Methodology for Fast and Facile Characterisation of Carbon-Based Electrodes Focused on Bioelectrochemical Systems Development and Scale Up
Abstract
:1. Introduction
2. Experimental
2.1. Methodology Proposal
2.2. Determination of Electroactive Area
2.3. Determination of Fractal Dimension (Df)
2.4. Method Validation
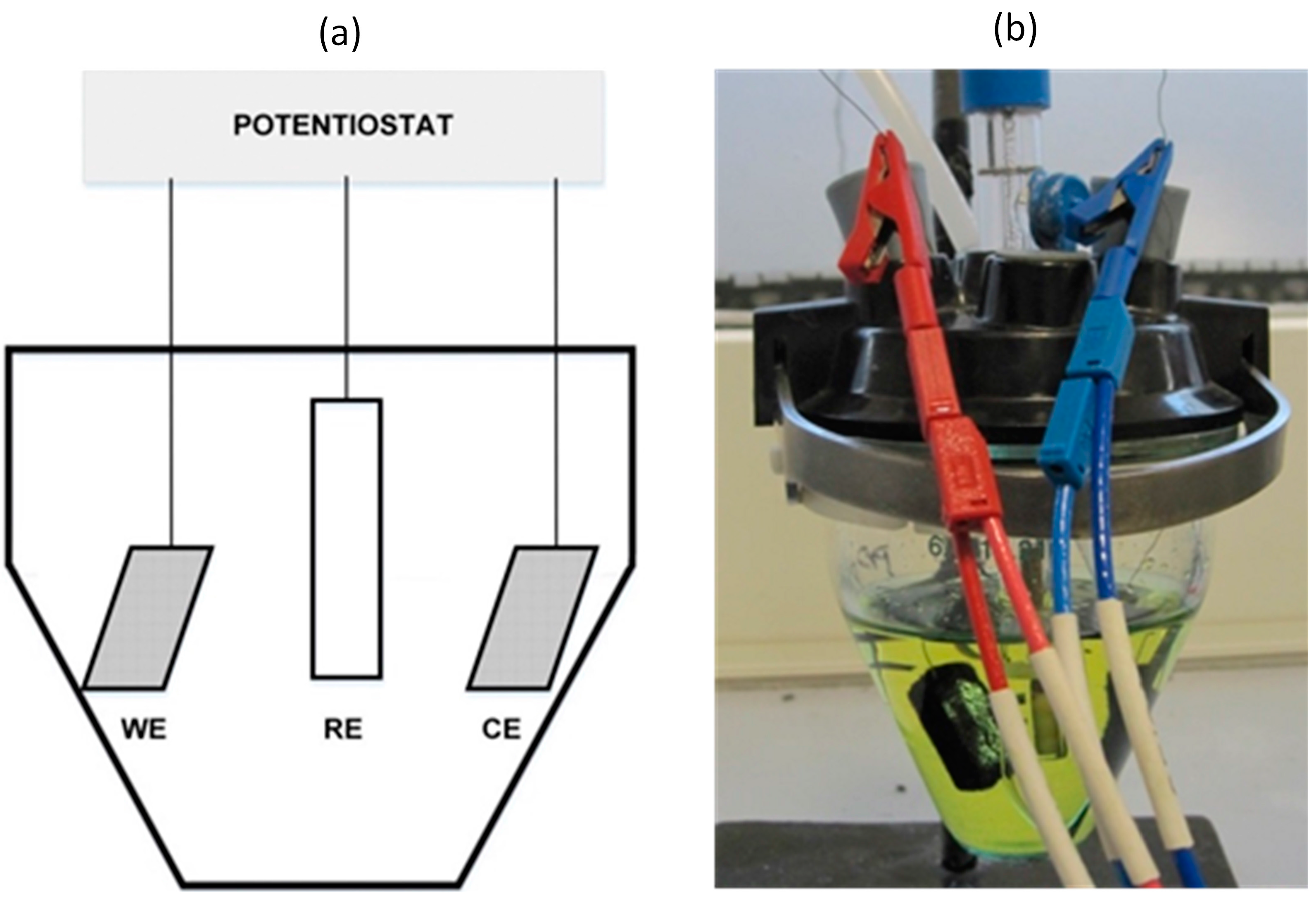
2.5. Cell Set-Up and Instrumentation
3. Results and Discussion
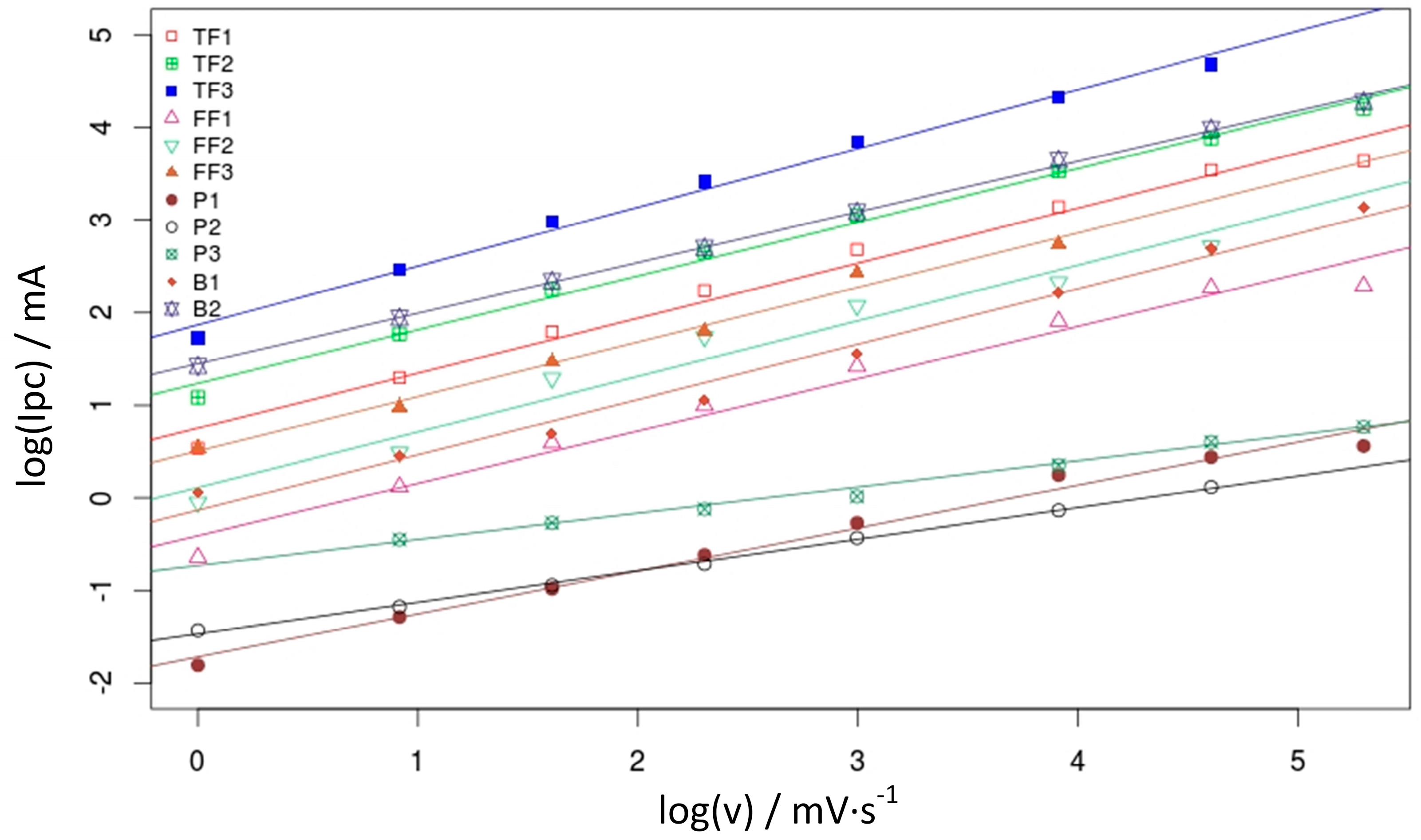
3.1. Estimation of Electroactive Area
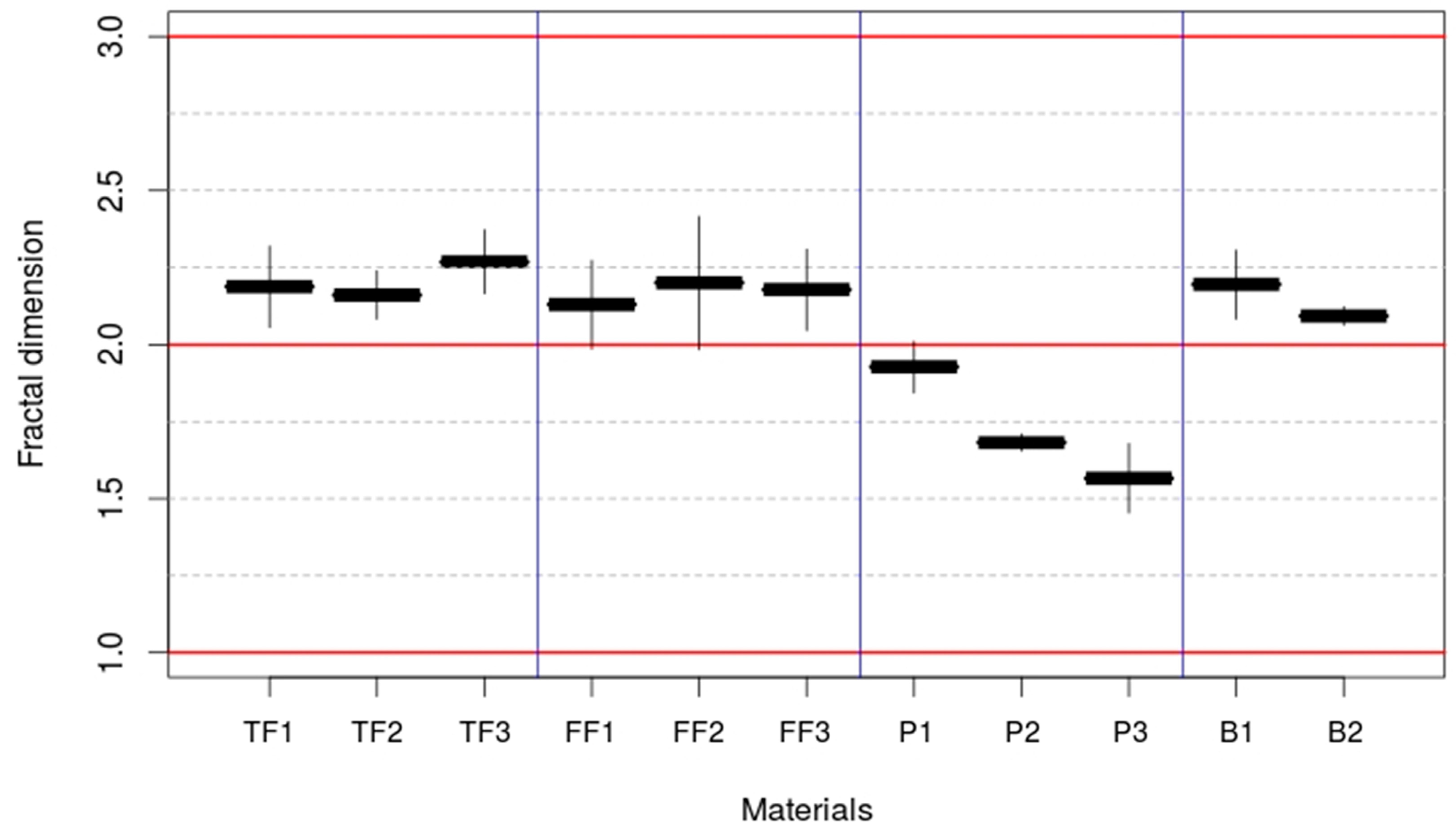
3.2. Determination of the Fractal Dimension
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Butti, S.K.; Velvizhi, G.; Sulonen, M.L.K.; Haavisto, J.M.; Koroglu, E.O.; Cetinkaya, A.Y.; Singh, S.; Arya, D.; Modestra, J.A.; Krishna, K.V.; et al. Microbial electrochemical technologies with the perspective of harnessing bioenergy: Maneuvering towards upscaling. Renew. Sustain. Energy Rev. 2016, 53, 462–476. [Google Scholar] [CrossRef]
- Escapa, A.; San-Martín, M.I.; Mateos, R.; Morán, A. Scaling-up of membraneless microbial electrolysis cells (MECs) for domestic wastewater treatment: Bottlenecks and limitations. Bioresour. Technol. 2015, 180, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Modin, O.; Wang, X.; Wu, X.; Rauch, S.; Fedje, K.K. Bioelectrochemical recovery of Cu, Pb, Cd, and Zn from dilute solutions. J. Hazard. Mater. 2012, 235–236, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Webster, D.P.; TerAvest, M.A.; Doud, D.F.R.; Chakravorty, A.; Holmes, E.C.; Radens, C.M.; Sureka, S.; Gralnick, J.A.; Angenent, L.T. An arsenic-specific biosensor with genetically engineered Shewanella oneidensis in a bioelectrochemical system. Biosens. Bioelectron. 2014, 62, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.W.; Ross, D.E.; Fichot, E.B.; Norman, R.S.; May, H.D. Electrosynthesis of commodity chemicals by an autotrophic microbial community. Appl. Environ. Microbiol. 2012, 78, 8412–8420. [Google Scholar] [CrossRef] [PubMed]
- Heidrich, E.S.; Dolfing, J.; Scott, K.; Edwards, S.R.; Jones, C.; Curtis, T.P. Production of hydrogen from domestic wastewater in a pilot-scale microbial electrolysis cell. Appl. Microbiol. Biotechnol. 2013, 97, 6979–6989. [Google Scholar] [CrossRef] [PubMed]
- Ieropoulos, I.A.; Stinchcombe, A.; Gajda, I.; Forbes, S.; Merino-Jimenez, I.; Pasternak, G.; Sanchez-Herranz, D.; Greenman, J. Pee power urinal—Microbial fuel cell technology field trials in the context of sanitation. Environ. Sci. Water Res. Technol. 2016, 2, 336–343. [Google Scholar] [CrossRef]
- ElMekawy, A.; Srikanth, S.; Vanbroekhoven, K.; De Wever, H.; Pant, D. Bioelectro-catalytic valorization of dark fermentation effluents by acetate oxidizing bacteria in bioelectrochemical system (BES). J. Power Sources 2014, 262, 183–191. [Google Scholar] [CrossRef]
- Rabaey, K. Bioelectrochemical Systems: From Extracellular Electron Transfer to Biotechnological Application; IWA Publishing: London, UK, 2010. [Google Scholar]
- Escapa, A.; Mateos, R.; Martínez, E.J.; Blanes, J. Microbial electrolysis cells: An emerging technology for wastewater treatment and energy recovery. From laboratory to pilot plant and beyond. Renew. Sustain. Energy Rev. 2016, 55, 942–956. [Google Scholar] [CrossRef]
- Cui, H.-F.; Du, L.; Guo, P.-B.; Zhu, B.; Luong, J.H.T. Controlled modification of carbon nanotubes and polyaniline on macroporous graphite felt for high-performance microbial fuel cell anode. J. Power Sources 2015, 283, 46–53. [Google Scholar] [CrossRef]
- Bagotsky, V.S. Fundamentals of Electrochemistry; John Wiley & Sons: Hoboken, NJ, USA, 2005; Volume 44. [Google Scholar]
- Guo, K.; Prévoteau, A.; Patil, S.A.; Rabaey, K. Engineering electrodes for microbial electrocatalysis. Curr. Opin. Biotechnol. 2015, 33, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Strømme, M.; Niklasson, G.A.; Granqvist, C.G. Voltammetry on fractals. Solid State Commun. 1995, 96, 151–154. [Google Scholar] [CrossRef]
- Kant, R.; Singh, M.B. Generalization of Randles-Ershler admittance for an arbitrary topography electrode: Application to random finite fractal roughness. Electrochim. Acta 2015, 163, 310–322. [Google Scholar] [CrossRef]
- Pacios, M.; del Valle, M.; Bartroli, J.; Esplandiu, M.J. Electrochemical behavior of rigid carbon nanotube composite electrodes. J. Electroanal. Chem. 2008, 619–620, 117–124. [Google Scholar] [CrossRef]
- Neves, S.; Fonseca, C.P. Determination of fractal dimension of polyaniline composites by SAXS and electrochemical techniques. Electrochemi. Commun. 2001, 3, 36–43. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications; Wiley: New York, NY, USA, 1980; Volume 2. [Google Scholar]
- Zoski, C.G.C. Handbook of Electrochemistry; Elsevier: Amsterdam, The Netherlands, 2007; Volume 5. [Google Scholar]
- Panah, N.B.; Mahjani, M.G.; Jafarian, M. Correlation between irregular surface geometry and certain electrochemical quantities in poly-ortho-aminophenol. Progress Org. Coat. 2009, 64, 33–38. [Google Scholar] [CrossRef]
- Ammar, Y.; Swailes, D.; Bridgens, B.; Chen, J. Influence of surface roughness on the initial formation of biofilm. Surface Coat. Technol. 2015, 284, 410–416. [Google Scholar] [CrossRef]
- Mandelbrot, B.B. The Fractal Geometry of Nature/Revised and Enlarged Edition; WH Freeman and Co.: New York, NY, USA, 1983; p. 495. [Google Scholar]
- Avnir, D. Fractal Approach to Heterogeneous Chemistry; Wiley: New York, NY, USA, 1989. [Google Scholar]
- Kant, R. Theory for staircase voltammetry and linear scan voltammetry on fractal electrodes: Emergence of anomalous Randles–Sevik behavior. Electrochim. Acta 2013, 111, 223–233. [Google Scholar]
- Strømme, M.; Niklasson, G.A.; Granqvist, C.G. Determination of fractal dimension by cyclic I-V studies: The Laplace-transform method. Phys. Rev. B 1995, 52, 14192–14197. [Google Scholar] [CrossRef]
- Go, J.-Y.; Pyun, S.-I.; Hahn, Y.-D. A study on ionic diffusion towards self-affine fractal electrode by cyclic voltammetry and atomic force microscopy. J. Electroanal. Chem. 2003, 549, 49–59. [Google Scholar] [CrossRef]
- Dhar, B.R.; Gao, Y.; Yeo, H.; Lee, H.-S. Separation of competitive microorganisms using anaerobic membrane bioreactors as pretreatment to microbial electrochemical cells. Bioresour. Technol. 2013, 148, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.E.G.; Davies, T.J.; de B. Baynes, N.; Nichols, R.J. The electrochemical characterisation of graphite felts. J. Electroanal. Chem. 2015, 747, 29–38. [Google Scholar] [CrossRef]
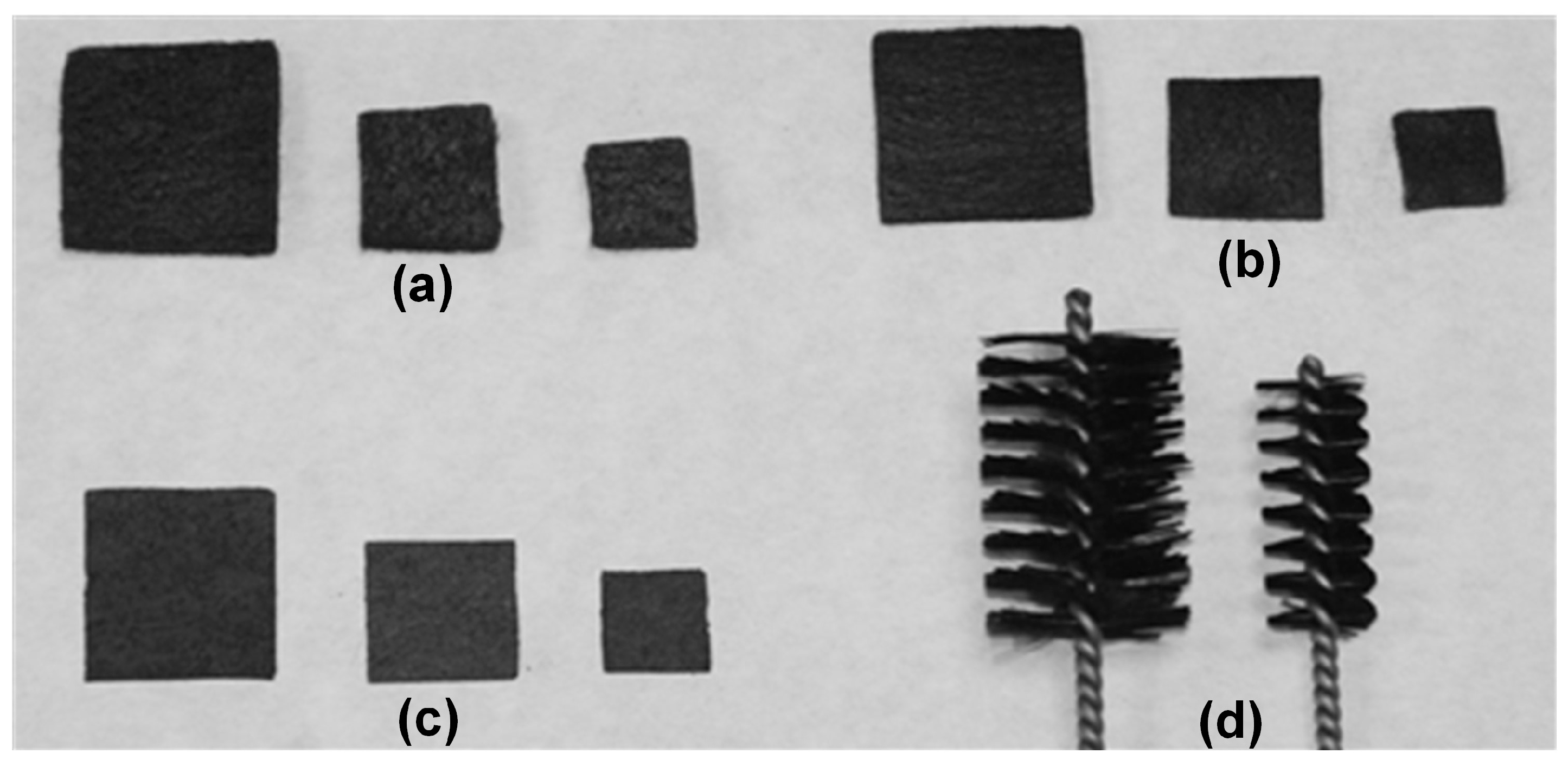


| Code | Material | Size | Apparent Surface (cm2) |
|---|---|---|---|
| TF1 | Thick carbon felt | 1 cm width; 1 cm length; 5 mm thickness | 1 |
| TF2 | Thick carbon felt | 1.5 cm width; 1.5 cm length; 5 mm thickness | 2.25 |
| TF3 | Thick carbon felt | 2 cm width; 2 cm length; 5 mm thickness | 4 |
| FF1 | Fine carbon felt | 1 cm width; 1 cm length; 2 mm thickness | 1 |
| FF2 | Fine carbon felt | 1.5 cm width; 1.5 cm length; 2 mm thickness | 2.25 |
| FF3 | Fine carbon felt | 2 cm width; 2 cm length; 2 mm thickness | 4 |
| P1 | Carbon paper | 1 cm width; 1 cm length | 1 |
| P2 | Carbon paper | 1.5 cm width; 1.5 cm length | 2.25 |
| P3 | Carbon paper | 2 cm width; 2 cm length | 4 |
| B1 | Carbon brush | 1 cm diameter; 2.5 cm height | 1.87 |
| B2 | Carbon brush | 2 cm diameter; 3 cm height | 5.33 |
| Electrodes | Mean Ohmic Drop (Ω) | Standard Error |
|---|---|---|
| TF1 | 22.50 | 0.010 |
| TF2 | 16.65 | 0.011 |
| TF3 | 10.09 | 0.012 |
| FF1 | 34.08 | 0.011 |
| FF2 | 18.05 | 0.011 |
| FF3 | 20.02 | 0.013 |
| P1 | 18.29 | 0.011 |
| P2 | 14.05 | 0.010 |
| P3 | 9.81 | 0.012 |
| B1 | 13.71 | 0.009 |
| B2 | 8.16 | 0.010 |
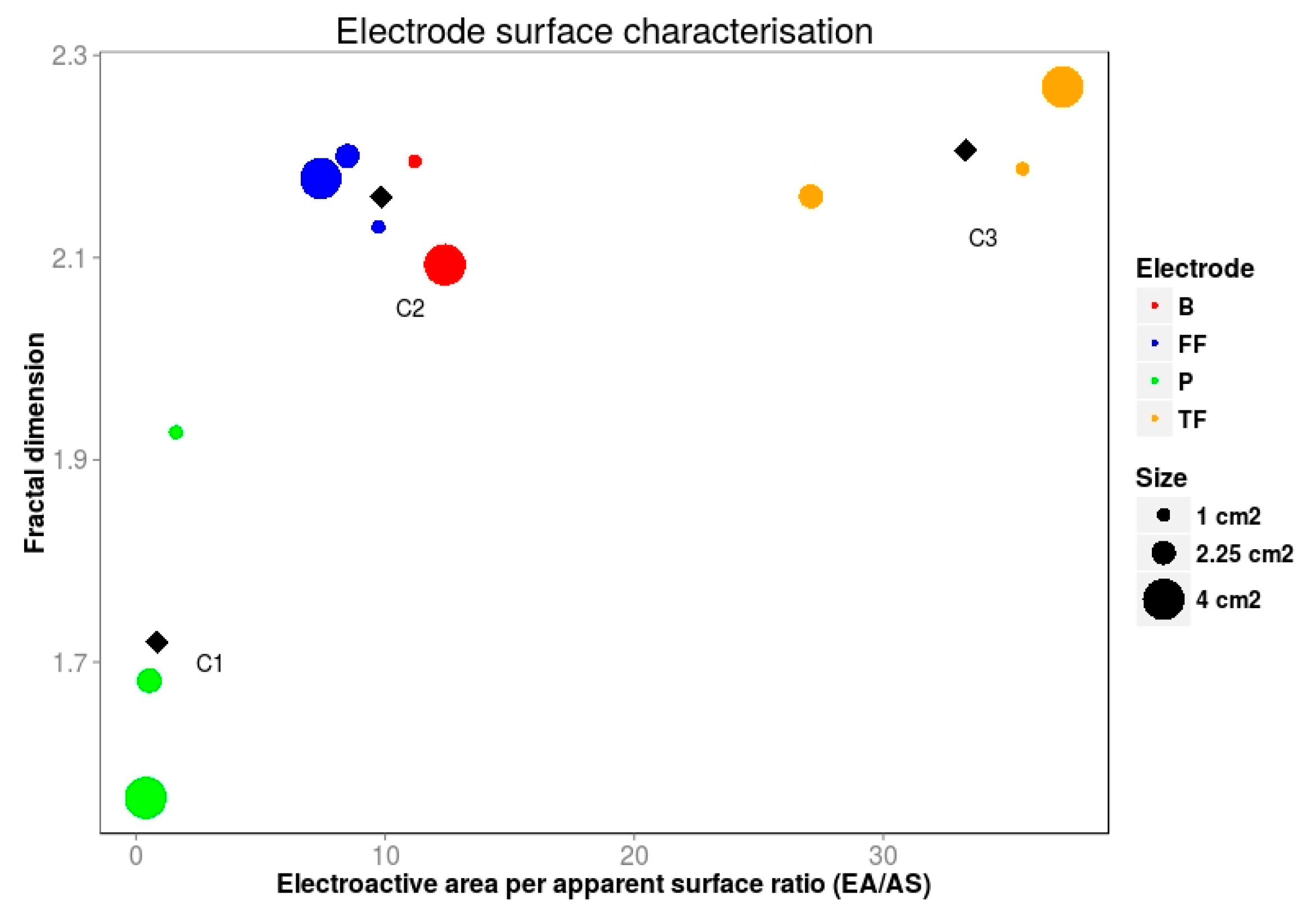
| Material | Slope | Electroactive Area (cm2) | Electroactive Area per Apparent Surface Area Ratio (EA/AS) |
|---|---|---|---|
| TF1 | 9.44 × 10−2 | 37.16 | 37.2 |
| TF2 | 1.55 × 10−1 | 61.03 | 27.1 |
| TF3 | 3.62 × 10−1 | 142.47 | 35.6 |
| FF1 | 2.47 × 10−2 | 9.73 | 9.73 |
| FF2 | 4.85 × 10−2 | 19.10 | 8.49 |
| FF3 | 7.53 × 10−2 | 29.66 | 7.42 |
| P1 | 4.07 × 10−3 | 1.60 | 1.60 |
| P2 | 3.11 × 10−3 | 1.22 | 0.54 |
| P3 | 3.91 × 10−3 | 1.54 | 0.39 |
| B1 | 5.31 × 10−2 | 20.91 | 11.2 |
| B2 | 1.68 × 10−1 | 65.97 | 12.4 |
| Electrode | Experimental Range υ (mV·s−1) | Fractal Parameter (α) | Correlation Coefficient (R2) |
|---|---|---|---|
| TF1 | 1–200 | 0.594 ± 0.066 | 0.981 |
| TF2 | 1–200 | 0.580 ± 0.039 | 0.993 |
| TF3 | 1–100 | 0.634 ± 0.052 | 0.992 |
| FF1 | 1–200 | 0.565 ± 0.072 | 0.975 |
| FF2 | 1–200 | 0.600 ± 0.108 | 0.961 |
| FF3 | 1–50 | 0.589 ± 0.066 | 0.989 |
| P1 | 1–200 | 0.464 ± 0.042 | 0.987 |
| P2 | 1–100 | 0.341 ± 0.014 | 0.998 |
| P3 | 2.5–200 | 0.283 ± 0.026 | 0.998 |
| B1 | 1–200 | 0.598 ± 0.056 | 0.986 |
| B2 | 1–200 | 0.547 ± 0.015 | 0.999 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateos, R.; Alonso, R.M.; Escapa, A.; Morán, A. Methodology for Fast and Facile Characterisation of Carbon-Based Electrodes Focused on Bioelectrochemical Systems Development and Scale Up. Materials 2017, 10, 79. https://doi.org/10.3390/ma10010079
Mateos R, Alonso RM, Escapa A, Morán A. Methodology for Fast and Facile Characterisation of Carbon-Based Electrodes Focused on Bioelectrochemical Systems Development and Scale Up. Materials. 2017; 10(1):79. https://doi.org/10.3390/ma10010079
Chicago/Turabian StyleMateos, Raúl, Raúl M. Alonso, Adrián Escapa, and Antonio Morán. 2017. "Methodology for Fast and Facile Characterisation of Carbon-Based Electrodes Focused on Bioelectrochemical Systems Development and Scale Up" Materials 10, no. 1: 79. https://doi.org/10.3390/ma10010079
APA StyleMateos, R., Alonso, R. M., Escapa, A., & Morán, A. (2017). Methodology for Fast and Facile Characterisation of Carbon-Based Electrodes Focused on Bioelectrochemical Systems Development and Scale Up. Materials, 10(1), 79. https://doi.org/10.3390/ma10010079










