Critical Filler Concentration in Sulfated Titania-Added Nafion™ Membranes for Fuel Cell Applications
Abstract
:1. Introduction
2. Material and Methods
2.1. Membrane Preparation
2.2. Characterization Techniques
3. Results and Discussion
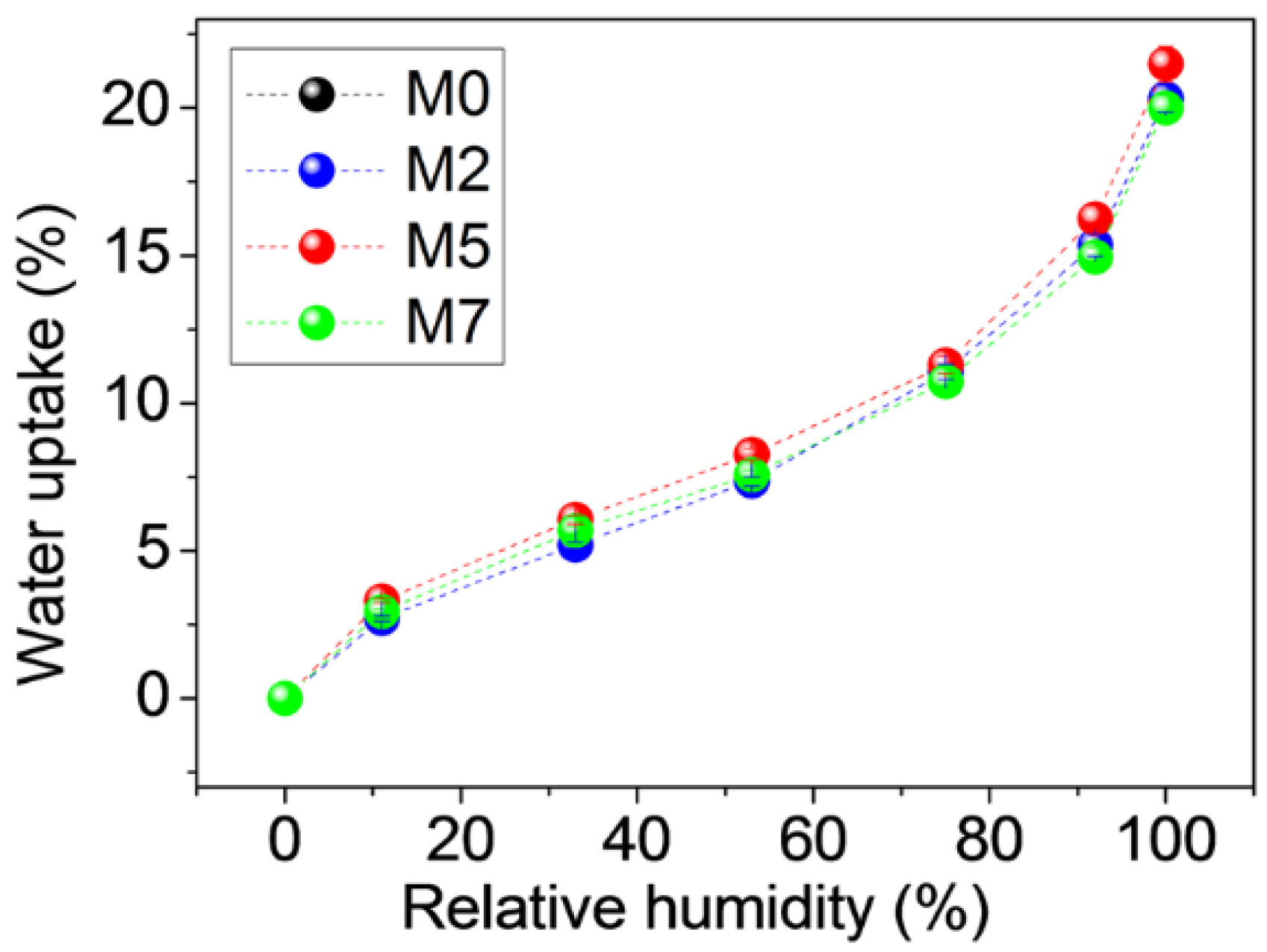
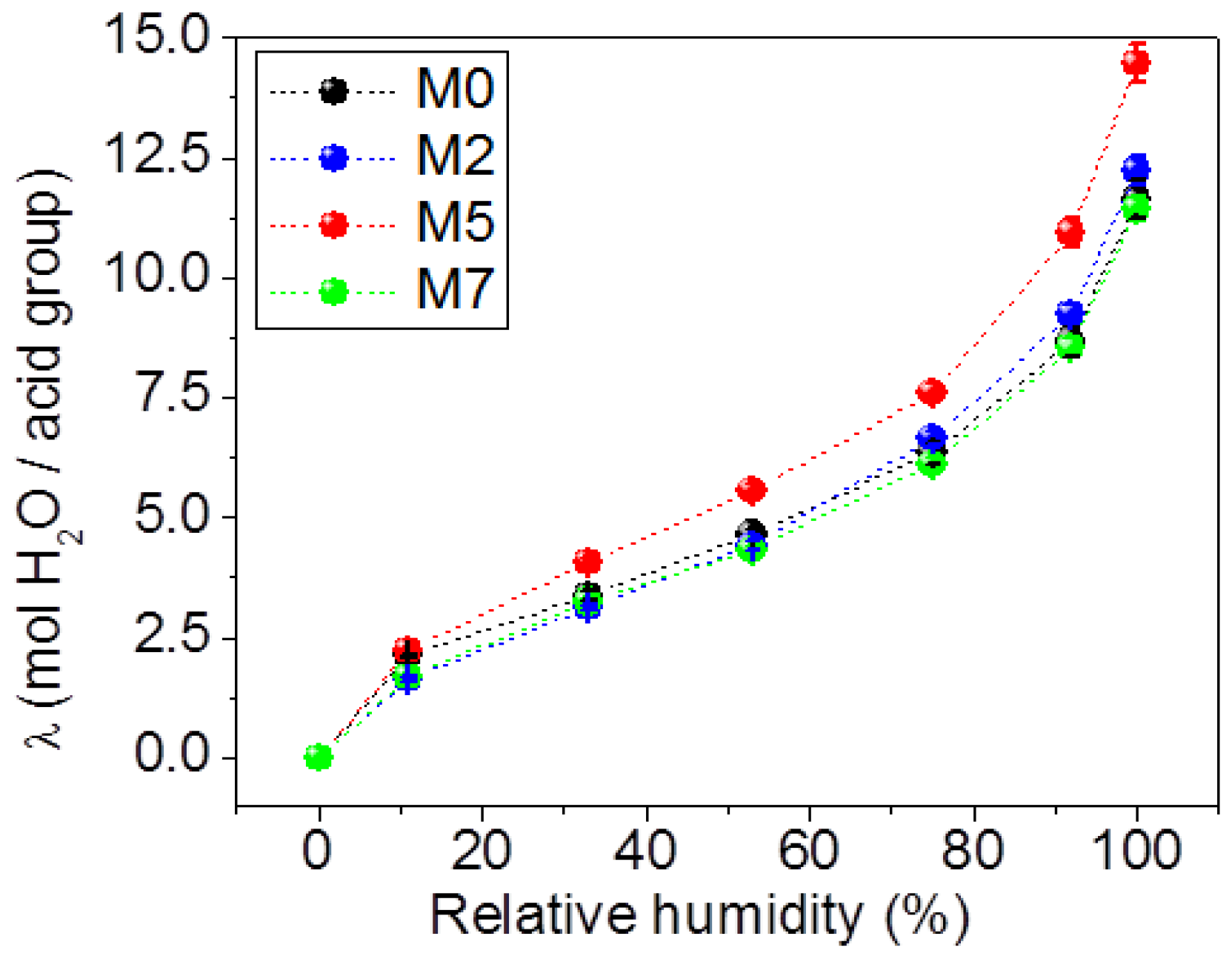
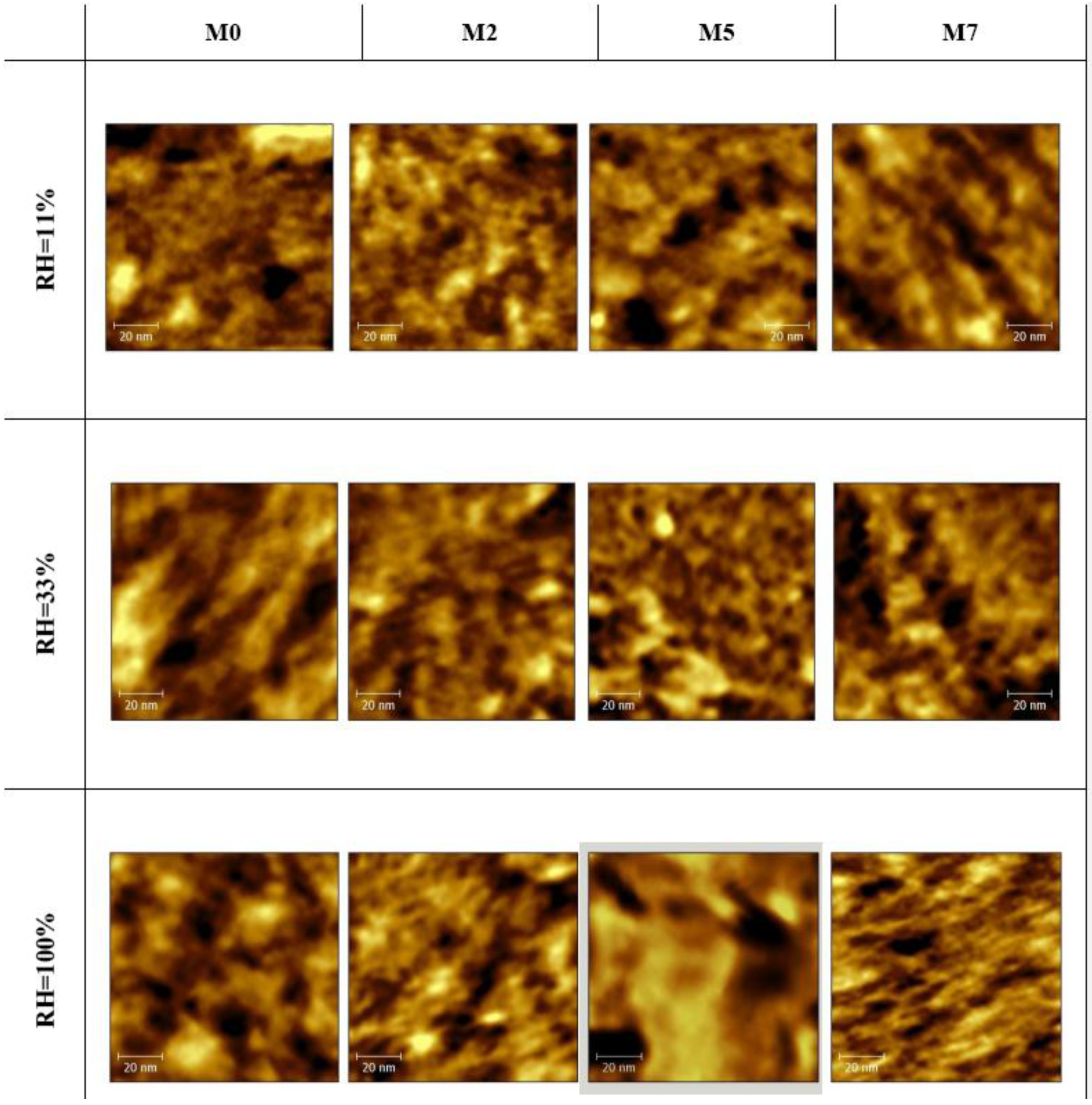
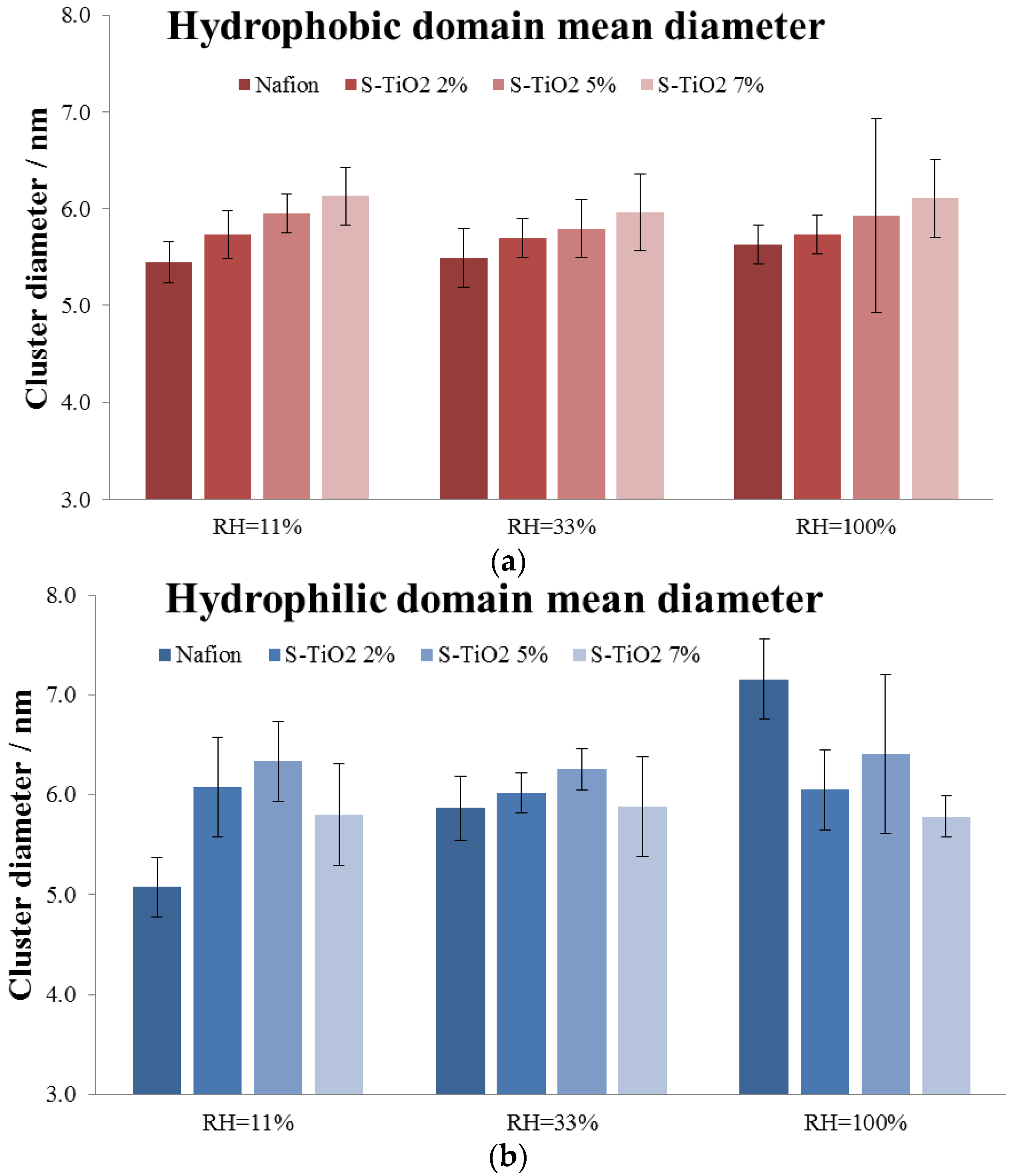
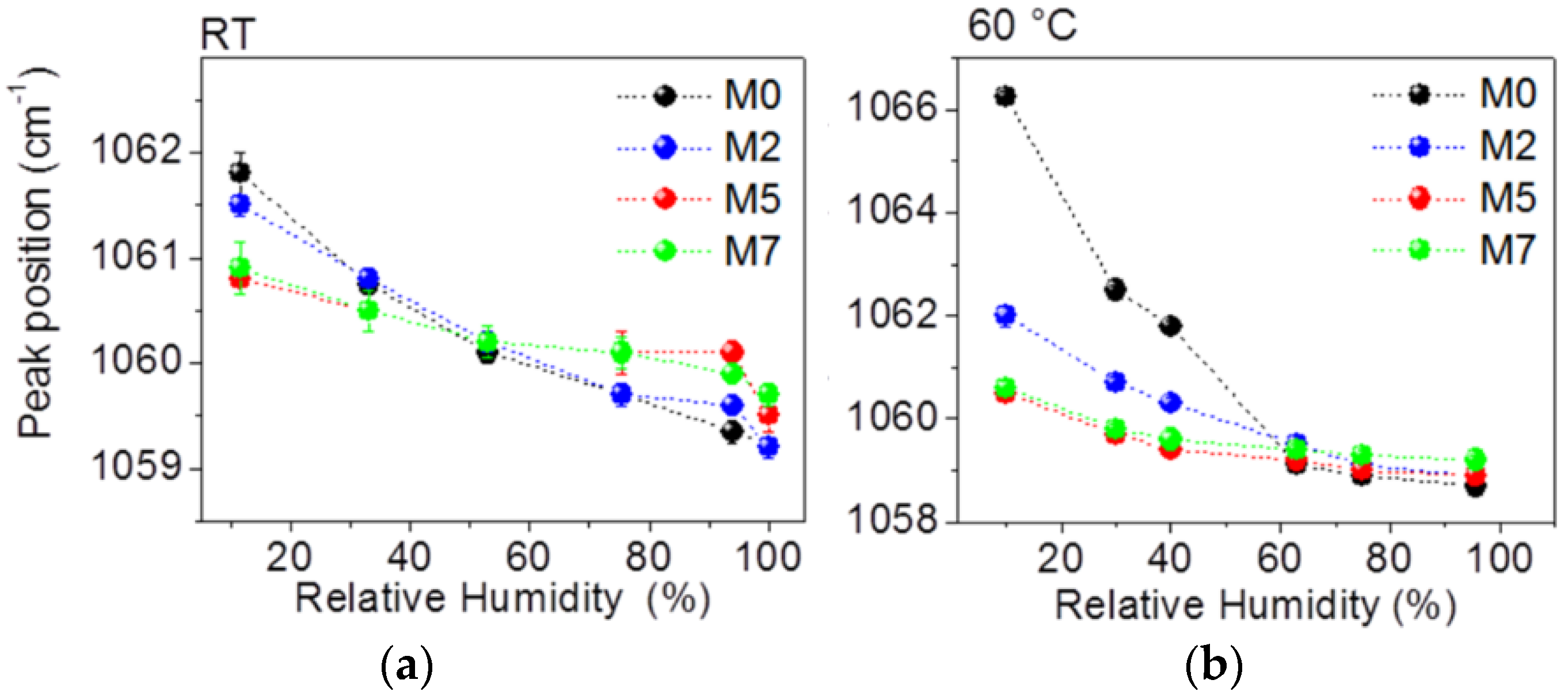
3.1. Water Absorption and Morphological Characterization of the Membranes
- (1)
- An increase in the hydrophobic domain size at increasing oxide loading, independent on the membrane hydration. In particular, the sizes of the hydrophobic domains are very similar for all samples and range between 5.5 nm and 6.2 nm for all the membranes at all RH conditions.
- (2)
- A non-monotonic trend for the hydrophilic domain size at increasing oxide loading and RH condition.
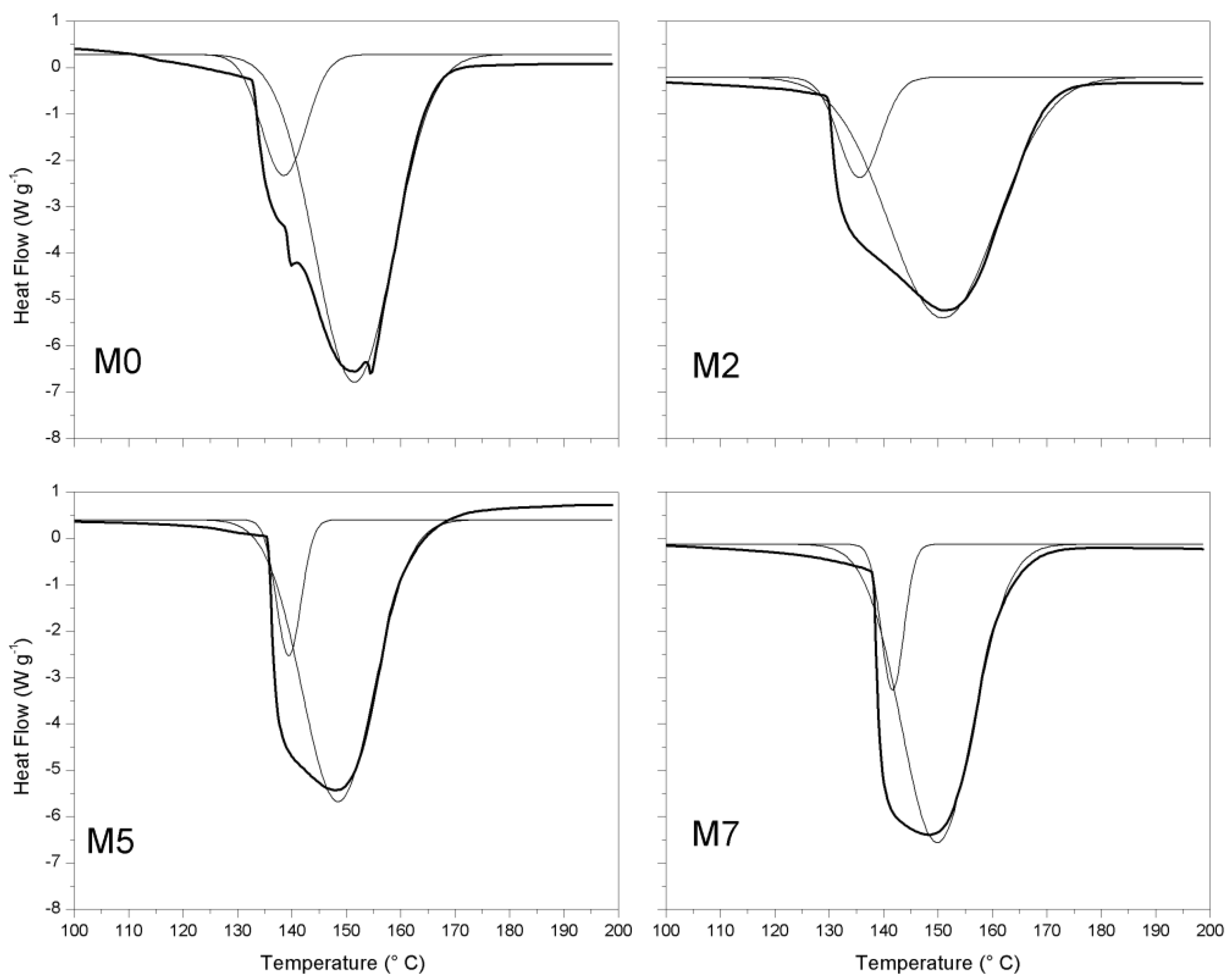
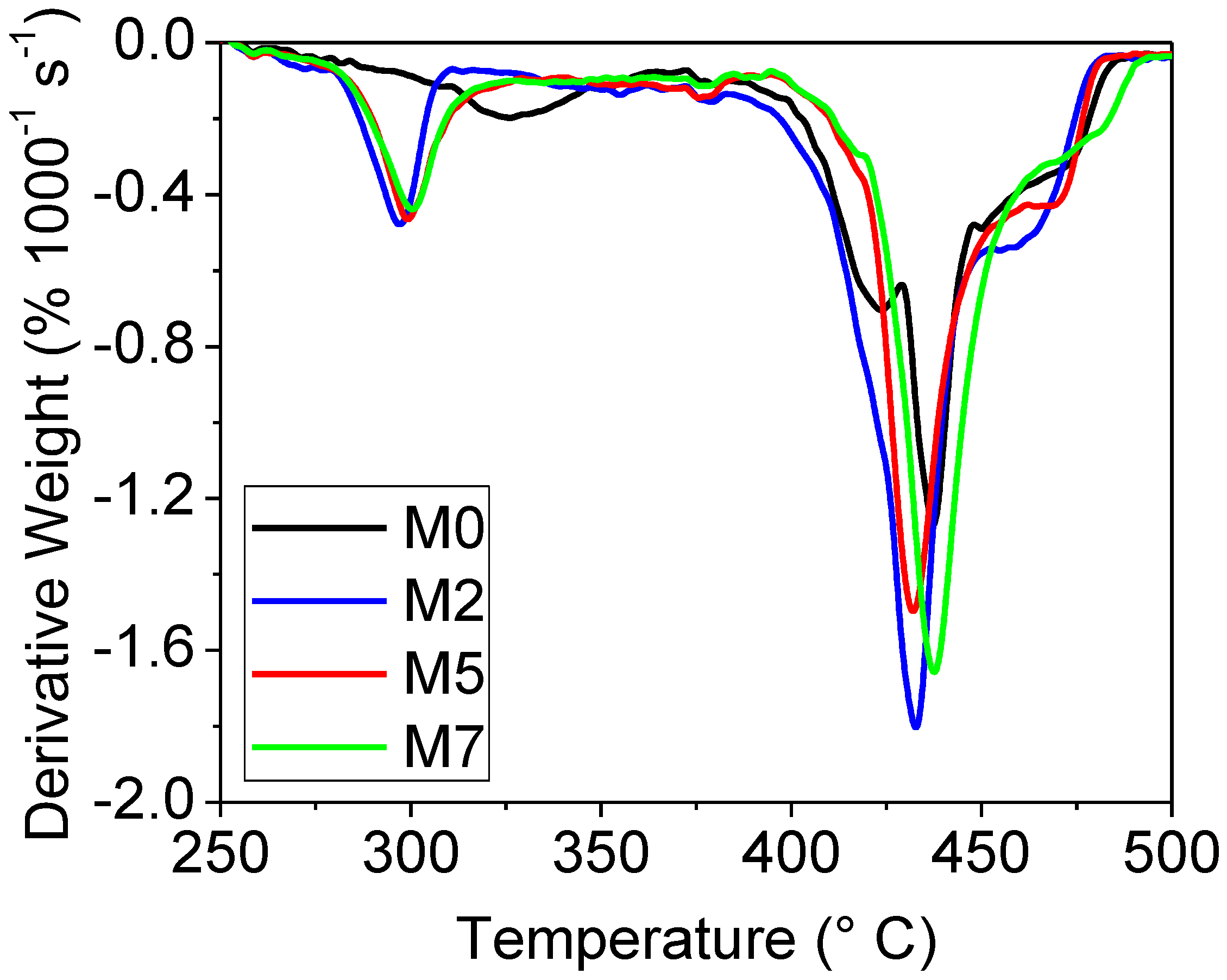
3.2. Thermal Properties of the Membranes
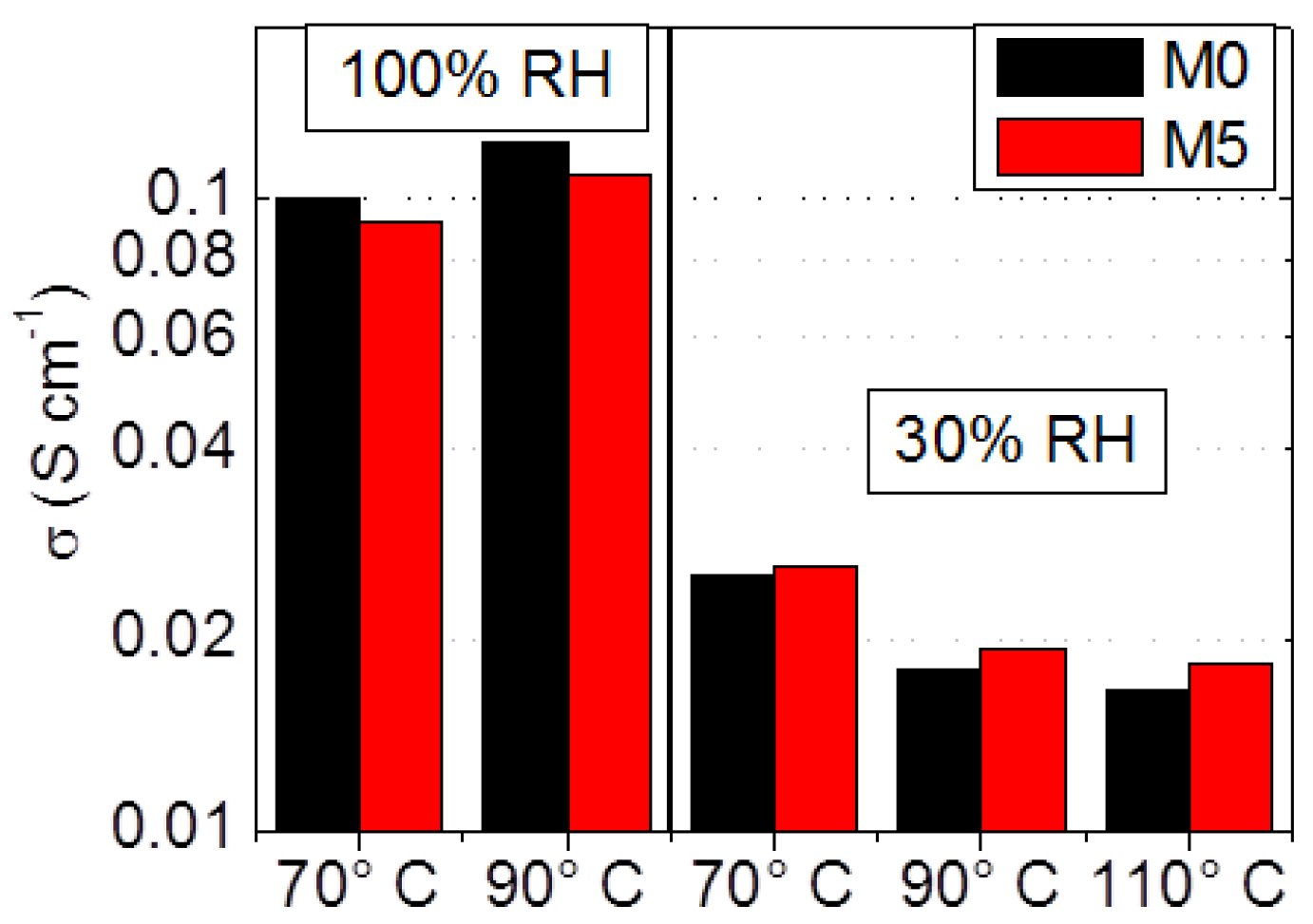
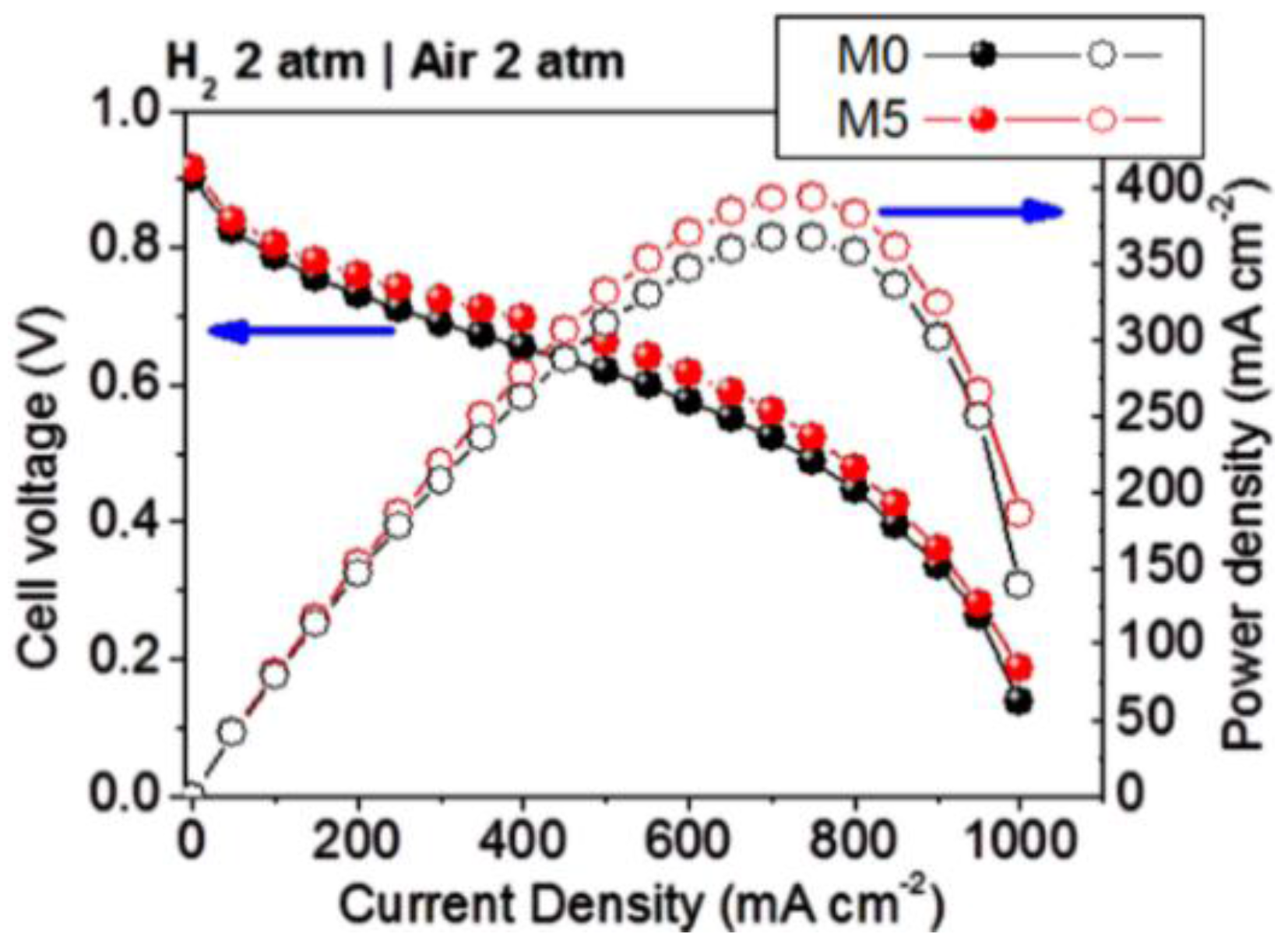
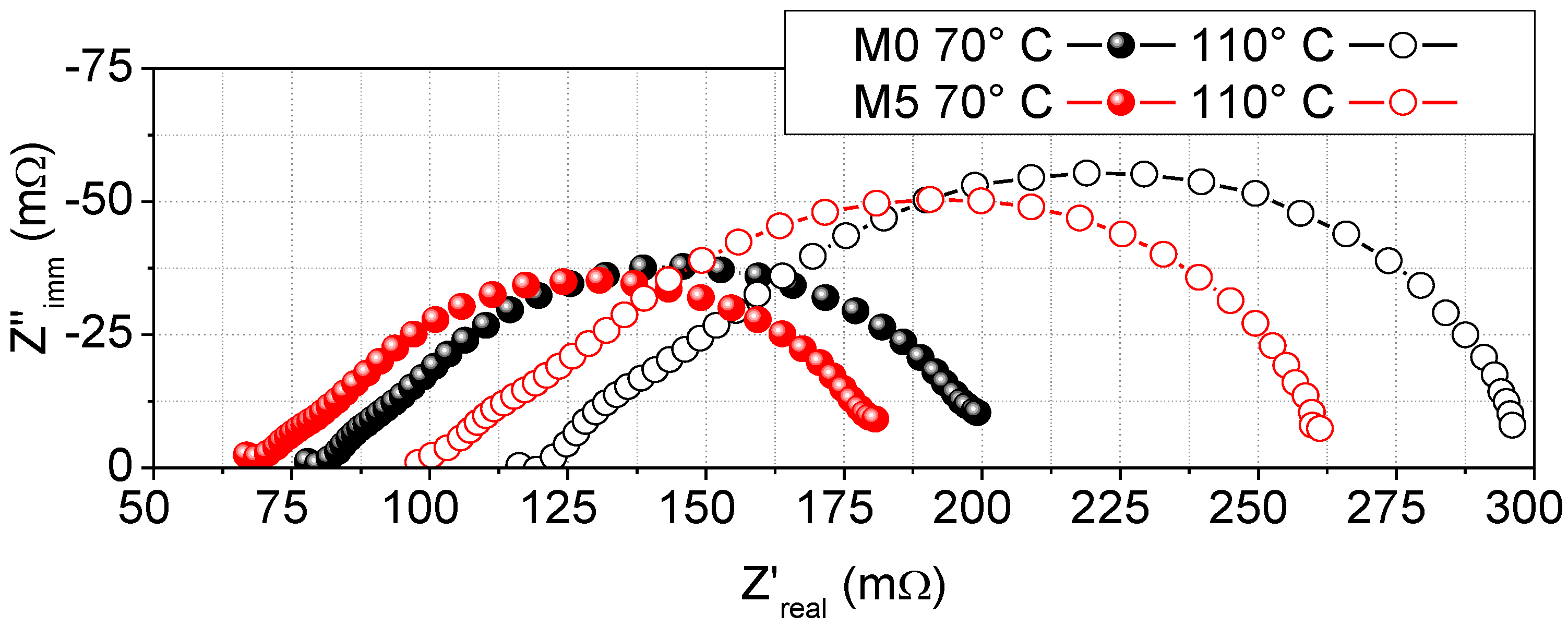
3.3. Ionic Conductivity and Hydrogen Fuel Cells Performances
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, Q.; Jensen, J.O.; Savinell, R.F.; Bjerrum, N.J. High temperature proton exchange membranes based on polybenzimidazoles for fuel cells. Prog. Polym. Sci. 2009, 34, 449–477. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Xie, Z.; Zhang, J.; Tang, Y.; Song, C.; Navessin, T.; Shi, Z.; Song, D.; Wang, H.; Wilkinson, D.P.; et al. High temperature PEM fuel cells. J. Power Sources 2006, 160, 872–891. [Google Scholar] [CrossRef]
- Springer, T.E.; Zawodzinski, T.A.; Gottesfeld, S. Polymer electrolyte fuel cell model. J. Electrochem. Soc. 1991, 138, 2334–2342. [Google Scholar] [CrossRef]
- Sumner, J.J.; Creager, S.E.; Ma, J.J.; DesMarteau, D.D. Proton conductivity in Nafion® 117 and in a novel bis[(perfluoroalkyl)sulfonyl]imide ionomer membrane. J. Electrochem. Soc. 1998, 145, 107–110. [Google Scholar] [CrossRef]
- Malhotra, S.; Datta, R. Membrane-supported nonvolatile acidic electrolytes allow higher temperature operation of proton-exchange membrane fuel cells. J. Electrochem. Soc. 1997, 144, 23–26. [Google Scholar] [CrossRef]
- Giffin, G.A.; Piga, M.; Lavina, S.; Navarra, M.A.; D’Epifanio, A.; Scrosati, B.; Di Noto, V. Characterization of sulfated-zirconia/Nafion® composite membranes for proton exchange membrane fuel cells. J. Power Sources 2012, 198, 66–75. [Google Scholar] [CrossRef]
- Wu, Y.N.; Liao, S.J. Review of SO42−/MxOy solid superacid catalysts. Front. Chem. Eng. China 2009, 3, 330–343. [Google Scholar] [CrossRef]
- Hino, M.; Arata, K.J. Synthesis of solid superacid catalyst with acid strength of H0 ≤ –16.04. J. Chem. Soc. Chem. Commun. 1980, 18, 851–852. [Google Scholar] [CrossRef]
- Reddy, B.M.; Patil, M.K. Organic syntheses and transformations catalyzed by sulfated zirconia. Chem. Rev. 2009, 109, 2185–2208. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.P.; Yang, R.T. Selective catalytic reduction of no with NH3 on SO4−2/TiO2 superacid catalyst. J. Catal. 1993, 139, 277–288. [Google Scholar] [CrossRef]
- Hino, M.; Arata, K. Catalytic activity of iron oxide treated with wulfate ion for dehydration of 2-propanol and ethanol and polymericazion of isobuthyl vinyl ether. Chem. Lett. 1979, 8, 477–480. [Google Scholar] [CrossRef]
- Arata, K.; Hino, M. Reaction of butane to isobutane catalyzed by the solid superacid of HfO2 treated with sulfate ion. React. Kinet. Catal. Lett. 1984, 25, 143–145. [Google Scholar] [CrossRef]
- Matsuhashi, H.; Hino, M.; Arata, K. Synthesis of solid superacid of silica treated with sulfuryl chloride. Catal. Lett. 1991, 8, 269–271. [Google Scholar] [CrossRef]
- Chavan, S.; Zubaidha, P.; Dantale, S.; Keshavaraja, A.; Ramaswamy, A.; Ravindranathan, T. Facile deprotection of allyl esters mediated by solid superacid (sulphated SnO2). Tetrahedron Lett. 1996, 37, 237–240. [Google Scholar] [CrossRef]
- Smirnova, M.; Toktarev, A.; Ayupov, A.; Echevsky, G. Sulfated alumina and zirconia in isobutane/butene alkylation and n-pentane isomerization: Catalysis, acidity, and surface sulfate species. Catal. Today 2010, 152, 17–23. [Google Scholar] [CrossRef]
- Navarra, M.A.; Croce, F.; Scrosati, B. New, high temperature superacid zirconia-doped Nafion™ composite membranes. J. Mater. Chem. 2007, 17, 3210–3215. [Google Scholar] [CrossRef]
- Wang, C.; Chalkova, E.; Lee, J.K.; Fedkin, M.V.; Komarneni, S.; Lvov, S.N. Composite membranes with sulfonic and phosphonic functionalized inorganics for reduced relative humidity PEM fuel cells. J. Electrochem. Soc. 2011, 158, B690–B697. [Google Scholar] [CrossRef]
- Branchi, M.; Sgambetterra, M.; Pettiti, I.; Panero, S.; Navarra, M.A. Functionalized Al2O3 particles as additives in proton-conducting polymer electrolyte membranes for fuel cell applications. Int. J. Hydrog. Energy 2015, 40, 14757–14767. [Google Scholar] [CrossRef]
- Scipioni, R.; Gazzoli, D.; Teocoli, F.; Palumbo, O.; Paolone, A.; Ibris, N.; Brutti, S.; Navarra, M.A. Preparation and characterization of nanocomposite polymer membranes containing functionalized SnO2 additives. Membranes 2014, 4, 123–142. [Google Scholar] [CrossRef] [PubMed]
- Brutti, S.; Scipioni, R.; Navarra, M.A.; Panero, S.; Allodi, V.; Giarola, M.; Mariotto, G. SnO2-Nafion® nanocomposite polymer electrolytes for fuel cell applications. Int. J. Nanothecnol. 2014, 11, 882–896. [Google Scholar] [CrossRef]
- Hwang, K.; Kim, J.; Kim, S.; Byun, H. Preparation of polybenzimidazole-based membranes and their potential applications in the fuel cell system. Energies 2014, 7, 1721–1732. [Google Scholar] [CrossRef]
- Ma, H.; Cheng, W.; Fang, F.; Hsu, C.; Lin, C. Compact design of 10 kW proton exchange membrane fuel cell stack systems with microcontroller units. Energies 2014, 7, 2498–2514. [Google Scholar] [CrossRef]
- Rabbani, A.; Rokni, M. Modeling and analysis of transport processes and efficiency of combined SOFC and PEMFC systems. Energies 2014, 7, 5502–5522. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Cho, C.D. Experimental investigation and discussion on the mechanical endurance limit of Nafion membrane used in proton exchange membrane fuel cell. Energies 2014, 7, 6401–6411. [Google Scholar] [CrossRef]
- Jang, H.; Sutradhar, S.; Yoo, J.; Ha, J.; Pyo, J.; Lee, C.; Ryu, T.; Kim, W. Synthesis and characterization of sulfonated poly(phenylene) containing a non-planar structure and dibenzoyl groups. Energies 2016, 9. [Google Scholar] [CrossRef]
- Liang, B.; Jiang, Q.B.; Tang, S.Q.; Li, S.L.; Chen, X. Porous polymer electrolytes with high ionic conductivity and good mechanical property for rechargeable batteries. J. Power Sources 2016, 307, 320–328. [Google Scholar] [CrossRef]
- Bella, F.; Colò, F.; Nair, J.; Gerbaldi, C. Photopolymer electrolytes for sustainable, upscalable, safe, and ambient-temperature sodium-ion secondary batteries. ChemSusChem 2015, 8, 3668–3676. [Google Scholar] [CrossRef] [PubMed]
- Imperiyka, M.; Ahmad, A.; Hanifah, S.A.; Bella, F. A UV-prepared linear polymer electrolyte membrane for dye-sensitized solar cells. Phys. B Condens. Matter 2014, 450, 151–154. [Google Scholar] [CrossRef]
- Anothumakkool, B.; Torris AT, A.; Veeliyath, S.; Vijayakumar, V.; Badiger, M.V.; Kurungot, S. High-performance flexible solid-state supercapacitor with an extended nanoregime interface through in situ polymer electrolyte generation. ACS Appl. Mater. Interfaces 2016, 8, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Sgambetterra, M.; Panero, S.; Hassoun, J.; Navarra, M.A. Hybrid membranes based on sulfated titania nanoparticles as low-cost proton conductors. Ionics 2013, 19, 1203–1206. [Google Scholar] [CrossRef]
- Allodi, V.; Brutti, S.; Giarola, M.; Sgambetterra, M.; Navarra, M.A.; Panero, S.; Mariotto, G. Structural and spectroscopic characterization of sulfated TiO2-added nanocomposite Nafion membranes for fuel cell applications. Polymers 2016, 8. [Google Scholar] [CrossRef] [Green Version]
- Nicotera, I.; Kosma, V.; Simari, C.; Ranieri, G.A.; Sgambetterra, M.; Panero, S.; Navarra, M.A. An NMR study on the molecular dynamic and exchange effects in composite Nafion/sulfated titania membranes for PEMFCs. Int. J. Hydrog. Energy 2015, 40, 14651–14660. [Google Scholar] [CrossRef]
- Greenspan, L. Humidity fixed points of binary saturated aqueous solutions. J. Res. Natl. Bur. Stand. A Phys. Chem. 1977, 81A, 89–95. [Google Scholar] [CrossRef]
- Adjemian, K.T.; Dominey, R.; Krishnan, L.; Ota, H.; Majsztrik, P.; Zhang, T.; Mann, J.; Kirby, B.; Gatto, L.; Velo-Simpson, M.; et al. Function and characterization of metal oxide−Nafion composite membranes for elevated-temperature H2/O2 PEM fuel cells. Chem. Mater. 2006, 18, 2238–2248. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, G.; Jin, W.; Hou, H.; Wang, S.; Xin, Q. Nafion® and nano-size TiO2–SO42− solid superacid composite membrane for direct methanol fuel cell. J. Membr. Sci. 2008, 313, 336–343. [Google Scholar] [CrossRef]
- Kreuer, K.D. The role of internal pressure for the hydration and transport properties of ionomers and polyelectrolytes. Solid State Ion. 2013, 252, 93–101. [Google Scholar] [CrossRef]
- Bussian, D.A.; O’Dea, J.R.; Metiu, H.; Buratto, S.K. Nanoscale current imaging of the conducting channels in proton exchange membrane fuel cells. Nano Lett. 2007, 7, 227–232. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Kusoglu, A.; Lucas, I.T.; Clark, K.; Weber, A.Z.; Kostecki, R. Correlating humidity-dependent ionically conductive surface area with transport phenomena in proton-exchange membranes. J. Phys. Chem. B 2011, 115, 11650–11657. [Google Scholar] [CrossRef] [PubMed]
- James, P.J.; Antognozzi, M.; Tamayo, J.; McMaster, T.J.; Newton, J.M.; Miles, M.J. Interpretation of Contrast in Tapping Mode AFM and Shear Force Microscopy. A Study of Nafion. Langmuir 2001, 17, 349–360. [Google Scholar] [CrossRef]
- James, P.J.; Elliot, J.A.; McMaster, T.J.; Newton, J.M.; Elliott, A.M.S.; Hanna, S.; Miles, M.J. Hydration of Nafion studied by AFM and X-ray scattering. J. Mater. Sci. 2000, 35, 5111–5119. [Google Scholar] [CrossRef]
- Kunimatzu, K.; Bae, B.; Miyatake, K.; Uchida, H.; Watanabe, M. ATR-FTIR study of water in Nafion membrane combined with proton conductivity measurements during hydration/dehydration cycle. J. Phys. Chem. B 2011, 115, 4315–4321. [Google Scholar] [CrossRef] [PubMed]
- Haraa, M.; Inukai, J.; Miyatake, K.; Uchidaa, H.; Watanabe, M. Temperature dependence of the water distribution inside a Nafion membrane in an operating polymer electrolyte fuel cell. A micro-Raman study. Electrochim. Acta 2011, 58, 449–455. [Google Scholar] [CrossRef]
- De Almeida, S.H.; Kawano, Y. Thermal behavior of Nafion membranes. J. Therm. Anal. Calorim. 1999, 58, 569–577. [Google Scholar] [CrossRef]
- Navarra, M.A.; Abbati, C.; Scrosati, B. Properties and fuel cell performance of a Nafion-based, sulfated zirconia-added, composite membrane. J. Power Sources 2008, 183, 109–113. [Google Scholar] [CrossRef]
- Guo, B.; Tay, S.W.; Liu, Z.; Hong, L. Assimilation of highly porous sulfonated carbon nanospheres into Nafion® matrix as proton and water reservoirs. Int. J. Hydrog. Energy 2012, 37, 14482–14491. [Google Scholar] [CrossRef]
- Gierke, T.D.; Munn, E.; Wilson, F.C. The morphology in Nafion† perfluorinated membrane products, as determined by wide- and small-angle X-ray studies. J. Polym. Sci. Polym. Phys. Ed. 1981, 19, 1687–1704. [Google Scholar] [CrossRef]
- Samms, S.R.; Wasmus, S.; Savinell, R.F. Thermal stability of Nafion® in simulated fuel cell environments. J. Electrochem. Soc. 1996, 143, 1498–1504. [Google Scholar] [CrossRef]
- Rhee, C.H.; Kim, Y.; Lee, J.S.; Kim, H.K.; Chang, H. Nanocomposite membranes of surface-sulfonated titanate and Nafion® for direct methanol fuel cells. J. Power Sources 2006, 159, 1015–1024. [Google Scholar] [CrossRef]
- Zawodzinski, T.A.; Neeman, M.; Sillerud, L.O.; Gottesfeld, S. Determination of water diffusion coefficients in perfluorosulfonate ionomeric membranes. J. Phys. Chem. 1991, 95, 6040–6044. [Google Scholar] [CrossRef]
- Zawodzinski, T.A.; Derouin, C.; Radzinski, S.; Sherman, R.J.; Smith, V.T.; Springer, T.E.; Gottesfeld, S. Water uptake by and transport through Nafion® 117 membranes. J. Electrochem. Soc. 1993, 140, 1041–1047. [Google Scholar] [CrossRef]

| Salts for Saturated Aqueous Solution | RH | |
|---|---|---|
| 25 °C | 60 °C | |
| LiCl | 11% | 11% |
| MgCl2 | 33% | 30% |
| Mg(NO3)2 | 53% | 40% |
| NaCl | 75% | 62% |
| KNO3 | 94% | 75% |
| K2SO4 | - | 96% |
| H2O | 100% | - |
| Sample | Filler content a | l b | IEC c |
|---|---|---|---|
| M0 | - | 100 | 0.97 ± 0.02 |
| M2 | 2 | 95 | 0.92 ± 0.01 |
| M5 | 5 | 95 | 0.82 ± 0.01 |
| M7 | 7 | 90 | 0.97 ± 0.02 |
| Sample | Tonset a (°C) | ΔH b (J gpol−1) |
|---|---|---|
| M0 | 130 ± 1 | 468 ± 12 |
| M2 | 132 ± 1 | 450 ± 11 |
| M5 | 135 ± 1 | 410 ± 9 |
| M7 | 137 ± 2 | 408 ± 9 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sgambetterra, M.; Brutti, S.; Allodi, V.; Mariotto, G.; Panero, S.; Navarra, M.A. Critical Filler Concentration in Sulfated Titania-Added Nafion™ Membranes for Fuel Cell Applications. Energies 2016, 9, 272. https://doi.org/10.3390/en9040272
Sgambetterra M, Brutti S, Allodi V, Mariotto G, Panero S, Navarra MA. Critical Filler Concentration in Sulfated Titania-Added Nafion™ Membranes for Fuel Cell Applications. Energies. 2016; 9(4):272. https://doi.org/10.3390/en9040272
Chicago/Turabian StyleSgambetterra, Mirko, Sergio Brutti, Valentina Allodi, Gino Mariotto, Stefania Panero, and Maria Assunta Navarra. 2016. "Critical Filler Concentration in Sulfated Titania-Added Nafion™ Membranes for Fuel Cell Applications" Energies 9, no. 4: 272. https://doi.org/10.3390/en9040272
APA StyleSgambetterra, M., Brutti, S., Allodi, V., Mariotto, G., Panero, S., & Navarra, M. A. (2016). Critical Filler Concentration in Sulfated Titania-Added Nafion™ Membranes for Fuel Cell Applications. Energies, 9(4), 272. https://doi.org/10.3390/en9040272








