3.1. Microstructural Observation
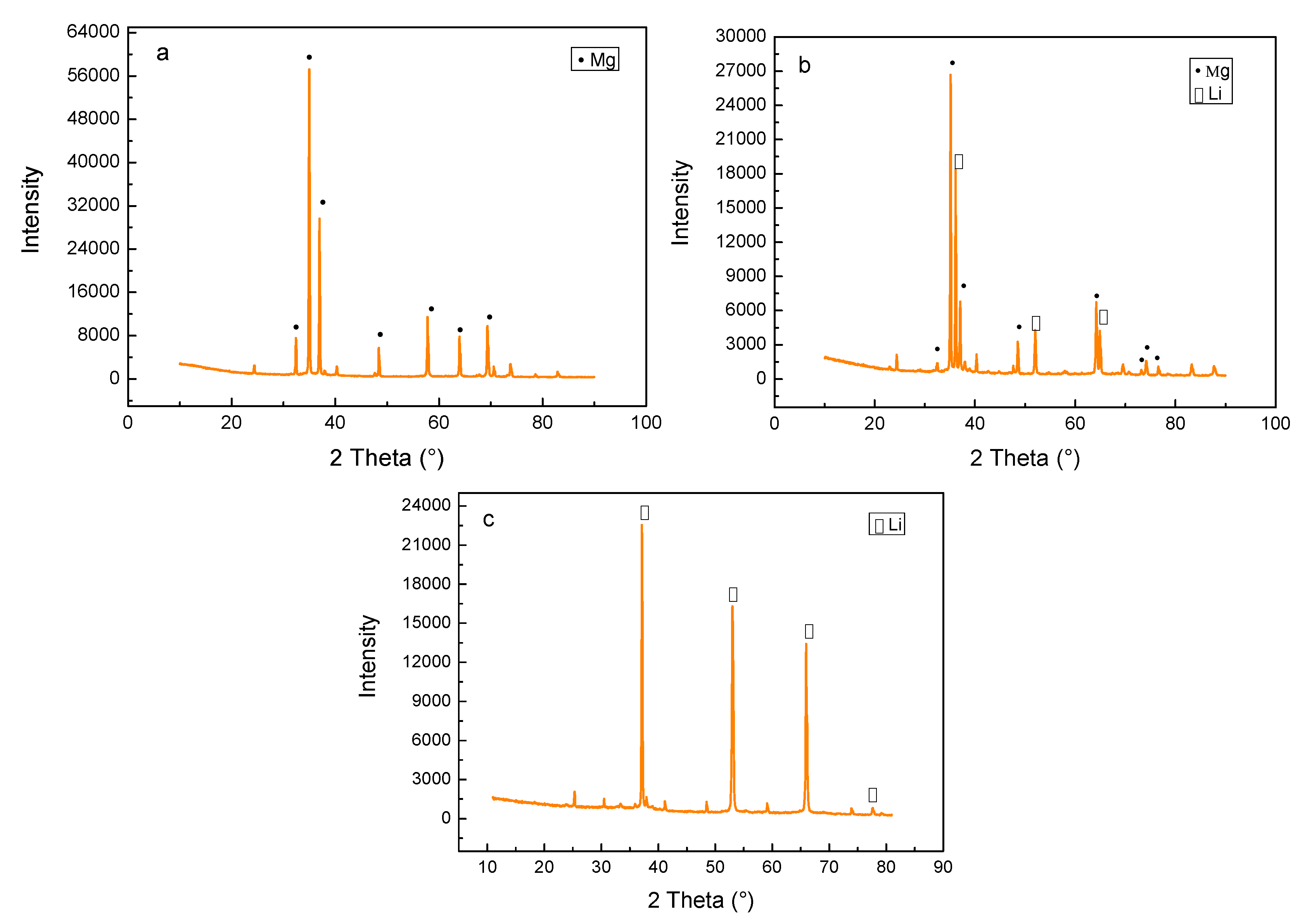
Figure 2 shows the XRD patterns of the as-cast alloys. It indicates that a transformation between the lattice structures took place with increasing Li content. When the Li content was increased to 5%, the Mg-5Li-3Al-2Zn-0.2Y alloy was mainly composed of α-Mg (hcp) phase, as shown in
Figure 2a. However, when the Li addition increased to 8%, some Li matrix peaks with relatively high intensity emerged, implying that the Mg-8Li-3Al-2Zn-0.2Y alloy was mainly composed of α-Mg+β-Li dual-phase, as shown in
Figure 2b. When the Li content was further increased to 11%, the previous α-Mg peak was mostly replaced by the β-Li peak, deducing that the Mg-11Li-3Al-2Zn-0.2Y alloy primarily consisted of β-Li phase, as shown in
Figure 2c.
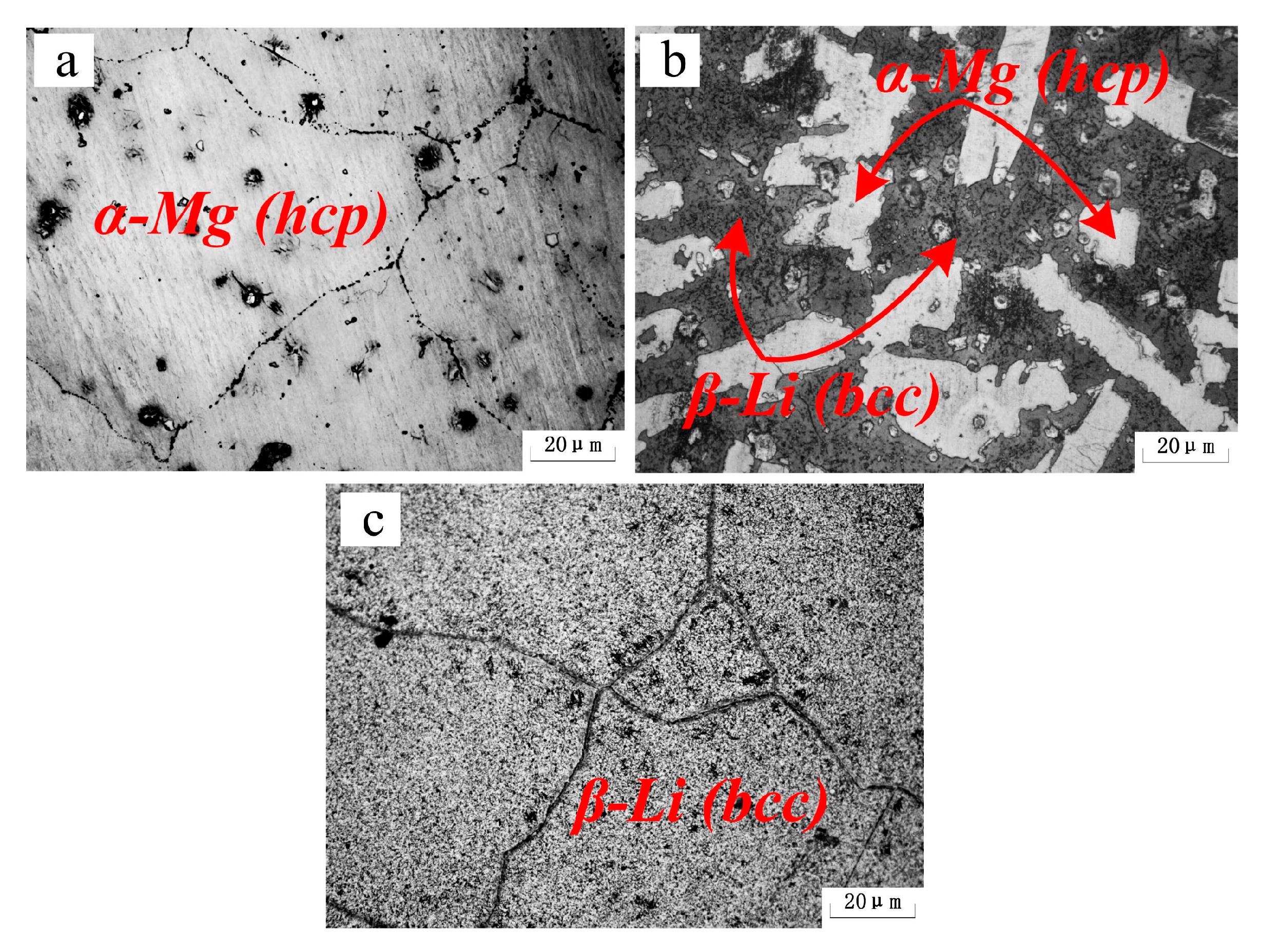
Figure 3 shows the microstructures of the as-cast alloys. Based on the previous XRD patterns, the microstructural evolution confirmed that the increasing Li element transformed the Mg lattice structure from hcp to bcc.
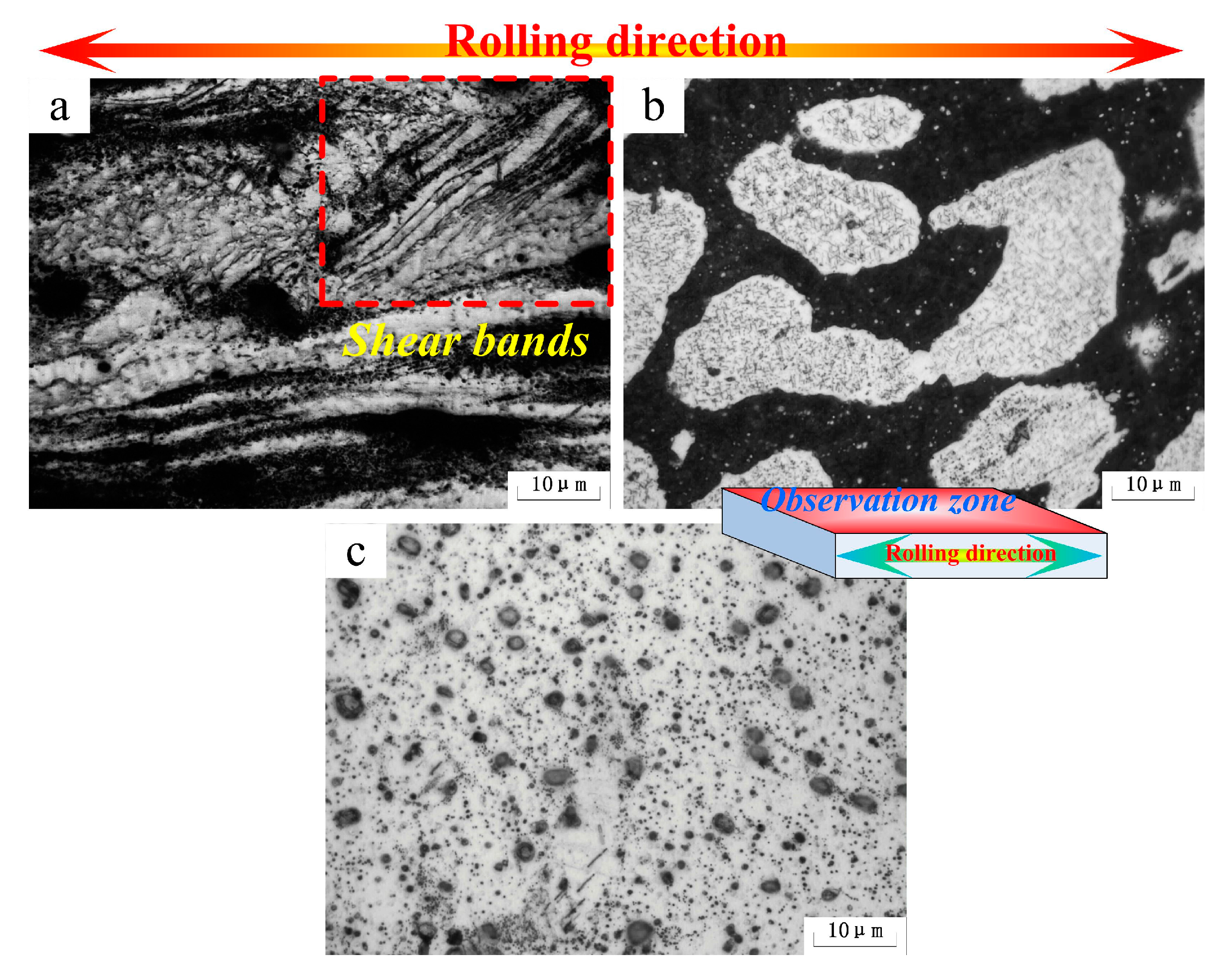
Figure 4 shows the microstructures of the as-rolled alloys. In Mg-5Li-3Al-2Zn-0.2Y (see
Figure 4a), the α-Mg (white zone) and mixed secondary (dark gray zone) phases were both elongated and well distributed along the rolling direction. Moreover, some shear bands aligned with the rolling direction were presented in the α-Mg matrix, indicating that the α-Mg alloy revealed the poor ability of plastic deformation at low temperature. This shear deformation resulted from the incomplete slip between the crystal lattices during the deformation process. The basal slip was the predominant slip mode during the early deformation. However, the normal slip deformation could not continue after basal slip finishing as the non-basal slips were difficult to activate at low temperature. Hence, the shear deformation emerged and then took over the primary deformation mode in the later deformation process.
In Mg-8Li-3Al-2Zn-0.2Y (see
Figure 4b), the β-Li phase (black zone) was obviously elongated along the rolling direction and shear bands were not found. It indicated that the plasticity of the β-Li matrix was much better than that of the α-Mg matrix. The difference between them was ascribed to the characteristics of both crystal structures. The β-Li with the bcc structure possessed many more slip systems and a symmetrical crystal structure compared with α-Mg. Hence, the coordinating deformation and dislocation movements between grains in the β-Li matrix are an advantage compared to the α-Mg matrix. Throughout the rolling process, no shear deformation could be observed in the α-Mg phase. It illustrated that the increasing Li element gradually improved the plasticity of the α-Mg matrix. The reason for this improvement was that the Li addition effectively reduced the parameter c/a axial ratio of the Mg crystal structure, promoting the non-basal slips, such as {10-10} prismatic and {10-12} pyramidal slips [
2]. Thereby, the deformation of the α-Mg matrix would be relatively amenable in the rolling process. In Mg-11Li-3Al-2Zn-0.2Y (see
Figure 4c), the markedly elongated β-Li microstructure was well distributed along the rolling direction. The results confirmed that the β-Li phase possessed a relatively outstanding plasticity.
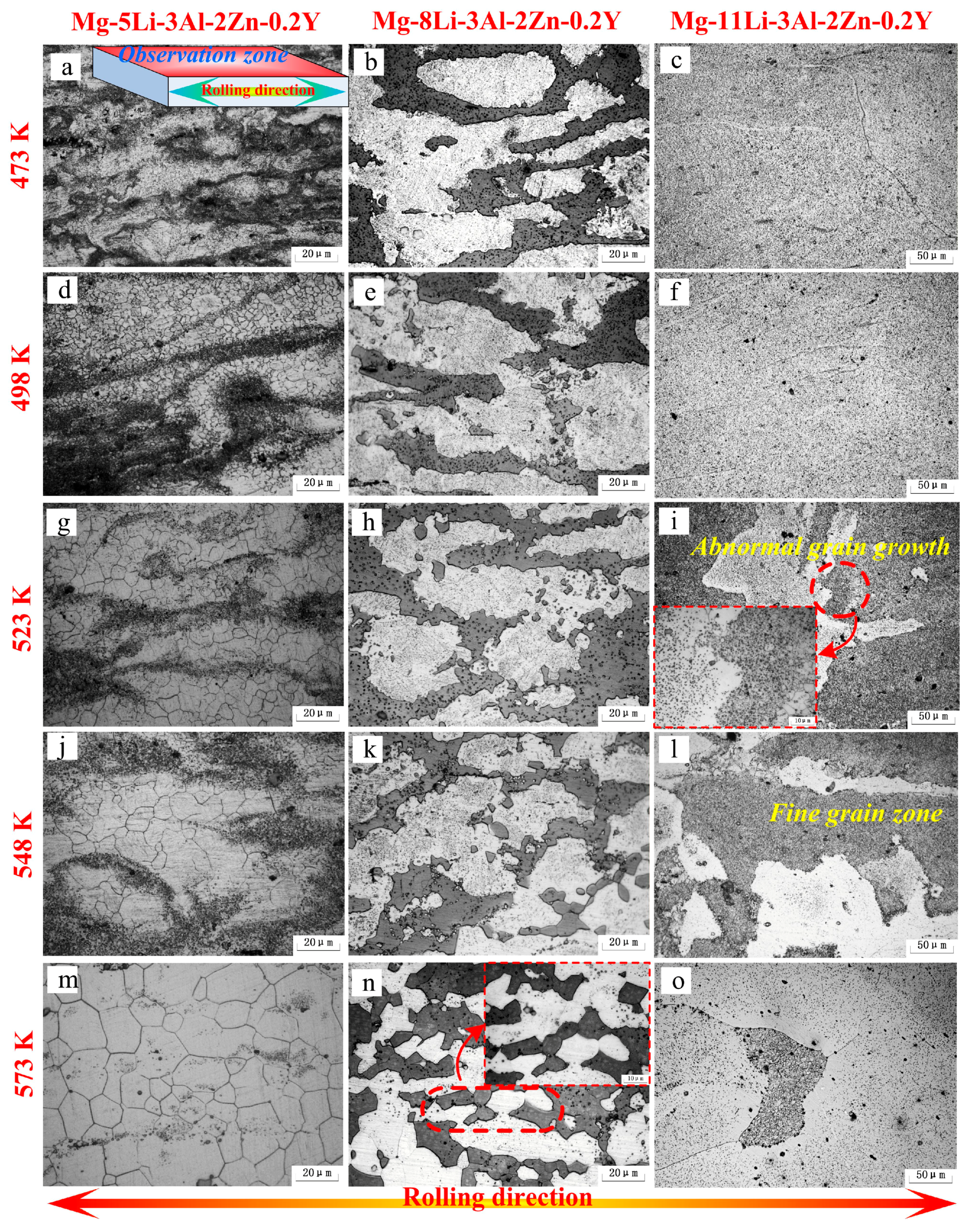
Figure 5 shows the microstructural evolution of the as-rolled alloys during annealing at different temperatures for 24 h (473 K–573 K). In Mg-5Li-3Al-2Zn-0.2Y, a small number of fine equiaxed grains at 473 K were presented in the matrix, indicating that static recrystallization behavior occurred at approximately 473 K. As indicated in
Figure 5a, the deformed microstructure still occupied the most area in the matrix. At 498 K, a great number of small equiaxed grains emerged from the matrix and the mean grain size was measured as 3.1 μm. Additionally, the previous deformed microstructure was apparently substituted by the recrystallized microstructure, illustrating that the recrystallization behavior had taken place, as shown in
Figure 5d. With the annealing temperature increasing to 523 K, the grain growth phenomenon was gradually manifested, in which the mean grain size was measured as 8.9 μm, as shown in
Figure 5g. In the range of 548 K–573 K (see
Figure 5j,m), the coarse grains and clear grain boundaries emerged, wherein the mean grain size at 548 K and 573 K was 13.5 μm and 20.3 μm, respectively.
Considering the recrystallization and grain growth, the grain (nuclei) growth speed could be characterized by Equation (1). As indicated in Equation (1), the grain (nuclei) growth speed was directly proportional to deformation energy. It illustrated that serious deformation would contribute to accelerating grain growth at the same condition, which could be explained by the fact that the deformed zone with high energy would provide a fast channel for the atomic diffusion to promote highly frequent nucleation. Equation (1) could be further simplified as Equation (2). From the Equation (2), the decreasing growth activation energy would accelerate the grain (nuclei) growth, which could be explained by the fact that the relatively high dislocation density could reduce barriers over which the atoms would stride in order to diffuse thoroughly. Hence, the relatively low activation energy would be in favor of grain growth. The mechanisms of recrystallization and grain growth behaviors should be attributed to the transition process from high free energy to low free energy.
where
is the grain (nuclei) growth speed,
is the diffusion coefficient at the grain boundary,
is the interface width,
is a constant,
is the deformation energy,
is the gas constant, and
is the temperature.
where
is the growth activation energy.
In Mg-8Li-3Al-2Zn-0.2Y, in the annealing period of 473 K–498 K, the two matrices did not generate clear microstructural evolution, wherein the elongated α and β phases were still distributed along the rolling direction, as shown in
Figure 5b,e. However, a little variation was gradually exhibited around the β phase edge where the small β grains with a granule shape emerged at 523 K, as shown in
Figure 5h. It indicated that the recrystallization behavior in the β matrix might take place at approximately 523 K. With the temperature increasing to 548 K, the recrystallized β grains became more numerous than before, as shown in
Figure 5k. When the alloy was annealed at 573 K, the mean grain size of β was measured as 15.6 μm, as shown in
Figure 5n. The whole recrystallization process in the β matrix should be ascribed to a recrystallization mechanism where the combination between the subgrains and dislocation absorbing was the main evolution process. In addition, the above–mentioned combination resulted from the transformation among the grain boundary category from a low angle to high angle [
18].
In Mg-11Li-3Al-2Zn-0.2Y, no obvious microstructural variation could be found below 498 K, as shown in
Figure 5c,f. When annealing treatment was set at 523 K (see
Figure 5i), a certain number of tremendous grains emerged around the fine grain zone, which were almost 100 times as big as the fine grains. With the increased annealing temperature (548 K), this phenomenon became more apparent, as shown in
Figure 5l. With annealing at 573 K, the coarse grains were predominant, and simultaneously a small number of residual fine grains were presented in the matrix, as shown in
Figure 5o. This behavior mentioned above belonged to the abnormal grain growth whose activation could be characterized by the driving force during the abnormal grain growth (see Equation (3)). As indicated in Equation (3), the activation of abnormal grain growth was the result of an interfacial energy difference between grains. Furthermore, the grain size in the matrix was closely associated with the interfacial energy. Obviously, the size of advantageous grain was coarser than that of fine grain. Hence,
p > 0 and the abnormal grain growth would be activated.
where
the is driving force,
is the interfacial energy difference,
is the mean grain diameter of fine grain,
is the grain diameter of advantageous grain, and
is a constant.
In the stable recrystallized matrix, a small number of advantageous grains were treated as the nuclei for abnormal grain growth. These advantageous grains could grow preferentially because of the inconsistently dissolved secondary phases. Moreover, their morphology category almost belonged to the polyhedron (>6) whose surface was revealed as concave in favor of grain boundary diffusion. In the growing process, the existence of a particular orientation difference contributed to enhancing the migration velocity to effectively promote the abnormal grain growth.
Figure 6 shows the XRD patterns of the as-rolled alloys during annealing for 24 h (473 K–573 K). In Mg-5Li-3Al-2Zn-0.2Y (see
Figure 6a), with increasing temperature, no evident phase evolution could be observed. In Mg-8Li-3Al-2Zn-0.2Y (see
Figure 6b), the peak altitude of AlLi phase manifested a little fluctuation with increasing temperature. However, the other phases remained stable. In Mg-11Li-3Al-2Zn-0.2Y (see
Figure 6c), the AlLi peak variation was consistent with that in Mg-8Li-3Al-2Zn-0.2Y, indicating that the elevating temperature changed the peak of AlLi phase. The above-mentioned results illustrated that the annealing temperature might influence the AlLi phase solubility in the matrix.
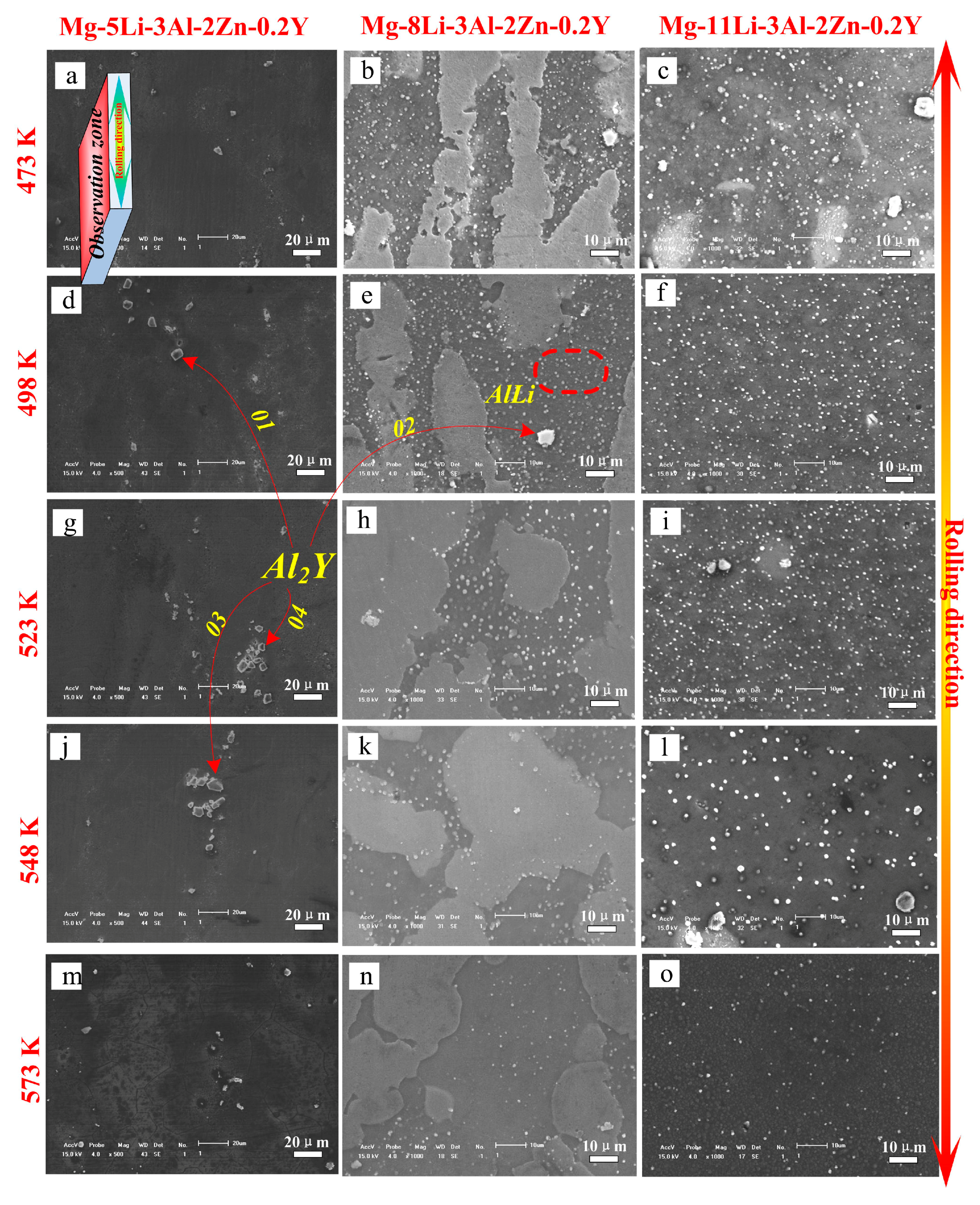
Figure 7 shows the SEM results of the as-rolled alloys during annealing for 24 h (473 K–573 K). In Mg-5Li-3Al-2Zn-0.2Y, the crushed Al
2Y phase was steadily distributed in the matrix with increasing temperature. Meanwhile, no other obvious change was observed in the matrix, as shown in
Figure 7a,d,g,j,m. The EDS results were obtained to make a better analysis for Al
2Y phase, as shown in
Figure 8. The Al
2Y stability that would not be affected by the annealing temperature should be attributed to its special crystal structure. The Al
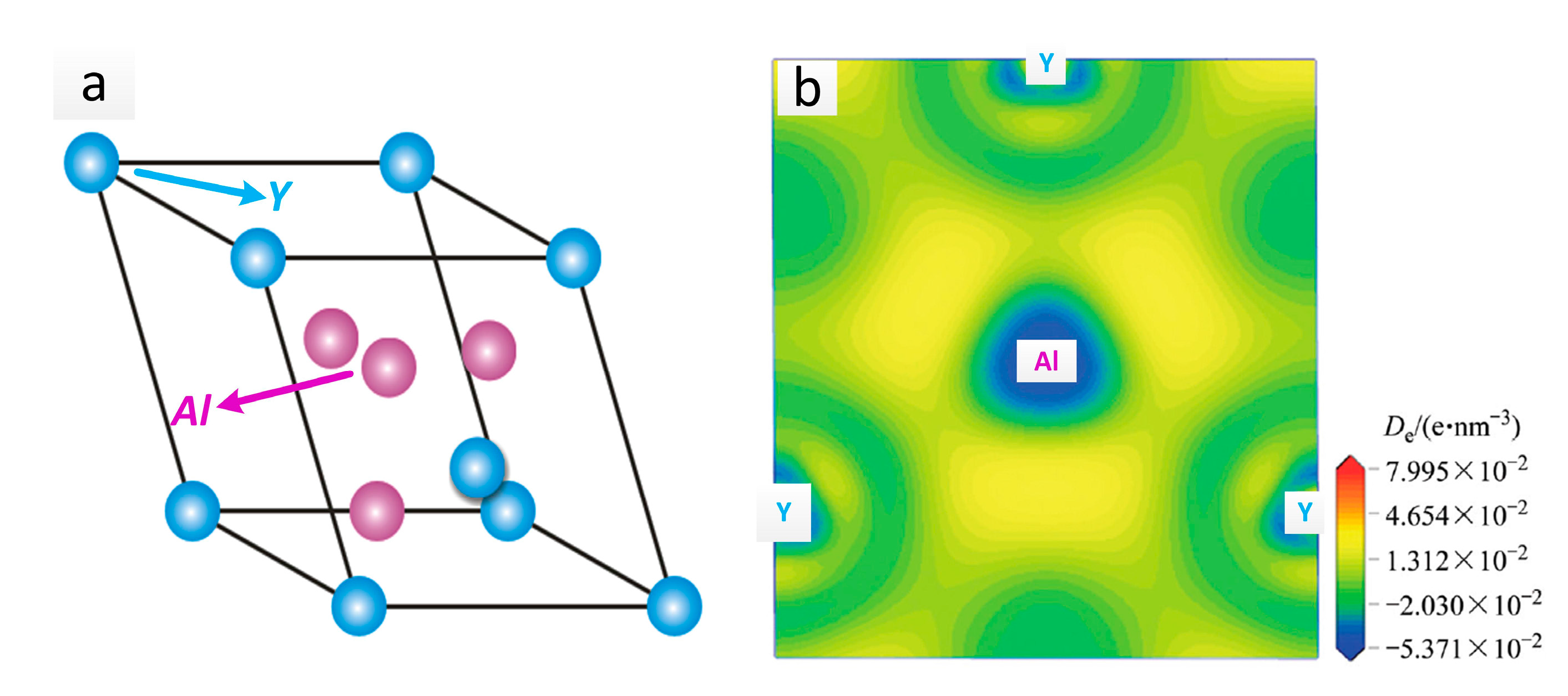
2Y crystal structure and its electronic density difference (De) of the (111) plane are shown in
Figure 9a,b [
19]. The stability mechanism resulted from an intense interaction between the valence electron orbits of the Al and Y atoms, in which a sort of Laves phase structure was made. Additionally, the metallic, covalent, and ionic bonds involved in the Al
2Y lattice structure also played a very important role. The crystal structure parameters of Al
2Y are listed in
Table 1 [
19].
In Mg-8Li-3Al-2Zn-0.2Y, a great many dispersed white granules were well distributed in the β matrix at 473 K (see
Figure 7b). With annealing at 498 K, these dispersed granules became less numerous. In the annealing period of 523–548 K (see
Figure 7h,k), the number of white granules apparently decreased, implying that they decomposed and dissolved into the β matrix. According to the previous XRD analysis, these dissolved granules were considered as AlLi phase. At 573 K (see
Figure 7n), a small amount of residue was only maintained in the microstructure. Furthermore, a similar dissolution law occurred in the Mg-11Li-3Al-2Zn-0.2Y alloy, as shown in
Figure 7c,f,i,l,o.
The atomic solid solubility in the matrix could be analyzed by the Hume-Rothery empirical rule, which was named the “15%” rule (see Equation (4)). As indicated in Equation (4), when
δ is more than 15%, the solubility of the dissolved atom in the matrix is extremely low because the relatively big radius difference limits the atomic dissolution, promoting the formation of an intermetallic compound. According to the calculation,
δ on Li-Al was only 5.9%. Thereby, Al solubility in the β matrix was relatively high. In addition, the bonding energy between the Al and Li atoms in the intermetallic compound was relatively low. Therefore, based on the above-mentioned analysis, the increased temperature would promote the decomposition and dissolution of AlLi phase during the annealing process. The solid solution law with the annealing process is shown in
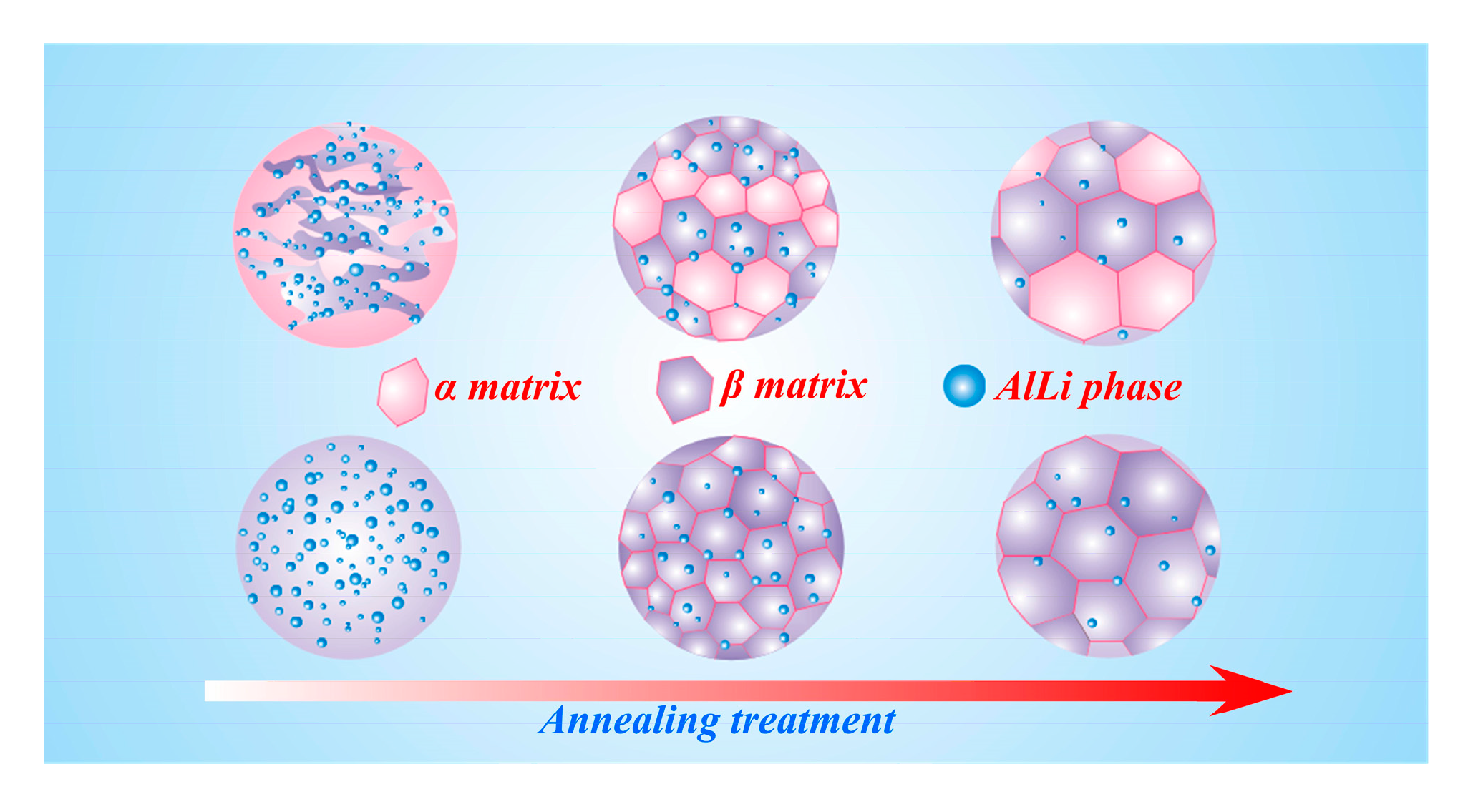
Figure 10. It described the microstructural evolution law in the α and β matrices. In this evolution, the recrystallization phenomenon gradually emerged in the two matrices during annealing treatment. Meanwhile, the amount of AlLi phase in the β matrix decreased gradually, indicating that the solid solution behavior in the β matrix could be gradually activated by an increasing temperature. The recrystallization behavior should be ascribed to the dislocation density reduction and grain boundary migration. In addition, the increasing temperature accelerated the atomic diffusion and promoted the solid solution process.
where
is the matrix atom diameter and
is the dissolved atom diameter.
3.2. Mechanical Properties
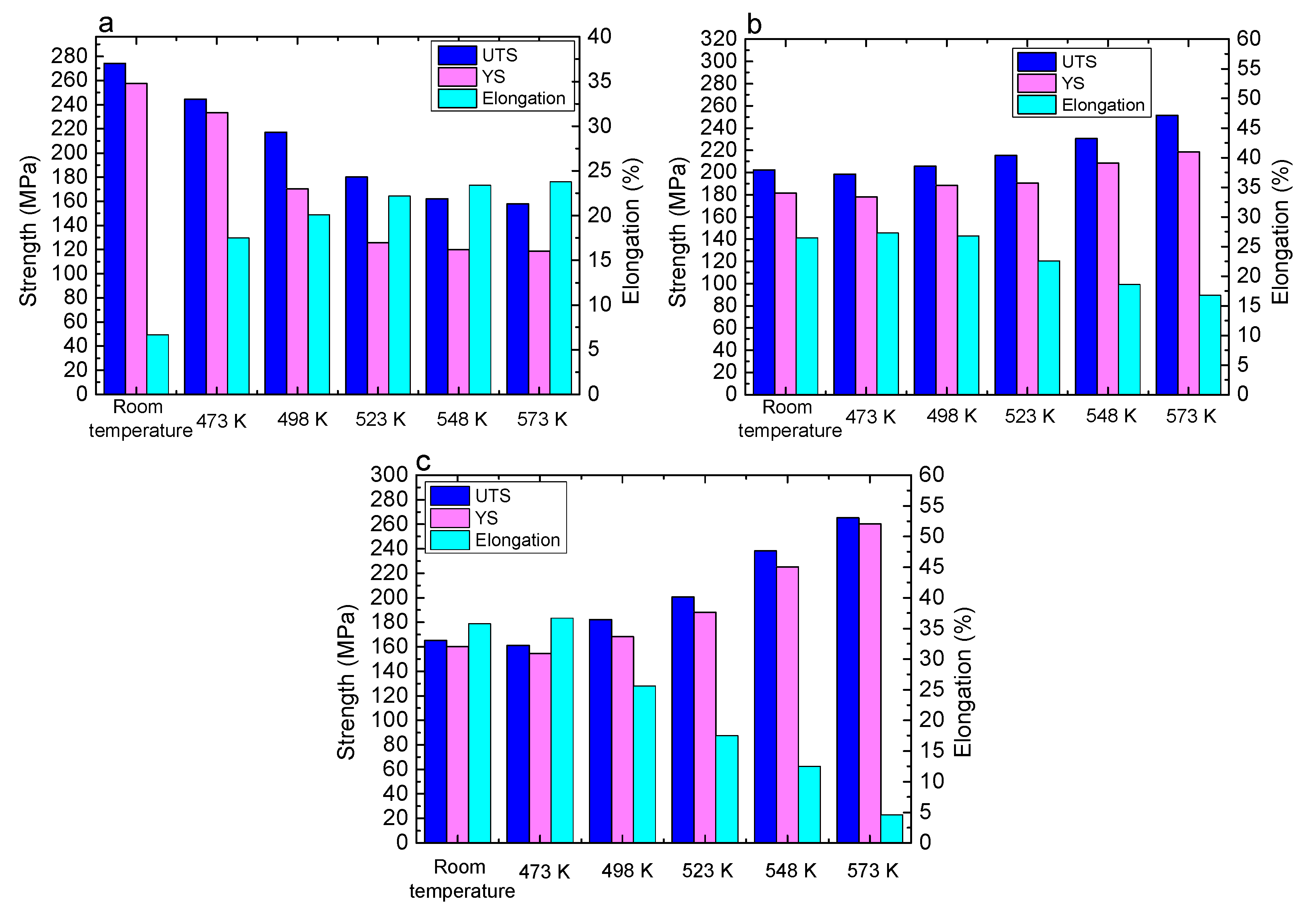
Figure 11 shows the mechanical properties of the three alloys at different conditions. In Mg-5Li-3Al-2Zn-0.2Y (see
Figure 11a), during annealing (473 K–573 K), the strength revealed the declining trend with increasing temperature. The ultimate tensile strength decreased from the original value of 274.3 MPa (at room temperature) to 157.8 MPa (at 573 K). In contrast, the elongation increased from 6.7% to 23.8%. This variation should be ascribed to the recrystallization softening effect. In the recrystallization process, the large number of dislocations was absorbed to effectively promote the grain boundary diffusion in which no strengthening factor was generated simultaneously. Furthermore, the reduction of dislocation density also contributed to enhancing the crystal slips. Thereby, the tensile properties of the Mg-5Li-3Al-2Zn-0.2Y alloy exhibited the weakening law during annealing treatment.
In Mg-8Li-3Al-2Zn-0.2Y (see
Figure 11b), the tensile properties of the alloy were strengthened by increasing the temperature during annealing. The ultimate tensile strength increased from the original value of 202.2 MPa (at room temperature) to 251.6 MPa (at 573 K). At the same time, the elongation decreased from 26.5% to 16.8%. There existed a similar variation law arising in the tensile properties of the Mg-11Li-3Al-2Zn-0.2Y alloy (see
Figure 11c), wherein the ultimate tensile strength increased from the original value of 182.3 MPa (at room temperature) to 265.3 MPa (at 573 K), and simultaneously the elongation decreased from 25.6% to 4.5%.
This phenomenon whereby the annealing process improved the properties of the alloy should be ascribed to the effect of solid solution strengthening. This solid solution mechanism could be measured by the interaction energy between the dissolved atom and dislocation, as shown in Equation (5). Equation (5) could be further simplified as Equation (6).
where
is the interaction energy,
is the normal stress on the dislocation,
is the poisson ratio,
is the shear modulus,
is the burgers vector,
is the distance between the point defect and dislocation,
is the lattice volume change,
is the radius of the matrix atom,
is the radius of the dissolved atom, and
is
y/
r.
As indicated by Equations (5) and (6), the attraction between the dissolved atoms and dislocations will occur when
E is positive. In contrast, the repulsion between the dissolved atoms and dislocations will occur when
E is negative. Furthermore,
E was inversely proportional to
r, indicating that the smaller the distance between the point defect and dislocation, the higher the value of |
E|. In addition, the strengthening mechanism can be explained by the dissolved atoms, which had a “pinning” effect on the dislocations. The theory was that the dissolved atoms were segregated around the dislocation line because of the elastic interaction between the dissolved atoms and dislocations. Thereby, the dislocation movements changed the equilibrium position of dissolved atoms, which gave rise to enhancing the system energy and restraining dislocation movements. This restraining extent could be defined by pinning stress (
τ), as shown in Equations (7) and (8).
where
is the pinning stress,
is the highest force on the dislocation, and
is the radius of edge dislocation.
3.3. Establishing Mathematical Relationship
To quantitatively measure the strengthening effect, the relationship between the microstructure and mechanical properties should be proposed and determined. Hence, the fitting method with a high accuracy is used to determine their relationship models. The fitting accuracy is measured in terms of the relative error (
RE), the mean relative error (
MRE), and the mean square error (
MS), as shown in Equation (9). The strength and AlLi volume fraction at different temperatures are listed in
Table 2.
where
is the experimental value,
is the calculated value from the model, and
is the experimental number.
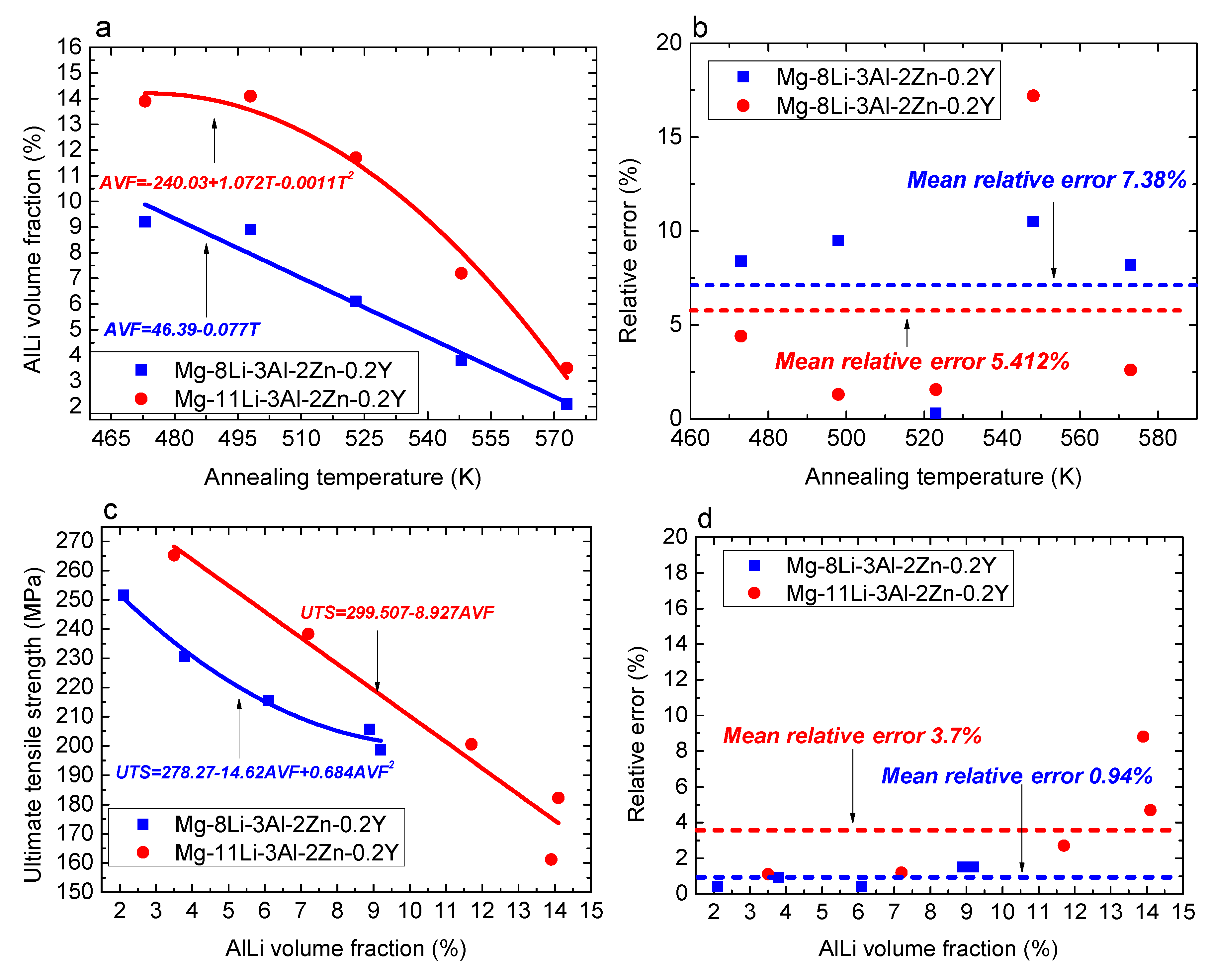
Figure 12 shows the fitting relationship results of Mg-8Li-3Al-2Zn-0.2Y and Mg-11Li-3Al-2Zn-0.2Y. As indicated in
Figure 12a, based on the evolution characteristic between the AlLi volume fraction and annealing temperature, the fitting method adopted the linear fitting and quadratic polynomial fitting, respectively. The fitting equations of Mg-8Li-3Al-2Zn-0.2Y and Mg-11Li-3Al-2Zn-0.2Y are shown in Equation (10). According to the fitting accuracy (see
Figure 12b), the mean relative error for Mg-8Li-3Al-2Zn-0.2Y and Mg-11Li-3Al-2Zn-0.2Y was 7.38% and 5.412%, respectively. The fitting results indicated that the effect of the annealing temperature on the dissolution number of AlLi phase in Mg-8Li-3Al-2Zn-0.2Y was relatively more remarkable than that in Mg-11Li-3Al-2Zn-0.2Y. Based on the analysis, α-Mg matrix occupied a certain amount of area in the Mg-8Li-3Al-2Zn-0.2Y alloy. Furthermore, a large amount of AlLi phase was distributed in the β-Li matrix instead of the α-Mg matrix. Thereby, compared with Mg-11Li-3Al-2Zn-0.2Y, the AlLi phase dissolution in Mg-8Li-3Al-2Zn-0.2Y was more sensitive to the annealing temperature.
Figure 12c shows the fitting results between the ultimate tensile strength and AlLi volume fraction, wherein the fitting equations are shown in Equation (11). The corresponding mean relative error for Mg-8Li-3Al-2Zn-0.2Y and Mg-11Li-3Al-2Zn-0.2Y was 0.94% and 3.7%, respectively, as shown in
Figure 12d. Compared with the previous fitting results, the mean relative error for Mg-8Li-3Al-2Zn-0.2Y and Mg-11Li-3Al-2Zn-0.2Y decreased by 6.44% and 1.712%, respectively, indicating that the fitting accuracy increased. It also indicated that the relationship between the strength and dissolution number was much closer.
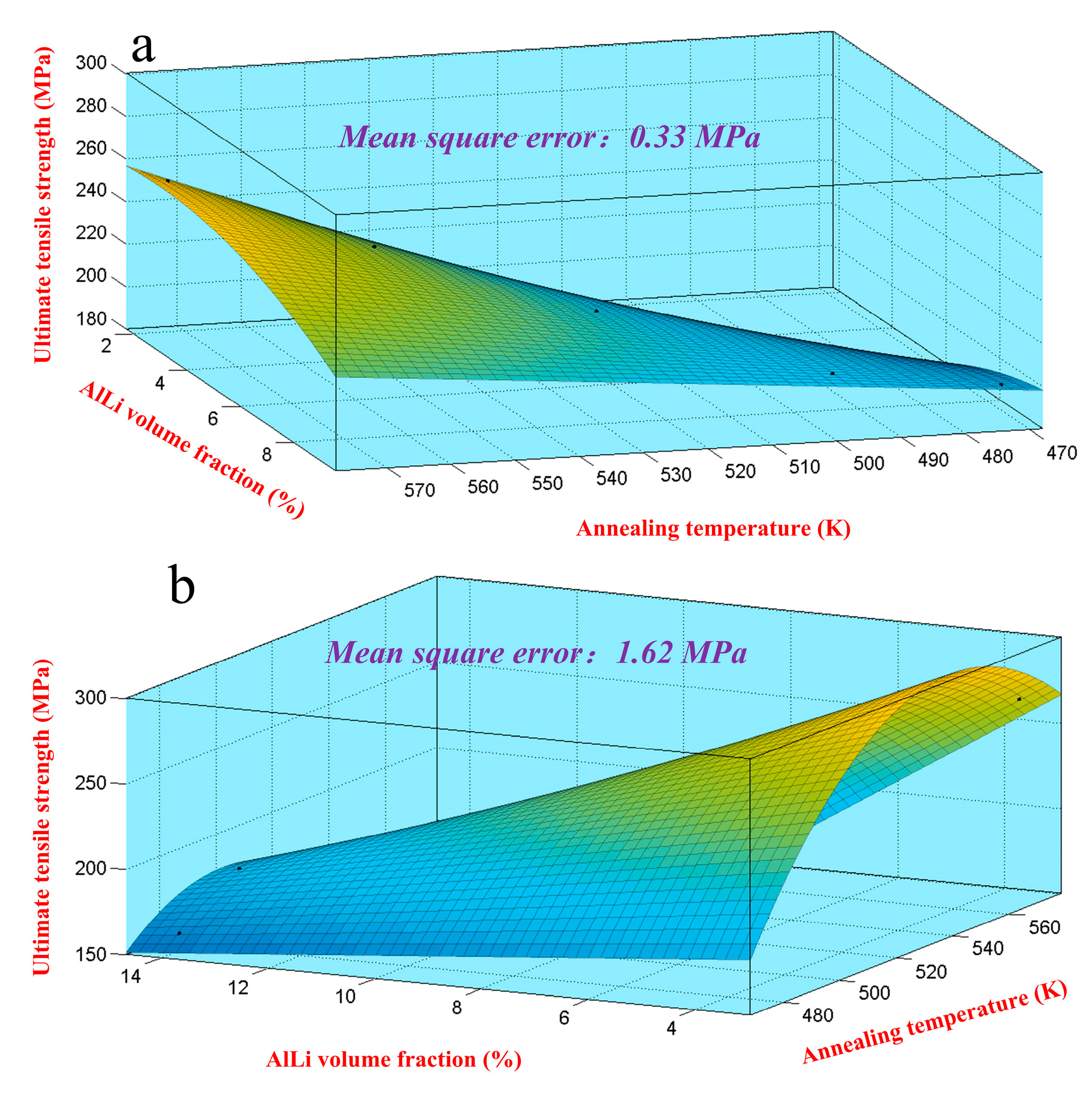
To accurately make a quantitative analysis for the strength on multi-condition, the relationship between the ultimate tensile strength, dissolution number, and annealing temperature should be proposed and determined. The fitting map of ultimate tensile strength under different volume fractions and temperatures is shown in
Figure 13. The corresponding fitting equations are shown in Equation (12). Considering the fitting accuracy, the mean square error (
MS) was calculated by Equation (9). The result manifested that the mean square error for Mg-8Li-3Al-2Zn-0.2Y and Mg-11Li-3Al-2Zn-0.2Y was 0.33 MPa and 1.62 MPa, respectively. Thereby, the fitting accuracy of their relationship models was relatively high.
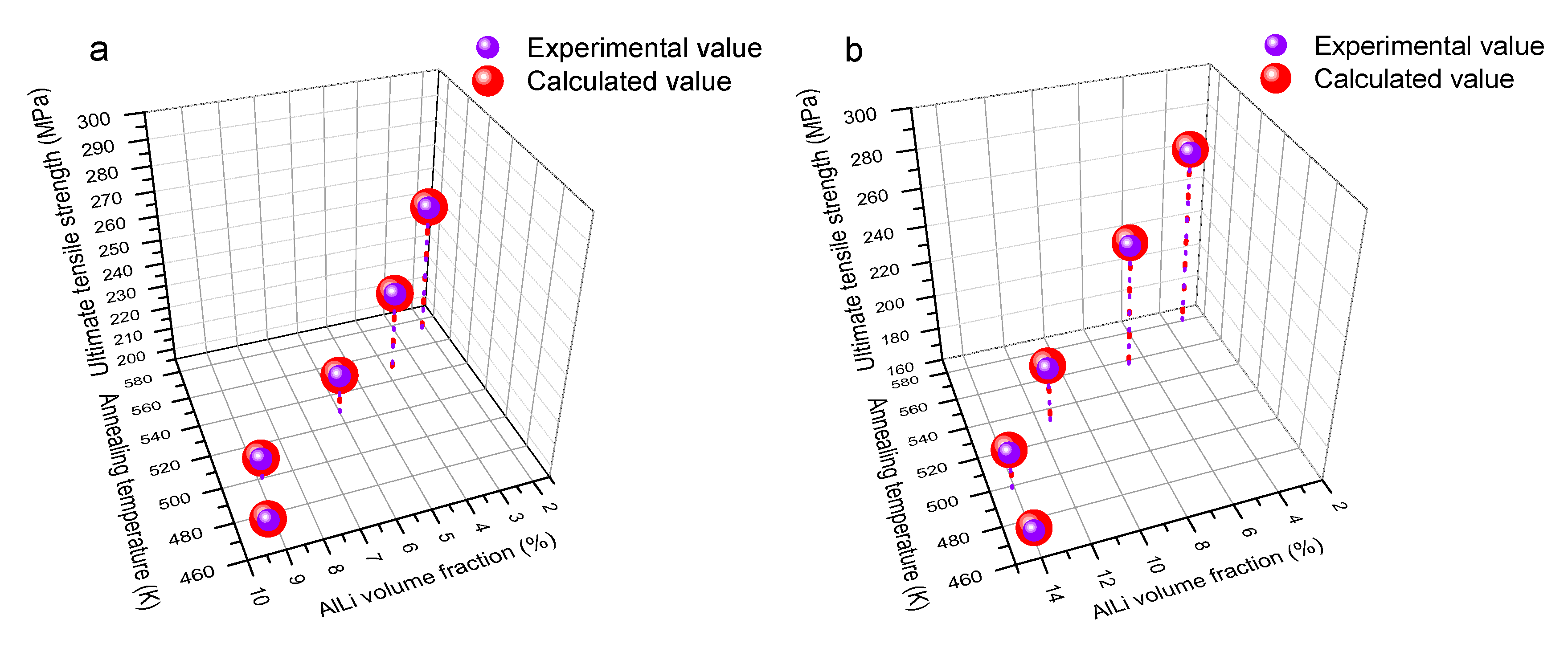
Figure 14 shows the comparison between the experimental value and calculated value of Equation (12). The comparison results also verified the relatively high fitting accuracy on their relationship models.