Novel Magnetic Zinc Oxide Nanotubes for Phenol Adsorption: Mechanism Modeling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Magnetic Zinc Oxide Nanotubes
2.2. Characterization of Magnetic ZnO Nanotubes
2.3. Batch Investigation of Magnetic Zinc Oxide Nanotubes
2.4. Kinetics and Thermodynamics Studies
3. Results and Discussion
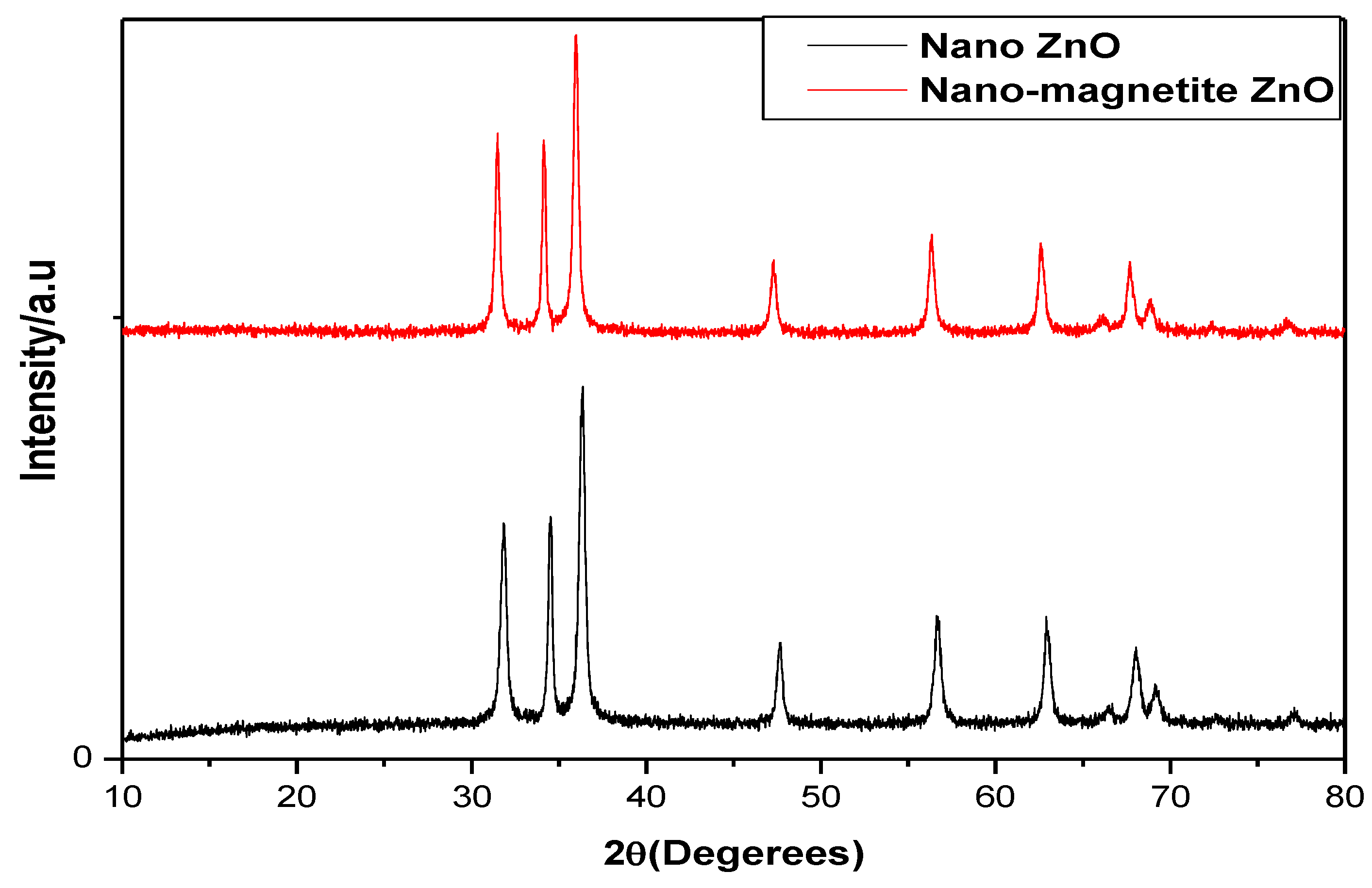
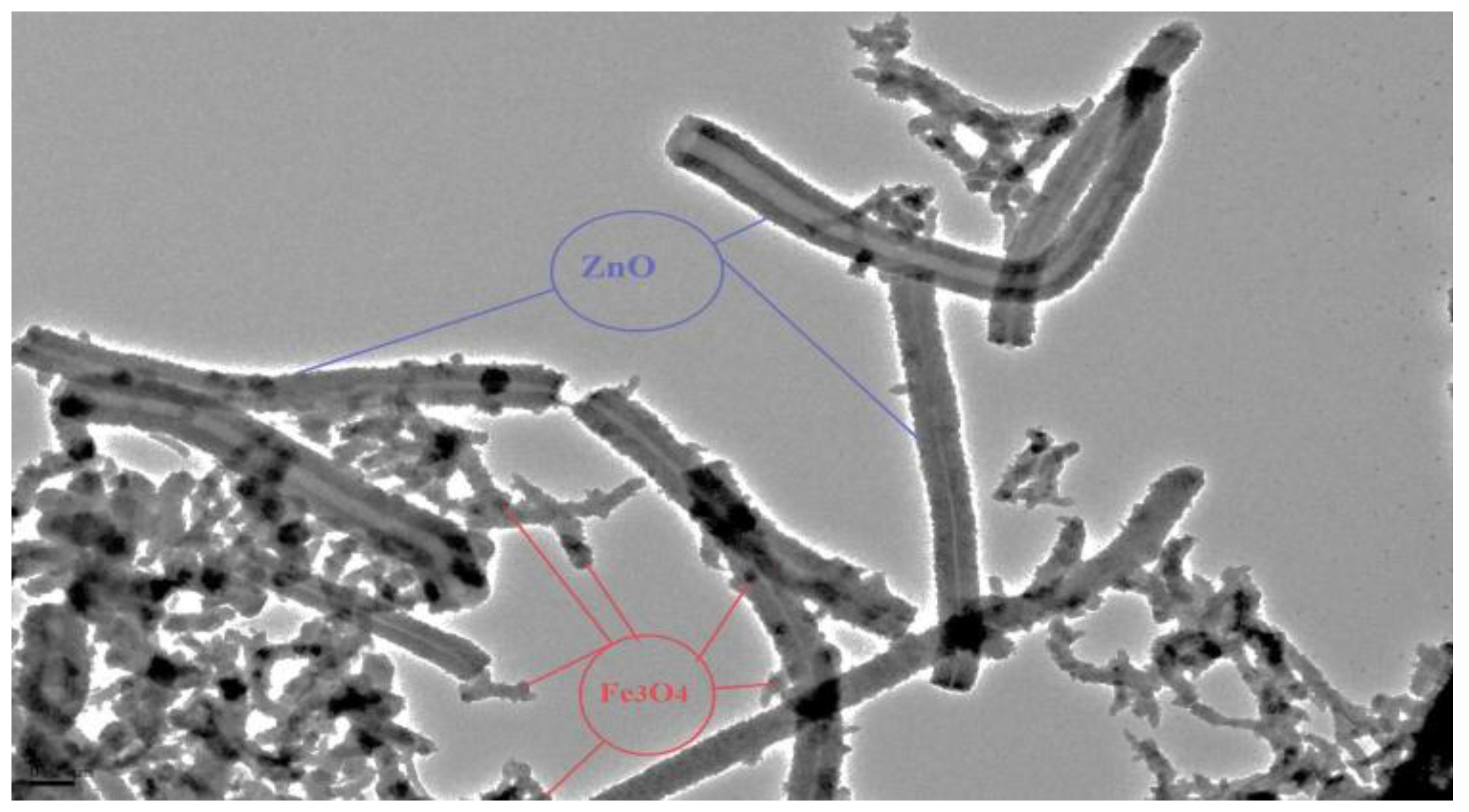
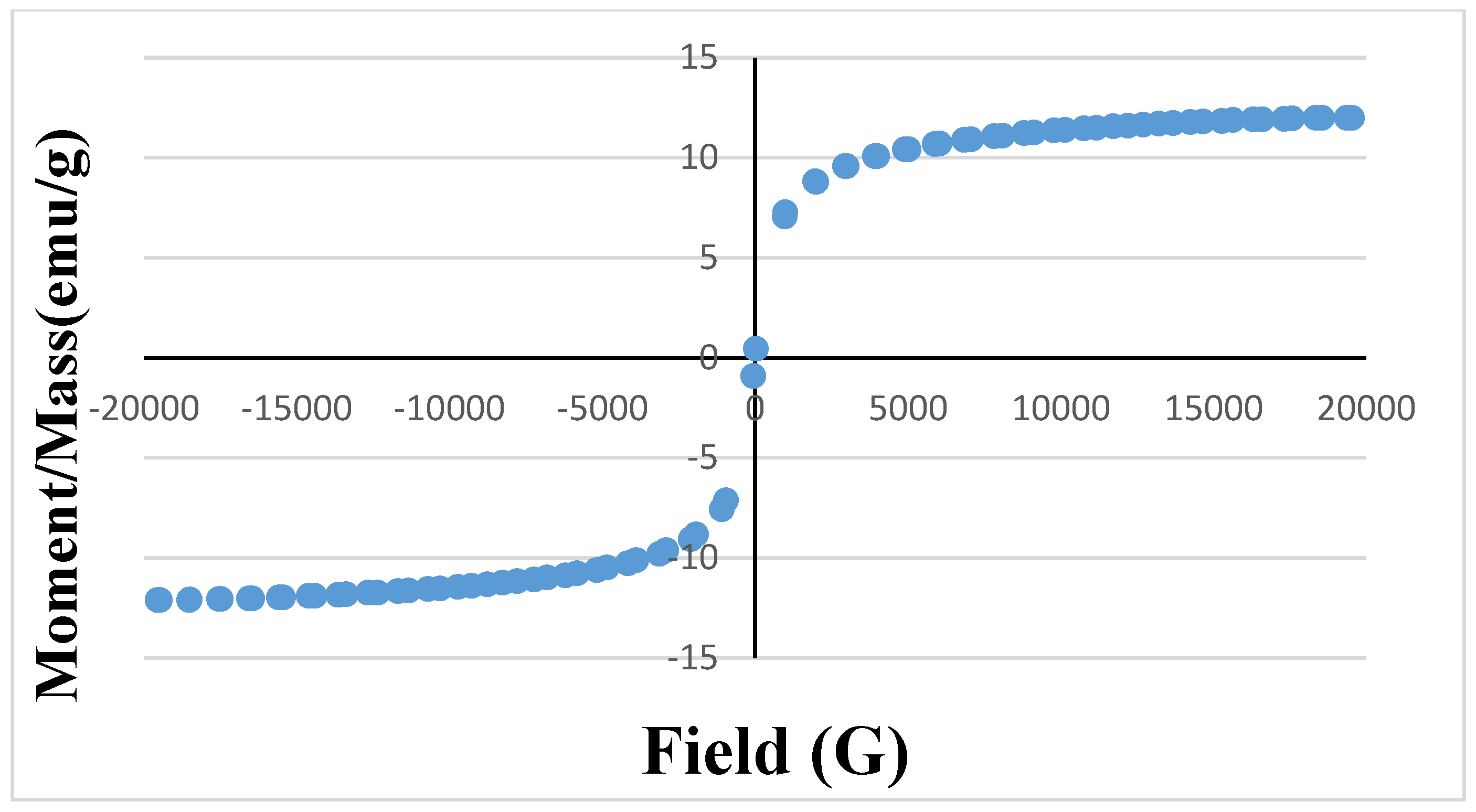
3.1. Characterization of Hollow Structured Magnetic Zinc Oxide Nanotubes
3.2. Magnetic Zinc Oxide Nanotubes for Phenol Removal
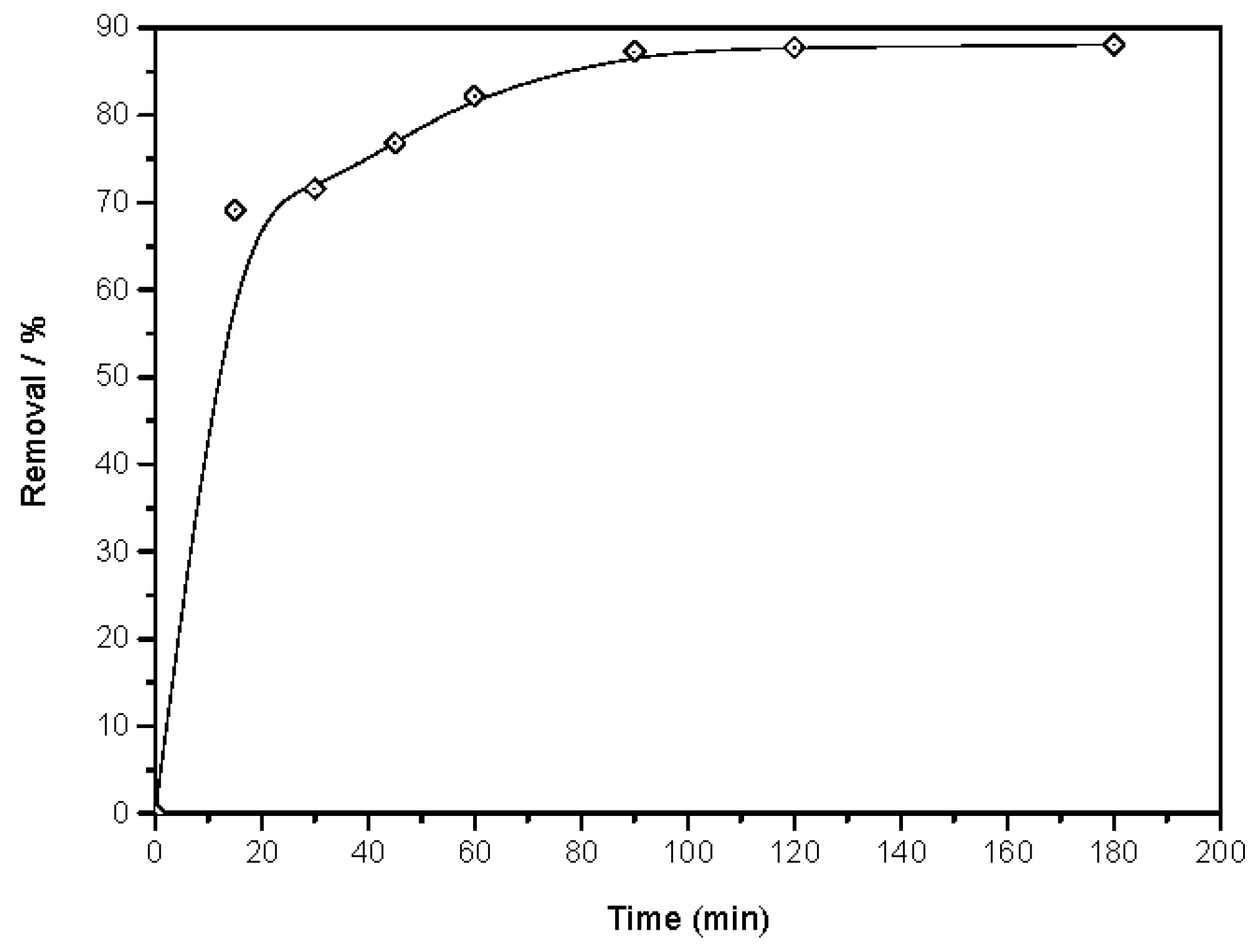
3.2.1. Influence of Contact Time on the Phenol Sorption Process on to Magnetic ZnO Nanotubes
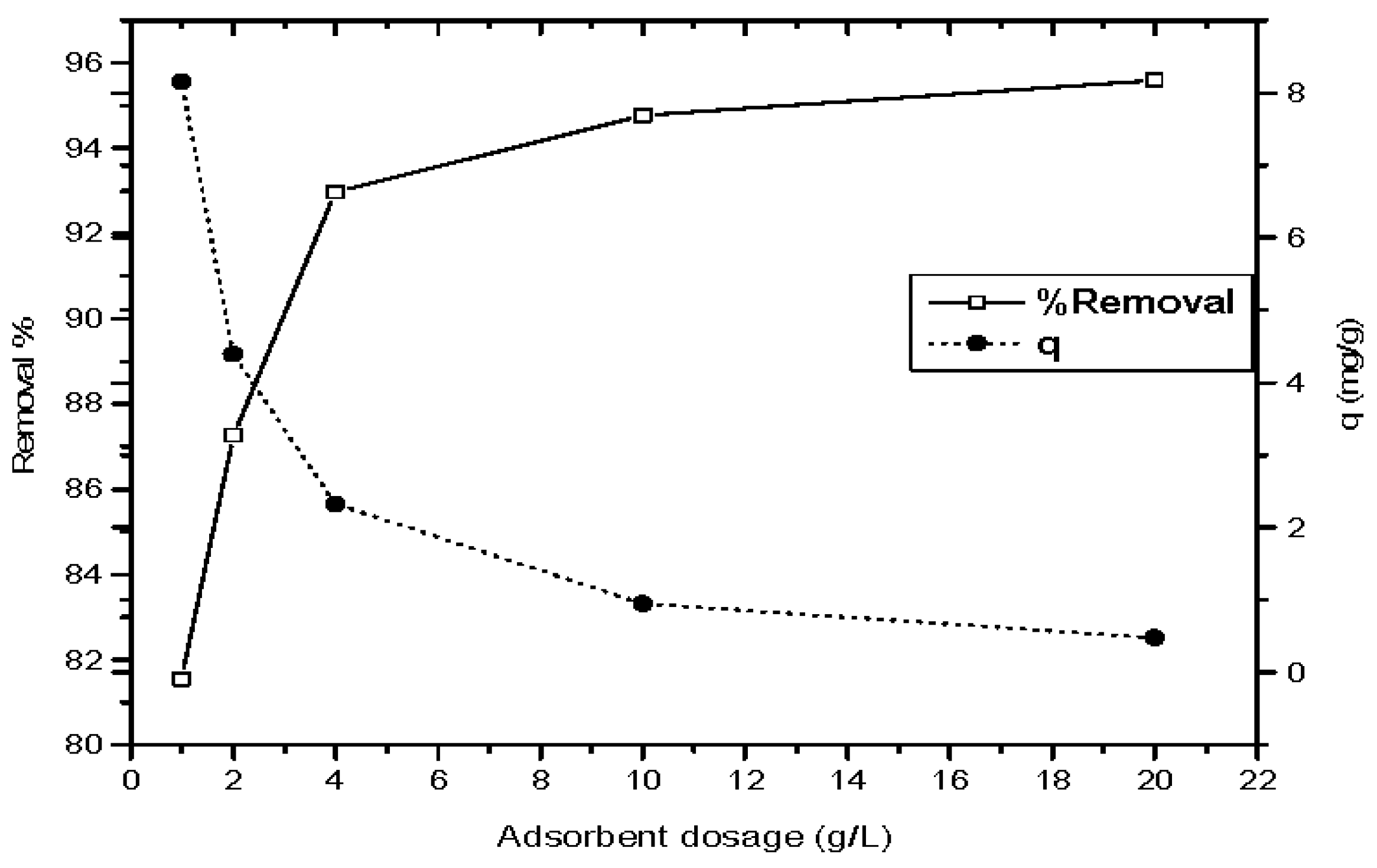
3.2.2. Influence of Magnetic Nano-ZnO Dosage on the Phenol Adsorption Process
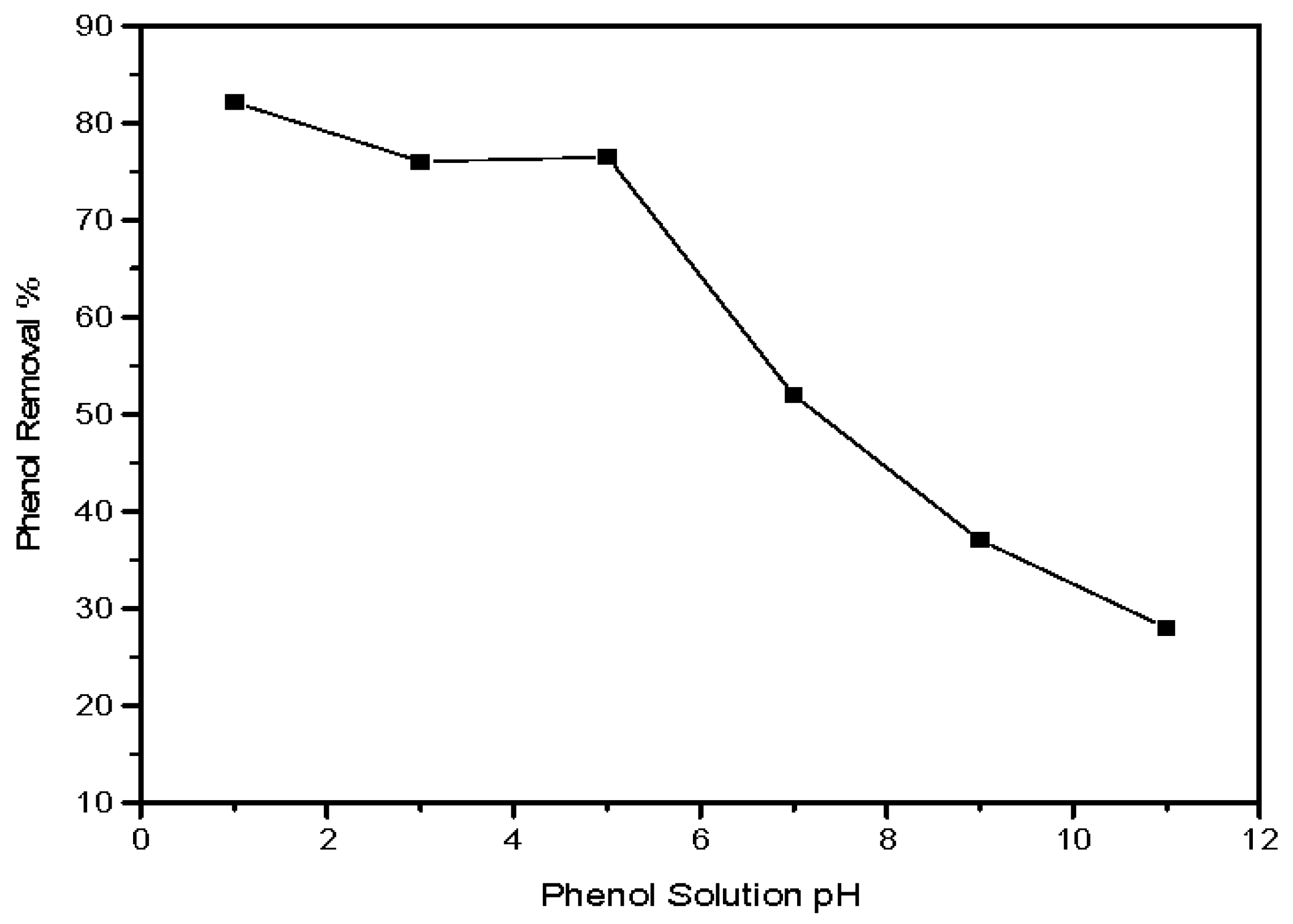
3.2.3. Influence of Initial pH on the Phenol Adsorption Process
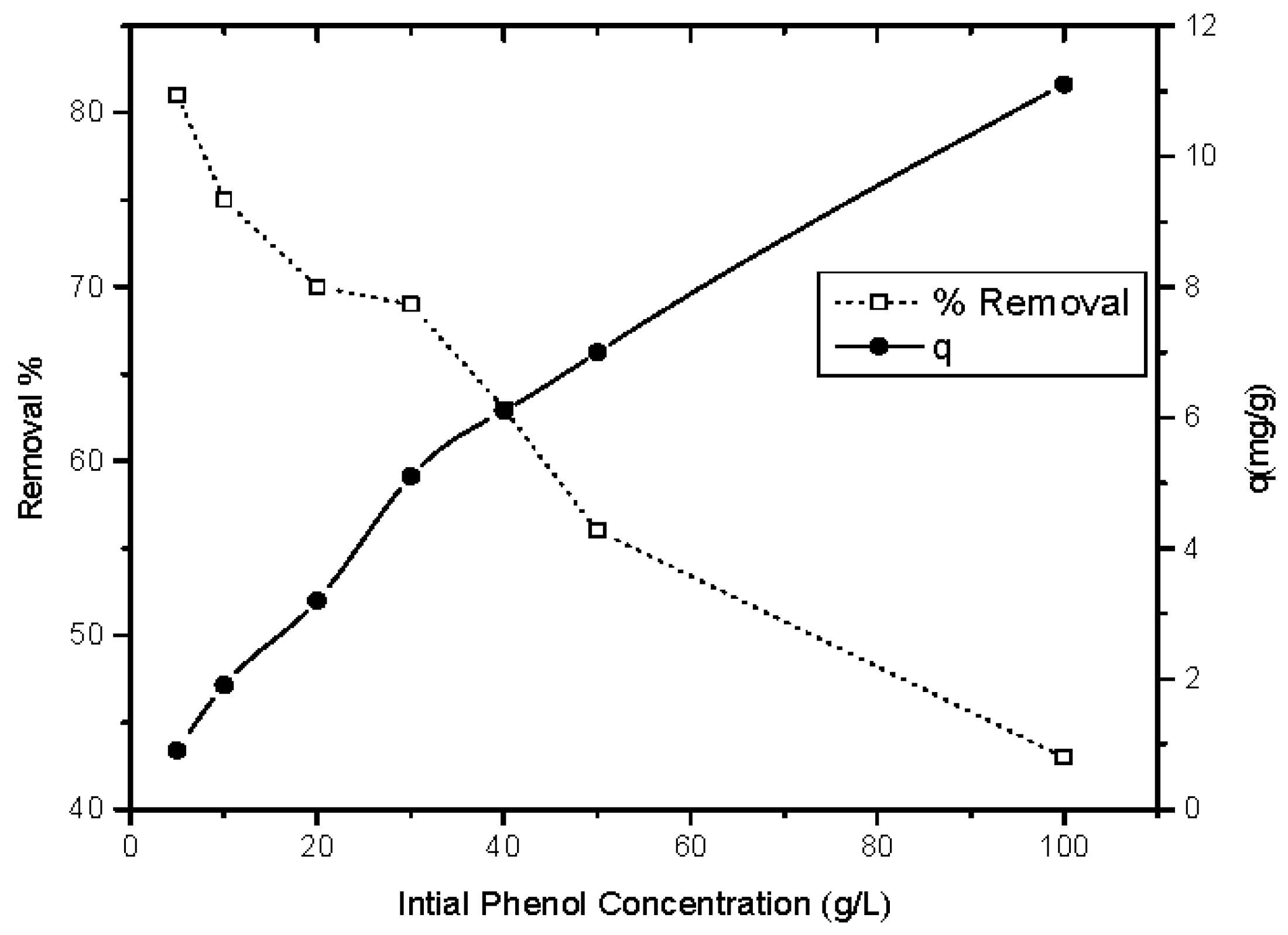
3.2.4. Influence of Initial Phenol Concentration on the Adsorption Process
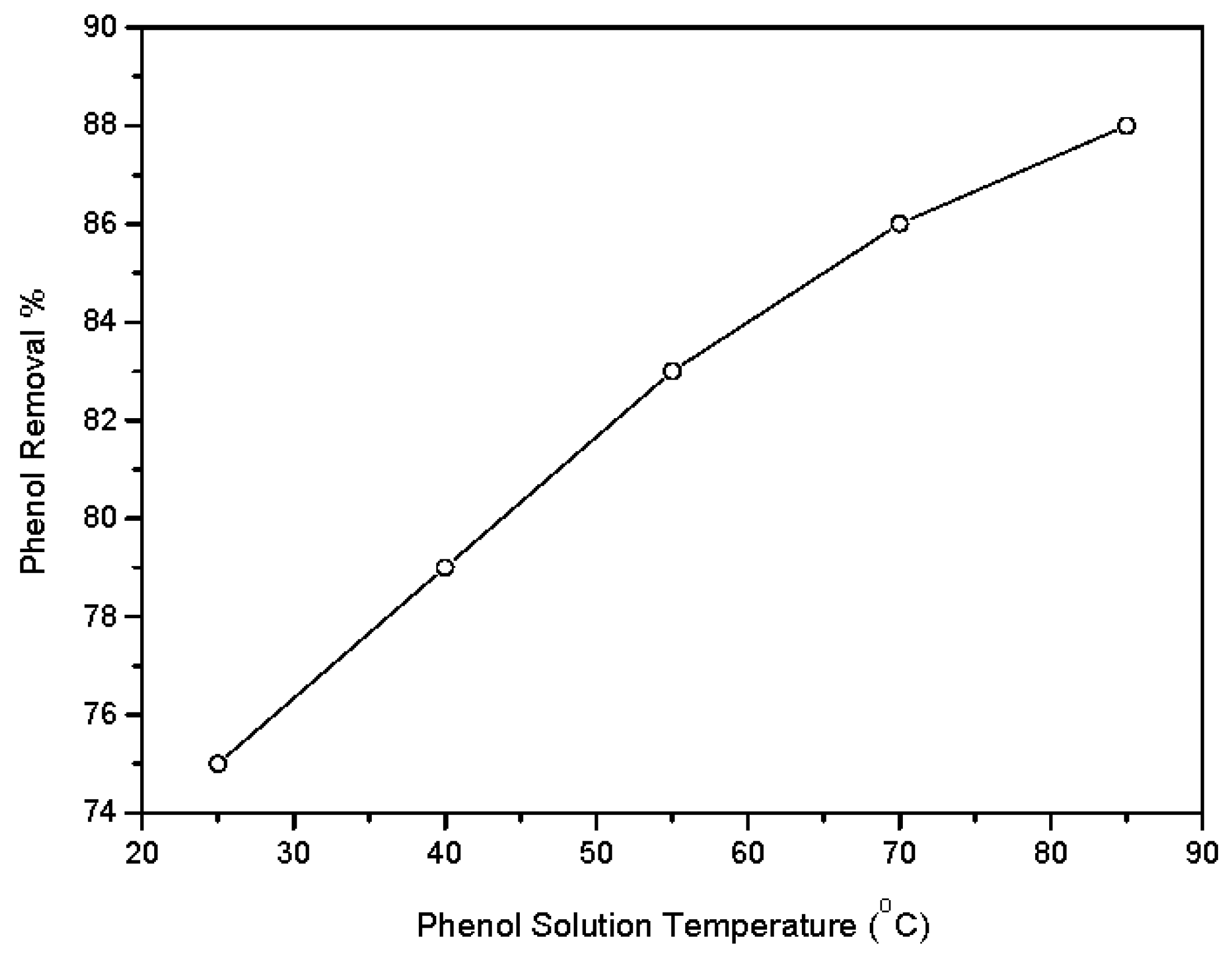
3.2.5. Influence of Solution Temperature on the Phenol Adsorption Process
3.3. Thermodynamics and Equilbrium Modeling
3.3.1. Equilibrium Isotherm Analysis for the Phenol Sorption Process on to Magnetic ZnO Nanotubes
3.3.2. Kinetic Model of Phenol Adsorption Process on to Hollow Magnetic ZnO Nanotubes
3.4. Phenol Desorption Process
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Elkady, M.F.; Hassan, H.S.; Hashim, A. Immobilization of magnetic nanoparticles onto amine-modified nano-silica gel for copper ions remediation. Materials 2016, 9, 460. [Google Scholar] [CrossRef] [PubMed]
- Elkady, M.F.; Hassan, H.S.; Salama, E. Sorption profile of phosphorus ions onto ZnO nanorods synthesized via sonic technique. J. Eng. 2016, 2016, 2308560. [Google Scholar] [CrossRef]
- Elkady, M.F.; Hassan, H.S.; Hamad, H. Effect of superparamagnetic nanoparticles on the physicochemical properties of nano hydroxyapatite for groundwater treatment: Adsorption mechanism of Fe(II) and Mn(II). RSC Adv. 2016, 6, 82244–82259. [Google Scholar] [CrossRef]
- El-Aassar, M.R.; Elkady, M.F.; Hassan, H.S.; Al-Deyab, S.S. Synthesis and characterization of surface modified electrospun poly (acrylonitrile-co-styrene) nanofibers for dye decolorization. J. Taiwan Inst. Chem. Eng. 2016, 58, 274–282. [Google Scholar] [CrossRef]
- Elkady, M.F.; Hassan, H.S.; El-Sayed, E.M. Basic Violet Decolourization Using Alginate Immobilized Nanozirconium Tungestovanadate Matrix as Cation Exchanger. J. Chem. 2015, 2015, 385741. [Google Scholar] [CrossRef]
- Elkady, M.F.; EL-Sayed, E.; Farag, H.; Zaatout, A. Assessment of novel synthetized nanozirconium tungstovanadate as cation exchanger for lead ion decontamination. J. Nanomater. 2014, 2014, 149312. [Google Scholar] [CrossRef]
- Villegas, L.G.C.; Mashhadi, N.; Chen, M.; Mukherjee, D.; Taylor, K.E.; Biswas, N. A Short Review of Techniques for Phenol Removal from Wastewater. Curr. Pollut. Rep. 2016, 2, 157–167. [Google Scholar] [CrossRef]
- Dougna, A.A.; Gombert, B.; Kodom, T.; Djaneye-Boundjou, G.; Boukari, S.O.B.; Leitner, N.K.V.; Bawa, L.M. Photocatalytic removal of phenol using titanium dioxide deposited on different substrates: Effect of inorganic oxidants. J. Photochem. Photobiol. A 2015, 305, 67–77. [Google Scholar] [CrossRef]
- Elkady, M.F.; Hassan, H.S. Invention of hollow zirconium tungesto-vanadate at nanotube morphological structure for radionuclides and heavy metal pollutants decontamination from aqueous solutions. Nanoscale Res. Lett. 2015, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Huitle, C.A.; Ferro, S. Electrochemical oxidation of organic pollutants for the wastewater treatment: Direct and indirect processes. Chem. Soc. Rev. 2006, 35, 1324–1340. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, B.; Yue, Q.; Wang, Y.; Yang, Z. Removal of acid and direct dye by epichlorohydrin-dimethylamine: Flocculation performance and floc aggregation. Bioresour. Technol. 2012, 113, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.; Wu, F.; Luo, F.; Liu, Z. Photodegradation of dyes in aqueous solutions containing Fe(III)-oxalato complexes. Chemosphere 1997, 35, 2697–2706. [Google Scholar]
- Liotta, L.F.; Gruttadauria, M.; DiCarlo, G.; Perrini, G.; Librando, V. Heterogeneous catalytic degradation of phenolic substrates: Catalysts activity. J. Hazard. Mater. 2009, 162, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Faria, P.C.C.; Orfao, J.J.M.; Pereira, M.F.R. Mineralization of substituted aromatic compounds by ozonation catalyzed by cerium oxide and a cerium oxide-activated carbon composite. Catal. Lett. 2009, 127, 195–203. [Google Scholar] [CrossRef]
- El Ashtoukhy, E.S.Z. Loofa egyptiaca as a novel adsorbent for removal of direct blue dye from aqueous solution. J. Environ. Manag. 2009, 90, 2755–2761. [Google Scholar] [CrossRef] [PubMed]
- Deniz, F.; Karaman, S. Removal of Basic Red 46 Dye from aqueous solution by pine tree leaves. Chem. Eng. J. 2011, 170, 67–74. [Google Scholar] [CrossRef]
- Vieira, A.P.; Santana, S.A.A.; Bezerra, C.W.B.; Silva, H.A.S.; Chaves, J.A.P.; Melo, J.C.P.; Filho, E.C.S.; Airold, C. Kinetics and thermodynamics of textile dye adsorption from aqueous solutions using babassu coconut mesocarp. J. Hazard. Mater. 2009, 166, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Hameed, B.H.; El-Khaiary, M.I. Removal of phenol from aqueous solutions by adsorption onto activated carbon prepared from biomass material. J. Hazard. Mater. 2008, 159, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Orlando, U.S.; Okuda, T.; Nishijima, W. Chemical properties of anion exchangers prepared from waste natural materials. React. Funct. Polym. 2003, 55, 311–318. [Google Scholar] [CrossRef]
- Gong, R.M.; Ding, Y.; Li, M.; Yang, C.; Liu, H.; Sun, Y. Utilization of powdered peanut hull as biosorbent for removal of anionic dyes from aqueous solution. Dyes Pigments 2005, 64, 187–192. [Google Scholar] [CrossRef]
- Robinson, T.; Chandran, B.; Nigam, P. Removal of dyes from a synthetic textile dye effluent by biosorption on apple pomace and wheat straw. Water Res. 2002, 36, 2824–2830. [Google Scholar] [CrossRef]
- Li, L.H.; Xiao, J.; Liu, P.; Yang, G.W. Super adsorption capability from amorphousization of metal oxide nanoparticles for dye removal. Sci. Rep. 2015, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.S.; Elkady, M.F.; Hafez, E.E.; Salama, E. Novel antibacterial zinc oxide polymeric nanocomposite membrane as wound dress. Nanosci. Nanotechnol. Asia 2017, 7, 62–72. [Google Scholar] [CrossRef]
- Lu, H.; Wang, J.; Stoller, M.; Wang, T.; Bao, Y.; Hao, H. An overview of nanomaterials for water and wastewater treatment. Adv. Mater. Sci. Eng. 2016, 2016, 4964828. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef] [PubMed]
- Hasanpoor, M.; Aliofkhazraei, M.; Delavari, H. Microwave-assisted synthesis of zinc oxide nanoparticles. Procedia Mater. Sci. 2015, 11, 320–325. [Google Scholar] [CrossRef]
- Kong, X.; Duan, Y.; Peng, P.; Qiu, C.; Wu, L.; Liu, L.; Zheng, W. A novel route to prepare ZnO nanotubes by using microwave irradiation method. Chem. Lett. 2007, 36, 428–429. [Google Scholar] [CrossRef]
- Shaaban, E.R.; El-Hagary, M.; Moustafa, E.; Hassan, H.S.; Ismail, Y.A.M.; Ismail, M.E.; Ali, A.S. Structural, linear and nonlinear optical properties of co-doped ZnO thin films. Appl. Phys. A 2016, 122, 1–20. [Google Scholar] [CrossRef]
- Elkady, M.F.; Hassan, H.S. Equilibrium and dynamic profiles of azo dye sorption onto innovative nano-zinc oxide biocomposite. Curr. Nanosci. 2015, 11, 805–814. [Google Scholar] [CrossRef]
- Hassan, H.S.; Elkady, M.F.; Farghali, A.A.; Salem, A.M.S.; Abd El-Hamid, A.I. Fabrication of novel magnetic zinc oxide cellulose acetate hybrid nano-fiber to be utilized for phenol decontamination. J. Taiwan Inst. Chem. Eng. 2017, 78, 307–316. [Google Scholar] [CrossRef]
- Nikazar, M.; Alizadeh, M.; Lalavi, R.; Rostami, M.H. The optimum conditions for synthesis of Fe3O4/ZnO core/shell magnetic nanoparticles for photodegradation of phenol. J. Environ. Health Sci. Eng. 2014, 12, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.C. Bifunctional core-shell nanocomposite Mndoped ZnO/Fe3O4 for photodegradation of reactive blue 198 dye. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, 1–6. [Google Scholar] [CrossRef]
- Elkady, M.F.; Hassan, H.S.; Hafez, E.E.; Fouad, A. Construction of zinc oxide into different morphological structures to be utilized as antimicrobial agent against multidrug resistant bacteria. Bioinorg. Chem. Appl. 2015, 2015, 536854. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Patel, D.; Choi, E.S.; Baek, M.J.; Chang, Y.; Kim, T.J.; Lee, G.H. Salt effects on the physical properties of magnetite nanoparticles synthesized at different NaCl concentrations. Colloids Surf. 2010, 367, 41–46. [Google Scholar] [CrossRef]
- Malayeri, H.Z.; Ayati, B.; Ganjidoust, H. Photocatalytic phenol degradation by immobilized nano ZnO. Water Environ. Res. 2014, 86, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Latif, M.; Ibrahim, A.M.; El-Kady, M.F. Adsorption equilibrium, kinetics and thermodynamics of methylene blue from aqueous solutions using biopolymer oak sawdust composite. J. Am. Sci. 2010, 6, 267–283. [Google Scholar]
- Thinakaran, N.; Baskaralingam, P.; Pulikesi, M.; Panneerselvam, P.; Sivanesan, S. Removal of acid violet 17 from aqueous solution by adsorption on to activated carbon prepared from sunflower seed hull. J. Hazard. Mater. 2008, 151, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Parida, K.M.; Pradhan, A.C. Removal of phenolic compounds from aqueous solutions by adsorption onto manganese nodule leached residue. J. Hazard. Mater. 2010, 173, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Elkady, M.F.; El-Aassar, M.R.; Hassan, H.S. Adsorption profile of basic dye onto novel fabricated carboxylated functionalized co-polymer nanofibers. Polymers 2016, 8, 177. [Google Scholar] [CrossRef]
- Alkaram, U.F.; Mukhlis, A.A.; Al-Dujaili, A.H. The removal of phenol from aqueous solutions by adsorption using surfactant-modified bentonite and kaolinite. J. Hazard. Mater. 2009, 169, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Özer, A.; Dursun, G. Removal of methylene blue from aqueous solution by dehydrated wheat bran carbon. J. Hazard. Mater. 2007, 146, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Ozkaya, B. Adsorption and desorption of phenol on activated carbon and a comparison of isotherm models. J. Hazard. Mater. 2007, 129, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Qadeer, R.; Rehan, A.H. A study of the adsorption of phenol by activated carbon from aqueous solutions. Turk. J. Chem. 2002, 26, 357–361. [Google Scholar]
- Rengaraj, S.; Moon, S.; Sivabalan, R.; Arabindoo, B.; Murugesan, V. Removal of phenol from aqueous solution and resin manufacturing industry wastewater using an agricultural waste: Rubber seed coat. J. Hazard. Mater. 2002, 89, 185–196. [Google Scholar] [CrossRef]

| Temp.(K) | 1000/T | CBe | CAe | Kc | lnKc | ΔG0 (kJ·mol−1) | E0 (kJ·mol−1) | ΔH0 (kJ·mol−1) | ΔS0 (J·mol−1·K−1) |
|---|---|---|---|---|---|---|---|---|---|
| 298 | 3.356 | 8.775 | 1.225 | 7.163 | 1.969 | −4.878 | 2.501 | 23.495 | 96.027 |
| 313 | 3.195 | 9.134 | 0.866 | 10.547 | 2.356 | −6.119 | 2.626 | ||
| 328 | 3.049 | 9.642 | 0.358 | 26.933 | 3.293 | −8.983 | 2.750 | ||
| 343 | 2.915 | 9.678 | 0.322 | 30.056 | 3.403 | −9.706 | 2.875 | ||
| 358 | 2.793 | 9.709 | 0.291 | 33.364 | 3.508 | −10.44 | 3.000 |
| Isotherms | Parameters | Value |
|---|---|---|
| Langmuir | qm (mg/g) k (L/mg) R2 | 20.408 0.107 0.966 |
| Freundlich | KF (mg/g)(L/mg)1/n 1/nF R2 | 1.122 0.612 0.978 |
| Temkin | A (L/g) B (J/mol) R2 | 2.722 3.164 0.923 |
| Kinetic Model | Parameter | Value |
|---|---|---|
| Pseudo-first-order | qexp (mg/g) | 4.388 |
| qtheor (mg/g) | 1.817 | |
| K1 (min−1) | 0.033 | |
| R2 | 0.872 | |
| Pseudo-second-order | qexp (mg/g) | 4.388 |
| qtheor (mg/g) | 4.405 | |
| K2 (g/mg·min) | 0.042 | |
| R2 | 0.994 | |
| Elovich kinetic model | qexp (mg/g) | 4.388 |
| qtheor (mg/g) | 4.196 | |
| α (mg/g·min) | 2.158 | |
| β (g/mg) | 0.453 | |
| R2 | 0.878 |
| Sodium Hydroxide Concentration | Percentage Phenol Desorbed |
|---|---|
| 0.05 N | 24.2 |
| 0.1 N | 30.7 |
| 0.15 N | 47.8 |
| 0.2 N | 68 |
| 0.25 N | 70 |
| 0.3 N | 73.55 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elkady, M.F.; Hassan, H.S.; Amer, W.A.; Salama, E.; Algarni, H.; Shaaban, E.R. Novel Magnetic Zinc Oxide Nanotubes for Phenol Adsorption: Mechanism Modeling. Materials 2017, 10, 1355. https://doi.org/10.3390/ma10121355
Elkady MF, Hassan HS, Amer WA, Salama E, Algarni H, Shaaban ER. Novel Magnetic Zinc Oxide Nanotubes for Phenol Adsorption: Mechanism Modeling. Materials. 2017; 10(12):1355. https://doi.org/10.3390/ma10121355
Chicago/Turabian StyleElkady, Marwa F., Hassan Shokry Hassan, Wael A. Amer, Eslam Salama, Hamed Algarni, and Essam Ramadan Shaaban. 2017. "Novel Magnetic Zinc Oxide Nanotubes for Phenol Adsorption: Mechanism Modeling" Materials 10, no. 12: 1355. https://doi.org/10.3390/ma10121355
APA StyleElkady, M. F., Hassan, H. S., Amer, W. A., Salama, E., Algarni, H., & Shaaban, E. R. (2017). Novel Magnetic Zinc Oxide Nanotubes for Phenol Adsorption: Mechanism Modeling. Materials, 10(12), 1355. https://doi.org/10.3390/ma10121355






