; Alini, M. Degradable polymeric materials for osteosynthesis: Tutorial. Eur. Cell. Mater. 2008, 16, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Gunatillake, P.A.; Adhikari, R. Biodegradable synthetic polymers for tissue engineering. Eur. Cell. Mater. 2003, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sohier, J.; Moroni, L.; van Blitterswijk, C.; de Groot, K.; Bezemer, J.M. Critical factors in the design of growth factor releasing scaffolds for cartilage tissue engineering. Expert Opin. Drug Deliv. 2008, 5, 543–566. [Google Scholar] [CrossRef] [PubMed]
- Szycher, M. Scycher’s Handbook of Polyurethanes; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Wang, Z.; Wan, P.; Ding, M.; Yi, X.; Li, J.; Fu, Q.; Tan, H. Synthesis and micellization of new biodegradable phosphorylcholine-capped polyurethane. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 2033–2042. [Google Scholar] [CrossRef]
- Stampfl, J.; Wöß, A.; Seidler, S.; Fouad, H.; Pisaipan, A.; Schwager, F.; Liska, R. Water soluble, photocurable resins for rapid prototyping applications. Macromol. Symp. 2004, 217, 99–108. [Google Scholar] [CrossRef]
- Claycomb, J.R.; Tran, J.Q.P. Introductory Biophysics: Perspectives on the Living State; Jones and Bartlett Learning: Burlington, MA, USA, 2010. [Google Scholar]
- Schuurman, W.; Levett, P.A.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.A.; Hutmacher, D.W.; Melchels, F.P.W.; Klein, T.J.; Malda, J. Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol. Biosci. 2013, 13, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chu, M.; Lin, C.; Chen, H.; Hung, H.; Sun, W.; Kao, W.; Hsu, S. Enhanced migration of Wharton’s Jelly mesenchymal stem cells grown on polyurethane nanocomposites. J. Med. Biol. Eng. 2013, 33, 139–148. [Google Scholar] [CrossRef]
- Rutjes, A.W.S.; Juni, P.; da Costa, B.R.; Trelle, S.; Nuesch, E.; Reichenbach, S. Viscosupplementation for osteoarthritis of the knee: A systematic review and meta-analysis. Ann. Intern. Med. 2012, 157, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Kon, E.; Roffi, A.; di Martino, A.; Marcacci, M. Scaffold-based repair for cartilage healing: A systematic review and technical note. Arthroscopy 2013, 29, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, M.J.; Caplan, A.I. Hyaluronic acid bonded to cell-culture surfaces stimulates chondrogenesis in stage 24 limb mesenchyme cell cultures. Dev. Biol. 1986, 114, 504–518. [Google Scholar] [CrossRef]
- Kim, I.L.; Mauck, R.L.; Burdick, J.A. Hydrogel design for cartilage tissue engineering: A case study with hyaluronic acid. Biomaterials 2011, 32, 8771–8782. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, A.; Whyte, G.P. One-stage cartilage repair using a hyaluronic acid–based scaffold with activated bone marrow-derived mesenchymal stem cells compared with microfracture: Five-year follow-up. Am. J. Sport. Med. 2016, 44, 2846–2854. [Google Scholar] [CrossRef] [PubMed]
- Bansil, R.; Yannas, I.V.; Stanley, H.E. Raman spectroscopy: A structural probe of glycosaminoglycans. Biochim. Biophys. Acta 1978, 541, 535–542. [Google Scholar] [CrossRef]
- Tan, H.; Li, H.; Rubin, J.P.; Marra, K.G. Controlled Gelation and Degradation Rates of Injectable Hyaluronic Acid-based Hydrogels through a Double Crosslinking Strategy. J. Tissue Eng. Regen. Med. 2011, 5, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Dånmark, S.; Finne-Wistrand, A.; Schander, K.; Hakkarainen, M.; Arvidson, K.; Mustafa, K.; Albertsson, A.-C. In vitro and in vivo degradation profile of aliphatic polyesters subjected to electron beam sterilization. Acta Biomater. 2011, 7, 2035–2046. [Google Scholar] [CrossRef] [PubMed]
- Nalluri, S.M.; Krishnan, G.R.; Cheah, C.; Arzumand, A.; Yuan, Y.; Richardson, C.A.; Yang, S.; Sarkar, D. Hydrophilic polyurethane matrix promotes chondrogenesis of mesenchymal stem cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 54, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.S.; Lee, E.A.; Yoon, J.J.; Park, T.G. Hyaluronic acid modified biodegradable scaffolds for cartilage tissue engineering. Biomaterials 2005, 26, 1925–1933. [Google Scholar] [CrossRef]
- Fan, H.; Hu, Y.; Zhang, C.; Li, X.; Lv, R.; Qin, L.; Zhu, R. Cartilage regeneration using mesenchymal stem cells and a PLGA-gelatin/chondroitin/hyaluronate hybrid scaffold. Biomaterials 2006, 27, 4573–4580. [Google Scholar] [CrossRef]
- Choong, Y.Y.C.; Maleksaeedi, S.; Eng, H.; Su, P.-C.; Wei, J. Curing characteristics of shape memory polymers in 3D projection and laser stereolithography. Virtual Phys. Prototyp. 2016, 1–8. [Google Scholar] [CrossRef]
- Zarek, M.; Layani, M.; Eliazar, S.; Mansour, N.; Cooperstein, I.; Shukrun, E.; Szlar, A.; Cohn, D.; Magdassi, S. 4D printing shape memory polymers for dynamic jewellery and fashionwear. Virtual Phys. Prototyp. 2016, 11, 263–270. [Google Scholar] [CrossRef]
- Leist, S.K.; Zhou, J. Current status of 4D printing technology and the potential of light-reactive smart materials as 4D printable materials. Virtual Phys. Prototyp. 2016, 11, 249–262. [Google Scholar] [CrossRef]
- Afseth, N.K.; Segtnan, V.H.; Wold, J.P. Raman spectra of biological samples: A study of preprocessing methods. Appl. Spectrosc. 2006, 60, 1358–1367. [Google Scholar] [CrossRef]
- Fu, S.; Ni, P.; Wang, B.; Chu, B.; Peng, J.; Zheng, L.; Zhao, X.; Luo, F.; Wei, Y.; Qian, Z. In vivo biocompatibility and osteogenesis of electrospun poly(epsilon-caprolactone)-poly(ethylene glycol)-poly(epsilon-caprolactone)/nano-hydroxyapatite composite scaffold. Biomaterials 2012, 33, 8363–8371. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, P.B.; Solursh, M.; Reiter, R.S. Stage-related capacity for limb chondrogenesis in cell culture. Dev. Biol. 1977, 60, 69–82. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shie, M.-Y.; Chang, W.-C.; Wei, L.-J.; Huang, Y.-H.; Chen, C.-H.; Shih, C.-T.; Chen, Y.-W.; Shen, Y.-F. 3D Printing of Cytocompatible Water-Based Light-Cured Polyurethane with Hyaluronic Acid for Cartilage Tissue Engineering Applications. Materials 2017, 10, 136. https://doi.org/10.3390/ma10020136
Shie M-Y, Chang W-C, Wei L-J, Huang Y-H, Chen C-H, Shih C-T, Chen Y-W, Shen Y-F. 3D Printing of Cytocompatible Water-Based Light-Cured Polyurethane with Hyaluronic Acid for Cartilage Tissue Engineering Applications. Materials. 2017; 10(2):136. https://doi.org/10.3390/ma10020136
Chicago/Turabian StyleShie, Ming-You, Wen-Ching Chang, Li-Ju Wei, Yu-Hsin Huang, Chien-Han Chen, Cheng-Ting Shih, Yi-Wen Chen, and Yu-Fang Shen. 2017. "3D Printing of Cytocompatible Water-Based Light-Cured Polyurethane with Hyaluronic Acid for Cartilage Tissue Engineering Applications" Materials 10, no. 2: 136. https://doi.org/10.3390/ma10020136
APA StyleShie, M.-Y., Chang, W.-C., Wei, L.-J., Huang, Y.-H., Chen, C.-H., Shih, C.-T., Chen, Y.-W., & Shen, Y.-F. (2017). 3D Printing of Cytocompatible Water-Based Light-Cured Polyurethane with Hyaluronic Acid for Cartilage Tissue Engineering Applications. Materials, 10(2), 136. https://doi.org/10.3390/ma10020136







