Synthesis and Characterization of TiO2 Nanoparticles for the Reduction of Water Pollutants
Abstract
:1. Introduction
2. Methods
2.1. Synthesis
2.2. Dye Degradation Tests
2.3. Bacteriological Tests
2.4. Characterization
3. Results and Discussion
3.1. Diffractometric Analysis
3.2. Morphological Analysis
3.3. Dye Degradation Tests
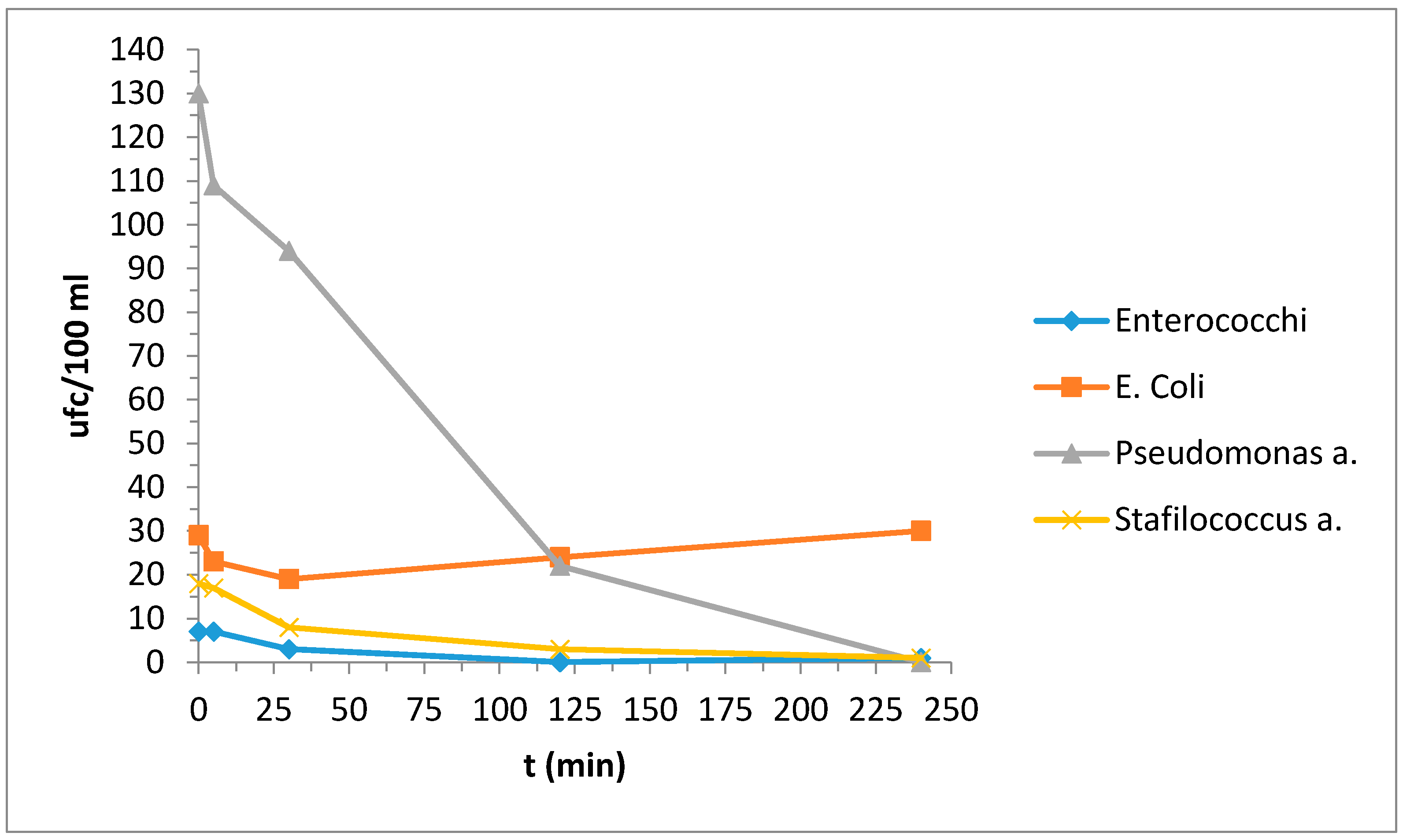
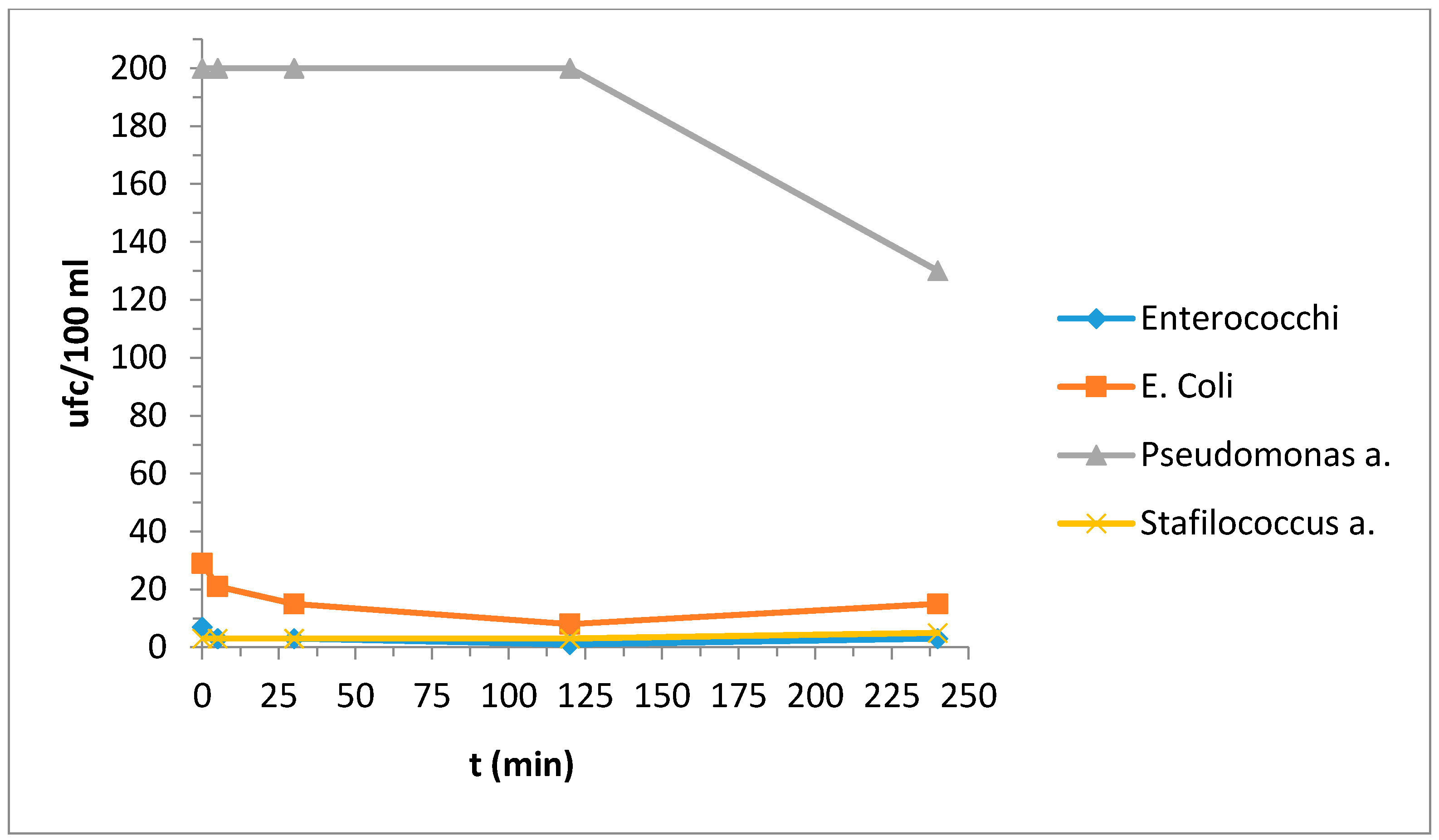
3.4. Bacteriological Tests
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Shipra, M.; Gupta, E.; Manoj, T. A review of TiO2 nanoparticles. Chin. Sci. Bull. 2011, 56, 1639–1657. [Google Scholar]
- Lan, Y.; Lu, Y.; Ren, Z. Mini review on photocatalysis of titanium dioxide nanoparticles and their solar application. Nano Energy 2013, 2, 1031–1045. [Google Scholar] [CrossRef]
- Park, H.; Kim, H.I.; Moon, G.H.; Choi, W. Photoinduced charge transfer processes in solar photocatalysisbased on modified TiO2. Energy Environ. Sci. 2016, 9, 411–433. [Google Scholar] [CrossRef]
- Martinez, T.; Bertron, A.; Ringot, E.; Escadeillas, G. Degradation of NO using Photocatalytic coatings applied to different substates. Build. Environ. 2014, 71, 176–182. [Google Scholar]
- Carp, O.; Huisman, C.L.; Raller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Taoda, H. Development of TiO2 photocatalysts suitable for practical use and their application in environmental cleanup. Res. Chem. Intermed. 2008, 34, 417–426. [Google Scholar] [CrossRef]
- Hussain, M.; Russo, N.; Saracco, G. Photocatalytic abatement of VOCs by novel optimized TiO2 nanoparticels. Chem. Eng. J. 2011, 116, 138–149. [Google Scholar] [CrossRef]
- Manoj, A.L.; Shaji, V.; Santhosh, S. Photocatalytic Water Treatment by Titanium Dioxide: Recent Updates. Catalysts 2012, 2, 572–601. [Google Scholar]
- Motta, F.; Strini, A.; Carraro, E.; Bonetta, S. Photocatalytic bacterial inactivation by TiO2-coated surfaces. AMB Express 2013, 3, 1–8. [Google Scholar]
- Herrmann, J.M.; Guillard, C.; Pichat, P. Heterogeneous photocatalysis: An emerging technology for water treatment. Catal. Today 1993, 17, 7–20. [Google Scholar] [CrossRef]
- Ambrus, Z.; Mogyorosi, K.; Szalai, A.; Alapi, T.; Demeter, K.; Dombi, A.; Sipos, P. Substrate-dependent photocatalytic activity of nanocrystalline TiO2 with tailor-made rutile to anatase ratio. Appl. Catal. A 2008, 340, 153–161. [Google Scholar] [CrossRef]
- Inagaki, M.; Nakazawa, Y.; Hirano, M.; Kobayashi, Y.; Toyoda, M. Preparation of stable anatase-type TiO2 and its photocatalytic performance. Int. J. Inorg. Mater. 2001, 3, 809–811. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Mills, A.; Hunte, S.L. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Kim, S.H.; Ngo, H.H.; Shon, H.K.; Vigneswaran, S. Adsorption and photocatalysis kinetics of herbicide onto titanium oxide and powdered activated carbon. Sep. Purif. Technol. 2008, 58, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Vo, P.T.; Ngo, H.H.; Guo, W.; Zhou, J.L.; Nguyen, P.D.; Listowski, A.; Wang, X.C. A mini-review on the impacts of climate change on wastewater reclamation and reuse. Sci. Total Environ. 2014, 494–495, 9–17. [Google Scholar]
- De la Cruz, N.; Romero, V.; Dantas, R.F.; Marco, P.; Bayarri, B.; Giménez, J.; Esplugas, S. o-Nitrobenzaldehyde actinometry in the presence of suspended TiO2 for photocatalytic reactors. Catal. Today 2013, 209, 209–214. [Google Scholar] [CrossRef]
- Zhang, G.; Kim, G.; Choi, W. Visible light driven photocatalysis mediated via ligand-to-metal charge transfer (LMCT): An alternative approach to solar activation of titania. Energy Environ. Sci. 2014, 7, 954–966. [Google Scholar] [CrossRef]
- De Filipo, G.; Palermo, A.M.; Rachiele, F. Preventing fungal growth in wood by titanium dioxide nanoparticles. Int. Biodeterior. Biodegrad. 2013, 85, 217–222. [Google Scholar] [CrossRef]
- Robertson, P.K.J.; Robertson, J.M.C.; Bahnemann, D.W. Removal of microorganism and their chemical metabolites from water using semiconductors photocatalysis. J. Hazard. Mater. 2012, 211–212, 161–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dvoranova, D.; Brezova, V.; Mazur, M.; Malati, M.A. Investigation of metal-doped titanium dioxide photocatalysts. Appl. Catal. B 2002, 37, 91–105. [Google Scholar] [CrossRef]
- Reddy, K.M.; Baruwati, B.; Jayalakshmi, M. S-,N-and C-doped titanium dioxide nanoparticles: Synthesis, characterization and redox charge transfer study. J. Solid State Chem. 2005, 178, 3352–3358. [Google Scholar] [CrossRef]
- Lee, H.U.; Lee, S.C.; Choi, S.; Son, B. Efficient visible-light induced photocatalysis on nanoporous nitrogen-doped titanium dioxide catalysts. Chem. Eng. J. 2013, 228, 756–764. [Google Scholar] [CrossRef]
- Jo, W.K.; Kim, J. Application of visible light photocatalysis with nitrogen-doped or unmodified titanium dioxide for control of indoor-level volatile organic compounds. J. Hazard. Mater. 2009, 164, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Zadeh, E.K.; Zebarjad, S.M.; Janghorban, K. Optimization of synthesis conditions of N-doped TiO2 nanoparticles using Taguchi robust design. Mater. Chem. Phys. 2017, 201, 69–77. [Google Scholar] [CrossRef]
- Mezni, A.; Saber, N.B.; Ibrahim, M.M.; Kemary, M.E.; Aldalbahi, A.; Smiri, L.S.; Altalhi, T. Facile synthesis of highly thermally stable TiO2 photocatalysts. New J. Chem. 2017, 41, 5021–5030. [Google Scholar] [CrossRef]
- Ojeda, M.; Kumar, D.K.; Chen, B.; Xuan, J.; Maorto-Valer, M.M.; Leung, D.Y.C.; Wang, H. Polymeric templating synthesis of Anatase TiO2 nanoparticles from low-cost inorganic titanium sources. Chem. Select 2017, 2, 702–706. [Google Scholar]
- Su, C.; Hong, B.Y.; Tseng, C.M. Sol-gel preparation and photocatalysis of titanium dioxide. Catal. Today 2004, 96, 119–126. [Google Scholar] [CrossRef]
- Yin, H.; Wada, Y.; Kitamura, T.; Kambe, S.; Murasawa, S.; Mori, H.; Sakata, T.; Yanagida, S. Hydrothermal synthesis of nanosized anatase and rutile TiO2 using amorphous phase TiO2. J. Mater. Chem. A 2001, 11, 1694–1703. [Google Scholar] [CrossRef]
- Yamashita, H.; Honda, M.; Harada, M.; Ichihashi, Y.; Anpo, M.; Hirao, T.; Itoh, N.; Iwamoto, N. Preparation of titanium oxide photocatalysts anchored on porous silica glass by a metal ion-implantation method and their photocatalytic reactivities for the degradation of 2-propanol diluted in water. J. Phys. Chem. B 1998, 102, 10707–10711. [Google Scholar] [CrossRef]
- Miao, L.; Jin, P.; Kaneko, K.; Terai, A.; Nabatova-Gabain, N.; Tanemura, S. Preparation and characterization of polycrystalline anatase and rutile TiO2 thin films by RF magnetron sputtering. Appl. Surf. Sci. 2003, 212, 255–263. [Google Scholar] [CrossRef]
- Kominami, H.; Kato, J.I.; Murakami, S.Y.; Kera, Y.; Inoue, M.; Inui, T.; Ohtani, B. Synthesis of titanium (IV) oxide of ultra-high photocatalytic activity: High temperature hydrolysis of titanium alkoxides with water liberated homogeneously from solvent alcohols. J. Mol. Catal. A Chem. 1999, 144, 165–171. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.; Zhang, J.; Schwank, J.W. A review on TiO2 based nanotubes synthetized via hydrothermal method: formation mechanism, structure modification and photocatalytic application. Catal. Today, 2014, 225, 34–51. [Google Scholar] [CrossRef]
- Suyama, Y.; Kato, A. TiO2 produced by vapor-phase oxygenolysis of TiCI4. J. Am. Ceram. Soc. 1976, 59, 146–149. [Google Scholar] [CrossRef]
- Akhtar, M.K.; Vemury, S.; Pratsinis, S.E. Competition between TiCl4 hydrolysis and oxidation and its effect on product TiO2 powder. AIChE J. 1994, 40, 1183–1192. [Google Scholar] [CrossRef]
- Ani, J.K.; Savithri, S.; Surender, G.D. Characteristics of titania nanoparticles synthesized through low temperature aerosol process. Aerosol Air Qual. Res. 2005, 5, 1–13. [Google Scholar]
- Kirkbir, F.; Komiyama, H. Low temperature synthesis of TiO2 by vapor-phase hydrolysis of titanium isopropoxide. Chem. Lett. 1988, 5, 791–794. [Google Scholar] [CrossRef]
- Comparelli, R.; Fanizza, E.; Curri, M.L. Photocatalytic degradation of azo dyes by organic-capped anatase TiO2 nanocrystals immobilized onto substrates. Appl. Catal. B Environ. 2005, 55, 81–91. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO). Quanti-Tray®, Standard 9308-2:2012; ISO: Geneva, Switzerland, 2012. [Google Scholar]
- JCPDS (Joint Committee on Powder Diffraction Standards) International Center for Diffraction Data. PCPFWIN, version 2.3; JCPDS International Center for Diffraction Data: Swarthmore, PA, USA, 2002. [Google Scholar]
- Patterson, A. The scherrer formula for X-ray particle size determination, Phys. Rev. 1939, 56, 978–982. [Google Scholar]
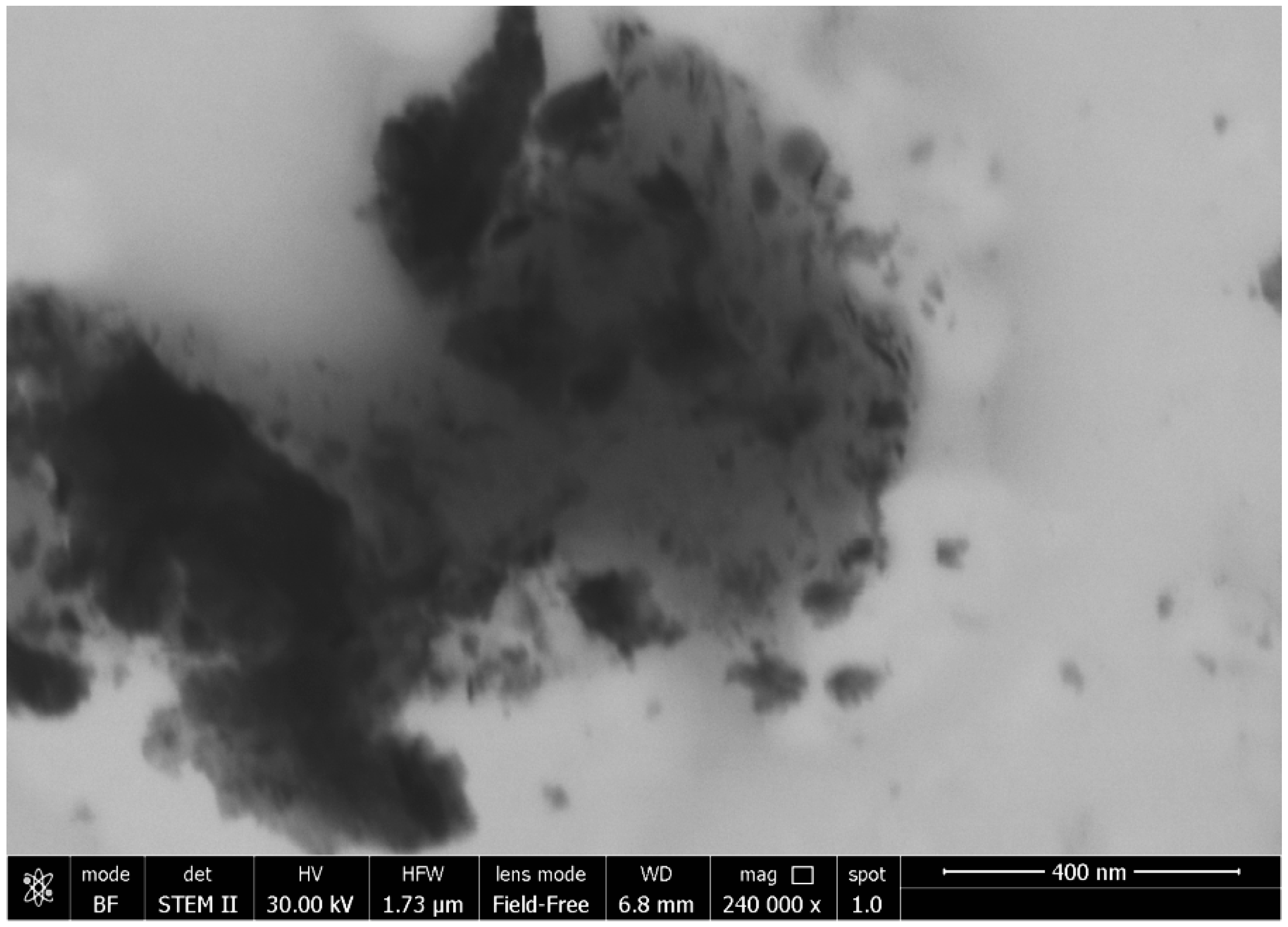
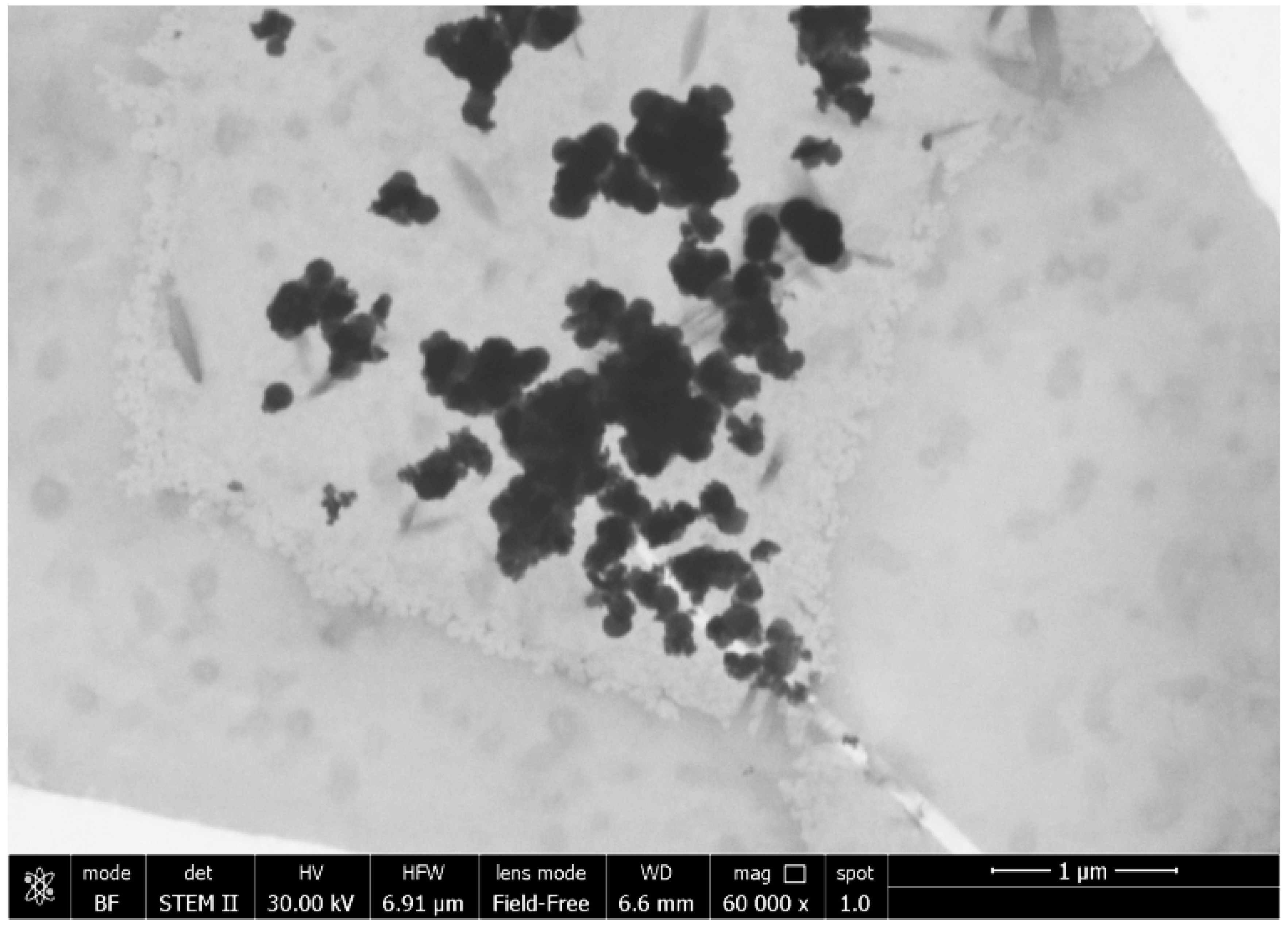
| Compounds | Amounts |
|---|---|
| TiCl4 | 5 mL |
| C2H5OH | 50 mL |
| H2O | 200 mL |
| Compounds | Amounts |
|---|---|
| C12H28O4Ti | 50 mL |
| H2O | 200 mL |
| CO(NH2)2 | 1 g |
| Compounds | Amounts |
|---|---|
| C12H28O4Ti | 3.17 mL |
| C3H8O | 9.50 mL |
| CH3COOH | 10 mL |
| CH3OH | 24 mL |
| Molar Ratios C12H28O4Ti:CO(NH2)2:NH4Cl | Compounds | Amounts |
|---|---|---|
| 10:1:0 | C12H28O4Ti | 50 mL |
| CO(NH2)2 | 1 g | |
| H2O | 200 mL | |
| 2:1:0 | C12H28O4Ti | 50 mL |
| CO(NH2)2 | 5 g | |
| H2O | 200 mL | |
| 10:1:0.52 | C12H28O4Ti | 10 mL |
| CO(NH2)2 | 0.2 g | |
| NH4Cl | 0.95 g | |
| H2O | 40 mL | |
| 2:1:0.52 | C12H28O4Ti | 10 mL |
| CO(NH2)2 | 40 mL | |
| NH4Cl | 0.2 g | |
| H2O | 1.02 g |
| Anatase | Synthesis 1 | Synthesis 2 | Synthesis 3 | ||
|---|---|---|---|---|---|
| d (Å) | 2θ | I (%) | I (%) | I (%) | I (%) |
| 3.52 | 25.25 | 100 | 100 | 100 | – |
| 1.89 | 47.97 | 35 | 14 | 23 | – |
| 2.38 | 37.80 | 28 | 25 | 32 | – |
| Anatase | Synthesis 4 Molar Ratio C12H28O4Ti:CO(NH2)2:NH4Cl Temperature | |||||||
|---|---|---|---|---|---|---|---|---|
| 10:1:0 50 °C | 2:1:0 50 °C | 10:1:0 r.t. | 2:1:0 r.t. | 10:1:0.52 50 °C | 2:1:0.52 50 °C | |||
| d (Å) | 2θ | I (%) | I (%) | |||||
| 3.52 | 25.25 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 1.89 | 47.97 | 35 | 32 | 29 | 48 | 19 | 20 | 61 |
| 2.38 | 37.80 | 28 | 32 | 30 | 70 | 27 | 19 | 57 |
| Molar Ratio, C12H28O4Ti:CO(NH2)2:NH4Cl Temperature | ||||||
|---|---|---|---|---|---|---|
| 10:1:0 50 °C | 2:1:0 50 °C | 10:1:0 r.t. | 2:1:0 r.t. | 10:1:0.52 50 °C | 2:1:0.52 50 °C | |
| Shape | Irregular | Spherical | Irregular | Spherical | Quite spherical | Quite spherical |
| Distribution | Irregular, aggregates | Regular | Irregular, aggregates | Irregular, aggregates | Irregular, aggregates | Irregular, aggregates |
| Molar Ratios, C12H28O4Ti:CO(NH2)2:NH4Cl Temperature | ||||||
|---|---|---|---|---|---|---|
| 10:1:0 50 °C | 2:1:0 50 °C | 10:1:0 r.t. | 2:1:0 r.t. | 10:1:0.52 50 °C | 2:1:0.52 50 °C | |
| XRD (nm) | 27 | 27 | 27 | 27 | 28 | 28 |
| FESEM (nm) | 30 | 30 | 40 | 40 | 40 | 30 |
| (a) | ||
| Molar Ratios | Dyes | |
| Methyl Orange | Bromothymol Blue | |
| 10:1 50 °C | Total degradation after two hours | Total degradation after two hours |
| 2:1 50 °C | Total degradation after four hours | Total degradation after four hours |
| 10:1 r.t. | Total degradation after one hour | Total degradation after one hour |
| 2:1 r.t. | Partial degradation after four hours | Partial degradation after four hours |
| (b) | ||
| Molar Ratios | Dyes | |
| Methyl Orange | Bromothymol Blue | |
| 10:1:0 | Low degradation | Total degradation after ½ h |
| 2:1:0 | Total degradation after ½ h | Total degradation after ½ h |
| 10:1:0.52 | No degradation | No degradation |
| 2:1:0.52 | No degradation | Low degradation |
| (a) | |||||
| Bacterial Strains | t (min) | ||||
| 0 | 5 | 30 | 120 | 240 | |
| Enterococchi | 7 | 7 | 3 | 0 | 1 |
| E. coli | 29 | 23 | 19 | 24 | 30 |
| Pseudomonas a. | 130 | 109 | 94 | 22 | 0 |
| Stafilococcus a. | 18 | 17 | 8 | 3 | 1 |
| (b) | |||||
| Bacterial Strains | t (min) | ||||
| 0 | 5 | 30 | 120 | 240 | |
| Enterococchi | 7 | 3 | 3 | 1 | 3 |
| Escherichia coli | 29 | 21 | 15 | 8 | 15 |
| Pseudomonas a. | 200 | 200 | 200 | 200 | 130 |
| Stafilococcus a. | 3 | 3 | 3 | 3 | 5 |
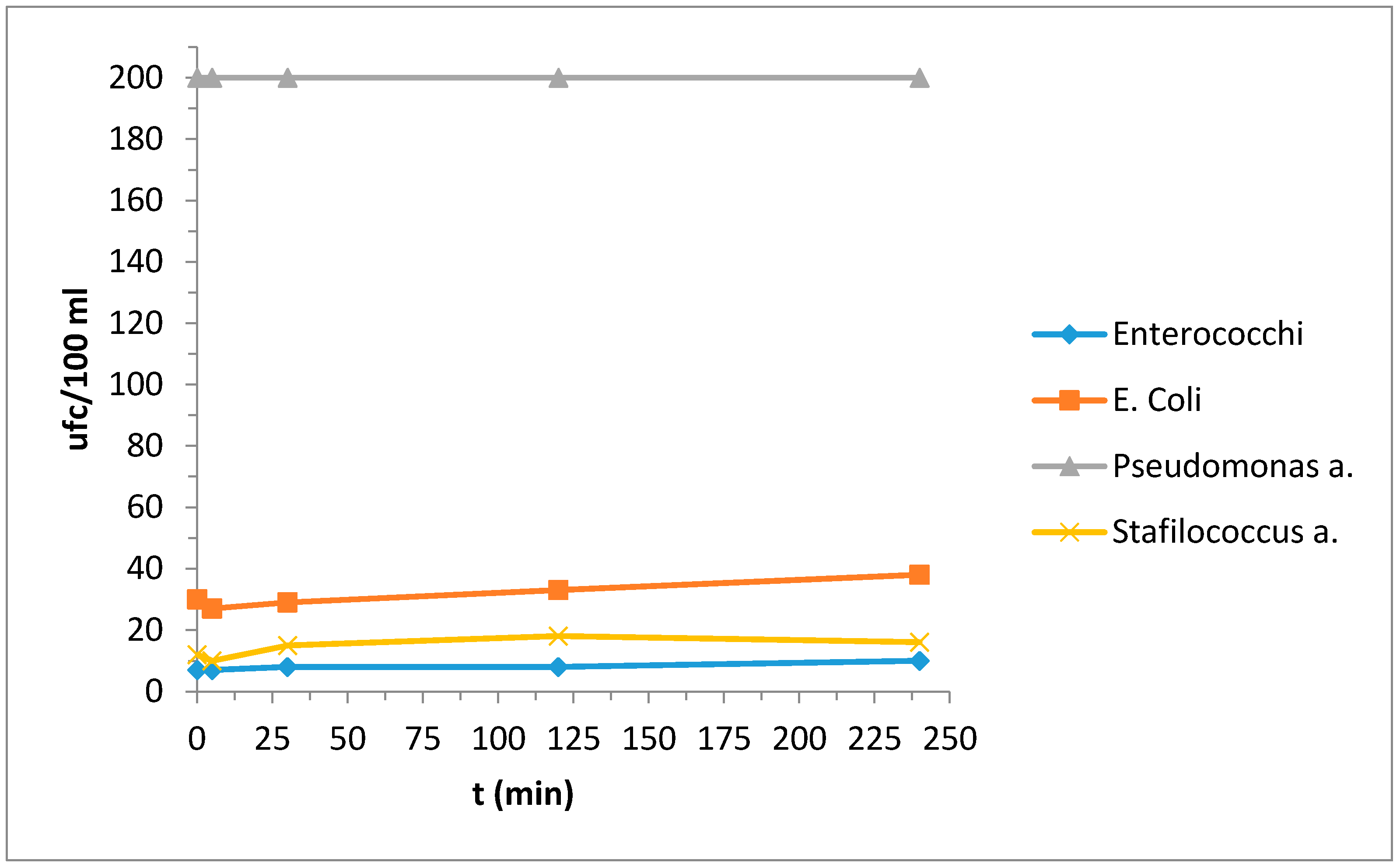
| Bacterial Colonies | t (min) | ||||
|---|---|---|---|---|---|
| 0 | 5 | 30 | 120 | 240 | |
| Enterococchi | 7 | 7 | 8 | 8 | 10 |
| Escherichia coli | 30 | 27 | 29 | 33 | 38 |
| Pseudomonas aeruginosa | 200 | 200 | 200 | 200 | 200 |
| Stafilococcus aureus | 12 | 10 | 15 | 18 | 16 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lusvardi, G.; Barani, C.; Giubertoni, F.; Paganelli, G. Synthesis and Characterization of TiO2 Nanoparticles for the Reduction of Water Pollutants. Materials 2017, 10, 1208. https://doi.org/10.3390/ma10101208
Lusvardi G, Barani C, Giubertoni F, Paganelli G. Synthesis and Characterization of TiO2 Nanoparticles for the Reduction of Water Pollutants. Materials. 2017; 10(10):1208. https://doi.org/10.3390/ma10101208
Chicago/Turabian StyleLusvardi, Gigliola, Corrado Barani, Federica Giubertoni, and Giulia Paganelli. 2017. "Synthesis and Characterization of TiO2 Nanoparticles for the Reduction of Water Pollutants" Materials 10, no. 10: 1208. https://doi.org/10.3390/ma10101208
APA StyleLusvardi, G., Barani, C., Giubertoni, F., & Paganelli, G. (2017). Synthesis and Characterization of TiO2 Nanoparticles for the Reduction of Water Pollutants. Materials, 10(10), 1208. https://doi.org/10.3390/ma10101208





