Supercritical CO2-Assisted Spray Drying of Strawberry-Like Gold-Coated Magnetite Nanocomposites in Chitosan Powders for Inhalation
Abstract
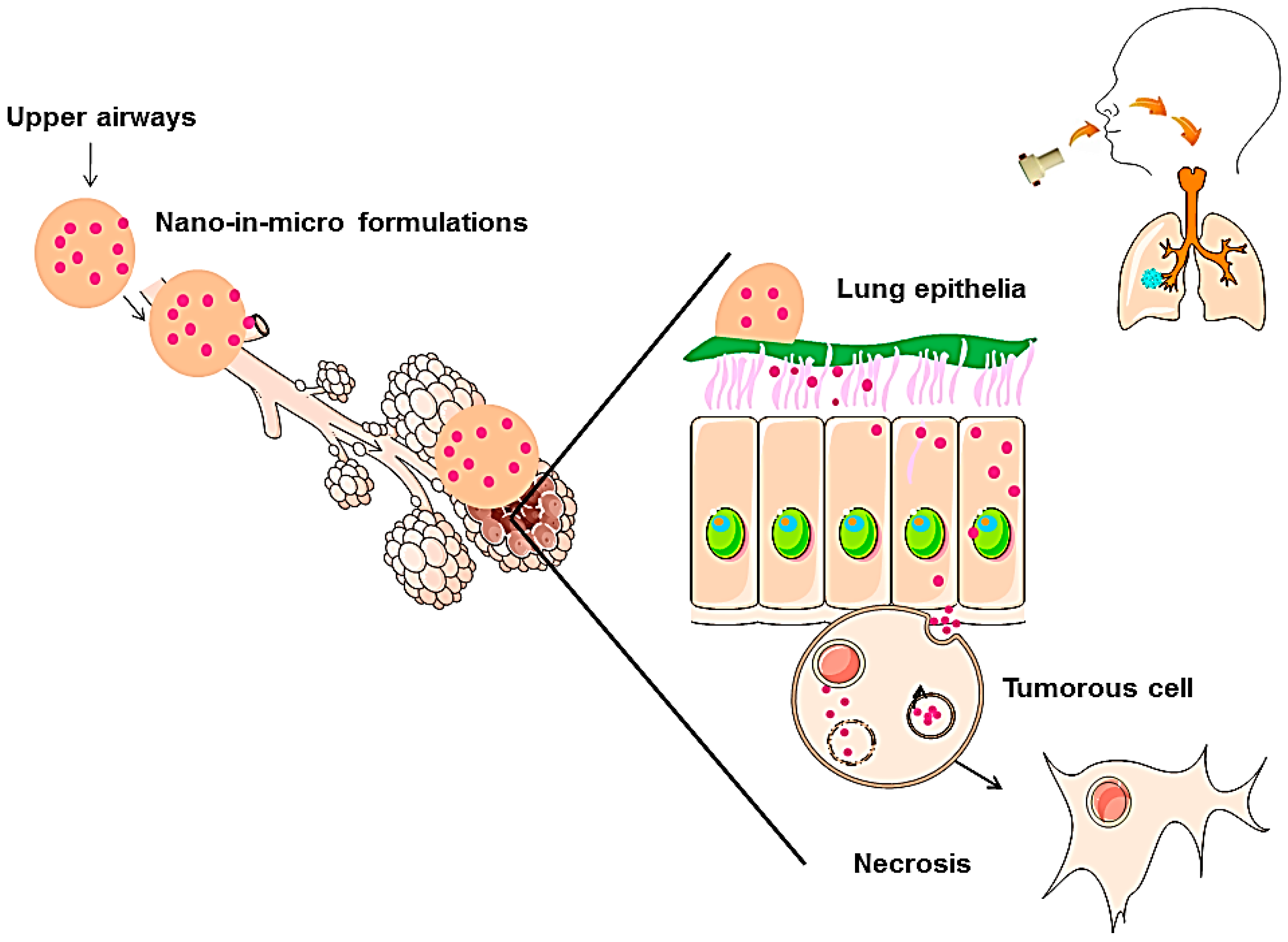
:1. Introduction
2. Results and Discussion
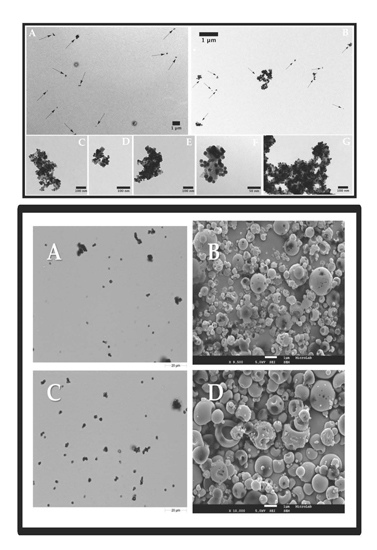
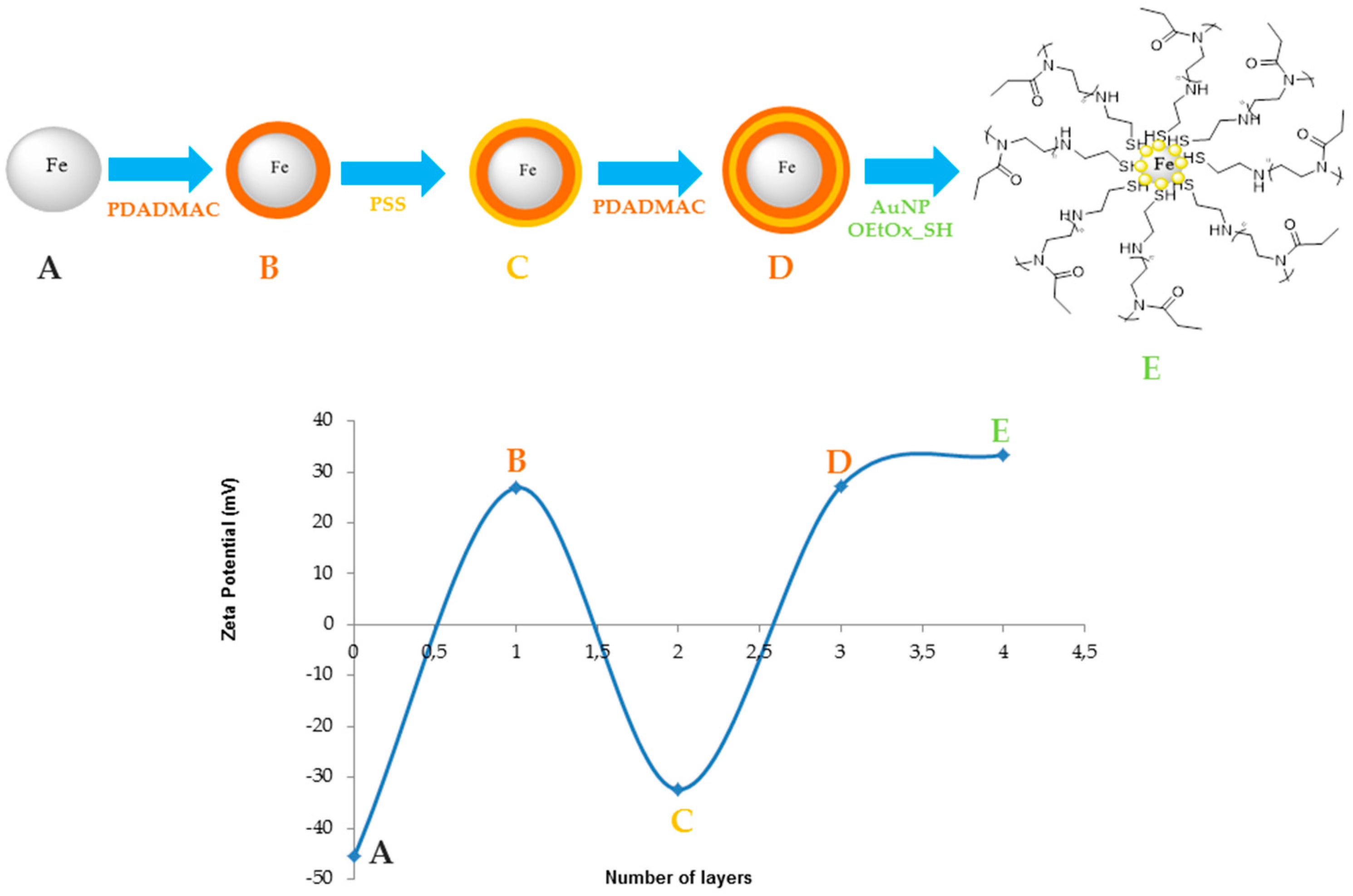
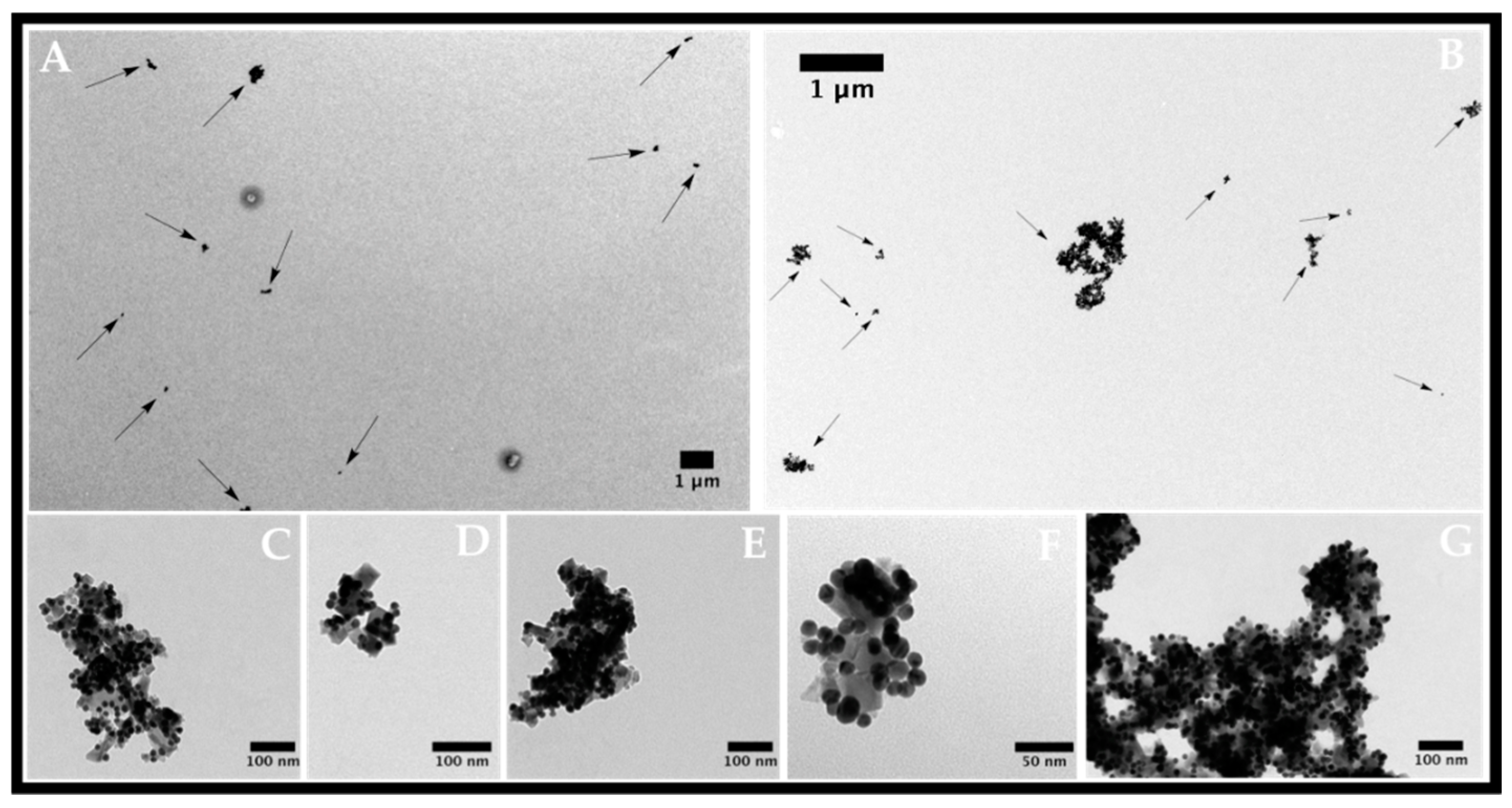
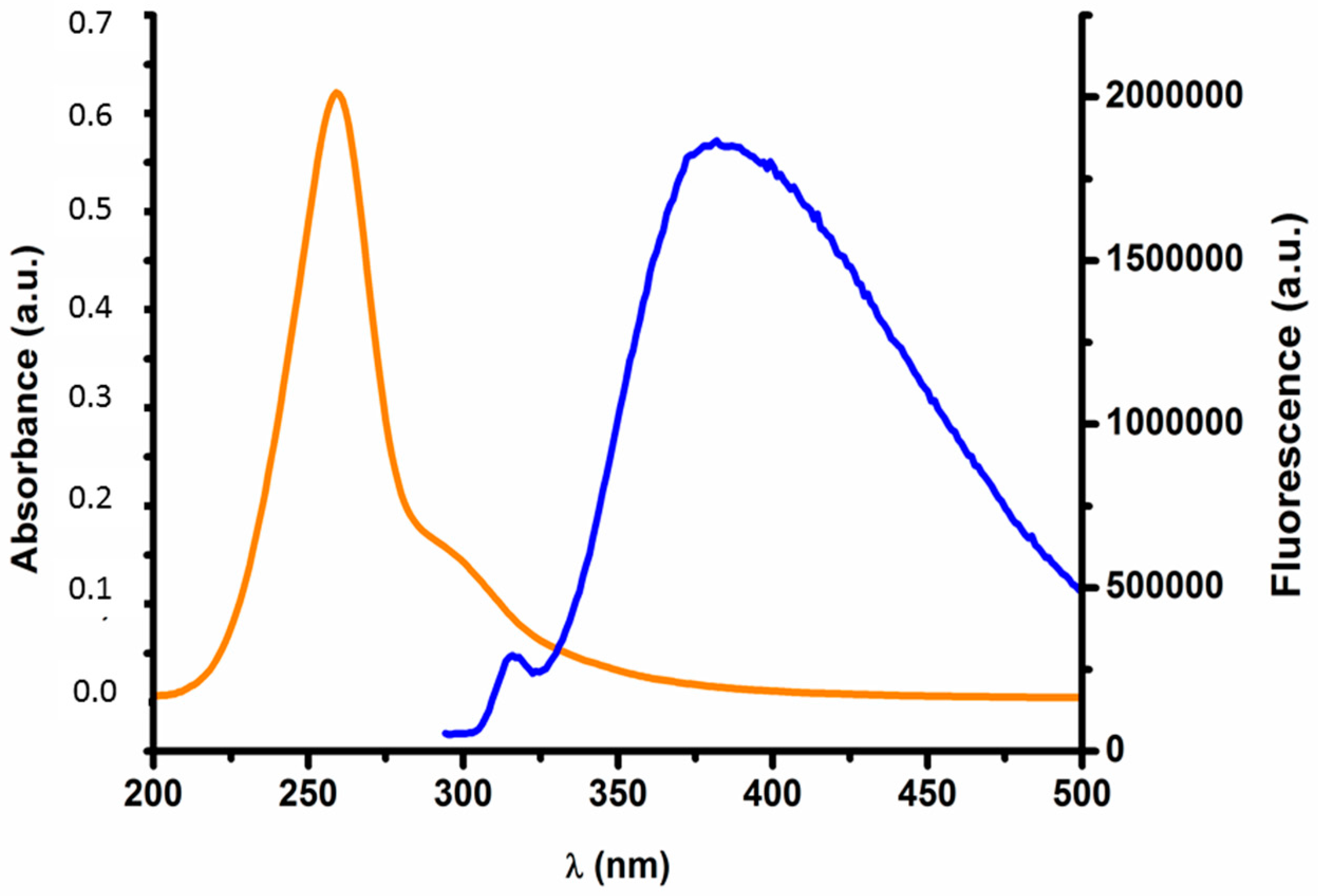
2.1. Nanoparticle Characterization
2.2. Characterization of Nano-in-Micro Formulations
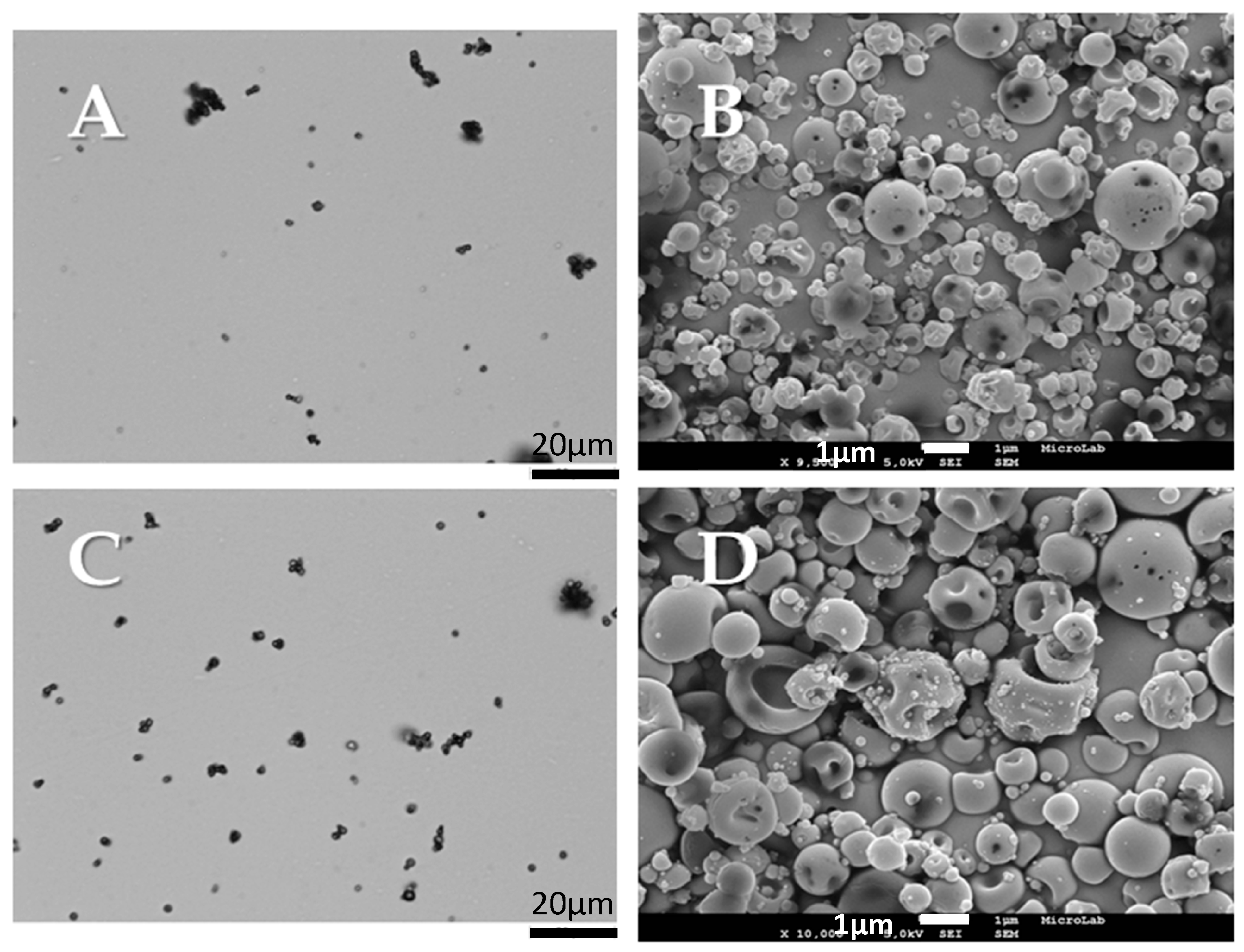
2.2.1. Morphological and Physical-Chemical Properties
2.2.2. Aerodynamic Performance
2.2.3. Entrapment Efficiency
2.2.4. In Vitro Cumulative Studies
3. Materials and Methods
3.1. Materials
3.2. Synthesis of the Gold Coated Magnetite (Fe@Au)
3.3. Synthesis of the Living Oligooxazoline
3.3.1. End-Capping of Living Oligo(2-ethyl-2-oxazoline)
3.3.2. Nanocomposite Functionalization with OPOX-SH
3.4. Nanoparticle Characterization
3.5. Production and Characterization of the Nano-in-Micro Formulations
3.5.1. SASD Apparatus: Particle Production
3.5.2. Particle Size Analysis and Morphological Assessment
Morphology G3 Analysis
Scanning Electron Microscopy
Fourier Transformed Infrared Spectra
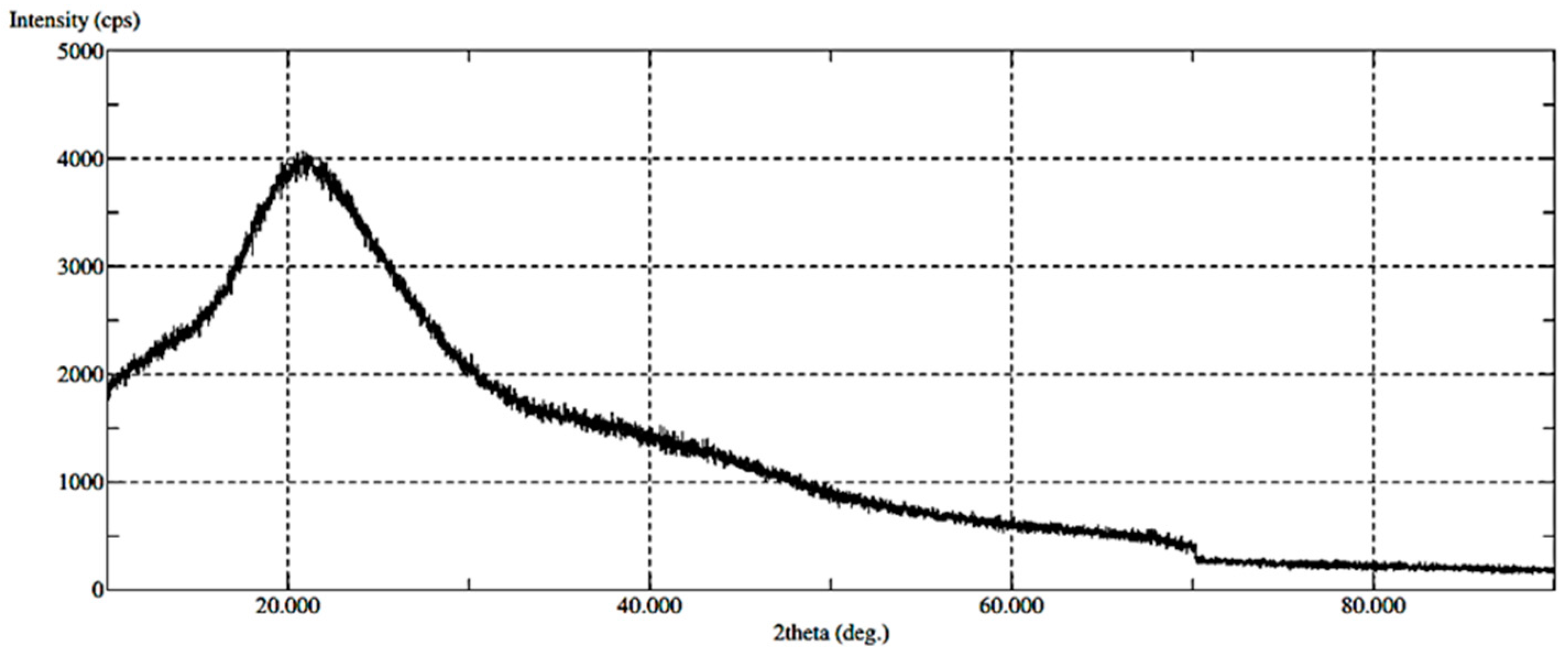
X-ray Diffraction (XRD)
Karl Fischer Coulometric Titration
3.5.3. Aerodynamic Performance
3.5.4. Entrapment Efficiency
3.5.5. In Vitro Cumulative Release Studies
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SASD | Supercritical Assisted Spray Drying |
| scCO2 | Supercritical Carbon Dioxide |
| FT-IR | Fourier Transform Infra Red |
| TEM | Transmission Electron Microscopy |
| SEM | Scanning Electron Microscopy |
| DDS | Drug Delivery System |
| CHT | Chitosan |
| NP | Nanoparticles |
| IBP | Ibuprofen |
| PDADMAC | Poly-diallyldimethylammonium chloride |
| PSS | Poly-sodium 4-styrenesulfonate |
| FPF | Fine Particle Fraction |
| EF | Emitted Fraction |
| RF | Respirable Fraction |
| MMAD | Mass Median Aerodynamic Diameter |
| GSD | Geometric Standard Deviation |
| ACI | Anderson Cascade Impactor |
References
- Sanders, M. Pulmonary Drug Delivery: An Historical Overview. In Controlled Pulmonary Drug Delivery; Springer: New York, NY, USA, 2011; pp. 51–73. [Google Scholar]
- Provencio, M.; Isla, D.; Sánchez, A.; Cantos, B. Inoperable stage III non-small cell lung cancer: Current treatment and role of vinorelbine. J. Thorac. Dis. 2011, 3, 197–204. [Google Scholar] [PubMed]
- Bailey, M.M.; Berkland, C.J. Nanoparticle formulations in pulmonary drug delivery. Med. Res. Rev. 2009, 29, 196–212. [Google Scholar] [CrossRef] [PubMed]
- Buttini, F.; Colombo, P.; Rossi, A.; Sonvico, F.; Colombo, G. Particles and powders: Tools of innovation for non-invasive drug administration. J. Control. Release 2012, 161, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Odziomek, M.; Sosnowski, T.R.; Gradoń, L. Conception, preparation and properties of functional carrier particles for pulmonary drug delivery. Int. J. Pharm. 2012, 433, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Patil, J.S.; Sarasija, S. Pulmonary drug delivery strategies: A concise, systematic review. Lung India 2012, 29, 44–49. [Google Scholar] [PubMed]
- Patel, A.; Patel, M.; Yang, X.; Mitra, A.K. Recent Advances in Protein and Peptide Drug Delivery—A Special Emphasis on Polymeric Nanoparticles. Protein Pept. Lett. 2014, 21, 1102–1120. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Han, G.; Ghosh, P.; Rotello, V.M. Functionalized gold nanoparticles for drug delivery. Nanomedicine 2007, 2, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Credi, A.; Park, C.; Youn, H.; Kim, H.; Noh, T.; Kook, H.; Oh, T.; Park, J.; Kook, Y.H.; et al. Cyclodextrin-covered gold nanoparticles for targeted delivery of an anti-cancer drug. J. Mater. Chem. 2009, 19, 2261–2440. [Google Scholar]
- Grenha, A.; Remuñán-López, C.; Carvalho, E.L.S.; Seijo, B. Microspheres containing lipid/chitosan nanoparticles complexes for pulmonary delivery of therapeutic proteins. Eur. J. Pharm. Biopharm. 2008, 69, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.; Rafael, D.; Videira, M.; Ferreira, D.; Sosnik, A.; Sarmento, B. Nanotechnology and pulmonary delivery to overcome resistance in infectious diseases. Adv. Drug Deliv. Rev. 2013, 65, 1816–1827. [Google Scholar] [CrossRef] [PubMed]
- Barnaby, S.N.; Sita, T.L.; Petrosko, S.H.; Stegh, A.H.; Mirkin, C.A. Nanotechnology-Based Precision Tools for the Detection and Treatment of Cancer. Cancer Treat. Res. 2015, 166, 293–322. [Google Scholar]
- Rana, S.; Bajaj, A.; Mout, R.; Rotello, V.M. Monolayer coated gold nanoparticles for delivery applications. Adv. Drug Deliv. Rev. 2012, 64, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, K.; Sarkar, S.; Rao, K.J.; Paria, S. Core/shell nanoparticles in biomedical applications. Adv. Colloid Interface Sci. 2014, 209, 8–39. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.Z.; Akhter, S.; Jain, G.K.; Rahman, M.; Pathan, S.A.; Ahmad, F.J.; Khar, R.K. Metallic nanoparticles: Technology overview & drug delivery applications in oncology. Expert Opin. Drug Deliv. 2010, 7, 927–942. [Google Scholar] [PubMed]
- Takami, T.; Murakami, Y. Development of PEG-PLA/PLGA microparticles for pulmonary drug delivery prepared by a novel emulsification technique assisted with amphiphilic block copolymers. Colloids Surf. B Biointerfaces 2011, 87, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Fang, I.J.; Trewyn, B.G. Application of Mesoporous Silica Nanoparticles in Intracellular Delivery of Molecules and Proteins, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Yang, Y.; Bajaj, N.; Xu, P.; Ohn, K.; Tsifansky, M.D.; Yeo, Y. Development of highly porous large PLGA microparticles for pulmonary drug delivery. Biomaterials 2009, 30, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.S.; Tavares, M.T.; Aguiar-Ricardo, A. Sustainable strategies for nano-in-micro particle engineering for pulmonary delivery. J. Nanopart. Res. 2014, 16, 2602. [Google Scholar] [CrossRef]
- Huynh, N.T.; Roger, E.; Lautram, N.; Benoît, J.-P.; Passirani, C. The rise and rise of stealth nanocarriers for cancer therapy: Passive versus active targeting. Nanomedicine 2010, 5, 1415–1433. [Google Scholar] [CrossRef] [PubMed]
- Koo, O.M.; Rubinstein, I.; Onyuksel, H. Role of nanotechnology in targeted drug delivery and imaging: A concise review. Nanomed. Nanotechnol. Biol. Med. 2005, 1, 193–212. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.H.; Salabas, E.L.; Schuth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chemi. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Spasova, M.; Salgueiriño-Maceira, V.; Schlachter, A.; Hilgendorff, M.; Giersig, M.; Liz-Marzán, L.M.; Farle, M. Magnetic and optical tunable microspheres with a magnetite/gold nanoparticle shell. J. Mater. Chem. 2005, 15, 2095–2098. [Google Scholar] [CrossRef]
- Araújo, J.E.; Lodeiro, C.; Capelo, J.L.; Rodríguez-González, B.; Santos, A.A.D.; Santos, H.M.; Fernandez-Lodeiro, J. Novel nanocomposites based on a strawberry-like gold-coated magnetite (Fe@Au) for protein separation in multiple myeloma serum samples. Nano Res. 2015, 8, 1189–1198. [Google Scholar] [CrossRef]
- Silva, A.S.; Silva, M.C.; Miguel, S.P.; Bonifácio, V.D.B.; Correia, I.J.; Aguiar-Ricardo, A. Nanogold Poxylation: Towards always-on fluorescent lung cancer targeting. RSC Adv. 2016, 6, 33631–33635. [Google Scholar] [CrossRef]
- Restani, R.B.; Conde, J.; Pires, R.F.; Martins, P.; Fernandes, A.R.; Baptista, P.V.; Bonifácio, V.D.B.; Aguiar-Ricardo, A. POxylated Polyurea Dendrimers: Smart Core-Shell Vectors with IC50 Lowering Capacity. Macromol. Biosci. 2015, 15, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.; Ke, P.C.; Davis, T.P.; Kempe, K. Poly(2-oxazoline)-based micro- and nanoparticles: A review. Eur. Polym. J. 2016. [Google Scholar] [CrossRef]
- Patton, J.S. Unlocking the opportunity of tight glycaemic control: Inovative delivery of insulin via the lung. Diabetes Obes. Metab. 2005, 7, S5–S8. [Google Scholar] [CrossRef] [PubMed]
- Smola, M.; Vandamme, T.; Sokolowski, A. Nanocarriers as pulmonary drug delivery systems to treat and to diagnose respiratory and non respiratory diseases. Int. J. Nanomed. 2008, 3, 1–19. [Google Scholar] [CrossRef]
- Wang, Y.-B.; Watts, A.B.; Peters, J.I.; Liu, S.; Batra, A.; Williams, R.O. In vitro and in vivo performance of dry powder inhalation formulations: Comparison of particles prepared by thin film freezing and micronization. AAPS PharmSciTech 2014, 15, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Cabral, R.P.; Sousa, A.; Silva, A.S.; Paninho, A.I.; Temtem, M.; Costa, E.; Casimiro, T. Design of experiments approach on the preparation of dry inhaler chitosan composite formulations by supercritical CO2-assisted spray-drying. J. Supercrit. Fluids 2016, 116, 26–35. [Google Scholar] [CrossRef]
- Illum, L. Chitosan and its use as a pharmaceutical excipient. Pharm. Res. 1998, 15, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Grenha, A.; Seijo, B. The potential of chitosan for pulmonary drug delivery. J. Drug Deliv. Sci. Technol. 2010, 20, 33–43. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Peniche, C.; Arguelles-Monal, W.; Peniche, H.; Acosta, N. Chitosan: An Attractive Biocompatible Polymer for Microencapsulation. Macromol. Biosci. 2003, 3, 511–520. [Google Scholar] [CrossRef]
- Adami, R.; Liparoti, S.; Reverchon, E. A new supercritical assisted atomization configuration, for the micronization of thermolabile compounds. Chem. Eng. J. 2011, 173, 55–61. [Google Scholar] [CrossRef]
- Porta, G.D.; De Vittori, C.; Reverchon, E. Supercritical assisted atomization: A novel technology for microparticles preparation of an asthma-controlling drug. AAPS PharmSciTech 2005, 6, E421–E428. [Google Scholar]
- Shoyele, S.A.; Cawthorne, S. Particle engineering techniques for inhaled biopharmaceuticals. Adv. Drug Deliv. Rev. 2006, 58, 1009–1029. [Google Scholar] [CrossRef] [PubMed]
- Mero, A.; Pasut, G.; Via, L.D.; Fijten, M.W.M.; Schubert, U.S.; Hoogenboom, R.; Veronese, F.M. Synthesis and characterization of poly(2-ethyl 2-oxazoline)-conjugates with proteins and drugs: Suitable alternatives to PEG-conjugates? J. Control. Release 2008, 125, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Correia, V.G.; Bonifácio, V.D.; Raje, V.P.; Casimiro, T.; Moutinho, G.; da Silva, C.L.; Pinho, M.G.; Aguiar-Ricardo, A. Oxazoline-Based Antimicrobial Oligomers: Synthesis by CROP Using Supercritical CO2. Macromol. Biosci. 2011, 11, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Terada, H. Endocytosis of Particle Formulations by Macrophages and Its Application to Clinical Treatment. In Molecular Regulation of Endocytosis; INTECH Open Access Publisher: Rijeka, Croatia, 2012; pp. 413–428. [Google Scholar]
- Lambert, A.R.; Shaughnessy, P.O.; Tawhai, M.H.; Hoffman, E.A.; Lin, C. Regional deposition of particles in an image-based airway model: Large-eddy simulation and left-right lung ventilation asymmetry. Aerosol Sci. Technol. 2011, 45, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Chow, A.H.L.; Tong, H.H.Y.; Chattopadhyay, P.; Shekunov, B.Y. Particle engineering for pulmonary drug delivery. Pharm. Res. 2007, 24, 411–437. [Google Scholar] [CrossRef] [PubMed]
- Restani, R.B.; Correia, V.G.; Bonifácio, V.D.B.; Aguiar-Ricardo, A. Development of functional mesoporous microparticles for controlled drug delivery. J. Supercrit. Fluids 2010, 55, 333–339. [Google Scholar] [CrossRef]
- Caruso, F.; Lichtenfeld, H.; Giersig, M.; Mohwald, H. Electrostatic self-assembly of silica nanoparticle-polyelectrolyte multilayers on polystyrene latex particles. J. Am. Chem. Soc. 1998, 120, 8523–8524. [Google Scholar] [CrossRef]
- Caruso, F.; Caruso, R.A.; Möhwald, H. Nanoengineering of inorganic and hybrid hollow spheres by colloidal templating. Science 1998, 282, 1111–1114. [Google Scholar] [CrossRef] [PubMed]
- Caruso, F. Hollow capsule processing through colloidal templating and self-assembly. Chemistry 2000, 6, 413–419. [Google Scholar] [CrossRef]
- Salgueirino-Maceira, V.; Correa-Duarte, M.A.; Farle, M.; López-Quintela, A.; Sieradzki, K.; Diaz, R. Bifunctional Gold-Coated Magnetic Silica Spheres Bifunctional Gold-Coated Magnetic Silica Spheres. Chem. Mater. 2006, 18, 2701–2706. [Google Scholar] [CrossRef]
- De Macedo, C.V.; da Silva, M.S.; Casimiro, T.; Cabrita, E.J.; Aguiar-Ricardo, A. Boron trifluoride catalyzed polymerisation of 2-substituted-2-oxazolines in supercritical carbon dioxide. Green Chem. 2007, 9, 948–953. [Google Scholar] [CrossRef]
- Bosquillon, C.; Lombry, C.; Préaat, V.; Vanbever, R. Influence of formulation excipients and physical characteristics of inhalation dry powders on their aerosolization performance. J. Control. Release 2001, 70, 329–339. [Google Scholar] [CrossRef]
- Kinnunen, H.; Hebbink, G.; Peters, H.; Shur, J.; Price, R. An investigation into the effect of fine lactose particles on the fluidization behaviour and aerosolization performance of carrier-based dry powder inhaler formulations. AAPS PharmSciTech 2014, 15, 898–909. [Google Scholar] [CrossRef] [PubMed]
- De Boer, A.H.; Gjaltema, D.; Hagedoorn, P.; Frijlink, H.W. Can “extrafine” dry powder aerosols improve lung deposition? Eur. J. Pharm. Biopharm. 2015, 96, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.P. Dry powder inhalers for optimal drug delivery. Expert Opin. Biol. Ther. 2004, 4, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Buttini, F.; Brambilla, G.; Copelli, D.; Sisti, V.; Balducci, A.G.; Bettini, R.; Pasquali, I. Effect of Flow Rate on In Vitro Aerodynamic Performance of NEXThaler® in Comparison with Diskus® and Turbohaler® Dry Powder Inhalers. J. Aerosol Med. Pulm. Drug Deliv. 2016, 28, 167–178. [Google Scholar] [CrossRef] [PubMed]

| Sample | Dv,50 (µm) | Span | Water Content (%) |
|---|---|---|---|
| CHT | 2.6 | 0.9 | 11.1 |
| CHT_Fe@Au_POX-SH | 2.9 | 0.8 | 11.7 |
| Assay | MMAD (μm) | RF (%) | FPF (%) | GSD (%) | EF (%) | Yield, ɳ (%) |
|---|---|---|---|---|---|---|
| CHT_NP | 1.5 ± 0.1 | 70.2 ± 0.3 | 55 ± 2 | 2.5 ± 0.1 | 97.50 ± 0.04 | 38 |
| CHT | 1.2 ± 0.2 | 65 ± 2 | 48 ± 2 | 3.4 ± 0.3 | 96.3 ± 0.1 | 60 |
| n | k (h−1) | % Mass Released | |
|---|---|---|---|
| pH 7.4 | 0.6 | 23.1 | 68.9 |
| pH 6.8 | 0.6 | 19.2 | 81.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.C.; Silva, A.S.; Fernandez-Lodeiro, J.; Casimiro, T.; Lodeiro, C.; Aguiar-Ricardo, A. Supercritical CO2-Assisted Spray Drying of Strawberry-Like Gold-Coated Magnetite Nanocomposites in Chitosan Powders for Inhalation. Materials 2017, 10, 74. https://doi.org/10.3390/ma10010074
Silva MC, Silva AS, Fernandez-Lodeiro J, Casimiro T, Lodeiro C, Aguiar-Ricardo A. Supercritical CO2-Assisted Spray Drying of Strawberry-Like Gold-Coated Magnetite Nanocomposites in Chitosan Powders for Inhalation. Materials. 2017; 10(1):74. https://doi.org/10.3390/ma10010074
Chicago/Turabian StyleSilva, Marta C., Ana Sofia Silva, Javier Fernandez-Lodeiro, Teresa Casimiro, Carlos Lodeiro, and Ana Aguiar-Ricardo. 2017. "Supercritical CO2-Assisted Spray Drying of Strawberry-Like Gold-Coated Magnetite Nanocomposites in Chitosan Powders for Inhalation" Materials 10, no. 1: 74. https://doi.org/10.3390/ma10010074
APA StyleSilva, M. C., Silva, A. S., Fernandez-Lodeiro, J., Casimiro, T., Lodeiro, C., & Aguiar-Ricardo, A. (2017). Supercritical CO2-Assisted Spray Drying of Strawberry-Like Gold-Coated Magnetite Nanocomposites in Chitosan Powders for Inhalation. Materials, 10(1), 74. https://doi.org/10.3390/ma10010074