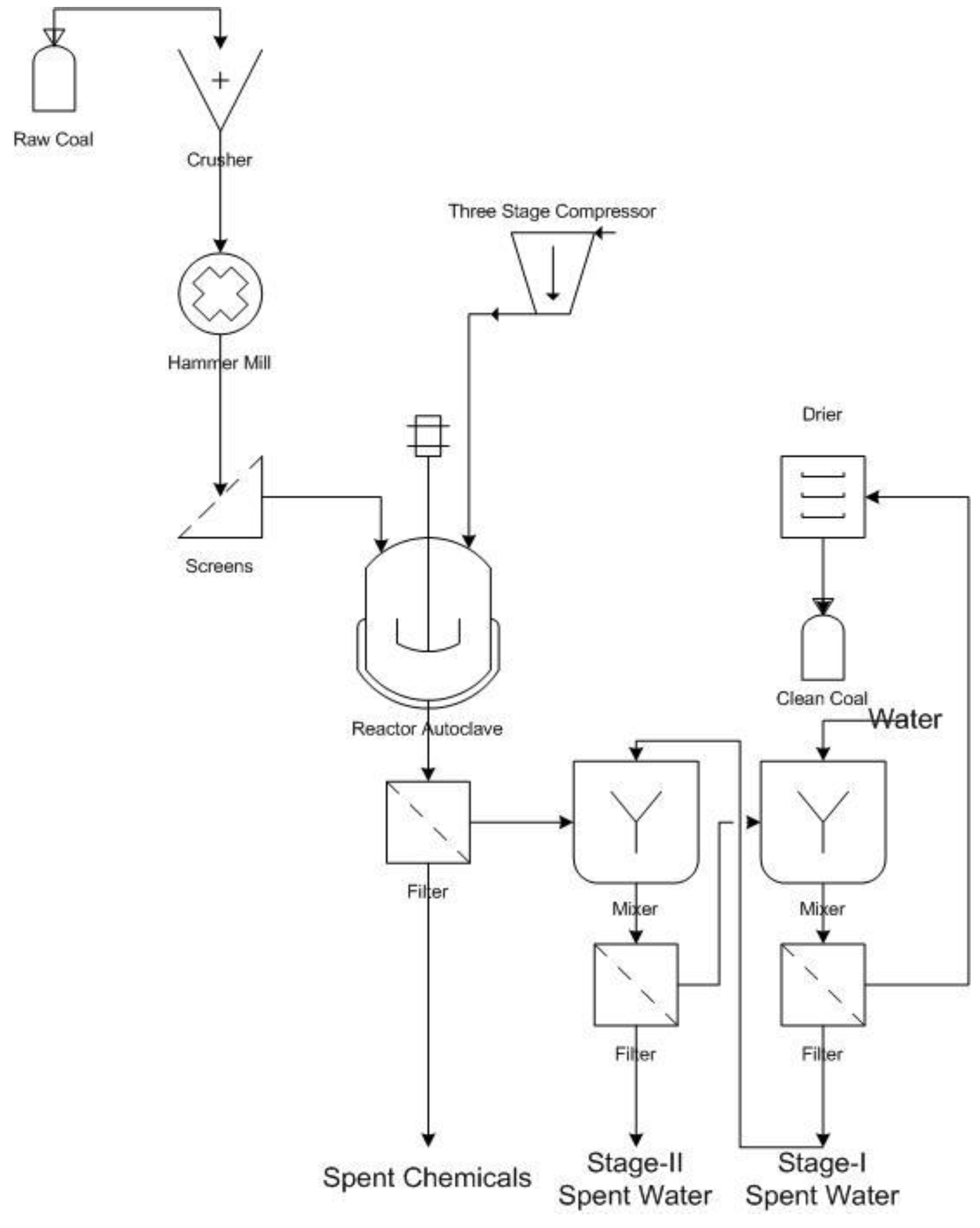
1. Introduction
High sulphur and ash-forming mineral content make the use of coal challenging in thermochemical conversion processes like combustion, gasification,
etc. [
1,
2]. Sulphur in coal has been recognized as one of the major impurities in coal [
3] and is present in different forms [
4]. The presence of these forms of sulphur makes it necessary to install different flue gas scrubbing systems in power generation systems [
5,
6,
7]. Chemical coal cleaning has its dominance over physical cleaning because of its significant reduction in organic sulphur along with the reduction in trace elements [
4,
8,
9].
Various chemicals are used to react selectively with the impurities present in the coal to convert them into a soluble fraction, to separate them from the main coal matrix [
10,
11]. Development of a chemical coal cleaning process dealing with relatively low product loss operating at ambient temperature could minimize the energy requirements and degradation of the coal matrix, requiring lower capital and operating costs [
12].
Oxydesulphurisation processes can effectively convert the sulphur into the form of soluble sulphates. Up to now, oxydesulphurisation research has mainly involved high temperature reactions. Yaman and Kucukbayrak [
13] studied the oxydesulphurisation behavior of a Turkish lignite under 0–1.5 MPa partial pressure of oxygen at 403 and 498 K using dilute alkaline solutions. Palmer
et al. [
14] investigated the chemical desulphurisation of coals using selective oxidation with a mixture of hydrogen peroxide in acetic acid. In addition, Palmer
et al. [
15,
16] characterized and studied the desulphurisation of oxidized and unoxidized coals via pyrolysis. Demirbas [
17] reported that alkaline desulphurisation is more effective in removing pyritic sulphur from coal.
Limited studies have been carried out on high pressure oxydesulphurisation of coal at ambient temperature while investigating the effect of alkali. This work aims to study the effect of high pressure for penetration to sulphur sites present deep in the coal matrix as a generic approach.
The technique investigated was using ambient temperature oxidant potassium permanganate (KMnO
4). The oxidising abilities and the kinetics of pyritic sulphur removal with this oxidant have been investigated by Cliffe and Syed, M.M. [
18]. The comparison with the other ambient temperature oxidants has shown that it oxidises sulphur more rapidly than the other oxidants [
19]. Previous work has shown that KMnO
4 will remove the majority of pyritic sulphur at ambient temperature and pressure but will not remove substantial amounts of organic sulphur. The problem with using KMnO
4 is that a layer of MnO
2 forms on the outside, restricting diffusion of oxygen to the sulphur, and it is postulated that the effect of pressure will increase the diffusion of oxygen.
Potassium permanganate, being a very strong oxidant, reacts by oxidizing the various sulphides present in coal to their sulphates which, being soluble in water or aqueous acid solutions, are washed out during the washing stages of the process. The two major types of inorganic-sulphur-carrying impurities in coal are iron pyrite and marcasite. Both of these sulphides have the same molecular formula,
i.e., FeS
2, but different molecular structures. The occurrence of marcasite is usually restricted to coals with sulphur contents >1% weight. Apart from these major sulphides there are some minor sulphides and sulphates also present in coal like pyrrhotite, galena, sphalerite and jarosite, anhydrite, gypsum
etc. [
20]. These sulphides, when reacted upon by potassium permanganate, follow an oxidation reduction reaction based upon the following two equations [
20];
According to Equations (1) and (2), potassium permanganate, while oxidising the sulphides (mainly pyrite) reduces to manganese dioxide and jarosite. These two compounds form an impervious ash layer over the sulphur sites, hence inhibiting the oxidation reaction and resulting in an increase in the ash content of the oxidised coal. Researchers have investigated different techniques in order to leach out this ash layer and have found that this ash layer can be removed by making use of some organic and inorganic acids. It was concluded that out of all the different acids investigated, a 14%
v/
v hydrochloric acid (HCl) solution was recommended [
20]; beyond this concentration there was no further effect on the ash content of the investigated coals. The same acid concentration was used to remove this ash layer in these investigations. This acid removed the manganese dioxide ash layer according to the following reaction [
14], without affecting the coal matrix.
The main coal used in this study was Prince of Wales and it was ground to a finer size (25 μm) to make access to the sulphur easier.
3. Results and Discussion
3.1. Effect of Pressure on Sulphur Removal
It has been established that the oxidation of pyritic sulphur is controlled by the diffusion of the oxygen through the ash layer to the sulphur site [
24]. This diffusion of the reactants through the ash layer can be enhanced by the increase in the system pressure. It has been found that the increase in pressure increased the sulphur removal in the case of the air water system. The oxidation by potassium permanganate produces the build-up of an ash layer on the surface of the pyrite, and in order to study the effect of pressure to cross this ash layer barrier and enhance the diffusional effects, a set of preliminary experiments was performed. The investigations were performed at five different pressures varying from atmospheric to 300 bar. The temperature of the system was kept constant at 25 °C while the reaction time was one hour. The impeller used was a 6-blade rushton turbine.
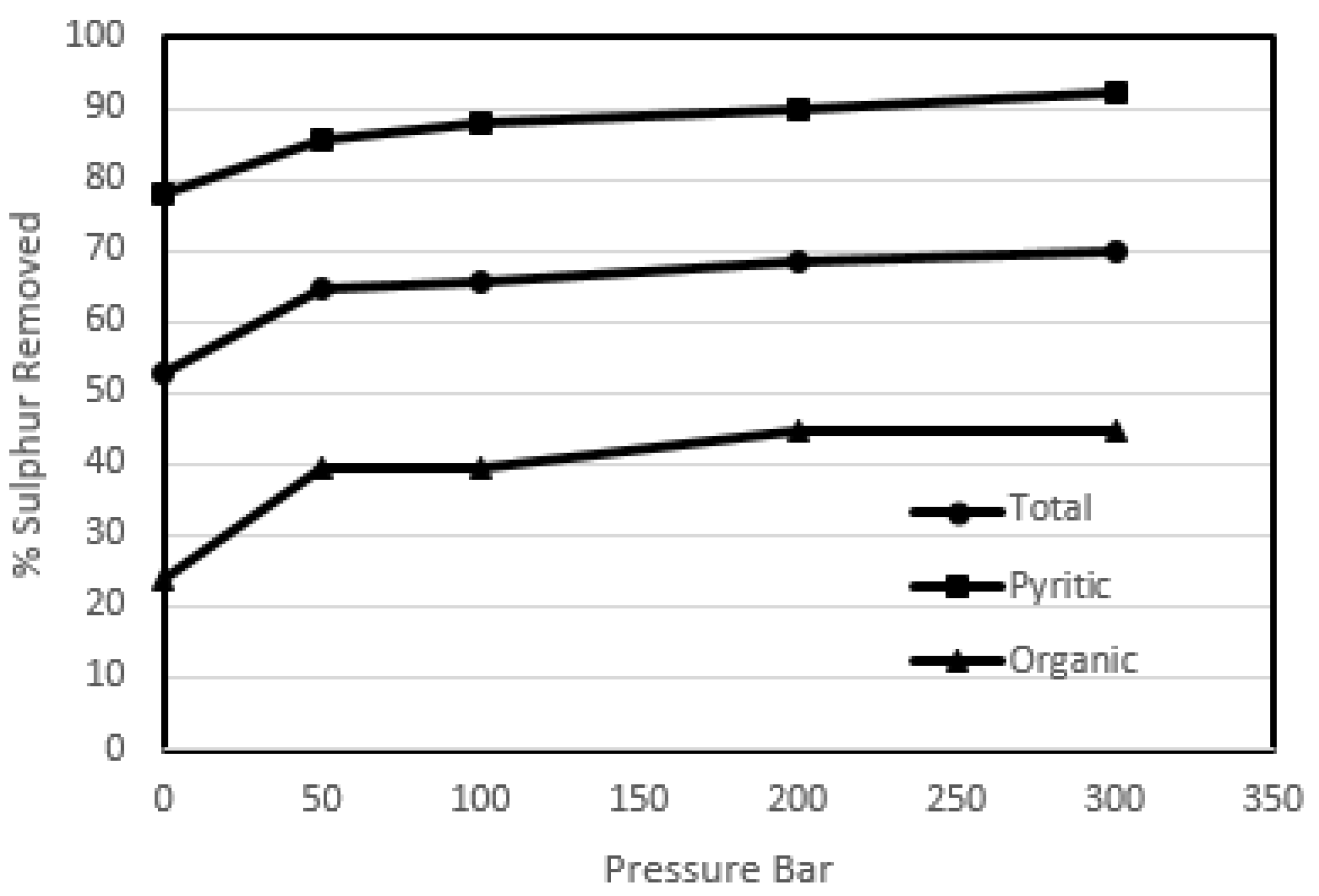
Figure 2 shows the effect of pressure on total, pyritic and the organic sulphur contents of the coal, while
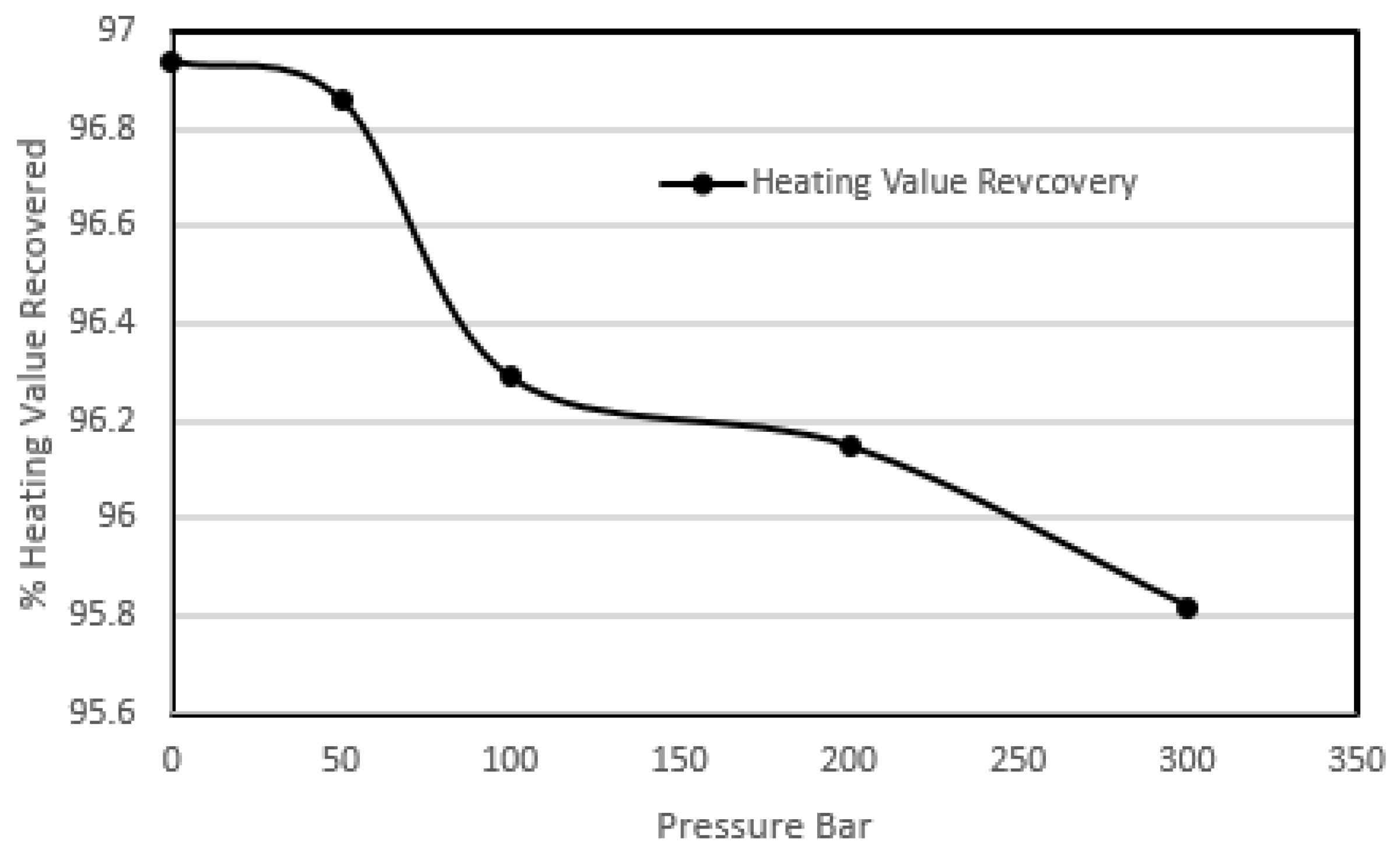
Figure 3 shows the heating value recoveries of these experiments.
The results of this study show that the effect of high pressure was quite pronounced on the sulphur removals not only on the pyritic sulphur but also on the organic sulphur content as well. The pyritic and the organic sulphur removal was enhanced from 77.78% to 92.30% and 23.73% to 44.66% respectively. While the heating value recoveries were still above 95%. The reason that could be attributed to this increase in removal is the fact that high pressure enhanced the diffusion of oxygen through the ash layer and coal matrix to the sulphur species. The results of the heating value recoveries proved that the ambient temperature oxidation did not destroy the coal matrix. The decrease in the gross calorific values were due to an increase in the oxygen content of the treated coal, but the overall heating value recoveries were high, hence the degradation of the coal did not take place.
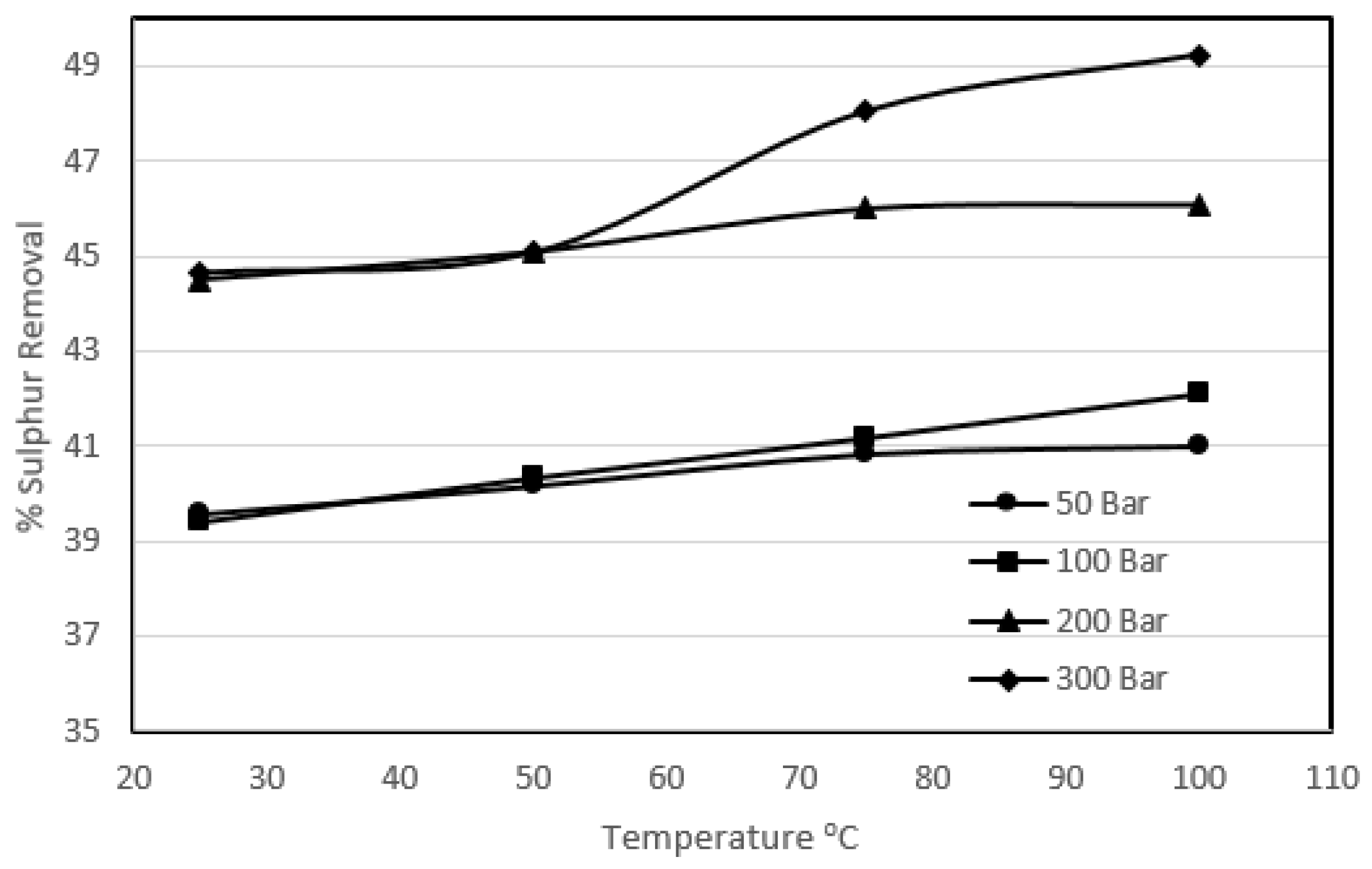
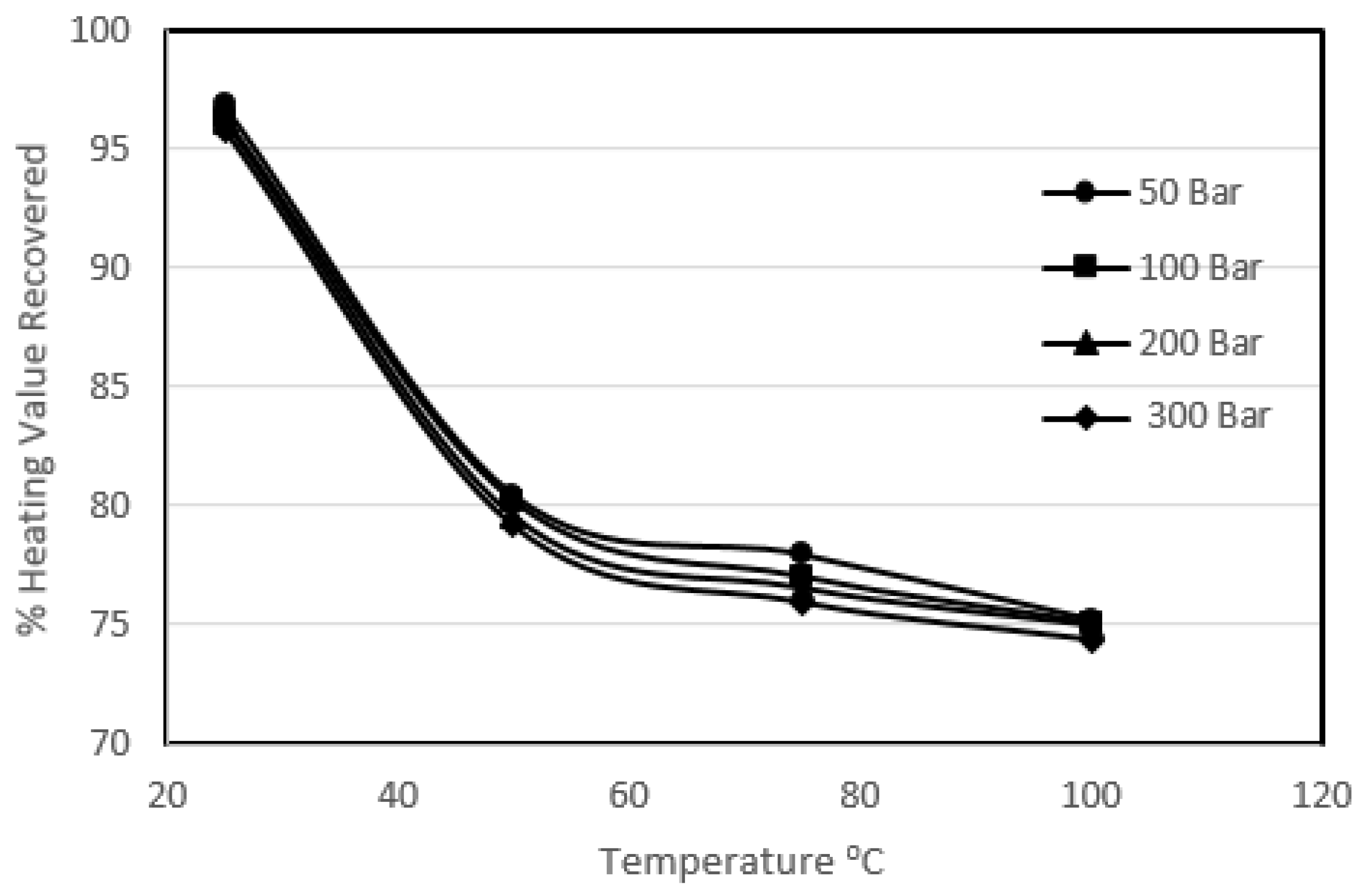
3.2. Effect of Temperature on Sulphur Removal
Although this part of the work is mainly concerned with the ambient temperature experiments, the previous research using ambient pressure and high temperature [
18] has shown the negligible effect of temperature on oxydesulphurisation using KMnO
4. It was observed that the high temperature reduced the calorific value due to attack of the oxidant on the carbon. The purpose of this study was to evaluate the idea that, although the high temperature will destroy the coal matrix, it would further enhance the organic sulphur removal under high pressure. The effect of temperature was investigated at four different pressures varying from 50 to 300 bar, and the temperature of the system varied from 25 to 100 °C for a particular set of experiments. The coal slurry concentration was 5%, and 10% KMnO
4 solution was used.
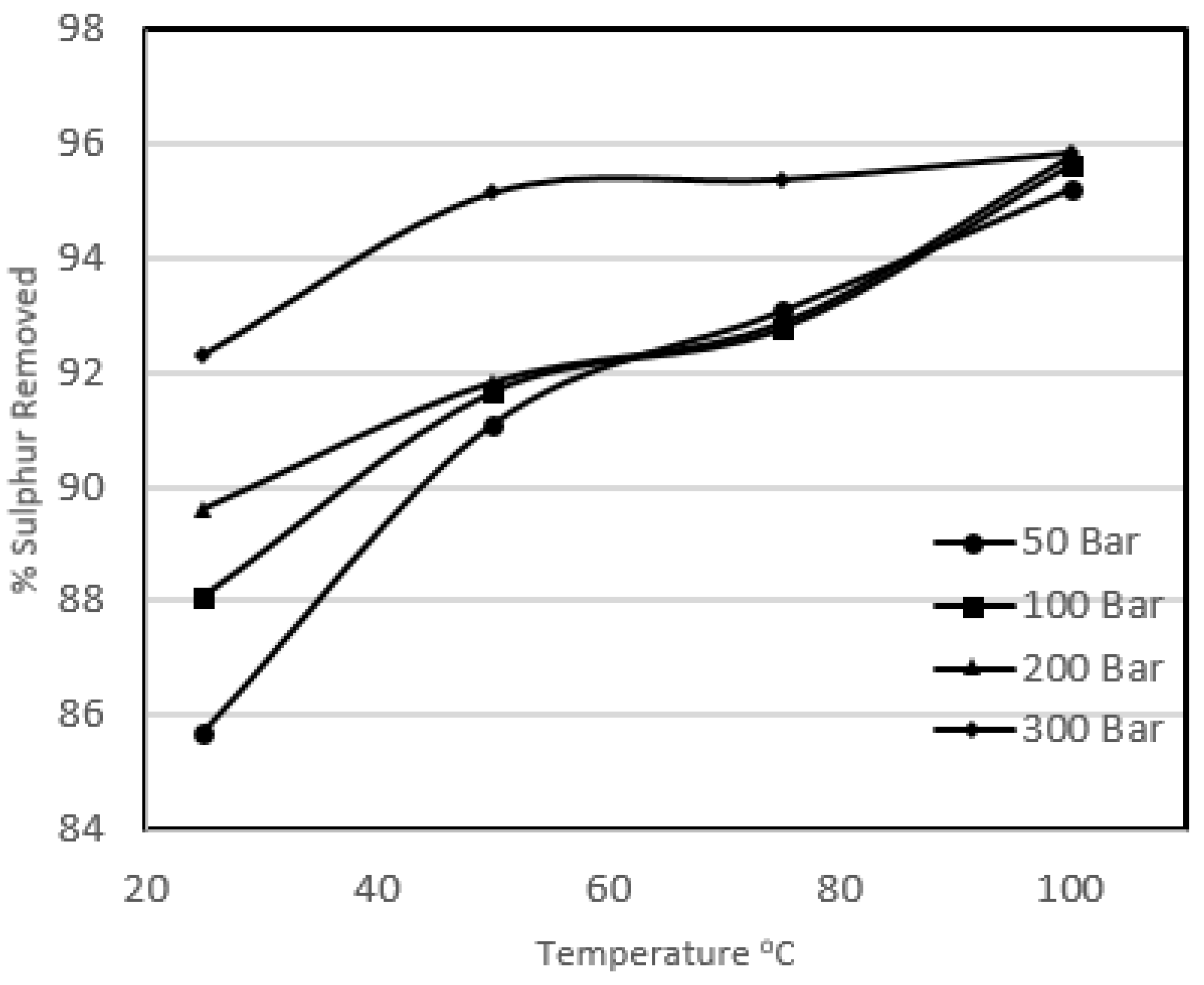
Figure 4 and
Figure 5 show the effect of temperature on pyritic and organic sulphur removal, while
Figure 6 shows the heating value recovery.
It can be seen that the increase in temperature enhanced the pyritic and organic sulphur removal, varying from 85.7% to 95.8% and 39.6% to 49.2% respectively. It was observed that there was generally a sharp increase in carbon loss with increasing temperature from 25 °C to 50 °C at a particular pressure. It was also observed that for the experiments carried out at 50 °C and above, all of the potassium permanganate present in the solution was destroyed, thus liberating all the available oxygen. The liberated oxygen attacked the coal and resulted in a great loss in calorific value recoveries. Beyond 50 °C the coal losses were not very significant, which defined the upper temperature range of potassium permanganate. However, the removal was enhanced with the increase in temperature beyond 50 °C but only slightly for a particular pressure. This slight increase in removal could be due to high temperature in the system as explained in the previous high-temperature studies, but this slight increase in removal was at the expense of a great loss in product recovery. The overall heating value recovery went down from 96.9% to 74.4%. It should be noted that this loss in heating value is greater than the loss observed in the extreme conditions of pressure in the previous sections. It can be concluded that the high temperature work with potassium permanganate oxidised coal vigorously, resulting in a great loss of heating values. The oxygen content of the treated coal samples also ended up high.
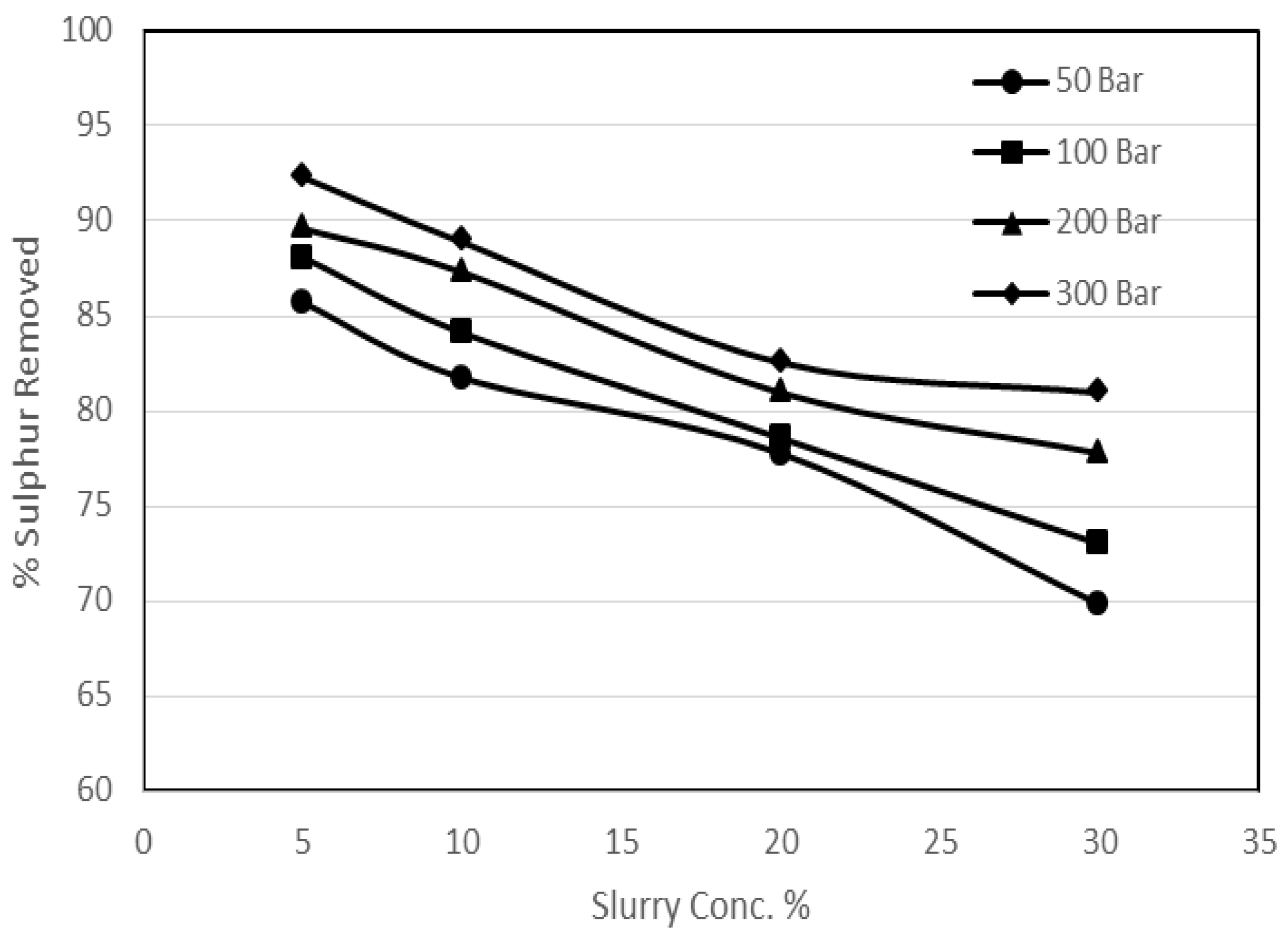
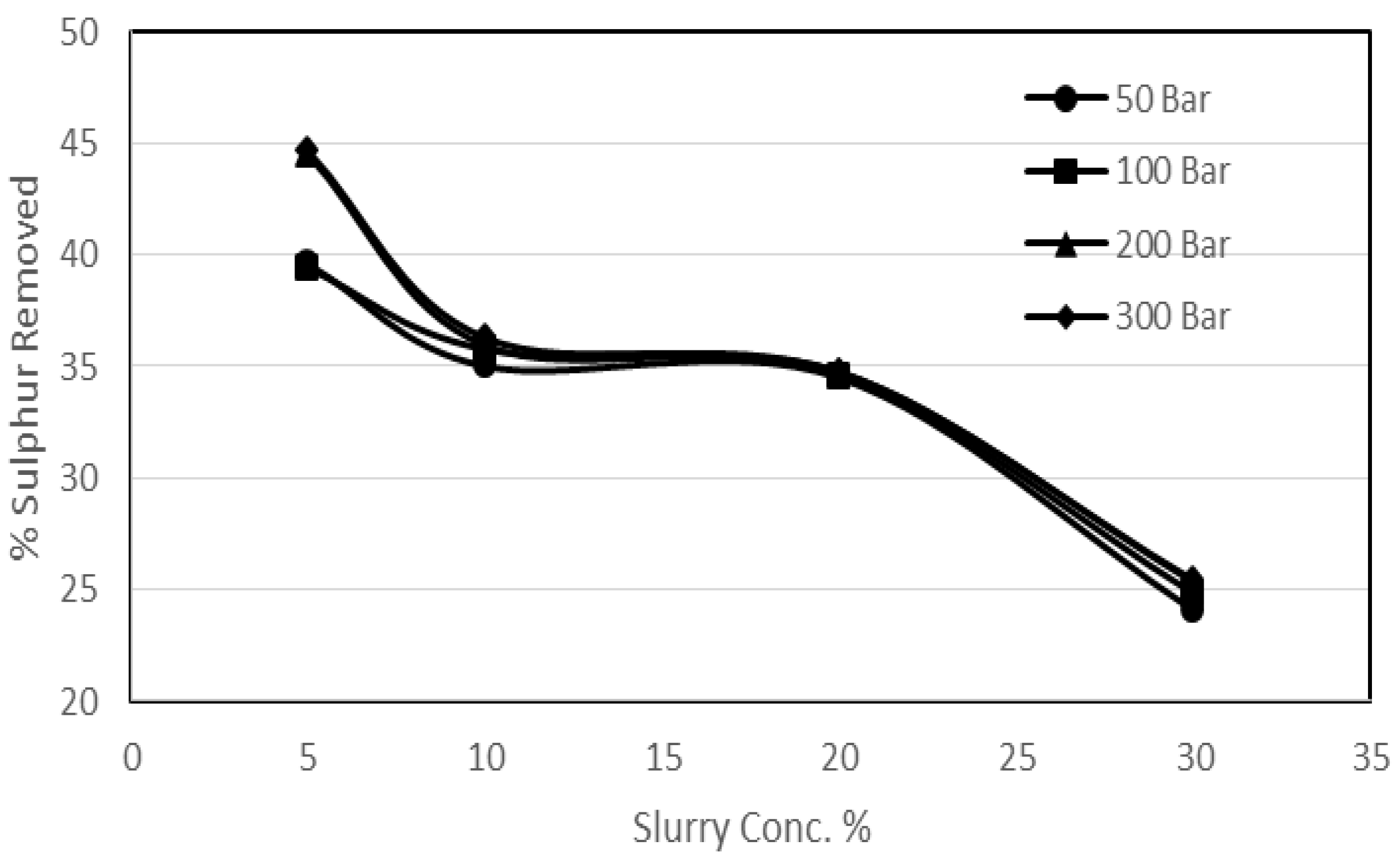
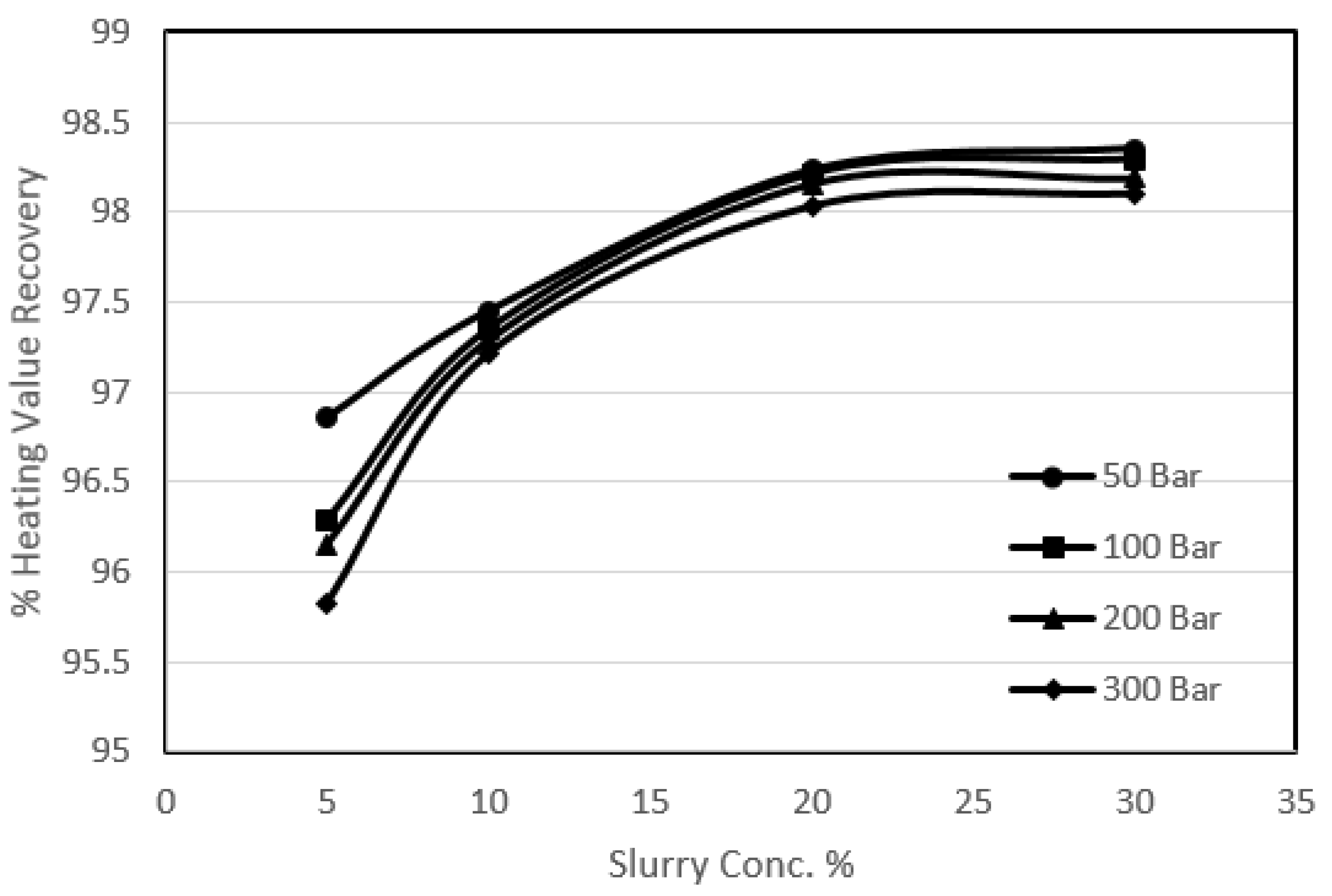
3.3. Effect of Coal Slurry Concentration on Sulphur Removal
In the case of KMnO
4, it has been found that an ash layer forms on the surface of the pyrite and coal due to the reduction of KMnO
4 to MnO
2. It has also been proven that the pressure enhances the diffusional effects. It was supposed that the high coal slurry concentration might incorporate a thinning effect on the ash layer, which would help diffusion. In order to investigate the effect of coal slurry concentration on the ash layer, a series of experimental runs was performed. In these investigations the effect of coal slurry concentration and the effect of pressure were studied. For the experimental runs the coal slurry concentrations used varied from 5% to 30%, whereas the range of the pressure was 50 to 300 bar at 25 °C.
Figure 7 and
Figure 8 show the effect of coal slurry concentration on pyritic and organic sulphur removal, while the effect on heating value recoveries can be seen in
Figure 9.
It can be seen from the experimental results that the effect of pressure was as usual; however, the effect of an increase in coal slurry concentration was a decrease in sulphur removal. The reason that could be attributed to this decrease is that, since the main oxygen participating substance for the reactions was potassium permanganate and it was observed that for a reaction with higher coal slurry concentration the oxidant consumption was more than a reaction with less slurry concentration, there was relatively less availability of the oxygen in the system for a reaction with high slurry concentration, which resulted in less removal. As far as the agitational and the diffusional effects are concerned these were observed, because there was quite a significant increase in the pyritic sulphur removal with the increase in pressure at a constant slurry concentration especially for higher slurry concentration experiments. But due to the increase in quantity of the coal the net effect was an overall decrease in removals. It was also observed that for a particular set of experiments with an increase in slurry concentration the oxygen content of the treated sample decreased, so the heating value recoveries rose. However, there was a declining trend in the calorific value recoveries with the increase in pressure which was the same as observed in the previous sections.
The conclusions from these investigations required more detailed investigation in order to differentiate between the effect of KMnO4 amounts versus the sulphur removals and the effect of agitation on reduction of ash layer to enhance sulphur removals. These two effects were investigated in detail and are reported in the following two sections.
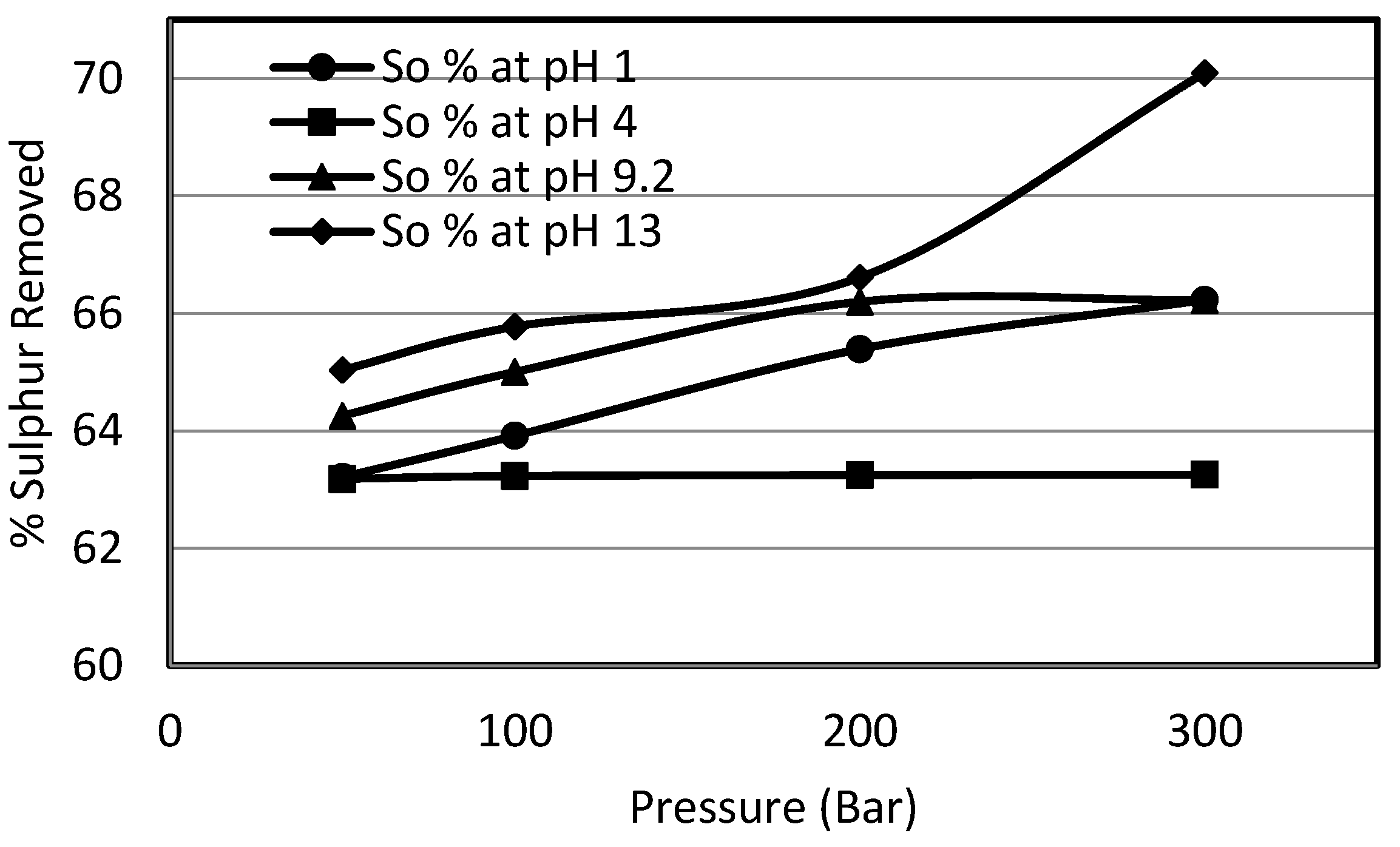
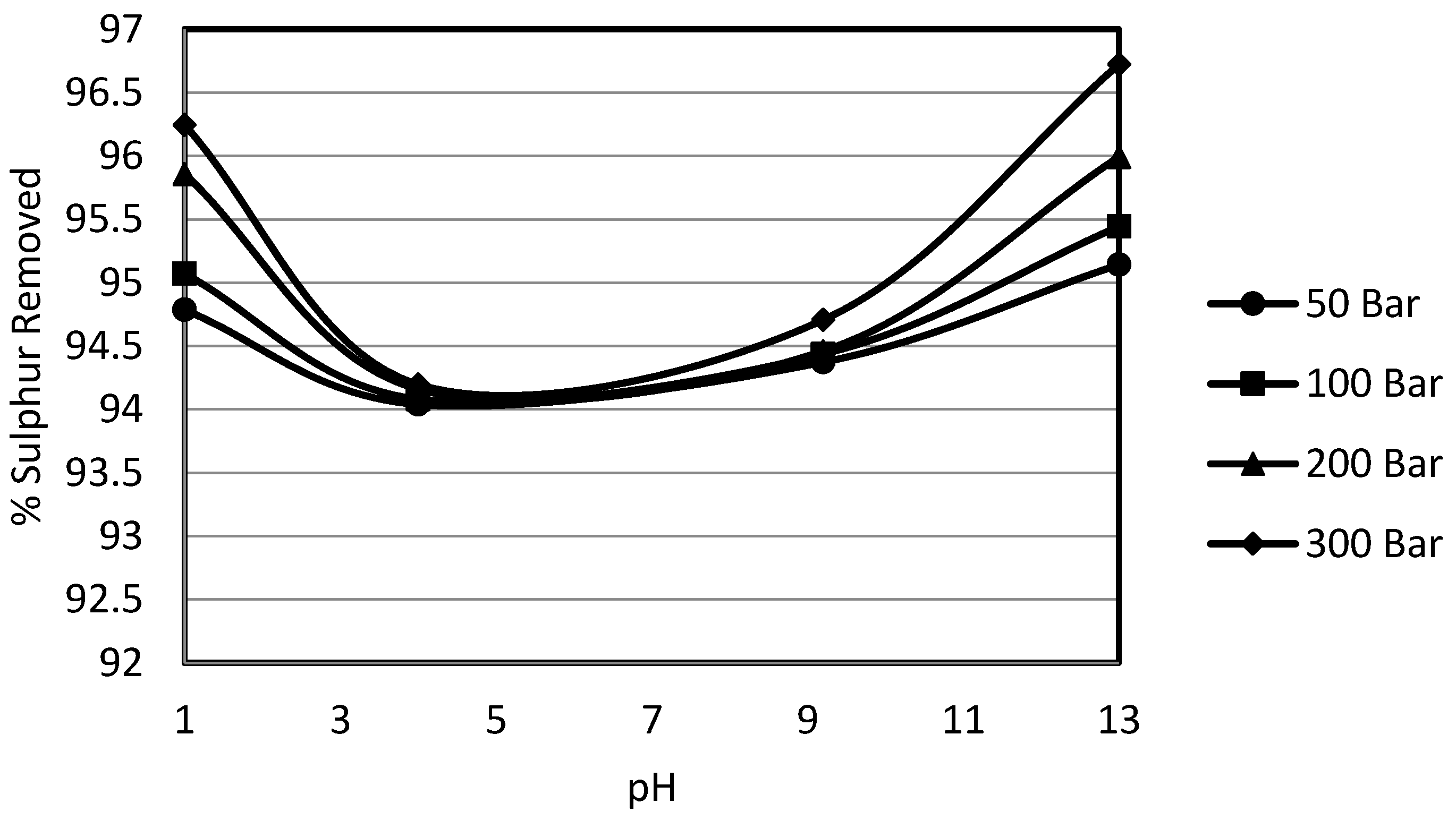
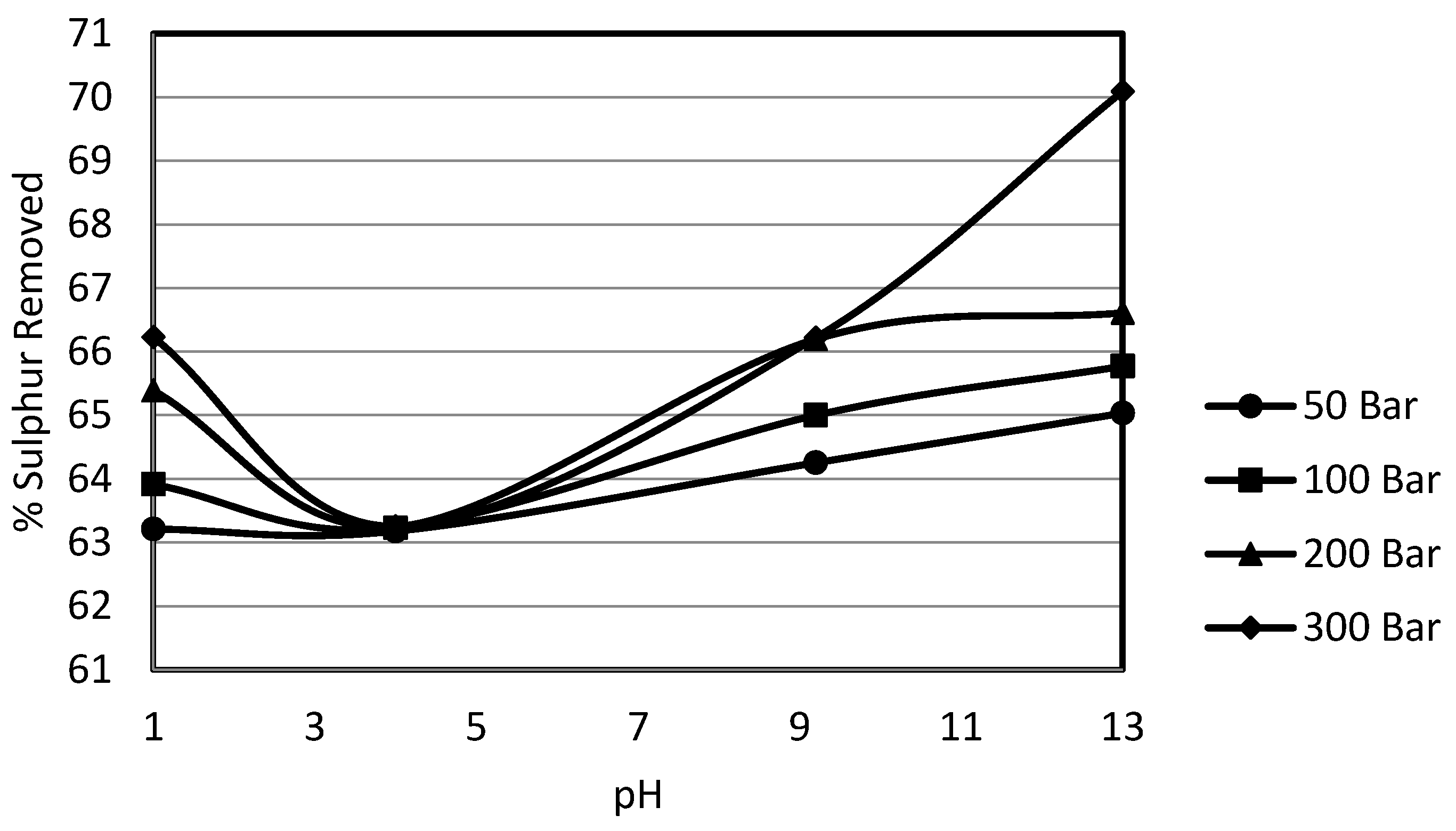
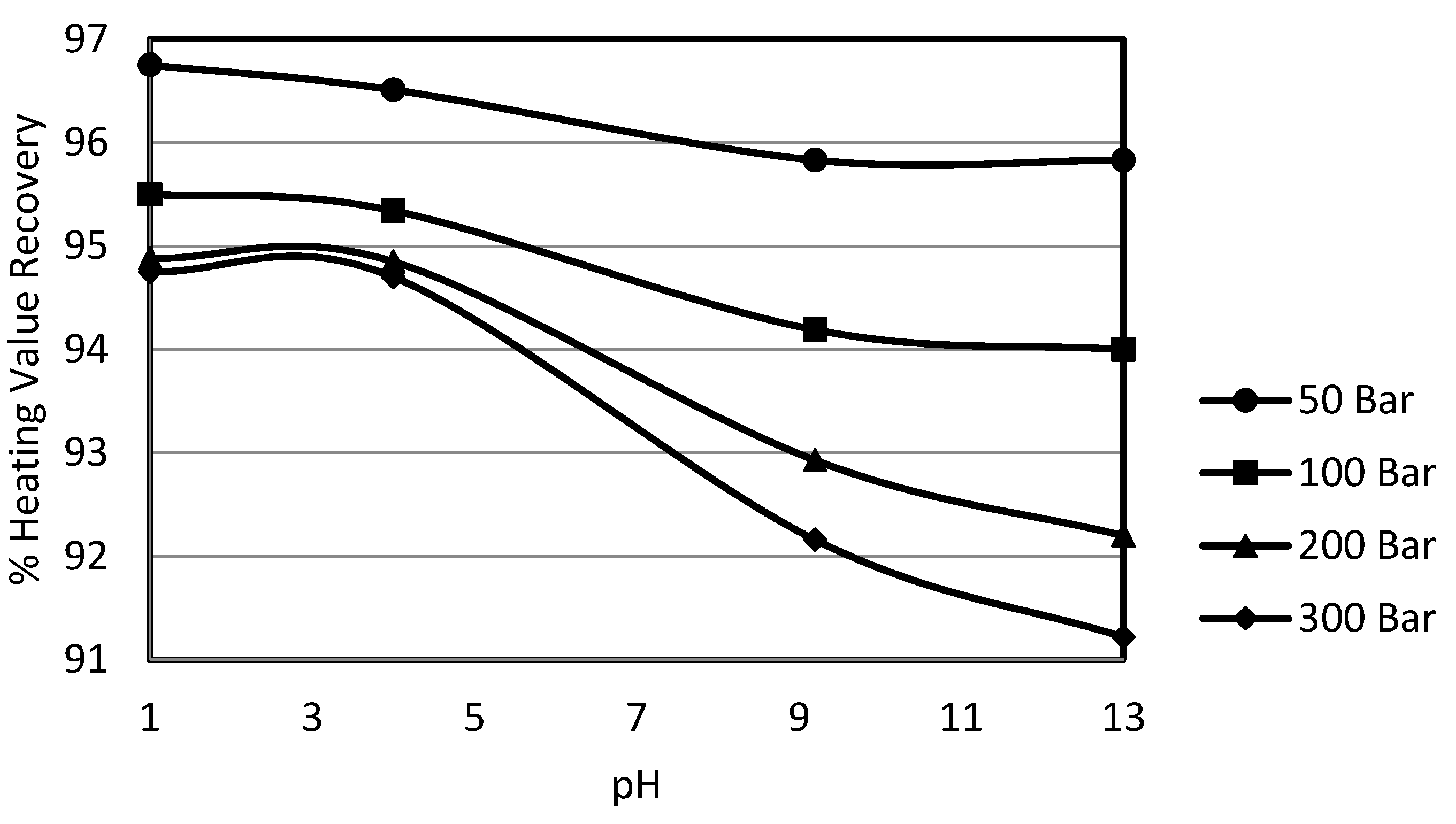
3.4. Effect of pH on Sulphur Removal
It was observed that for an experiment without pH control, the initial pH of the reaction mixture i.e., coal slurries in potassium permanganate solution, used to be 9.3. It was also observed that after the expiry of the reaction the pH went down to 8.2 to 8.3. This behaviour of change in pH led to the idea that the sulphur removal might be dependent on the solution pH and the control of pH might further enhance the organic desulphurisation abilities of potassium permanganate under high pressure.
In order to study the effect of constant pH, a wide range of pH buffers was selected. The 10% potassium permanganate solution for the experiments was prepared in the pH buffer solutions at ambient temperature. These buffer solutions were supplied by Whatman and were used as such for the preparation of solutions. The selected pHs were 1.0, 4.0, 9.2 and 13.0. This wide range of pH was selected to gain deeper insight into the effect of pH on the oxydesulphurisation of potassium permanganate, since it provided extreme acidic to mild acidic and mild alkaline to strong alkaline conditions. In order to study the effects of pressure at a constant pH, the system pressure was varied from 50 bar to 300 bar.
The experimental results show that sulphur removal was decreased by moving from the strong acidic conditions to the mild acidic conditions,
i.e., the variation in pH from 1 to 4 resulted in a decrease in sulphur removal. Sulphur removal was again enhanced in the alkaline region especially by the increase in pH from 9.2 to 13. This phenomenon could be explained by the fact that under mild acidic conditions, elemental sulphur formation takes place. This form of sulphur is not a water soluble form and thus is reported in total sulphur. When calculating the organic sulphur content by subtracting the inorganic sulphur content from the total sulphur, then this elemental sulphur formed gets accounted for as organic sulphur. It was noted by Attia and Fung [
25] that this phenomenon of elemental sulphur formation is minimised under extremely acidic conditions or in alkaline conditions during an oxydesulphurisation study using potassium permanganate as oxidant with the pH of the solution as variable. Overall, it was observed that there was a decrease in removal for the change in pH from 1 to 4 while the removal was enhanced again by the further increase in solution pH. It was also observed that sulphur removal was slightly higher compared in the case without pH control. Though the pyritic sulphur removals were high as usual, overall they varied around 95%. The only reason that could be attributed to higher removal is the higher removal of organic sulphur content. The higher organic sulphur removal could be explained as the removal of organic sulphur by the oxydesulphurisation technique, which is the result of different simple and or complex reactions that the sulphur species undergo. Permanganate reactivity depends to a great extent upon the reaction conditions, whether neutral, acidic or basic. This is usually due to a change incorporated in the organic substrate.
i.e., ionisation of molecules rather than to any alteration in the permanganate ion. The use of a constant pH buffer likely incorporated such a change in the organic substrates, which resulted in the high organic sulphur removals. Also the high rates of organic sulphur removal in the alkaline region could be due to the reduction of potassium permanganate to potassium manganate liberating oxygen. The potassium manganate formed, being able to liberate oxygen, further oxidises the sulphur. The organic sulphur removal was overall enhanced from 63% to 70%. This high removal could also be explained by heating value recoveries which went down to 91%, explaining how the high organic sulphur removal resulted in a drop of calorific value recoveries from 95% to 91%. This behaviour of heating value recoveries was similar to the one observed in the previous investigations on Mequinenza lignite.
The effect of pressure at any pH was quite strong other than the experiments conducted at pH 4, although there was a slight increase in the pyritic sulphur removal, but that effect was counterbalanced in total and organic sulphur contents due to elemental sulphur formation. The effect of pressure was strongest in the case of the experiments carried out at pH 13.
Figure 10 shows the effect of pressure on organic sulphur removal at the constant solution pH. The effect of pH on pyritic and organic sulphur removal is shown in
Figure 11 and
Figure 12, while the effect on calorific value recoveries can be observed in
Figure 13.
The results lead to the conclusion that the organic sulphur removal could be enhanced by potassium permanganate oxydesulphurisation under high pH conditions that could change the organic substrate to produce a completely soluble form of the parent sulphur compound. It can also be concluded that whenever the organic sulphur removal is enhanced, it results in some loss of calorific value recovery.
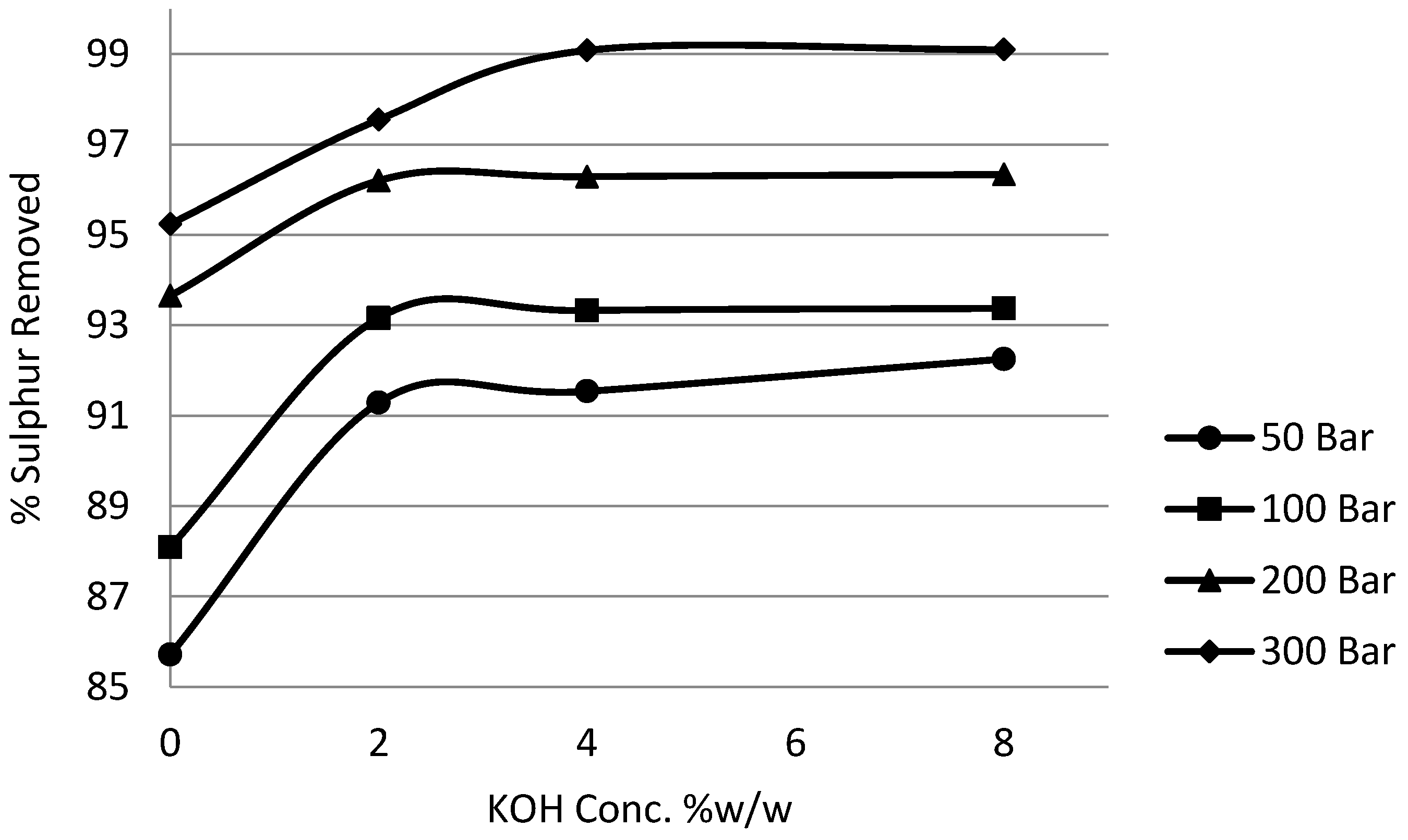
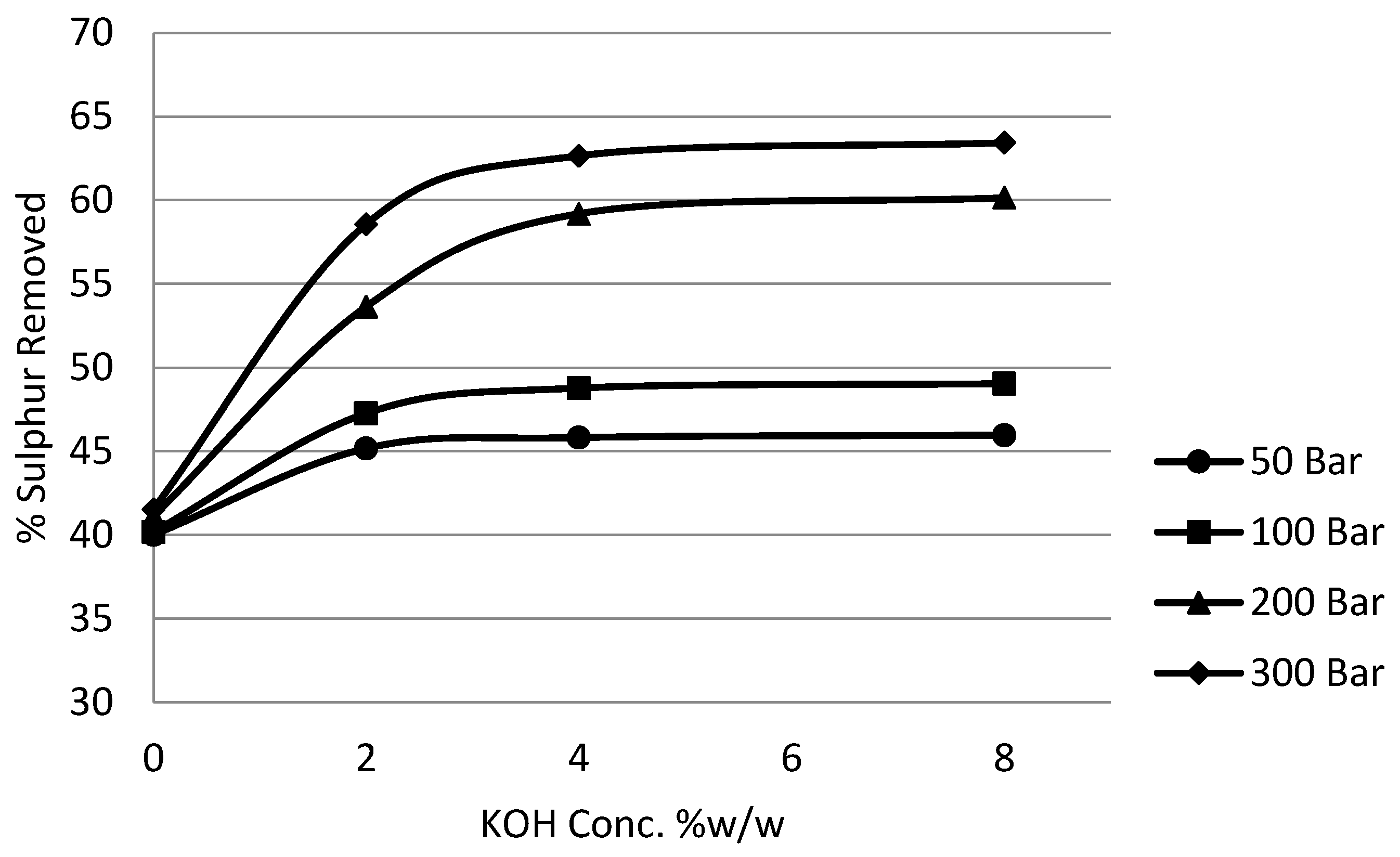
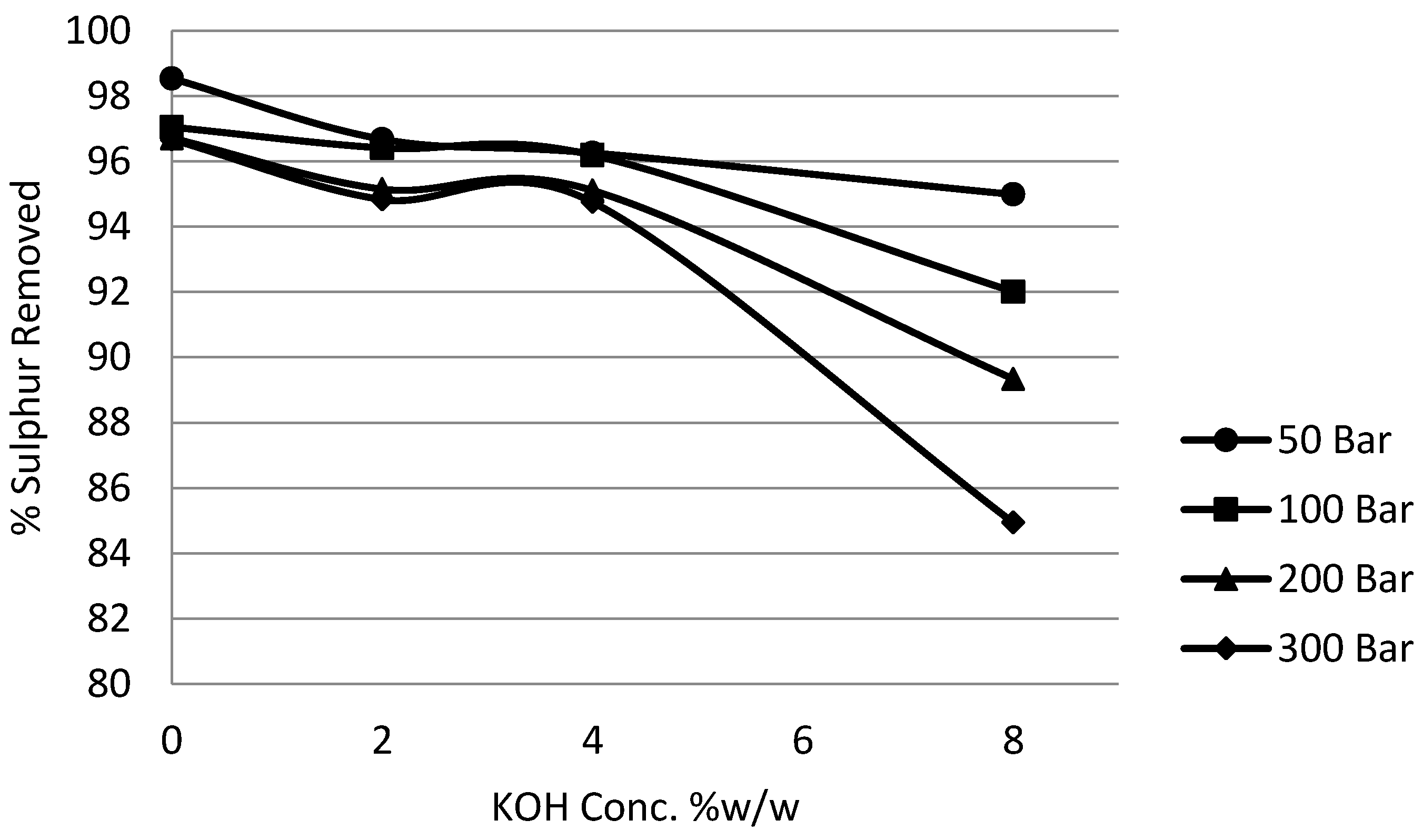
3.5. Effect of Potassium Hydroxide (KOH) on Potassium Permanganate Desulphurisation
It has been proven in the previous investigations of varying pH that the alkaline pH enhanced the removal either by incorporating some change in the organic matrix or due to potassium manganate formation [
26,
27]. The literature says that potassium permanganate oxidation of organic compounds is considerably faster in alkaline than in neutral solutions [
26]. Potassium permanganate has been used in the organic synthesis reactions. It has been observed in past that in the absence of alkaline conditions, complete oxidation reactions do not occur in most cases, e.g., the oxidation of thiols generally leads to the formation of disulphides, and the end product in this case should be sulphonic acid which does not occur in the absence of alkaline conditions. Mercaptans also form the corresponding sulphonic acids. In the case of an incomplete reaction the product obtained is a disulphide or a sulphone. The solubility data indicates that the solubility of an organic sulphone is very much similar to that of the parent sulphide [
28]. Hence, even though the different sulphides undergo the oxidation reaction, they are still not in a removable form. Apart from these reactions some organic sulphur species undergo exchange reactions for oxygen with potassium permanganate as oxidant. This exchange again takes place in alkaline media and the exchanged sulphur forms soluble sulphates [
27]. On the other hand, in the case of complex thiophenes, where sulphur is the part of the aromatic ring, no oxidation occurs [
29]. It should be noted that the mentioned reactions were investigated with pure compounds in the organic synthesis chemistry. Since coal is an aggregate of many simple and complex organic compounds, in order to see the validity of these reactions in the case of coal, a series of reactions were carried out on Prince of Wales coal samples. It should also be noted that coal is an insoluble material, so the different sulphur species present can be categorised depending upon their availability in mainly two forms,
i.e., available on the surface of the coal particle or embedded deep in the coal particle. In the case when a sulphur site is available on the surface, it is easy for the oxidant to oxidise that sulphur species, but for a site embedded deep in the coal particle it is necessary to enhance the diffusion of the reactant to the embedded sulphur site. This phenomenon of diffusion has been proven in the previous sections and can be enhanced by the increase in pressure. Prince of Wales sample was selected for its smaller particle size so that the diffusional effects would be more prominent.
In order to comprehensively study the effects of alkalinity and pressure, the concentration of potassium hydroxide was varied from 0% to 8% w/w and the pressure of the system was varied from 50 bar to 300 bar. Time, temperature, impeller speed, coal slurry concentration and potassium permanganate concentration parameters were fixed at one hour, 25 °C, 5 Hz, 5% and 10%, respectively.
Figure 14 and
Figure 15 show the effect of potassium hydroxide concentration on pyritic and organic sulphur removal, while the effect on heating value recoveries can be observed in
Figure 16.
It can be seen from the experimental results that the effect of potassium hydroxide was quite pronounced not only on organic sulphur but also on pyritic sulphur removal. Generally the removal was enhanced with the increase in the alkali concentration to 4% without a very significant loss in the calorific value recovery. Beyond 4% concentration there was a significant loss in heating value recoveries. It was also observed that the increase in removal beyond 4% was not very significant. Because at 8% potassium hydroxide concentration a major loss in calorific value was observed, the investigations were limited to this concentration. The reason that could be attributed to this loss in recoveries is the fact that, as explained earlier, the oxidation by potassium permanganate results in the lowering of the solution pH. Now the presence of a high concentration of potassium hydroxide maintained high pH conditions and at the same time kept the organic substrates well charged, which resulted in the oxidative distruction of organic material. It was observed that the use of potassium permanganate on the basis of reduction to manganese dioxide was also enhanced.
Another striking behaviour observed in these investigations was the wettability of coal. In the previous experiments, the coal wettability was quite different than in these experiments. This factor of wettability was very much enhanced, and the coal surface in the reaction mixture appeared to be well polished and shiny. This behaviour was never observed in the previous investigations. This behaviour was not due to oil formation or liquefaction of coal as there was never any difficulty observed at the filtration stage with these experiments. Another explanation for this behaviour is that, as explained above, the full oxidation of the organic sulphur species with potassium permanganate should lead to the formation of corresponding sulphonic acid. These sulphonic acids formed are crystalline solids, readily soluble in water and often hygroscopic. Because of the difficulty of isolation of the free radicals, they are usually encountered as alkali metal salts [
30]. These salts are sulphonates and are well known for their wetting properties [
26]. Due to the formation of these salts, sulphur species go into the solution and are removed.