Potential of Bioactive Glasses for Cardiac and Pulmonary Tissue Engineering
Abstract
1. Introduction
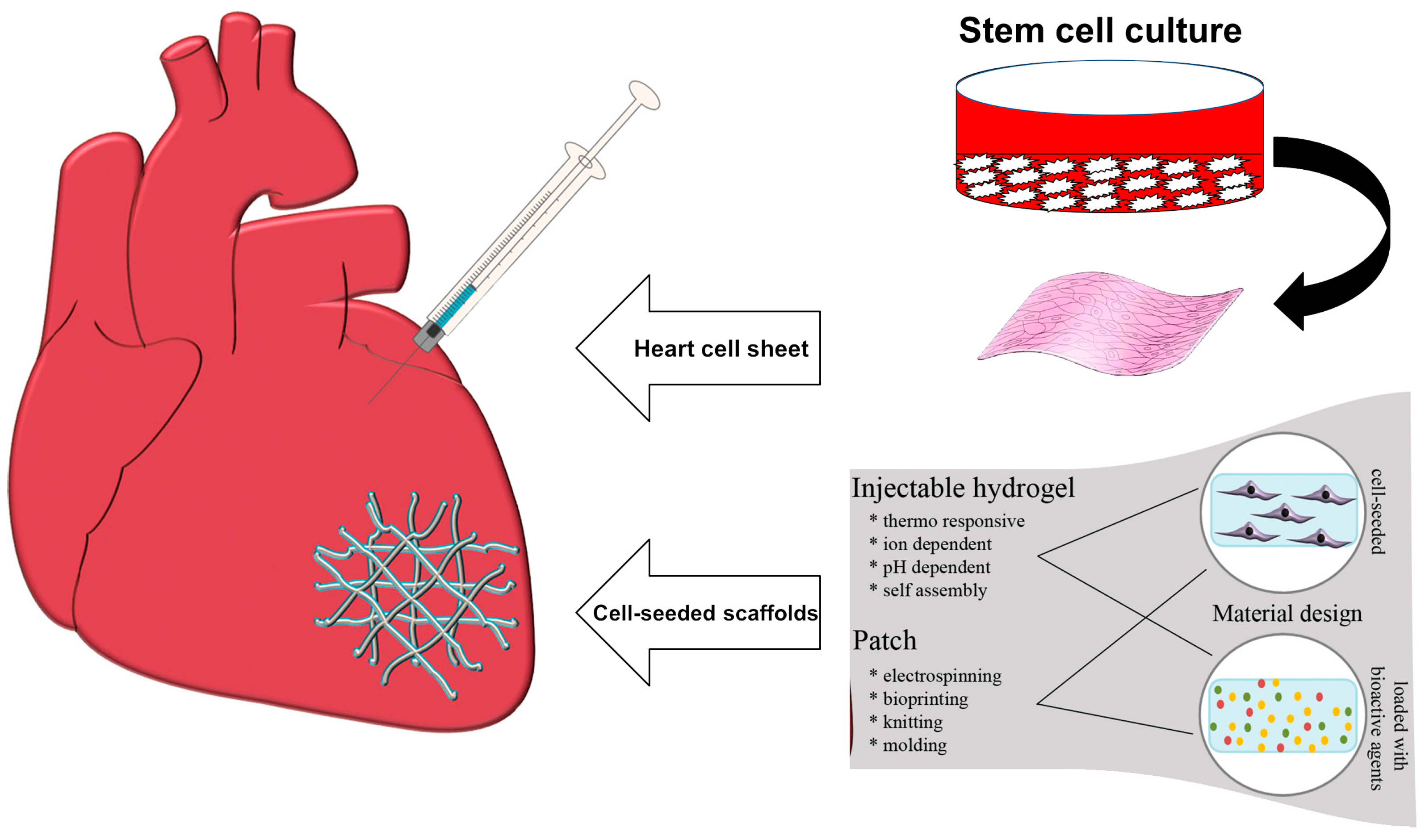
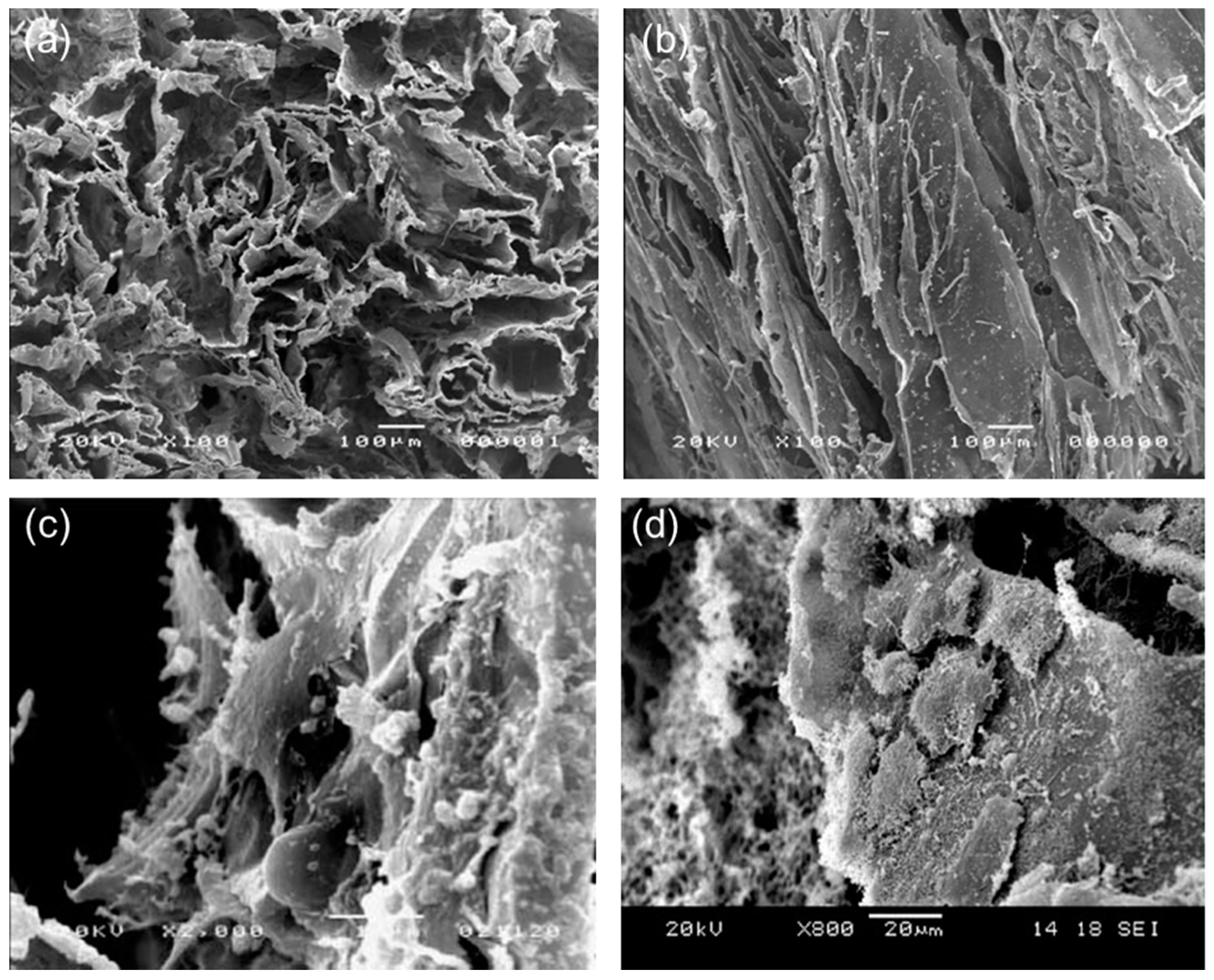
2. Applications in Cardiac Tissue Engineering
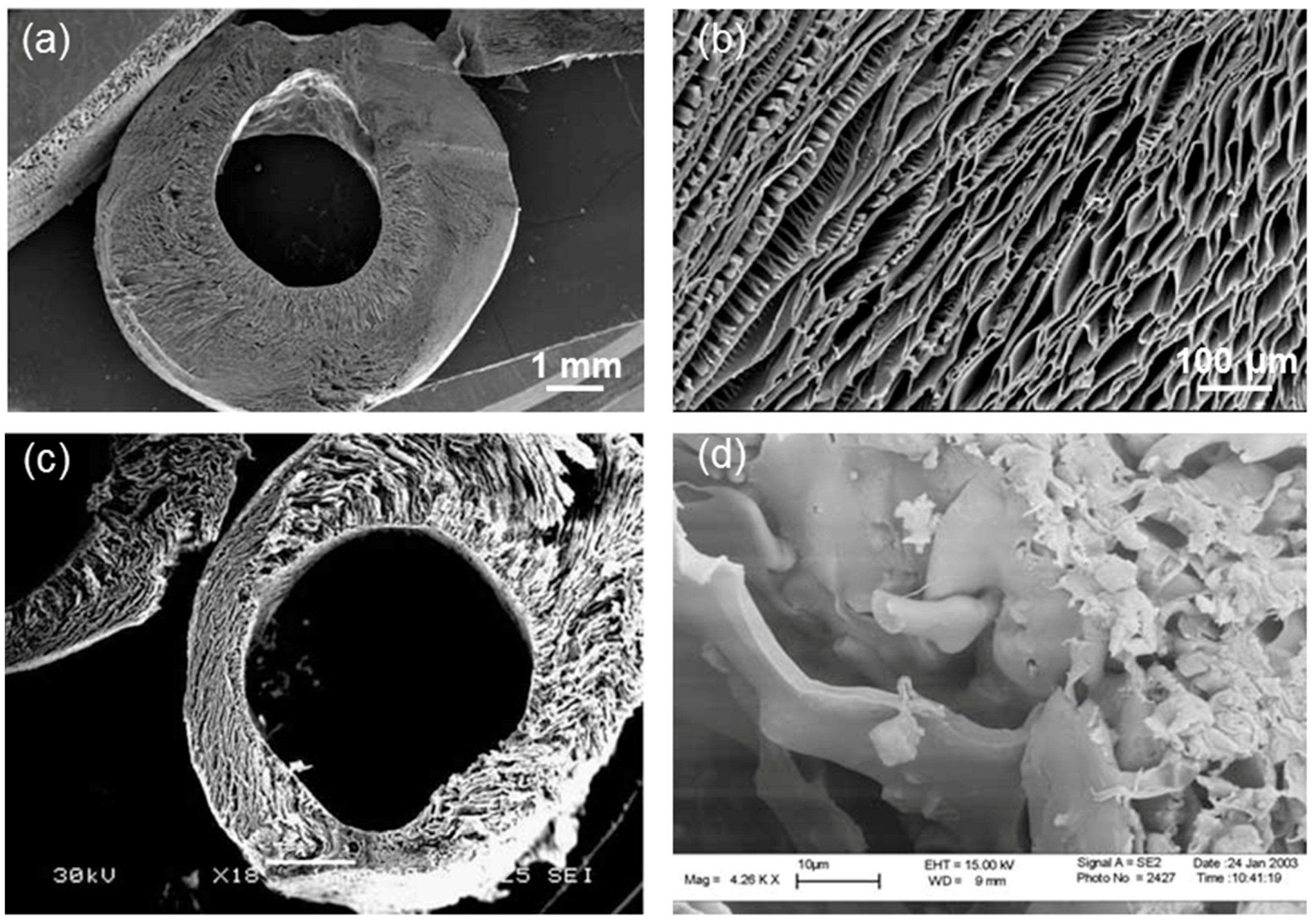
3. Applications in Lung Tissue Engineering
4. Discussion and Future Challenges
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Dawber, T.R.; Moore, F.E.; Mann, G.V., II. Coronary Heart Disease in the Framingham Study. Int. J. Epidemiol. 2015, 44, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Humbert, M.; Vachiery, J.-L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2015, 37, 67–119. [Google Scholar] [PubMed]
- Members, W.G.; Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Blaha, M.J.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Heart disease and stroke statistics—2014 update: A report from the American Heart Association. Circulation 2014, 129, e28–e292. [Google Scholar]
- Hashimoto, K.; Miyoshi, K.; Mizutani, H.; Otani, S.; Sugimoto, S.; Yamane, M.; Oto, T. Successful Lung Transplantation for Pulmonary Disease Associated With Erdheim-Chester Disease. Ann. Thorac. Surg. 2017, 104, e13–e15. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.J.; Glanville, A.R.; Aboyoun, C.; Belperio, J.; Benden, C.; Berry, G.J.; Hachem, R.; Hayes, D.; Neil, D.; Reinsmoen, N.L.; et al. Antibody-mediated rejection of the lung: A consensus report of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2016, 35, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Loupy, A.; Toquet, C.; Rouvier, P.; Beuscart, T.; Bories, M.; Varnous, S.; Guillemain, R.; Pattier, S.; Suberbielle, C.; Leprince, P.; et al. Late failing heart allografts: Pathology of cardiac allograft vasculopathy and association with antibody-mediated rejection. Am. J. Transplant. 2016, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- De Vlaminck, I.; Martin, L.; Kertesz, M.; Patel, K.; Kowarsky, M.; Strehl, C.; Cohen, G.; Luikart, H.; Neff, N.F.; Okamoto, J.; et al. Noninvasive monitoring of infection and rejection after lung transplantation. Proc. Natl. Acad. Sci. USA 2015, 112, 13336–13341. [Google Scholar] [CrossRef] [PubMed]
- Scarritt, M.E.; Pashos, N.C.; Bunnell, B.A. A review of cellularization strategies for tissue engineering of whole organs. Front. Bioeng. Biotechnol. 2015, 3, 43. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.; Puperi, D.S.; Kim, E.J.; Ayoub, S.; Shah, J.V.; Cuchiara, M.L.; West, J.L.; Grande-Allen, K.J. Anisotropic poly (ethylene glycol)/polycaprolactone hydrogel-fiber composites for heart valve tissue engineering. Tissue Eng. Part A 2014, 20, 2634–2645. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, M.Z.; Johnson, J.-A.; Sun, D.; Caritg, O.; Laresgoiti, U.; Brady, J.; Allen, G.; Giangreco, A.; Rawlins, E.L. Development of a genetically modifiable epithelial in-vitro culture system from human embryonic lung epithelial stem cells: Towards human lung regeneration in end-stage respiratory failure. Lancet 2017, 389, S74. [Google Scholar] [CrossRef]
- Garbern, J.C.; Lee, R.T. Cardiac stem cell therapy and the promise of heart regeneration. Cell Stem Cell 2013, 12, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Kotton, D.N.; Morrisey, E.E. Lung regeneration: Mechanisms, applications and emerging stem cell populations. Nat. Med. 2014, 20, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Clifford, D.M.; Fisher, S.A.; Brunskill, S.J.; Doree, C.; Mathur, A.; Watt, S.; Martin-Rendon, E. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst. Rev. 2012, 2. [Google Scholar] [CrossRef]
- Camci-Unal, G.; Annabi, N.; Dokmeci, M.R.; Liao, R.; Khademhosseini, A. Hydrogels for cardiac tissue engineering. NPG Asia Mater. 2014, 6, e99. [Google Scholar] [CrossRef]
- Boffito, M.; Sartori, S.; Ciardelli, G. Polymeric scaffolds for cardiac tissue engineering: Requirements and fabrication technologies. Polym. Int. 2014, 63, 2–11. [Google Scholar] [CrossRef]
- Mahoney, C.; Conklin, D.; Waterman, J.; Sankar, J.; Bhattarai, N. Electrospun nanofibers of poly (ε-caprolactone)/depolymerized chitosan for respiratory tissue engineering applications. J. Biomater. Sci. Polym. Ed. 2016, 27, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Leite, Á.J.; Mano, J. Biomedical applications of natural-based polymers combined with bioactive glass nanoparticles. J. Mater. Chem. B 2017. [Google Scholar] [CrossRef]
- Kargozar, S.; Lotfibakhshaiesh, N.; Ai, J.; Mozafari, M.; Milan, P.B.; Hamzehlou, S.; Barati, M.; Baino, F.; Hill, R.G.; Joghataei, M.T. Strontium- and cobalt-substituted bioactive glasses seeded with human umbilical cord perivascular cells to promote bone regeneration via enhanced osteogenic and angiogenic activities. Acta Biomater. 2017, 58, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Hashemian, S.J.; Soleimani, M.; Milan, P.B.; Askari, M.; Khalaj, V.; Samadikuchaksaraie, A.; Hamzehlou, S.; Katebi, A.R.; Latifi, N.; et al. Acceleration of bone regeneration in bioactive glass/gelatin composite scaffolds seeded with bone marrow-derived mesenchymal stem cells over-expressing bone morphogenetic protein-7. Mater. Sci. Eng. C 2017, 75, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Mozafari, M.; Hashemian, S.J.; Milan, P.B.; Hamzehlou, S.; Soleimani, M.; Joghataei, M.T.; Gholipourmalekabadi, M.; Korourian, A.; Mousavizadeh, K.; et al. Osteogenic potential of stem cells-seeded bioactive nanocomposite scaffolds: A comparative study between human mesenchymal stem cells derived from bone, umbilical cord Wharton’s jelly, and adipose tissue. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Splinter, R.J.; Allen, W.; Greenlee, T. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. Part A 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Baghbani, F.; Moztarzadeh, F.; Hajibaki, L.; Mozafari, M. Synthesis, characterization and evaluation of bioactivity and antibacterial activity of quinary glass system (SiO2-CaO-P2O5-MgO-ZnO): In vitro study. Bull. Mater. Sci. 2013, 36, 1339–1346. [Google Scholar] [CrossRef]
- Kargozar, S.; Lotfibakhshaiesh, N.; Ai, J.; Samadikuchaksaraie, A.; Hill, R.G.; Shah, P.A.; Milan, P.B.; Mozafari, M.; Fathi, M.; Joghataei, M.T. Synthesis, physico-chemical and biological characterization of strontium and cobalt substituted bioactive glasses for bone tissue engineering. J. Non-Cryst. Solids 2016, 449, 133–140. [Google Scholar] [CrossRef]
- Baino, F.; Fiorilli, S.L.; Mortera, R.S.; Onida, B.; Saino, E.; Visai, L.; Verné, E.; Vitale-Brovarone, C. Mesoporous bioactive glass as a multifunctional system for bone regeneration and controlled drug release. J. Appl. Biomater. Funct. Mater. 2012, 10, 12–21. [Google Scholar] [PubMed]
- Baino, F.; Vitale-Brovarone, C. Feasibility of glass-ceramic coatings on alumina prosthetic implants by airbrush spraying method. Ceram. Int. 2015, 41, 2150–2159. [Google Scholar] [CrossRef]
- Vergnol, G.; Ginsac, N.; Rivory, P.; Meille, S.; Chenal, J.M.; Balvay, S.; Chevalier, J.; Hartmann, D.J. In vitro and in vivo evaluation of a polylactic acid-bioactive glass composite for bone fixation devices. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Johari, B.; Kadivar, M.; Lak, S.; Gholipourmalekabadi, M.; Urbanska, A.M.; Mozafari, M.; Ahmadzadehzarajabad, M.; Azarnezhad, A.; Afshari, S.; Zargan, J.; et al. Osteoblast-seeded bioglass/gelatin nanocomposite: A promising bone substitute in critical-size calvarial defect repair in rat. Int. J. Artif. Organs 2016, 39, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Johari, B.; Ahmadzadehzarajabad, M.; Azami, M.; Kazemi, M.; Soleimani, M.; Kargozar, S.; Hajighasemlou, S.; Farajollahi, M.M.; Samadikuchaksaraei, A. Repair of rat critical size calvarial defect using osteoblast-like and umbilical vein endothelial cells seeded in gelatin/hydroxyapatite scaffolds. J. Biomed. Mater. Res. Part A 2016, 104, 1770–1778. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. Chronology of bioactive glass development and clinical applications. New J. Glass Ceram. 2013, 3, 67–73. [Google Scholar] [CrossRef]
- Westhauser, F.; Weis, C.; Prokscha, M.; Bittrich, L.A.; Li, W.; Xiao, K.; Kneser, U.; Kauczor, H.-U.; Schmidmaier, G.; Boccaccini, A.R.; et al. Three-dimensional polymer coated 45S5-type bioactive glass scaffolds seeded with human mesenchymal stem cells show bone formation in vivo. J. Mater. Sci. Mater. Med. 2016, 27, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, D.; Anesi, A.; Salvatori, R.; Chiarini, L.; Cannillo, V. A comparative in vivo evaluation of bioactive glasses and bioactive glass-based composites for bone tissue repair. Mater. Sci. Eng. C 2017, 79, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Durand, L.A.H.; Vargas, G.E.; Romero, N.M.; Vera-Mesones, R.; Porto-López, J.M.; Boccaccini, A.R.; Zago, M.P.; Baldi, A.; Gorustovich, A. Angiogenic effects of ionic dissolution products released from a boron-doped 45S5 bioactive glass. J. Mater. Chem. B 2015, 3, 1142–1148. [Google Scholar] [CrossRef]
- Guan, J.; Zhang, J.; Guo, S.; Zhu, H.; Zhu, Z.; Li, H.; Wang, Y.; Zhang, C.; Chang, J. Human urine-derived stem cells can be induced into osteogenic lineage by silicate bioceramics via activation of the Wnt/β-catenin signaling pathway. Biomaterials 2015, 55, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Naseri, S.; Lepry, W.C.; Nazhat, S.N. Bioactive glasses in wound healing: Hope or hype? J. Mater. Chem. B 2017, 5, 6167–6174. [Google Scholar] [CrossRef]
- Gerhardt, L.C.; Widdows, K.L.; Erol, M.M.; Nandakumar, A.; Roqan, I.S.; Ansari, T.; Boccaccini, A.R. Neocellularization and neovascularization of nanosized bioactive glass-coated decellularized trabecular bone scaffolds. J. Biomed. Mater. Res. Part A 2013, 101, 827–841. [Google Scholar] [CrossRef] [PubMed]
- Reffitt, D.; Ogston, N.; Jugdaohsingh, R.; Cheung, H.; Evans, B.A.J.; Thompson, R.; Powell, J.; Hampson, G. Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone 2003, 32, 127–135. [Google Scholar] [CrossRef]
- Lansdown, A.B. Calcium: A potential central regulator in wound healing in the skin. Wound Repair Regen. 2002, 10, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Durand, L.A.H.; Góngora, A.; López, J.M.P.; Boccaccini, A.R.; Zago, M.P.; Baldi, A.; Gorustovich, A. In vitro endothelial cell response to ionic dissolution products from boron-doped bioactive glass in the SiO2-CaO-P2O5-Na2O system. J. Mater. Chem. B 2014, 2, 7620–7630. [Google Scholar] [CrossRef]
- Dzondo-Gadet, M.; Mayap-Nzietchueng, R.; Hess, K.; Nabet, P.; Belleville, F.; Dousset, B. Action of boron at the molecular level. Biol. Trace Elem. Res. 2002, 85, 23–33. [Google Scholar] [CrossRef]
- Li, J.; Zhai, D.; Lv, F.; Yu, Q.; Ma, H.; Yin, J.; Yi, Z.; Liu, M.; Chang, J.; Wu, C. Preparation of copper-containing bioactive glass/eggshell membrane nanocomposites for improving angiogenesis, antibacterial activity and wound healing. Acta Biomater. 2016, 36, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.F.; Alshemary, A.Z.; Akram, M.; Kadir, A.; Rafiq, M.; Hussain, R. Bioactive Glass: An In-Vitro Comparative Study of Doping with Nanoscale Copper and Silver Particles. Int. J. Appl. Glass Sci. 2014, 5, 255–266. [Google Scholar] [CrossRef]
- Gérard, C.; Bordeleau, L.-J.; Barralet, J.; Doillon, C.J. The stimulation of angiogenesis and collagen deposition by copper. Biomaterials 2010, 31, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.P.; Rios, S.; Gonzalez, M. Modulation of the proliferation and differentiation of human mesenchymal stem cells by copper. J. Cell. Biochem. 2002, 85, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Nadworny, P.L.; Wang, J.; Tredget, E.E.; Burrell, R.E. Anti-inflammatory activity of nanocrystalline silver in a porcine contact dermatitis model. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Chaloupka, K.; Malam, Y.; Seifalian, A.M. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010, 28, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Dominic, E.A.; Reju, I.; Kaimal, B.; Kalarikkal, N.; Thomas, S. Investigation of angiogenesis and its mechanism using zinc oxide nanoparticle-loaded electrospun tissue engineering scaffolds. RSC Adv. 2014, 4, 51528–51536. [Google Scholar] [CrossRef]
- Yin, Y.; Cui, Q.; Li, Y.; Irwin, N.; Fischer, D.; Harvey, A.R.; Benowitz, L.I. Macrophage-derived factors stimulate optic nerve regeneration. J. Neurosci. 2003, 23, 2284–2293. [Google Scholar] [PubMed]
- Lang, C.; Murgia, C.; Leong, M.; Tan, L.-W.; Perozzi, G.; Knight, D.; Ruffin, R.; Zalewski, P. Anti-inflammatory effects of zinc and alterations in zinc transporter mRNA in mouse models of allergic inflammation. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2007, 292, L577–L584. [Google Scholar] [CrossRef] [PubMed]
- Valappil, S.P.; Ready, D.; Neel, E.A.A.; Pickup, D.M.; Chrzanowski, W.; O’Dell, L.A.; Newport, R.J.; Smith, M.E.; Wilson, M.; Knowles, J.C. Antimicrobial gallium-doped phosphate-based glasses. Adv. Funct. Mater. 2008, 18, 732–741. [Google Scholar] [CrossRef]
- Baino, F.; Ferraris, M.; Bretcanu, O.; Verné, E.; Vitale-Brovarone, C. Optimization of composition, structure and mechanical strength of bioactive 3-D glass-ceramic scaffolds for bone substitution. J. Biomater. Appl. 2013, 27, 872–890. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Novajra, G.; Miguez-Pacheco, V.; Boccaccini, A.R.; Vitale-Brovarone, C. Bioactive glasses: Special applications outside the skeletal system. J. Non-Cryst. Solids 2016, 432, 15–30. [Google Scholar] [CrossRef]
- Coraça-Huber, D.C.; Fille, M.; Hausdorfer, J.; Putzer, D.; Nogler, M. Efficacy of antibacterial bioactive glass S53P4 against S. aureus biofilms grown on titanium discs in vitro. J. Orthop. Res. 2014, 32, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Stoor, P.; Frantzen, J. Influence of bioactive glass S53P4 granules and putty on osteomyelitis associated bacteria in vitro. Biomed. Glasses 2017, 3, 79–85. [Google Scholar] [CrossRef]
- Zhang, D.; Leppäranta, O.; Munukka, E.; Ylänen, H.; Viljanen, M.K.; Eerola, E.; Hupa, M.; Hupa, L. Antibacterial effects and dissolution behavior of six bioactive glasses. J. Biomed. Mater. Res. Part A 2010, 93, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Miguez-Pacheco, V.; Hench, L.L.; Boccaccini, A.R. Bioactive glasses beyond bone and teeth: Emerging applications in contact with soft tissues. Acta Biomater. 2015, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. The future of bioactive ceramics. J. Mater. Sci. Mater. Med. 2015, 26, 86. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Greenspan, D. Interactions between bioactive glass and collagen: A review and new perspectives. J. Aust. Ceram. Soc. 2013, 49, 1–40. [Google Scholar]
- Laurance, J. Julia Margaret Polak. Lancet 2014, 384, 1342. [Google Scholar] [CrossRef]
- Gerber, Y.; Weston, S.A.; Enriquez-Sarano, M.; Berardi, C.; Chamberlain, A.M.; Manemann, S.M.; Jiang, R.; Dunlay, S.M.; Roger, V.L. Mortality associated with heart failure after myocardial infarction: A contemporary community perspective. Circ. Heart Fail. 2016, 9, e002460. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Yellon, D.M. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J. Clin. Investig. 2013, 123, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Poss, K.D. Cardiac regenerative capacity and mechanisms. Annu. Rev. Cell Dev. Biol. 2012, 28, 719–741. [Google Scholar] [CrossRef] [PubMed]
- Rane, A.A.; Christman, K.L. Biomaterials for the treatment of myocardial infarction: A 5-year update. J. Am. Coll. Cardiol. 2011, 58, 2615–2629. [Google Scholar] [CrossRef] [PubMed]
- Reis, L.A.; Chiu, L.L.; Feric, N.; Fu, L.; Radisic, M. Biomaterials in myocardial tissue engineering. J. Tissue Eng. Regen. Med. 2016, 10, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.-Z.; Harding, S.E.; Ali, N.N.; Lyon, A.R.; Boccaccini, A.R. Biomaterials in cardiac tissue engineering: Ten years of research survey. Mater. Sci. Eng. R Rep. 2008, 59, 1–37. [Google Scholar] [CrossRef]
- Hinderer, S.; Brauchle, E.; Schenke-Layland, K. Generation and assessment of functional biomaterial scaffolds for applications in cardiovascular tissue engineering and regenerative medicine. Adv. Healthc. Mater. 2015, 4, 2326–2341. [Google Scholar] [CrossRef] [PubMed]
- Vargas, G.E.; Durand, L.A.H.; Cadena, V.; Romero, M.; Mesones, R.V.; Mačković, M.; Spallek, S.; Spiecker, E.; Boccaccini, A.R.; Gorustovich, A.A. Effect of nano-sized bioactive glass particles on the angiogenic properties of collagen based composites. J. Mater. Sci. Mater. Med. 2013, 24, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Li, Y.; Miao, G.; Zhao, N.; Chen, X. Size control and biological properties of monodispersed mesoporous bioactive glass sub-micron spheres. RSC Adv. 2014, 4, 22678–22687. [Google Scholar] [CrossRef]
- Lukowiak, A.; Lao, J.; Lacroix, J.; Nedelec, J.-M. Bioactive glass nanoparticles obtained through sol-gel chemistry. Chem. Commun. 2013, 49, 6620–6622. [Google Scholar] [CrossRef] [PubMed]
- Erol-Taygun, M.; Zheng, K.; Boccaccini, A.R. Nanoscale bioactive glasses in medical applications. Int. J. Appl. Glass Sci. 2013, 4, 136–148. [Google Scholar]
- Ji, L.; Qiao, W.; Huang, K.; Zhang, Y.; Wu, H.; Miao, S.; Liu, H.; Dong, Y.; Zhu, A.; Qiu, D. Synthesis of nanosized 58S bioactive glass particles by a three-dimensional ordered macroporous carbon template. Mater. Sci. Eng. C 2017, 75, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, C.; Arroyo, F.; Balanda, C.; Neira, M.; von Marttens, A.; Caviedes, P.; Rodríguez, J.P.; Urra, C. The effect of the nanoscale structure of nanobioceramics on their in vitro bioactivity and cell differentiation properties. J. Nanomater. 2015, 16, 430. [Google Scholar] [CrossRef]
- Chen, Q.; Jin, L.; Cook, W.D.; Mohn, D.; Lagerqvist, E.L.; Elliott, D.A.; Haynes, J.M.; Boyd, N.; Stark, W.J.; Pouton, C.W.; et al. Elastomeric nanocomposites as cell delivery vehicles and cardiac support devices. Soft Matter 2010, 6, 4715–4726. [Google Scholar] [CrossRef]
- Barabadi, Z.; Azami, M.; Sharifi, E.; Karimi, R.; Lotfibakhshaiesh, N.; Roozafzoon, R.; Joghataei, M.T.; Ai, J. Fabrication of hydrogel based nanocomposite scaffold containing bioactive glass nanoparticles for myocardial tissue engineering. Mater. Sci. Eng. C 2016, 69, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Cohrs, N.H.; Schulz-Schönhagen, K.; Jenny, F.; Mohn, D.; Stark, W.J. Bioactive glass containing silicone composites for left ventricular assist device drivelines: Role of bioglass 45s5® particle size on mechanical properties and cytocompatibility. J. Mater. Sci. 2017, 52, 9023–9038. [Google Scholar] [CrossRef]
- Zhou, Z.; Xiang, L.; Ou, B.; Huang, T.; Zhou, H.; Zeng, W.; Liu, L.; Liu, Q.; Zhao, Y.; He, S. Biological assessment in-vivo of gel-HA scaffold materials containing nano-bioactive glass for tissue engineering. J. Macromol. Sci. Part A 2014, 51, 572–576. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Erol, M.; Stark, W.J.; Mohn, D.; Hong, Z.; Mano, J.F. Polymer/bioactive glass nanocomposites for biomedical applications: A review. Compos. Sci. Technol. 2010, 70, 1764–1776. [Google Scholar] [CrossRef]
- Liang, S.-L.; Cook, W.D.; Thouas, G.A.; Chen, Q.-Z. The mechanical characteristics and in vitro biocompatibility of poly (glycerol sebacate)-bioglass® elastomeric composites. Biomater. 2010, 31, 8516–8529. [Google Scholar] [CrossRef] [PubMed]
- Qazi, T.H.; Rai, R.; Dippold, D.; Roether, J.E.; Schubert, D.W.; Rosellini, E.; Barbani, N.; Boccaccini, A.R. Development and characterization of novel electrically conductive pani-pgs composites for cardiac tissue engineering applications. Acta Biomater. 2014, 10, 2434–2445. [Google Scholar] [CrossRef] [PubMed]
- Gorustovich, A.A.; Roether, J.A.; Boccaccini, A.R. Effect of bioactive glasses on angiogenesis: A review of in vitro and in vivo evidences. Tissue Eng. Part B Rev. 2009, 16, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Detsch, R.; Stoor, P.; Grünewald, A.; Roether, J.A.; Lindfors, N.C.; Boccaccini, A.R. Increase in VEGF secretion from human fibroblast cells by bioactive glass S53P4 to stimulate angiogenesis in bone. J. Biomed. Mater. Res. Part A 2014, 102, 4055–4061. [Google Scholar] [CrossRef] [PubMed]
- Petersen, T.H.; Calle, E.A.; Zhao, L.; Lee, E.J.; Gui, L.; Raredon, M.B.; Gavrilov, K.; Yi, T.; Zhuang, Z.W.; Breuer, C. Tissue-engineered lungs for in vivo implantation. Science 2010, 329, 538–541. [Google Scholar] [PubMed]
- Tan, A.; Romanska, H.; Lenza, R.; Jones, J.R.; Hench, L.L.; Polak, J.M.; Bishop, A. The effect of 58S bioactive sol-gel derived foams on the growth of murine lung epithelial cells. Key Eng. Mater. 2003, 240–242, 719–724. [Google Scholar] [CrossRef]
- Saravanapavan, P.; Verrier, S.; Hench, L.L. A549 lung carcinoma cells: Binary vs. ternary bioactive gel-glasses. Key Eng. Mater. 2004, 254–256, 781–784. [Google Scholar] [CrossRef]
- Verrier, S.; Blaker, J.J.; Maquet, V.; Hench, L.L.; Boccaccini, A.R. PDLLA/Bioglass® composites for soft-tissue and hard-tissue engineering: An in vitro cell biology assessment. Biomaterials 2004, 25, 3013–3021. [Google Scholar] [CrossRef] [PubMed]
- Boccaccini, A.R.; Blaker, J.J.; Maquet, V.; Day, R.; Jérôme, R. Preparation and characterisation of poly (lactide-co-glycolide) (PLGA) and PLGA/Bioglass® composite tubular foam scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2005, 25, 23–31. [Google Scholar] [CrossRef]
- Pires, E.G.; Bonan, R.F.; Rocha, Í.M.; Gonçalves, I.M.F.; de Souza, J.R.; Gonzales, L.H.V.; Junior, J.V.J.D.; Perez, D.E.D.; Tavares, P.C.B.; da Silva, S.M.; et al. Silver-doped 58S Bioactive Glass as an Anti-Leishmania Agent. Int. J. Appl. Glass Sci. 2017. [Google Scholar] [CrossRef]
- Wang, T.W.; Wu, H.C.; Wang, W.R.; Lin, F.H.; Lou, P.J.; Shieh, M.J.; Young, T.H. The development of magnetic degradable DP-Bioglass for hyperthermia cancer therapy. J. Biomed. Mater. Res. Part A 2007, 83, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Boccaccini, A.; Polak, J.; Bishop, A.; Maquet, V. Biocompatibility of poly-dl-lactic acid (pdlla) for lung tissue engineering. J. Biomater. Appl. 2006, 21, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Meseguer-Olmo, L.; Ros-Nicolás, M.; Clavel-Sainz, M.; Vicente-Ortega, V.; Alcaraz-Baños, M.; Lax-Pérez, A.; Arcos, D.; Ragel, C.; Vallet-Regí, M. Biocompatibility and in vivo gentamicin release from bioactive sol-gel glass implants. J. Biomed. Mater. Res. Part A 2002, 61, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.; Garino, J.; Flaitz, C.; Ducheyne, P. Excretion of resorption products from bioactive glass implanted in rabbit muscle. J. Biomed. Mater. Res. Part A 2005, 75, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R.; Brauer, D.S.; Hupa, L.; Greenspan, D.C. Bioglass and bioactive glasses and their impact on healthcare. Int. J. Appl. Glass Sci. 2016, 7, 423–434. [Google Scholar] [CrossRef]
- Jones, J.R.; Gentleman, E.; Polak, J. Bioactive glass scaffolds for bone regeneration. Elements 2007, 3, 393–399. [Google Scholar] [CrossRef]
- Gerhardt, L.-C.; Boccaccini, A.R. Bioactive glass and glass-ceramic scaffolds for bone tissue engineering. Materials 2010, 3, 3867–3910. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Vitale-Brovarone, C. Three-dimensional glass-derived scaffolds for bone tissue engineering: Current trends and forecasts for the future. J. Biomed. Mater. Res. Part A 2011, 97, 514–535. [Google Scholar] [CrossRef] [PubMed]
- Tallawi, M.; Rosellini, E.; Barbani, N.; Cascone, M.G.; Rai, R.; Saint-Pierre, G.; Boccaccini, A.R. Strategies for the chemical and biological functionalization of scaffolds for cardiac tissue engineering: A review. J. R. Soc. Interface 2015, 12, 20150254. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chang, J. Multifunctional mesoporous bioactive glasses for effective delivery of therapeutic ions and drug/growth factors. J. Controll. Release 2014, 193, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Fiorilli, S.; Vitale-Brovarone, C. Composite biomaterials based on sol-gel mesoporous silicate glasses: A review. Bioengineering 2017, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.-B.; Chen, J.-Y.; Feng, X.-X.; Chang, J. Symbolfabrication and characterization of chitosan/mesoporous bioactive glasses porous films. J. Clin. Rehabil. Tissue Eng. Res. 2011, 15, 7877–7880. [Google Scholar]
- Miguez-Pacheco, V.; Greenspan, D.; Hench, L.; Boccaccini, A. Bioactive glasses in soft tissue repair. Am. Ceram. Soc. Bull. 2015, 94, 27–31. [Google Scholar]
- Lin, C.; Mao, C.; Zhang, J.; Li, Y.; Chen, X. Healing effect of bioactive glass ointment on full-thickness skin wounds. Biomed. Mater. 2012, 7, 045017. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, L.; Wang, H.; Zhang, Y.; Cheng, X.; Zhou, N.; Rahaman, M.N.; Liu, Z.; Huang, W.; Zhang, C. Wound dressings composed of copper-doped borate bioactive glass microfibers stimulate angiogenesis and heal full-thickness skin defects in a rodent model. Biomaterials 2015, 53, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.T.; Peitl, O.; Zanotto, E.D.; Boccaccini, A.R. Novel Double-Layered Conduit Containing Highly Bioactive Glass Fibers for Potential Nerve Guide Application. Int. J. Appl. Glass Sci. 2016, 7, 183–194. [Google Scholar] [CrossRef]
- Koudehi, M.F.; Fooladi, A.A.I.; Mansoori, K.; Jamalpoor, Z.; Amiri, A.; Nourani, M.R. Preparation and evaluation of novel nano-bioglass/gelatin conduit for peripheral nerve regeneration. J. Mater. Sci. Mater. Med. 2014, 25, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Fan, W.; Chang, J.; Xiao, Y. Mesoporous bioactive glass scaffolds for efficient delivery of vascular endothelial growth factor. J. Biomater. Appl. 2013, 28, 367–374. [Google Scholar] [CrossRef] [PubMed]
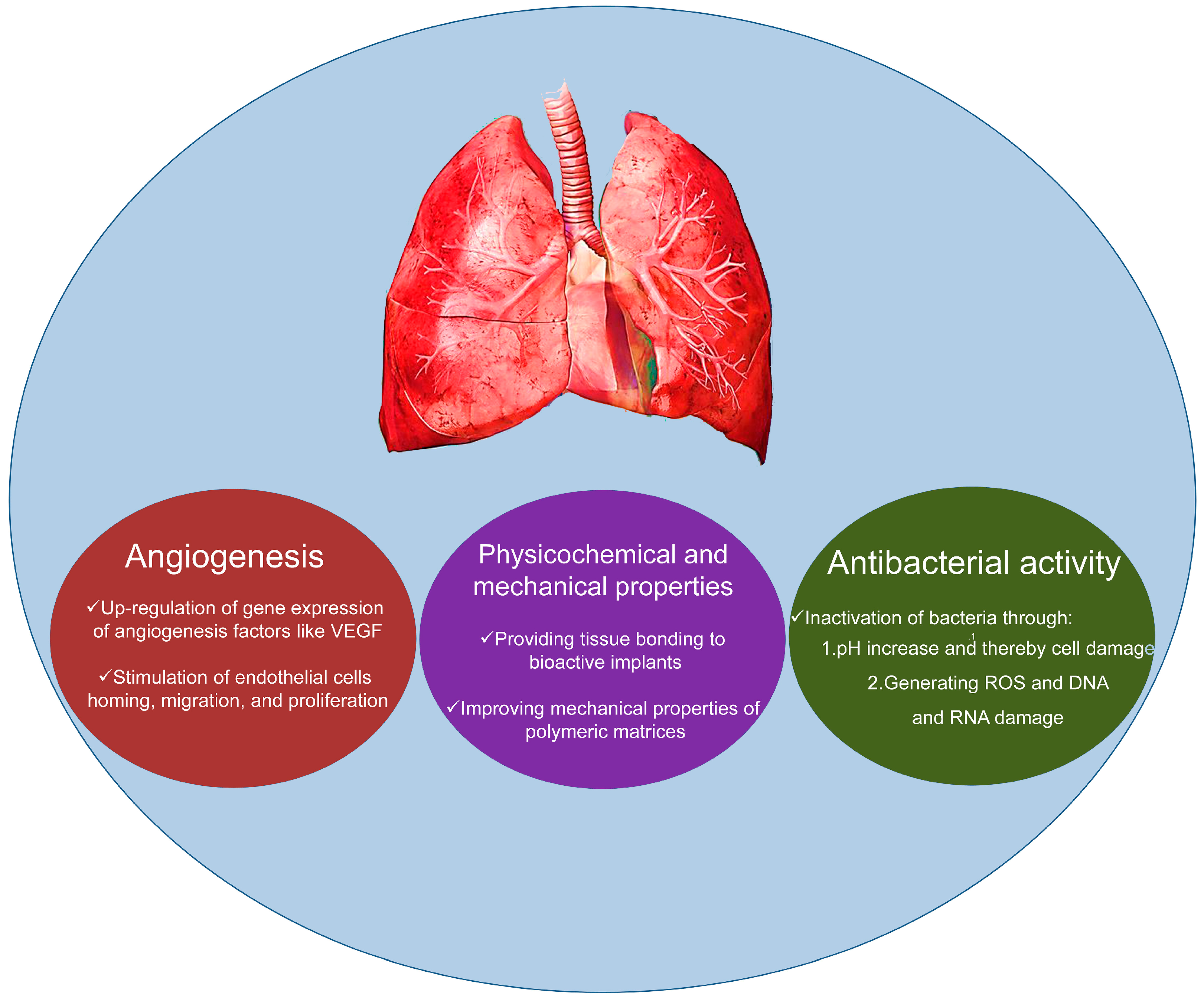
| Element | Effects | References |
|---|---|---|
| Si | -Promotes neovascularization. -Stimulates collagen type I formation. | [35,36] |
| Ca | -Promotes the migration and proliferation of epidermal cells. -Accelerates blood-clotting. | [37] |
| B | -Stimulates vascularization and angiogenesis. -Increases RNA synthesis in fibroblasts. | [38,39] |
| Cu | -Stimulates angiogenesis. -Antimicrobial property. | [40,41,42,43] |
| Ag+ | -Anti-inflammatory property. -Antimicrobial property. | [44,45,46] |
| Zn | -Stimulates angiogenesis. -Enhances nerve regeneration. -Anti-inflammatory property. -Enhances wound healing processes. | [47,48,49] |
| Ga | -Antimicrobial property. | [50] |
| Materials | In Vitro/In Vivo Tests | Remarks | References | |
|---|---|---|---|---|
| BG Name and Composition | Polymeric Matrix | |||
| 45S5 Bioglass® (45SiO2-24.5CaO-24.5Na2O-6P2O5 wt %) melt-derived particles | Poly(glycerol sebacate) | Human ESC derived cardiomyocytes | -Promoting cell differentiation | [73] |
| Fibroblasts | -Non-toxic | |||
| 45S5 Bioglass® (45SiO2-24.5CaO-24.5Na2O-6P2O5 wt %) melt-derived particles | Gelatin-collagen hydrogel | Endometrial stromal Stem cells (EnSCs) | -Inducing differentiation into cardiomyocytes -Increasing VEGF expression | [74] |
| L929 cells | -Good biocompatibility | |||
| 45S5 Bioglass® (45SiO2-24.5CaO-24.5Na2O-6P2O5 wt %) melt-derived particles | Silicone elastomer | Primary fibroblasts | -Improving mechanical properties -Improving cytocompatibility | [75] |
| Sol-gel BG nanoparticles (60SiO2-35CaO-5P2O5 mol %) | Gelatin/hyaluronic acid | Oral administration to rats | -No remarkable change in the morphology of heart tissue | [76] |
| Materials | In Vitro/ In Vivo Tests | Remarks | References | |
|---|---|---|---|---|
| BG Name and Composition | Polymeric Matrix | |||
| 58S (58SiO2-36CaO-6P2O5 mol %) sol-gel scaffold | - | MLE-12 cells | -Good biocompatibility further improved by laminin coating or amine functionalization | [83] |
| - | A549 cells | -Good biocompatibility | [84] | |
| 45S5 Bioglass® (45SiO2-24.5CaO-24.5Na2O-6P2O5 wt %) melt-derived particles | PDLLA | A549 cells | -Dose-dependent effect | [85] |
| PLGA | L929 cells | -Dose-dependent effect | [86] | |
| Ag-doped 58S sol-gel glass (58SiO2-(36-x)CaO-6P2O5-xAg2O, with x = 0, 1, 2 mol %) | - | A549 cells | -Non-toxic | [87] |
| Fe-doped sol-gel glass (basic composition: 8.4Na2O-40CaO-39.6SiO2-12P2O5 wt % doped with 0.2-1 wt % of Fe) | - | A549 cells | -Hyperthermic effect for possible application in lung cancer treatment | [88] |
| Sol-gel BG nanoparticles (60SiO2-35CaO-5P2O5 mol %) | Gelatin/hyaluronic acid | Oral administration to rats | -No remarkable change in the morphology of lung tissue | [76] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kargozar, S.; Hamzehlou, S.; Baino, F. Potential of Bioactive Glasses for Cardiac and Pulmonary Tissue Engineering. Materials 2017, 10, 1429. https://doi.org/10.3390/ma10121429
Kargozar S, Hamzehlou S, Baino F. Potential of Bioactive Glasses for Cardiac and Pulmonary Tissue Engineering. Materials. 2017; 10(12):1429. https://doi.org/10.3390/ma10121429
Chicago/Turabian StyleKargozar, Saeid, Sepideh Hamzehlou, and Francesco Baino. 2017. "Potential of Bioactive Glasses for Cardiac and Pulmonary Tissue Engineering" Materials 10, no. 12: 1429. https://doi.org/10.3390/ma10121429
APA StyleKargozar, S., Hamzehlou, S., & Baino, F. (2017). Potential of Bioactive Glasses for Cardiac and Pulmonary Tissue Engineering. Materials, 10(12), 1429. https://doi.org/10.3390/ma10121429






