Abstract
This paper reviews the serviceability of hydrous ethanol as a clean, cheap and green renewable substitute fuel for spark ignition engines and discusses the comparative chemical and physical properties of hydrous ethanol and gasoline fuels. The significant differences in the properties of hydrous ethanol and gasoline fuels are sufficient to create a significant change during the combustion phase of engine operation and consequently affect the performance of spark-ignition (SI) engines. The stability of ethanol-gasoline-water blends is also discussed. Furthermore, the effects of hydrous ethanol, and its blends with gasoline fuel on SI engine combustion characteristics, cycle-to-cycle variations, engine performance parameters, and emission characteristics have been highlighted. Higher water solubility in ethanol‑gasoline blends may be obviously useful and suitable; nevertheless, the continuous ability of water to remain soluble in the blend is significantly affected by temperature. Nearly all published engine experimental results showed a significant improvement in combustion characteristics and enhanced engine performance for the use of hydrous ethanol as fuel. Moreover, carbon monoxide and oxides of nitrogen emissions were also significantly decreased. It is also worth pointing out that unburned hydrocarbon and carbon dioxide emissions were also reduced for the use of hydrous ethanol. However, unregulated emissions such as acetaldehyde and formaldehyde were significantly increased.
1. Introduction
With the development of the global economy the consumption of crude oil products is currently increasing rapidly. The problems resulting from the prominent use of fossil fuels, such as global warming, depletion of fossil fuel resources, and environmental deterioration have become serious challenges threatening the continuous development and sustainable progress of human society. Therefore, research activities have been focused on the search for alternative energy sources for sustainable development of the global economy and society [1]. The promising substitutes for petroleum fuels include bio-fuels, mainly, biodiesel and ethanol [2].
Ethanol is a renewable energy source and can be produced from many bio-sources such as sugar cane, grain straw, maize and brown seaweed [3]. Ethanol, like most short-chain alcohols, is a flammable, volatile, and colorless liquid, having a chemical formula of C2H5OH. In general, ethanol burns cleaner than petroleum fuel. Current challenges for the ethanol industry include ways of sustaining the boost in production efficiency and yields, as well as the need to boost production from readily available and low-cost biomass feedstocks. The use of ethanol as fuel has the potential of reducing greenhouse gas emissions, as the plants used for its production absorb carbon dioxide (CO2) during the growth process. In recent times, ethanol has been commonly used as a fuel additive or an alternative fuel in spark ignition (SI) engines and compression-ignition (diesel) engines. The use of ethanol, particularly in SI engines, has become attractive due to its relatively high octane number and the fact that it burns clean [4,5]. By the start of the 21st century, large scale commercial use of ethanol as a fuel had started. Currently, there are three ways of using ethanol fuel in SI engines: the use of pure ethanol, ethanol-gasoline blends and the use of gasoline-ethanol dual-fuel port injection systems.
There are two types of ethanol that are used as blend stocks for gasoline fuel with every type of ethanol being defined according to whether it is one of two types: (i) Anhydrous ethanol and (ii) hydrous ethanol. The manufacture of anhydrous ethanol (water content less than 1%) is expensive as a large amount of energy is needed during the distillation and dehydration process involved in the production of the ethanol. It has been noticed that removing the last 5% of water from ethanol comes at huge energy costs. Hence the direct use of hydrous ethanol as a fuel would significantly improve the overall energy efficiency, making it more attractive as a fuel source [6,7,8,9]. It has been proposed that 37% of the production costs for anhydrous ethanol are related to water distillation and dehydration [10,11,12,13,14,15].
There are numerous research works and reviews regarding ethanol production [1,2,16,17,18,19,20,21,22,23,24,25], and its use in a gasoline engine [26,27]. A number of review articles have also presented the effect of ethanol-gasoline blends in SI engines in terms of combustion, performance, and emission characteristics [28,29,30,31,32]. However, a study of literature indicated that there was no separate review on hydrous ethanol fuel and its use in SI engines, and which comprehensively reported hydrous ethanol gasoline blend stability, combustion, engine performance and emission.
This review, therefore, discusses recent research on the stability of hydrous ethanol-gasoline blends. Furthermore, the study also outlines the effects of hydrous ethanol and its blends with gasoline fuel in spark ignition engines, with a focus on combustion characteristics, performance and emissions.
2. Properties of Hydrous Ethanol Fuel
The efficient burning of fuel in an engine mainly depends on the fuel’s physical and chemical properties. The physical and chemical properties that influence the performance of an engine using hydrous and anhydrous ethanol are given in Table 1.The comparative qualities of hydrous ethanol, anhydrous ethanol and gasoline are presented as follows:
- Using hydrous ethanol with gasoline can decrease operating costs of petroleum refineries, as these refineries could produce low-grade gasoline with a lower octane number.
- On the combustion characteristics, the flash point and auto-ignition temperature of hydrous ethanol is higher than anhydrous ethanol and gasoline, a characteristic that makes it relatively safer to store and transport.
- The latent heat of evaporation of hydrous ethanol is higher than anhydrous ethanol and gasoline; this causes the temperature of the intake manifold to be reduced, as a consequence of the larger magnitude of heat transfer from the intake manifold to the fuel during the evaporation process. This ultimately leads to an increased volumetric efficiency.
- The heating value of hydrous ethanol is lower than anhydrous ethanol and gasoline and therefore needs extra hydrous ethanol to produce the same level energy.
- The stoichiometric air–fuel ratio of hydrous ethanol is lower than the anhydrous ethanol and gasoline; therefore the required amount of air for complete combustion is lower for hydrous ethanol.
- The lower C/H atom ratio of hydrous ethanol decreases the adiabatic flame temperature.
- Hydrous ethanol has a higher octane number than anhydrous ethanol and gasoline. The higher the octane number, the higher the compression ratio that can be used without detonation.
- Hydrous ethanol has a higher laminar flame propagation speed than anhydrous ethanol and gasoline, which makes the combustion process end earlier and consequently improving the engine thermal efficiency. However, experimental results reported by Bradley et al. [33] indicate that the flame speed for hydrous ethanol was higher than that of anhydrous ethanol for low equivalence ratios. On the other hand, the flame speed of anhydrous ethanol remained higher for higher equivalence ratios and certain initial fuel droplet diameters, and for all the cases considered.

Table 1.
Comparison of gasoline, anhydrous ethanol and hydrous ethanol fuel properties.
3. Stability of Ethanol-Gasoline-Water Blends
An ethanol-gasoline blend is basically immiscible with water, although ethanol can be blended with both water and gasoline due to its polar and non-polar groups [36]. As water dissolves in gasoline, the highest amount of water that gasoline can efficiently absorb is reached, implying that with the equilibrium being attained at this point, any extra water will not be dissolved. This situation leads to the formation of two separate phases of different ethanol compositions. The solubility of water and the possibility of phase separation in alcohol‑gasoline blends would depend on the following factors: (i) The proportions of gasoline and alcohol; (ii) The fuel temperature; (iii) The composition of gasoline; (iv) The polarity of the alcohol and (iiv) the pressure of the fuel system [44,45,46,47,48].
Some studies have been conducted to investigate the stability of ethanol-gasoline-water mixtures and the identification of suitable co-solvents. Liu et al. [49] studied the stability of E10 hydrous ethanol-gasoline blends, via the application of a self-advanced composite emulsifier. According to the experimental results, it was stated that the stability of hydrous ethanol-gasoline blends can be enhanced effectively by using the emulsification approach involving the addition of intensifier and antifreeze. The increased ethanol concentration enhanced its low temperature stability. Kyriakides et al. [50] studied a gasoline-ethanol-water blend for stability at various temperatures (2, 10 and 18 °C) for three different water qualities, two gasoline compositions and three different additive types. The results showed the water tolerance in ethanol-gasoline blends was not affected by water quality. The study used tert-amyl methyl ether (TAME) or methyl-tert-butyl ether (MTBE) as additives. The use of additives indicated that it enhanced water tolerance in gasoline-ethanol blends. An amount of 2 vol/vol% of palmitic acid was sufficient to increase the water tolerance by up to 1% at a temperature of 16 °C.
Karaosmanoglu et al. [51] also studied the stability of gasoline-ethanol-water mixtures prepared by blending unleaded gasoline with 5%, 10%, 15%, and 20% (v/v) hydrous ethanol, respectively, and examined the link between the phase separation temperatures and the chemical compositions of gasoline. Four types of gasoline with 27.20%, 39.02%, 48.80%, and 59.94% (wt/wt) aromatic hydrocarbons, respectively, were used. The fuel blends obtained using these gasoline samples were referred to as B1, B2, B3, and B4 (see Table 2). Molasses fusel oil fraction (FOF) was used as additive by percentages in the order of 0%, 1%, and 3%. The results showed that increasing the amount of the fusel oil fraction in the hydrous ethanol-gasoline blend decreased the phase separation temperature of the blends. Furthermore, the chemical composition of the gasoline used is a significant factor for the stability of ethanol-gasoline blends. Higher aromatic content of gasoline leads to lower phase separation temperatures. It is worth pointing out that increasing the aromatic content in gasoline may increase the total emissions of HC, NOX and benzene [52,53].

Table 2.
Phase separation temperatures for ethanol-water-gasoline blends having different gasoline compositions. Reprinted and adapted with permission from Ref. [51]. Copyright (1996) American Chemical Society.
Rajan and Saniee [54] studied the miscibility characteristics of hydrous ethanol blended with gasoline as a way of reducing the cost of ethanol-gasoline blends for use as fuel for an SI engine by using a fixed percentage of water in ethanol. The empirical data showed that for a stable mixture, a reasonably small amount of gasoline, by volume, could be added. Muzikova et al. [55] also investigated the phase stability of gasoline blends with ethanol. The results showed that the ETBE can affect the blending ethanol with gasoline. ETBE reduced the phase separation temperature of the ethanol-gasoline blends. Fuel additives like methanol and n-butanol can help make the blends stable [56,57]. Ye et al. [58] studied the stability of an E10 hydrous ethanol-gasoline blend (5% water content in ethanol) by using emulsification technology. The results showed that with the addition of 2%–2.5% SPY emulsifier (self-advanced composite emulsifier), the blend remained stable for more than 30 days at a temperature ranging between −10 and 40 °C. About 12% water, by volume, can be added to E85 before phase separation occurs. In summary, higher water solubility in ethanol-gasoline blends may be useful and suitable. However, the ability to keep water in soluble in the blend is affected by temperature by a large extent. It is also worth pointing out that the solubility of water in the blend could be improved by using additives.
The use of ethanol-gasoline-water blended fuels as an alternative fuel has some limitations. The volatility problem can also be closely attributed to the high latent heat of vaporization of ethanol, which is a problem in cold weather conditions and will lead to difficulties in starting the engine from cold [59]. Additionally, for a two stroke engine, it has been demonstrated by Korotney [60] that if there is phase separation, the ethanol and water phases will compete for running through the engine with the other gasoline phase, and this will result in reduced lubrication and could have a damaging effect on the engine. Water has an undesirable effect on the engine. However, small amounts of water in solution with gasoline cannot cause any serious damage [61].
4. Combustion Characteristics and Performance of Hydrous Ethanol Fuel in Spark-Ignition Engines
4.1. Combustion Characteristics and Performance of Hydrous Ethanol Fuel Compared to Gasoline
The effects of hydrous ethanol and its blends with gasoline fuel on the combustion characteristics and performance of SI engines compared with gasoline fuel are discussed in this section, the summary of which is presented in Table 3 for different engine types, test conditions, and fuel blends.

Table 3.
Combustion and performance of hydrous ethanol fuel and its blends with gasoline in SI engine.
Experimental work carried out by Schifter et al. [6] investigated the effect of using gasoline‑ethanol mid-level blends (10%–40% anhydrous or hydrous (4% water content in ethanol)), in a single-cylinder SI engine. The results showed that higher pressures and lower intake manifold temperatures were obtained with the use of hydrous ethanol blends in comparison with the use of anhydrous ethanol blends and led to increased volumetric efficiency. Furthermore, hydrous ethanol blended gasoline decreased the combustion speed, combustion duration, and the heat release rate. In addition, the results revealed an increase in the indicated mean effective pressure (IMEP) for hydrous ethanol blends over anhydrous ethanol blends. For rich air-fuel ratios, the use of hydrous ethanol results in higher power outputs than anhydrous ethanol. Moreover, the fuel consumption by mass slightly increased using the hydrous ethanol. In the work of Clemente et al. [8], the peak torque and peak power for hydrous ethanol (7% water concentration) was enhanced by 9% and 14% respectively in comparison with the blend of gasohol (22% ethanol and 78% gasoline). The increase in peak torque and peak power output for hydrous ethanol is related to one reason, hydrous ethanol allows working with more advanced ignition points aiming for maximum brake torque (MBT) conditions, so a mixture with 78% gasoline was knock limited, while hydrous ethanol worked at the MBT operation point. The authors also described that the specific fuel consumption for hydrous ethanol was increased by 35% compared with the E22 fuel, because hydrous ethanol has a lower heating value than E22, with the brake mean effective pressure (BMEP) also being enhanced for hydrous ethanol (see Figure 1).
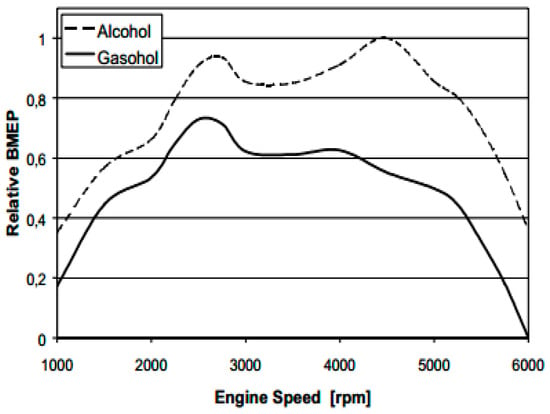
Figure 1.
Variation of brake mean effective pressure versus engine speed. Reprinted with permission from Ref. [8]. Copyright (2007) SAE International.
Olberding et al. [10] tested a transit van modified to run on gasoline or ethanol‑water fueled blends. The use of a 70% ethanol and 30% water fuel blend caused a considerable increase in the brake thermal efficiency in comparison with the use of gasoline fuel and attributed this improvement to reduced heat transfer losses due to lower burned gas temperature. Costa et al. [36] observed that for a speed over 4000 rpm, the use of hydrous ethanol (6.8% water) resulted in higher torque and power as compared with the gasoline ethanol blend (E22). In addition, the specific fuel consumption for the use of hydrous ethanol was around 54% higher than that for the use of gasoline-ethanol fuel blends.
Furthermore, the BMEP was higher when the gasoline-ethanol blend was used as fuel at low engine speeds and for high engine speeds, a higher BMEP was obtained when hydrous ethanol fuel was used. This phenomenon can be explained by the enhanced air-fuel mixing mechanism and improved combustion process at higher speeds. In addition, the increased amount of H, O, OH radicals from water dissociation also enhance the combustion process which results in higher BMEP for hydrous ethanol. Wang et al. [38] investigated the effects of hydrous ethanol (containing 5% water by volume) gasoline on the combustion and emission characteristics of a port fuel injection gasoline engine. The test engine was fueled by commercial gasoline, which also was the base fuel for the E10 and HE10 fuels. The results showed that for all the operating conditions, the HE10 fuel showed higher peak in-cylinder pressures (see Figure 2) and peak heat release rates than was possible for the E10 fuel.
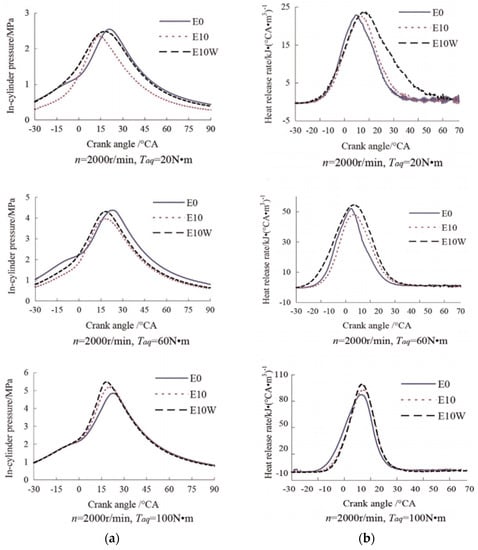
Figure 2.
The variation of (a) in-cylinder pressure (b) heat release rate with crank angle for a fixed speed of 2000 rpm, and engine torque of 20 Nm, 60 Nm, and 100 Nm. (E0 = 0% ethanol & 100% gasoline, E10W = HE10 = 10% hydrous ethanol & 90% gasoline, E10 = 10% ethanol & 90% gasoline) [38]. Copyright (2015) Elsevier Ltd.
The authors illustrated that water contained in hydrous ethanol directly led to a faster combustion and flame propagation and as well as an enhanced combustion process [38]. The combustion rate increased because of the improvement in chain reactions due to water addition was much more pronounced than the negative thermal effect for low percentages of water addition [62]. In addition, the increased amount of H, O, OH radicals from water dissociation also enhances the combustion process [63,64,65]. However, the effect of water on combustion characteristics of hydrous ethanol fuel is not fully understood. Further work on explain the physical and chemical kinetic roles of water on the combustion characteristics in engines is still in progress. It also found that at high load the peak in-cylinder pressure for HE10 was the higher than that of gasoline.
In an experimental study conducted by Melo et al. [39], a flex-fuel FIAT engine was tested by using hydrous ethanol gasoline blends to study the combustion and emissions characteristics of the engine. Gasoline (E25) was blended with hydrous ethanol by 30%, 50% and 80% volume. The results showed that the hydrous ethanol addition permitted the use of an over-advanced spark timing without the risk of knocking and as a consequence higher in-cylinder maximum pressures could be achieved. Melo et al. [66] tested a 1.4 liter, four-cylinder, flex-fuel engine, by applying different blends of gasoline and hydrous ethanol to investigate the effects of adding hydrous ethanol on the cycle-to-cycle variation (CCV) of the maximum in-cylinder pressure, IMEP and combustion duration parameters. The results showed that hydrous ethanol addition decreased the CCV for all the measured combustion parameters. Melo et al. [39,42] reported that as hydrous ethanol has a much lower heating value than gasoline, a higher specific fuel consumption was achieved with hydrous ethanol addition compared with gasoline. The energy efficiency increased for hydrous ethanol (HE100) as compared with gasoline (E25). Kyrialides et al. [50] also noticed an enhancement in torque for hydrous ethanol (HE40) over gasoline (E0). The fuel consumption was higher for HE40 due to the zero heating value of water. Venugopal et al. [67] found that for the lean combustion limit, hydrous ethanol gasoline blends performed better in terms of a lower COV of IMEP due to its larger flammability limit at part throttle operation compared to the use of gasoline. Furthermore, with lean mixtures at part throttle operation adapted for a port-injected engine fueled with 10% hydrous ethanol (containing 8.33% water by volume) gasoline blends produced a higher torque and power output compared with gasoline operation. In addition, the brake thermal efficiency for the use of hydrous ethanol blend (E10) was higher than gasoline (see Figure 3). These results may be attributed to the higher flame velocity of hydrous ethanol compare with gasoline.
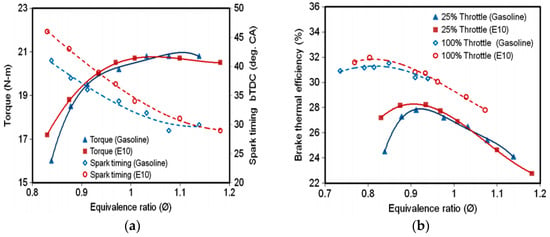
Figure 3.
The variation of (a) Torque (b) Brake thermal efficiency versus equivalence ratio, at a fixed speed of 2500 rpm and 25% throttle opening. Reprinted and adapted with permission from Ref. [67]. Copyright (2012) John Wiley and Sons.
Augoye and Aleiferis [68] studied a single-cylinder optical SI engine equipped with both Port Fuel Injection (PFI) and Direct Injection (DI). The content of hydrous ethanol and water in the fuel, by volume, was 6% and 10% respectively. iso-Octane and pure ethanol were used for the experiment. The results showed that both hydrous and anhydrous ethanol burned faster than gasoline and iso-octane for both PFI and DI operation (see Figure 4), the peak cylinder pressure and rate of combustion reduced with the water content in ethanol. This result may be attributed to low engine speed condition (1000 rpm) and used a fixed spark advance of 30° CA for all fuels. Furthermore, the COV in IMEP was larger for PFI engine than for the GDI engine for all fuels. Moreover, the hydrous ethanol reported higher COV in IMEP for the PFI engine.
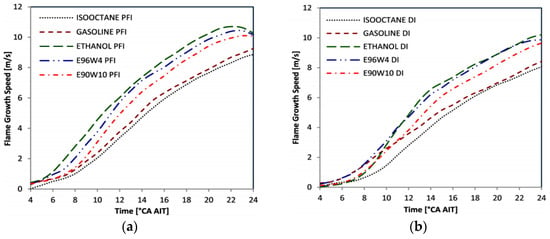
Figure 4.
Flame growth speed for (a) PFI and (b) DI operation, for the first 24° CA of combustion at a fixed engine speed of 1000 rpm and an equivalence ratio 1. Reprinted with permission from Ref. [68]. Copyright (2014) SAE International.
In another study, Chen et al. [69] conducted an experimental investigated on a four-cylinder SI engine equipped with PFI. It was found that for up to 14% water content in the fuel mixture, the torque reduced by approximately 10% for the use of aqueous alcohol. Amorim et al. [70] also conducted an experiment on a flex-fuel engine, with the engine being tested using CNG, 100% hydrous ethanol (containing 4% water by volume) and its 50% blend with gasoline E25 for a fixed compression ratio. The results of the experiment showed that hydrous ethanol produced the highest torque, power, and specific fuel consumption among the fuels. Costa et al. [71] conducted a study using a four cylinder (4C) flex fuel spark ignition (SI) engine. The torque and power for hydrous ethanol (water concentration 6.8%) was enhanced by 1.6% and 3.1% respectively at speed 4250 rev/min compared with the blend of 22% ethanol and 78% gasoline. The reason behind it is hydrous ethanol has a higher octane number than E22, when increasing the compression ratio the ignition timing had to be retarded to avoid knock for use E22, especially in the high engine speed. However, for low engine speed, torque and power were slightly lower when hydrous ethanol was used as fuel. The authors also described that increasing the compression ratio slightly decreased the SFC for hydrous ethanol and increased the brake thermal efficiency, it was also stated that increasing the CR slightly increased the BMEP for hydrous ethanol. It was concluded that compression ratio was a key parameter for improving brake thermal efficiency when using hydrous ethanol as fuel [71]. Zheng et al. [72] confirmed that CCV is lower with increased compression ratio, with the enhanced combustion characteristics. According to a study conducted by Young [73], there was 10% increase in power output for an identical fuel consumption and that a decrease in emissions from the engine could be achieved if the CCV could be reduced. The fuel type also affects the CCV and mainly influenced by the value of its laminar flame speed, the hydrous ethanol flame progresses much faster compared to gasoline. A higher combustion speed decreases the impact of turbulence and minimizes the CCV [74]. Addition of hydrous ethanol to gasoline will decrease the CCV in a spark-ignition engine.
In brief, as seen in Table 3, the engine speed, engine load, ignition time, and fuel blends type parameters had a significant effect on the engine combustion and performance. For gasoline engines retard spark timing at high load and speed to avoid knock, resulting in retarded combustion phasing. Blending hydrous ethanol with gasoline results in an increase in knock resistance [39]. The hydrous ethanol (containing lower water percentage by volume) gasoline fuel blends compared with the use of gasoline in SI engines increases the combustion efficiency [6,67], cylinder pressure [6,38,39,68], heat release rate [38], combustion temperature and cylinder temperature [6], and flame speed [67,68]. Furthermore, hydrous ethanol blended gasoline decreased the combustion duration [6], and reduced the cycle-to-cycle variation [66,67]. In addition, at low speed and low load conditions, the increased water content in ethanol fuel blends lead to reduce the peak cylinder pressure [68]. While, adding hydrous ethanol fuel to gasoline for use in SI engines slightly increases the engine torque [8,36,50,67,70,71], engine power [6,8,36,50,67,70,71], engine thermal efficiency [10,36,42,67,71], and brake mean effective pressure [6,36,71]. Clearly, Costa et al. [71] reported that increasing compression ratio slightly decreases SFC for hydrous ethanol compared to use of gasoline.
4.2. Combustion and Performance of Ethanol Fuel with Different Water Content
Summarized in Table 4 are the effects of ethanol with different water content on the combustion characteristics and performance of SI engines. The summary is for different engine types and test conditions.

Table 4.
Combustion and performance of ethanol fuel with diffrent water content in SI engine.
In different countries, the specification for the production of hydrous ethanol is such that the allowed percentage of water content in hydrous ethanol ranges from 4.0% to 5.0% by volume as limited by the ethanol water distillation azeotrope. Ethanol with different water content is not available on the market, and for this reason this theme has been added to the discussion on the investigating the effects of these blends on an engine performance. Many researchers have studied the effects of increasing the water content in ethanol fuel blends on the combustion and performance of spark ignition engines. Stone et al. [75] studied the effect of increasing the water content in ethanol on the coefficient of variation in indicated mean effective pressure (COV in IMEP) for GDI and PFI engines for rich and stoichiometric mixtures. The results indicated that for both GDI and PFI cases, the rich mixture had a lower COV in IMEP than that for stoichiometric operation, and the PFI had a lower COV in IMEP than the GDI engine. Furthermore, the elevated water content in ethanol fuel blends increases the COV in IMEP, adding water to ethanol increases the combustion duration. However, the reduction of combustion stability is not important with up to 30% water by volume [75]. An increase in combustion duration with an increased water content in ethanol (see Figure 5), leads to lower flame velocity and the resultant combustion degradation, there was no reduction in indicated thermal efficiency with an increased water content in ethanol [76].
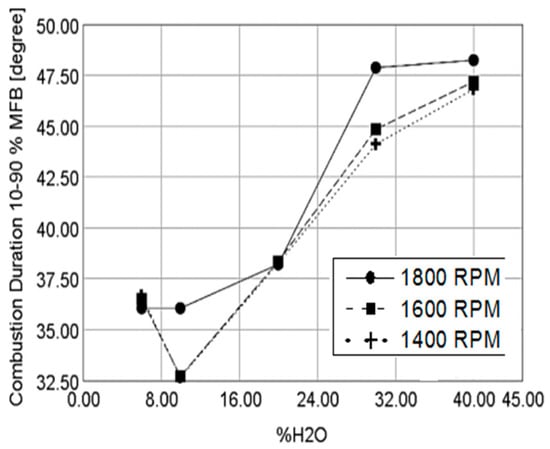
Figure 5.
Effect of increased water content in ethanol on combustion duration (10%–90% of mass fraction burned) Reprinted with permission from Ref. [76]. Copyright (2015) SAE International.
The presence of small quantities of water in ethanol resulted in faster combustion, but when the water content increased, adverse effects were observed, and the flame development rate decreased. The authors illustrated that as the water content increased, both dilution and chemical effects become more important and as a result the flame propagation velocity of the blend decreases [9]. The authors also investigated the pressure history as a function of time for different water contents, and the results revealed that the pressure reached a peak faster for small water contents than for the anhydrous ethanol. However, for high-water contents, the peak pressure was achieved later than with anhydrous ethanol. The authors illustrated that to the ethanol molecules being trapped in the large cavities within the water structure, which can lead to volume contraction of the ethanol–water blend [9]. In a related study, Ambros et al. [77] tested commercial hydrous ethanol blends ranging from 10% to 40% by volume prepared by the addition of water. The results showed that for an SI engine running under fixed ignition timing advance, high-water content in ethanol mixtures caused high pressures at the end of compression phase, with the in-cylinder pressures decreasing with an increased water content in the fuel. Under conditions of maximum brake torque (MBT), the in-cylinder pressures increased with addition water content in ethanol; the maximum pressure, maximum torque and maximum power occurred for the use of E70W30 (70% ethanol and 30% water). The increase in torque and power output is related to just one reason, the increase in water percentage allow to work with advanced ignition points aiming for maximum brake torque condition, so mixtures with 5% and 10% water were knock limited, while more hydrous mixtures worked in the MBT operation point. It was also found that any water addition beyond this 30% content had an adverse effect on engine performance. This was attributed to both dilution and chemical effects that become more important and as a result the flame propagation velocity of the blend decreases. The authors also found that the specific fuel consumption decreased with an increase in the water content in ethanol.
Furthermore, Lanzanova et al. [78] investigated the effect of water content (5% to 40% by volume)- ethanol blends in an Otto engine at maximum brake torque. The results revealed that the in-cylinder pressure was enhanced with the increase in water content in ethanol. By increasing the water content, advanced spark timing could be used without the occurrence of knock. The combustion duration was nearly unaffected with only an increase in ignition delay for up to 30% of water, it also found that until 30% of water content, by volume, is reached in ethanol, the engine burning rates remained almost constant, with this resulting in an increased brake thermal efficiency, brake mean effective pressure and a decreased brake specific fuel consumption (see Figure 6), these results attributed to the spark advance and its consequences in combustion as the water content increased.
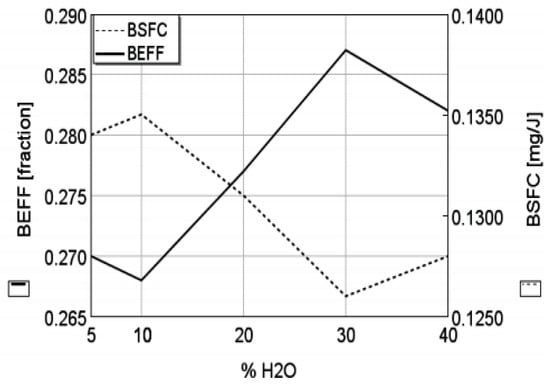
Figure 6.
Effect of increased water content in ethanol on the brake thermal efficiency and brake specific fuel consumption. Reprinted with permission from Ref. [78]. Copyright (2013) SAE International.
Brewster et al. [79] conducted an experimental study on anhydrous ethanol (E100) and hydrous ethanol containing different percentages of water (E93W7, E87W13, and E80W20) used as fuel for a four-cylinder turbo charged direct injection engine having a compression ratio of 10.4:1. The engine was operated at an engine speed of 2000 rpm, a lambda value equaling 1, and an intake pressure of 1 bar for a fixed ignition timing setting. The results showed that the addition of water reduces peak pressure and also slowed combustion, because some energy from the combustion of hydrous ethanol is lost to vaporization of the incompletely vaporized water present in the charge mixture [15]. It also found that at a speed of over 3500 rpm, the use of hydrous ethanol resulted in a higher brake torque output than that of pure ethanol, the increase in brake torque for hydrous ethanol can be explained by the enhanced air-fuel mixing mechanism and increased amount of H, O, OH radicals from water dissociation at higher speeds enhance the combustion process, with the brake thermal efficiency decreasing with an increase in the water content in ethanol. The reduction in brake thermal efficiency at fixed ignition timing may be attributed to a reduction in burn rate. Munsin et al. [80] studied the effect of different generator loads on engine performance using ethanol with 5% water content by volume (E95W5) at a constant speed of 3600 rpm and with stoichiometric air-fuel ratio. By increasing the generator load from 10% to 100%, the overall efficiency (electrical power output divided by fuel energy input) increased by 18%, while the BSFC decreased by 76%, hydrous ethanol contained 5% water can improved fuel economy only with proper engine hardware modifications. Furthermore, at a constant generator load and the addition of water ranging from 20% to 40% by volume in ethanol resulted in an increased BSFC by 75% because heating value per mass of hydrous ethanol is decreased with higher water content, while the overall efficiency decreased by 5% because of longer combustion duration.
In summary, and with reference to Table 4, the engine operating conditions and the percentages of water content in ethanol have a significant effect on the engine combustion and performance. The increase in water percentage (5%–30% by volume) in ethanol allow one to work with advanced ignition points, aiming maximum brake torque condition lead to increases the cylinder pressure [77,78,79]. Moreover, any water addition beyond this 30% content had an adverse effect on engine combustion [9,77,78], attributed to the ethanol molecules being trapped in the large cavities within the water structure and decreases in the laminar burning velocities with increased water content in ethanol. Finally, at fixed ignition timing setting conditions, the increased water content in ethanol fuel blends decreases the cylinder pressure [68,77], and combustion duration [68,79]. Moreover, an increased water content in ethanol fuel increases the cycle-to-cycle variations [68,75]. Furthermore, the increased water content in ethanol fuel blends used in SI engines increases the engine torque [79], and that maximum torque and maximum power are obtained for an optimal water content of 30%. In addition, the engine thermal efficiency under MBT condition was also obtained using a water content of 30% [76,78]. Finally, Lanzanova et al. [78] and Ambros et al. [77] found that for SI engine, running at MBT timing decreases SFC for hydrous ethanol. Munsin et al. [80] also noticed that by enlarging the generator load the BSFC reduced by about 76% for hydrous ethanol.
There are a number of combustion and performance issues that need further investigation and development. These possible future studies and suggestions are outlined as follows:
- The combustion characteristics of different water content with ethanol fuel needs to be explored further and understood in more detail with concrete evidence based on numerical and experimental methods.
- The combustion characteristics of high water content in ethanol-gasoline blends through the use of dual fuel injection still needs more investigation.
- Low temperature starting ability and turbocharger application for high-water content in ethanol for improving the specific output of SI engines needs to be investigated.
- There should be more research to deal with the reduction in the brake specific consumption for high water content in ethanol.
5. Emissions from Hydrous Ethanol Fuel in Spark-Ignition Engines
In general, the emissions from internal combustion engines fueled with alternative fuels are less toxic and reactive and consequently leads to a decreased production of ozone and an enhanced air quality [81]. Emissions are classified according to whether they are regulated or unregulated pollutants. Regulated pollutants include carbon monoxide (CO), carbon dioxide (CO2), nitrogen oxides (NOX), unburnt hydrocarbons (HC) and particulate matter (PM). On the other hand, unregulated pollutants, include aldehydes (RCHO), benzene, toluene, xylene (BTX), sulfur dioxide (SO2), among other pollutants [82].
5.1. Regulated Emissions (CO, CO2, NOX, HC, and PM)
In a study conducted by Schifter et al. [6] the use of mid-level hydrous ethanol (containing 4% water by volume)-gasoline blends and anhydrous gasoline blends were compared with the results indicating that 2% lesser NOX emissions were obtained for the use of hydrous ethanol‑gasoline blends than for the use of anhydrous ethanol‑gasoline blends. Clemente et al. [8] tested an engine running on hydrous ethanol with 7% water content by volume and gasohol. For the comparison of emissions, CO emissions resulting from the use of hydrous ethanol was less than that of gasohol by 41%, the reason behind its water present during combustion might act to decrease CO emissions by way of the water-gas shift mechanism, it also found that the HC produced from the use of hydrous ethanol was higher than that of gasohol by 56%. Trends in HC emissions are highly variable since it depends on charge heterogeneity [83], combustion chamber design [10], flame quenching [84] and other engine related parameters. However, hydrocarbon emissions would increase when the mixtures were down beyond the lean limit [74]. At high load, higher in-cylinder temperatures raised the conversion rates, resulting in lower total HC emissions compared to the low load case [84]. The residence time in the combustion system must be enough to accommodate ignition delay. Incomplete combustion of hydrous ethanol fuel can increase emissions of HC.
Olberding et al. [10] also studied the engine performance and emissions of a transit van, using hydrous ethanol (30% water content by volume) and compared the resulting emissions with that of gasoline. The use of hydrous ethanol has shown significant increases in HC emissions. Furthermore, CO produced from hydrous ethanol combustion under lean and stoichiometric conditions is relatively less at high loads and high speeds, while for stoichiometric condition the hydrous ethanol fuel produced high CO emissions at low speeds and low loads, due to lower in-cylinder temperature at low loads. It also found that the NOX being decreased by significant margins at most operating points. Wang et al. [38] compared the performance and emissions of gasoline and HE10 (containing 5% water by volume) at different loads and a constant speed of 2000 rpm. The HE10 fuel had a relatively less NOX emissions than the E0 fuel for all operating conditions as well a slower HC, CO, and CO2 emissions than the E0 fuel. Melo et al. [39] experimentally investigated the use of hydrous ethanol gasoline blends for different operation conditions of a 1.4 L flex-fuel FIAT engine. The results showed that the hydrous ethanol addition reduced CO, NOX and HC emissions, CO2 from hydrous ethanol increased, the higher oxygen content in the hydrous ethanol promotes the oxidation of CO to CO2. Costa et al. [36] found that NOX emission for hydrous ethanol was more than that of E22 due to advanced ignition timing for hydrous ethanol, therefore, higher peak temperature during burning and thereby increase the formation of NOX. Hydrous ethanol produced higher CO2 emission and lower CO over the whole speed range. The use of hydrous ethanol also decreases the HC emissions because of the chemical structure of hydrous ethanol having a lower carbon and hydrogen content.
Experiments conducted by Liu et al. [49] investigated the emissions of HE10 hydrous ethanol gasoline blends and gasoline. The results showed that for hydrous ethanol blends CO and HC emission were lower for all loads, while NOX emissions were reduced for low load engine operation. At idling speed, the CO, HC and NOX emissions were all reduced. NO and NOX emissions were reduced for the use of hydrous ethanol gasoline blended fuel (HE40).While, the HC, CO and CO2 emissions showed a small reduction for the use of hydrous ethanol containing fuels compared with gasoline [50]. The higher latent heat of evaporation of the ethanol blends, and water present in it resulted in lower in-cylinder temperature, which in turn resulted in lower NOX emissions (see Figure 7). HC emission was also lower for HE10 at 25% throttle opening, which was due to the presence of oxygen in the fuel and a better combustion rate [67]. Brewster et al. [79] investigated a spark ignition turbocharged engine fueled by anhydrous (El00) and hydrous (E93W7, E87W13, E80W20) ethanol fuels. The results showed that the emission of CO was unchanged by the water content in ethanol, with a NOX emission reduction with an increase water content in ethanol at a fixed ignition timing, the key is the existing water, which lowers the peak temperature during burning and slows down the procedure of NOX formation. For similar operating conditions, there was an increase in HC emissions with an increase in water content for the E93W7and E87W13 fuels may be resulted in incomplete combustion, the trend being largely independent of ignition timing (see Figure 8).
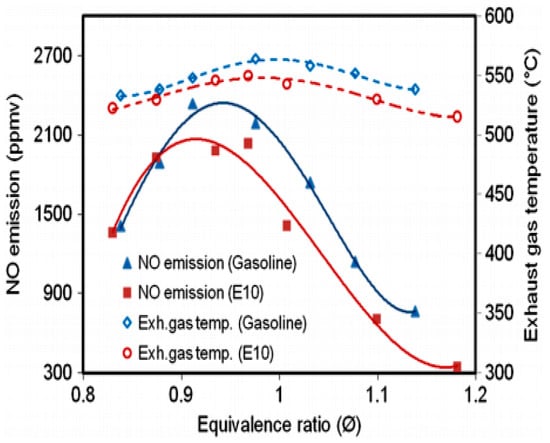
Figure 7.
NO emission and exhaust gas temperature versus equivalence ratio, for a fixed speed of 2500 rpm and a 25% throttle opening. Reprinted (adapted) with permission from Ref. [67]. Copyright (2012) John Wiley and Sons.
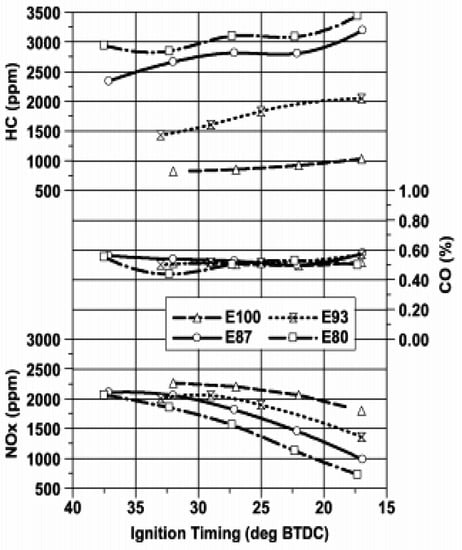
Figure 8.
Effect of increased water content in ethanol on exhaust emissions at 2000 rpm and 100 kPa intake manifold absolute pressure. Reprinted with permission from Ref. [79]. Copyright (2007) SAE International.
In another study Chen et al. [69] found that HC and CO emissions increased for the blends of E5 to E30 hydrous ethanol compared with gasoline and a slight decline in CO2 for hydrous ethanol. Munsin et al. [80] studied the effect of different loads on emission products using ethanol with 5% water content by volume (E95W5). By increasing the generator load from 10% to 100%, the CO and HC emissions before entering the catalytic convertor reduced by 76% and 63%, respectively, although there was an increase in the NOX emissions. This is due to the fact more fuel is injected and combusted in the cylinder when the generator load increases, which causes higher gas temperatures and results in more NOX formation in the engine cylinder, in addition the oxygen content of hydrous ethanol promotes combustion efficiency and reduces emissions of CO and HC, the catalytic convertor reduced the amount of CO, HC and NOX in the emissions by 78%–94%, 23%–61% and 29%–65%, respectively. The reduction conversation efficiency of the catalytic converter may be attributed to changing the ignition and injection system, produced higher exhaust gas temperature even using hydrous ethanol as fuel. Increasing operating temperature causes increased catalytic converter degradation, decreased oxygen storage capacity, decreased specific surface area and decreased conversion efficiency [85]. Furthermore, a high operating temperature can encourage the interaction between the Al2O3 support and precious metals, thus reducing conversion efficiency [85]. In addition, because of high oxygen concentration in the exhaust when ethanol is used, the NOX conversion of ethanol gasoline blends is lower relative to that of gasoline [86], and the differences in hydrous ethanol and gasoline combustion products composition may affect the conversion of unregulated emissions. It also reported that the addition of water from 20% to 40% by volume to ethanol resulted in incomplete combustion increasing the CO and HC emissions by 58% and 267%, respectively, while the NOX emissions decreased with increasing water content, the catalytic convertor reduced the amount of CO, HC and NOX in the emissions by 75%–89%, 10%–32% and 32%–50%, respectively. An increased water content in ethanol led to a decrease of the peak temperature during burning. The exhaust temperatures below 600 K would reduce the conversion efficiency of oxidation catalysts [84]. The catalytic convertor should be efficient enough to manage the emissions of hydrous ethanol. Peters et al. [87] investigated an analytical and experimental survey. The engine used was a single cylinder fueled by gasoline using a compression ratio of 8.0:1. The engine operated at a fixed speed of 1200 rpm and an 80 kPa intake manifold pressure, for varying equivalence ratio and ignition timing at MBT. Water was added in the form of emulsion with fuel at up to 40% by weight through the intake manifold. Water addition decreased the NO emissions, increased the emission of HC, with relatively no change in CO emission. As the water in ethanol content increases, CO and NOX emissions decreased while total HC increased [84]. More added water dilutes the fuel mixture and reduces the combustion temperature and lowers total HC oxidation. Suarez et al. [88] experimentally investigated the regulated and unregulated emissions from a Euro 5a flex-fuel light duty vehicle (FFV).The test was conducted at a temperature of 23 °C and −7 °C using nine different ethanol fuel blends. The results showed no differences on the emissions when hydrous ethanol blends were used instead of anhydrous ethanol blends. The use of HE85 and HE75 blends resulted in a decrease in NOX emissions (30%–55%) but increased the emissions of carbon monoxide. Hu et al. [89] studied the effect of E10 hydrous ethanol (5% water content by volume) gasoline blends on a gasoline engine. The results showed enhanced HC and CO emissions for the idle speed operating condition.
In conclusion, and with reference to Table 5, the hydrous ethanol and its blends with gasoline fuel used in SI engines decreased the nitrogen oxide (NOX) emissions [6,10,38,39,49,67,69,79,80,84,88], carbon monoxide (CO) emissions [10,36,38,39,49,50,84,89], carbon dioxide (CO2) emissions [38,50,69], and hydrocarbon (HC) emissions [36,38,39,49,50,67,89]. Furthermore, a few studies [79,80,84] have reported a slight increase in HC emissions due to the increase in water content in ethanol blends, while, NOX decreased with increased water content in ethanol blends. In the literature nothing was reported on the effect of hydrous ethanol on particulate matter (PM) emissions. Compared with gasoline fuel, hydrous ethanol has no aromatic content, high oxygen content, no C–C chemical bond. All of these factors may lead to the reduction of particulate matter emitted from hydrous ethanol. The effect of hydrous ethanol blends on Particulate Matter (PM) emissions from spark-ignition engines need to be investigated. In addition, the gaseous emissions emitted from different water content with ethanol fuel needs to be explored further and understood in more detail with concrete evidence based on numerical and experimental methods.

Table 5.
Exhaust emission products from hydrous ethanol fuel and its bends with gasoline in SI engines.
5.2. Unregulated Emissions (HCHO, CH3CHO)
Unregulated emissions include aldehydes, toluene, benzene, xylene (BTX) and sulfur dioxide (SO2). Aldehydes are acutely reactive organic compounds that take part in complex chemical reactions within the atmosphere. The emissions of aldehydes are higher for ethanol fuel due to the presence of the hydroxyl functional group (OH), not present in gasoline [90]. Mainly, formaldehyde (HCHO) and acetaldehyde (CH3CHO) in the hydrous state are significant emissions in internal combustion engines. The quantum of these emissions depend on the ethanol content, engine load and oxygen concentration [80,91].
A number of publications [8,39,80,88] have reported that acetaldehyde and formaldehyde emissions from hydrous ethanol fuel increased in comparison to the use of gasoline. This includes a study conducted by Clemente et al. [8] which concluded that the aldehyde from hydrous ethanol was higher than that for gasohol by 58%. Another interesting study was published by Melo et al. [42]. The results showed a significant increase in both acetaldehyde and formaldehyde as hydrous ethanol percentage, by volume, increased for all engine operating points (see Figure 9).
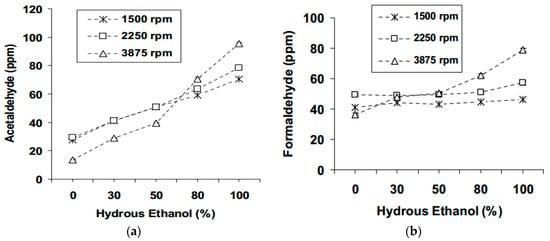
Figure 9.
Effect of increased hydrous ethanol content (water content from 6.5 up to 7.5% (wt/wt)) in gasoline on (a) Acetaldehyde (b) Formaldehyde emissions. Reprinted with permission from Ref. [42]. Copyright (2011) SAE International.
Munsin et al. [80] experimentally observed that in a one cylinder electronic fuel injection engine fueled by hydrous ethanol (E95W5) for an increasing load from 10% to 100% and at a constant speed of 3600 rpm, the acetaldehyde and formaldehyde were reduced by 49% and 40%, respectively, before the catalytic convertor, and reduced by 80%–90%, and 14%–30% after the catalytic convertor. For an increase in the water content in ethanol from 20% to 40% by volume at constant load (72% of rated power) the formaldehyde and acetaldehyde increased before the catalytic convertor by 150% and 275%, this results may attributed to the heat absorption by water vaporization causes a decrease of local adiabatic flame temperature that causes incomplete combustion, therefore increasing the formation of formaldehyde and acetaldehyde. In addition, the emissions of aldehydes are higher for hydrous ethanol fuel due to the larger amount of OH radicals provided by water reactions, which reduced by 8%–35% and 28%–44%, respectively, after the catalytic convertor. The use of hydrous ethanol gasoline blends resulted in an increase in the acetaldehyde emissions by a margin of up to 120% at a temperature of 23 °C and up to 400% at 7 °C, for acetaldehyde, leading to a severe increase of the ozone-forming potential [88].
6. Conclusions
Alternative fuels are becoming more relevant for vehicles because of the depletion of fossil fuel reserves and environmental concerns. Hydrous ethanol is considered as a green and clean renewable alternative fuel for spark-ignition engines. This study examined hydrous ethanol and its blends with gasoline, with specific focus on the following issues: (1) Properties of hydrous ethanol fuels; (2) Stability of ethanol-gasoline-water blends; (3) Combustion and performance of hydrous ethanol fuel in SI engines and (4) emissions from hydrous ethanol fuel in SI engines. Based on detailed literature reviews, it is found that:
- The use of ethanol with a high water content might be an environmentally friendlier and cheaper energy source than the use of gasoline. Hydrous ethanol mixed fuels can take advantage of a higher compression ratio in SI engines due to their higher octane number compared with gasoline.
- The temperature and the chemical composition of gasoline used are significant factors for the stability of the ethanol-water-gasoline blends. It is also worth pointing out that the solubility of water in the blend could be improved by using additives.
- In general, hydrous ethanol-gasoline blends used in SI engines improves the in-cylinder pressure, combustion efficiency, and flame speed. It also reduces the combustion duration, engine knocking tendency and combustion temperature. The engine performance results showed a remarkable improvement in engine torque, brake power, brake thermal efficiency, and brake mean effective pressure in SI engines fueled hydrous ethanol-gasoline blends. The presence of small quantities of water in ethanol blend resulted in faster combustion due to the improvement in chain reactions and increased amounts of H, O, OH radicals from water dissociation. Studies related to high water content in ethanol usage in SI engine demonstrates that laminar burning velocities decrease due to the dilution and chemical effects of water become more important. the increase in torque and power output is related to just one reason, the increase in water percentage allows engines to work with advanced ignition points aiming at maximum brake torque condition, so mixtures with 5% and 10% water were knock limited, while more hydrous mixtures worked at the MBT operation point. Any water addition beyond this 30% content had an adverse effect on engine performance. Thus, the E70W30 fuel appear to be a good substitute for renewable fuels market regarding engine performance and cost reduction. There should be more studies on the engine oil contamination and material compatibility for running an engine on E70W30 fuel.
- As the water content in ethanol effectively absorbs heat and lowers the peak temperature during the burning process, hydrous ethanol blends are more effective in NOX emission reduction compared with anhydrous ethanol blends, and gasoline. However, CO, CO2, and HC emissions were reduced by small margins for hydrous ethanol gasoline blends, because of the lower molar H/C ratio for hydrous ethanol compared with gasoline. In addition, high-water content in ethanol dilutes the fuel mixtures and lower combustion temperature, which leads to incomplete combustion resulting in higher HC. The residence time in a combustion system must be enough to accommodate ignition delay. Enhancements in the fuel-injection system are needed to enhance the spark ignition engine combustion process and emissions. Acetaldehyde and formaldehyde emissions from hydrous ethanol fuel are relatively higher in comparison with gasoline, due to the larger amount of OH radicals provided by water reactions and incomplete combustion. The exhaust temperature and oxygen concentration in the exhaust had a significant effect on the conversation efficiency, the catalytic convertor with high conversation efficiency is recommended for the use of hydrous ethanol in SI engines because it decreases the unregulated emissions, the catalytic convertor used in previous studies has not been finally optimized for this type of hydrous ethanol engine.
Acknowledgments
This work was supported by the “Fundamental Research Funds for Central Universities (WUT: 2015III040)”. Musaab O. El-Faroug acknowledges the Chinese Scholarship Council (CSC) for financial support for his Ph.D. studies in the form of CSC grant No. 2014GF145. Musaab O. El-Faroug also appreciates additional financial support from the Sudanese Government.
Author Contributions
This is to confirm that all the authors have contributed in diverse ways at all stages of the research including research design, data collection and final preparation of the manuscript. All authors have read and approved the final manuscript.
Conflicts of Interest
The authors declare no potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| B1–B4 | different gasoline composition |
| BMEP | brake mean effective pressure |
| BP | brake power |
| BSFC | brake specific fuel consumption |
| BTE | brake thermal efficiency |
| C | cylinder |
| CD | combustion duration |
| CE | combustion efficiency |
| CO | carbon monoxide |
| CO2 | carbon dioxide |
| COV in IMEP | coefficient of variation in indicated mean effective pressure |
| CP | cylinder pressure |
| CR | compression ratio |
| CT | cylinder temperature |
| DI | direct injection |
| E0 | 0% ethanol & 100% gasoline |
| E100 | 100% ethanol & 0% gasoline |
| E94W6 | 94% ethanol & 6% water |
| FC | fuel consumption |
| FFV | flex fuel vehicle |
| FIA | fixed ignition advance |
| FOF | fusel oil fraction |
| FS | flame speed |
| H2O | water |
| HC | unburned hydrocarbon |
| HC3CHO | formaldehyde |
| HCHO | acetaldehyde |
| HE | hydrous ethanol |
| HE10 | 10% hydrous ethanol & 90% gasoline |
| HRR | heat release rate |
| IMEP | indicated mean effective pressure |
| ITE | indicated thermal efficiency |
| MBT | maximum brake torque |
| N | engine speed |
| NOx | oxides of nitrogen |
| P | power |
| PFI | port fuel injection |
| PM | particulate matter |
| S | stroke |
| SI | spark ignition |
| T | torque |
| v/v | volume/volume |
| wt/wt | weight/weight |
References
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production. Renew. Sustain. Energy Rev. 2012, 16, 1462–1476. [Google Scholar] [CrossRef]
- Demirbas, A. Competitive liquid biofuels from biomass. Appl. Energy 2011, 88, 17–28. [Google Scholar] [CrossRef]
- Wallington, T.; Anderson, J.; Winkler, S. Comment on “natural and anthropogenic ethanol sources in north america and potential atmospheric impacts of ethanol fuel use”. Environ. Sci. Technol. 2013, 47, 2139–2140. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Choi, Y.; Kim, C.; Oh, S.; Lim, G.; Moriyoshi, Y. Performance and exhaust emission characteristics of a spark ignition engine using ethanol and ethanol-reformed gas. Fuel 2010, 89, 2118–2125. [Google Scholar] [CrossRef]
- Pang, X.; Mu, Y.; Yuan, J.; He, H. Carbonyls emission from ethanol-blended gasoline and biodiesel-ethanol-diesel used in engines. Atmos. Environ. 2008, 42, 1349–1358. [Google Scholar] [CrossRef]
- Schifter, I.; Diaz, L.; Gómez, J.; Gonzalez, U. Combustion characterization in a single cylinder engine with mid-level hydrated ethanol‑gasoline blended fuels. Fuel 2013, 103, 292–298. [Google Scholar] [CrossRef]
- Leng, R.; Wang, C.; Zhang, C.; Dai, D.; Pu, G. Life cycle inventory and energy analysis of cassava-based fuel ethanol in china. J. Clean. Prod. 2008, 16, 374–384. [Google Scholar] [CrossRef]
- Clemente, R.C.; Werninghaus, E.; Coelho, E.P.; Ferraz, L.A.S. Development of an Internal Combustion Alcohol Fueled Engine; Society of Automotive Engineers Papers: Warrendale, PA, USA, 2001. [Google Scholar]
- Rahman, K.M.; Kawahara, N.; Tsuboi, K.; Tomita, E. Combustion characteristics of wet ethanol ignited using a focused Q-switched ND: Yag nanosecond laser. Fuel 2016, 165, 331–340. [Google Scholar] [CrossRef]
- Olberding, J.; Beyerlein, D.C.S.; Steciak, J.; Cherry, M. Dynamometer Testing of an Ethanol-Water Fueled Transit Van; Society of Automotive Engineers Papers: Warrendale, PA, USA, 2005. [Google Scholar]
- Martinez-Frias, J.; Aceves, S.M.; Flowers, D.L. Improving ethanol life cycle energy efficiency by direct utilization of wet ethanol in HCCI engines. J. Energy Resour. Technol. 2007, 129, 332–337. [Google Scholar] [CrossRef]
- Shapouri, H.; Duffield, J.A.; Graboski, M.S. Estimating the Net Energy Balance of Corn Ethanol; Agricultural Economic Report; Economic Research Service: Washington, DC, USA, 1995.
- Shapouri, H.; Duffield, J.; Wang, M. The energy balance of corn ethanol revisited. Trans. ASAE 2003, 46, 959. [Google Scholar] [CrossRef]
- Saxena, S.; Schneider, S.; Aceves, S.; Dibble, R. Wet ethanol in hcci engines with exhaust heat recovery to improve the energy balance of ethanol fuels. Appl. Energy 2012, 98, 448–457. [Google Scholar] [CrossRef]
- Mack, J.H.; Flowers, D.L.; Aceves, S.M.; Dibble, R.W. Direct use of wet ethanol in a homogeneous charge compression ignition (HCCI) engine: Experimental and numerical results. In Proceedings of the 2007 Fall Meeting of the Western States Section of the Combustion Institute Sandia National Laboratories, Livermore, CA, USA, 16–17 October 2007.
- Balat, M.; Balat, H. Recent trends in global production and utilization of bio-ethanol fuel. Appl. Energy 2009, 86, 2273–2282. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Guimarães, P.M.; Silva, J.P.A.; Carneiro, L.M.; Roberto, I.C.; Vicente, A.; Domingues, L.; Teixeira, J.A. Technological trends, global market, and challenges of bio-ethanol production. Biotechnol. Adv. 2010, 28, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Azadi, H.; de Jong, S.; Derudder, B.; De Maeyer, P.; Witlox, F. Bitter sweet: How sustainable is bio-ethanol production in brazil? Renew. Sustain. Energy Rev. 2012, 16, 3599–3603. [Google Scholar] [CrossRef]
- Ganguly, A.; Chatterjee, P.; Dey, A. Studies on ethanol production from water hyacinth—A review. Renew. Sustain. Energy Rev. 2012, 16, 966–972. [Google Scholar] [CrossRef]
- Asgher, M.; Ahmad, Z.; Iqbal, H.M.N. Alkali and enzymatic delignification of sugarcane bagasse to expose cellulose polymers for saccharification and bio-ethanol production. Ind. Crops Prod. 2013, 44, 488–495. [Google Scholar] [CrossRef]
- Shen, F.; Hu, J.; Zhong, Y.; Liu, M.L.; Saddler, J.N.; Liu, R. Ethanol production from steam-pretreated sweet sorghum bagasse with high substrate consistency enzymatic hydrolysis. Biomass Bioenergy 2012, 41, 157–164. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, M.; Wu, Z. Pretreatment of sugarcane bagasse with NH4OH–H2O2 and ionic liquid for efficient hydrolysis and bioethanol production. Bioresour. Technol. 2012, 119, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Matsakas, L.; Christakopoulos, P. Ethanol production from enzymatically treated dried food waste using enzymes produced on-site. Sustainability 2015, 7, 1446–1458. [Google Scholar] [CrossRef]
- Cotana, F.; Cavalaglio, G.; Pisello, A.L.; Gelosia, M.; Ingles, D.; Pompili, E. Sustainable ethanol production from common reed (phragmites australis) through simultaneuos saccharification and fermentation. Sustainability 2015, 7, 12149–12163. [Google Scholar] [CrossRef]
- Scully, S.M.; Orlygsson, J. Recent advances in second generation ethanol production by thermophilic bacteria. Energies 2015, 8, 1–30. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, N.; Prasad, R. Anhydrous ethanol: A renewable source of energy. Renew. Sustain. Energy Rev. 2010, 14, 1830–1844. [Google Scholar] [CrossRef]
- García, C.A.; Manzini, F.; Islas, J. Air emissions scenarios from ethanol as a gasoline oxygenate in mexico city metropolitan area. Renew. Sustain. Energy Rev. 2010, 14, 3032–3040. [Google Scholar] [CrossRef]
- Manzetti, S.; Andersen, O. A review of emission products from bioethanol and its blends with gasoline. Background for new guidelines for emission control. Fuel 2015, 140, 293–301. [Google Scholar] [CrossRef]
- Masum, B.; Masjuki, H.; Kalam, M.; Fattah, I.R.; Palash, S.; Abedin, M. Effect of ethanol‑gasoline blend on nox emission in si engine. Renew. Sustain. Energy Rev. 2013, 24, 209–222. [Google Scholar] [CrossRef]
- Niven, R.K. Ethanol in gasoline: Environmental impacts and sustainability review article. Renew. Sustain. Energy Rev. 2005, 9, 535–555. [Google Scholar] [CrossRef]
- Bergthorson, J.M.; Thomson, M.J. A review of the combustion and emissions properties of advanced transportation biofuels and their impact on existing and future engines. Renew. Sustain. Energy Rev. 2015, 42, 1393–1417. [Google Scholar] [CrossRef]
- Stein, R.A.; Anderson, J.E.; Wallington, T.J. An overview of the effects of ethanol-gasoline blends on si engine performance, fuel efficiency, and emissions. SAE Int. J. Engines 2013, 6, 470–487. [Google Scholar] [CrossRef]
- Bradley, D.; Lawes, M.; Liao, S.; Saat, A. Laminar mass burning and entrainment velocities and flame instabilities of i-octane, ethanol and hydrous ethanol/air aerosols. Combus. Flame 2014, 161, 1620–1632. [Google Scholar] [CrossRef]
- Yüksel, F.; Yüksel, B. The use of ethanol–gasoline blend as a fuel in an si engine. Renew. Energy 2004, 29, 1181–1191. [Google Scholar] [CrossRef]
- Commercial Alcohols, Material Safety Data Sheet No. 1001. Available online: http://www.bme.mcgill.ca/REKLAB/manual/MSDS/Materials%20List/ethanolMSDS.pdf (accessed on 5 September 2016).
- Costa, R.C.; Sodré, J.R. Hydrous ethanol vs. Gasoline-ethanol blend: Engine performance and emissions. Fuel 2010, 89, 287–293. [Google Scholar] [CrossRef]
- Li, Y.; Nithyanandan, K.; Zhang, J.; Lee, C.-F.; Liao, S. Combustion and Emissions Performance of a Spark Ignition Engine Fueled with Water containing Acetone-Butanol-Ethanol and Gasoline Blends; Society of Automotive Engineers Papers: Warrendale, PA, USA, 2015. [Google Scholar]
- Wang, X.; Chen, Z.; Ni, J.; Liu, S.; Zhou, H. The effects of hydrous ethanol gasoline on combustion and emission characteristics of a port injection gasoline engine. Case Stud. Ther. Eng. 2015, 6, 147–154. [Google Scholar] [CrossRef]
- De Melo, T.C.C.; Machado, G.B.; Belchior, C.R.; Colaço, M.J.; Barros, J.E.; de Oliveira, E.J.; de Oliveira, D.G. Hydrous ethanol‑gasoline blends‑combustion and emission investigations on a flex-fuel engine. Fuel 2012, 97, 796–804. [Google Scholar] [CrossRef]
- Equistar Chemicals, LP. Available online: http://itecref.com/pdf/Ethyl_Alcohol_Handbook_Equistar.pdf (accessed on 5 September 2016).
- Delgado, R.C.; Araujo, A.S.; Fernandes, V.J. Properties of brazilian gasoline mixed with hydrated ethanol for flex-fuel technology. Fuel Process. Technol. 2007, 88, 365–368. [Google Scholar] [CrossRef]
- De Melo, T.C.C.; Machado, G.B.; de Oliveira, E.J.; Belchior, C.R.P.; Jos, M.; de Oliveira, D.G. Different Hydrous Ethanol-Gasoline Blends-FTIR Emissions of a Flex-Fuel Engine and Chemical Properties of the Fuels; Society of Automotive Engineers Papers: Warrendale, PA, USA, 2011. [Google Scholar]
- Koç, M.; Sekmen, Y.; Topgül, T.; Yücesu, H.S. The effects of ethanol‑unleaded gasoline blends on engine performance and exhaust emissions in a spark-ignition engine. Renew. Energy 2009, 34, 2101–2106. [Google Scholar] [CrossRef]
- Mueller, S.A.; Anderson, J.E.; Wallington, T.J.; Hammond, R.M. A classroom demonstration of water-induced phase separation of alcohol‑gasoline biofuel blends. J. Chem. Educ. 2009, 86, 1045. [Google Scholar] [CrossRef]
- French, R.; Malone, P. Phase equilibria of ethanol fuel blends. Fluid Phase Equilibria 2005, 228, 27–40. [Google Scholar] [CrossRef]
- Stephenson, R.M. Mutual solubilities: Water-ketones, water-ethers, and water-gasoline-alcohols. J. Chem. Eng. Data 1992, 37, 80–95. [Google Scholar] [CrossRef]
- Treatise on Alcohol-Blended Gasoline: Phase Separation and Alcohol Monitors. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.547.1368&rep=rep1&type=pdf (accessed on 5 September 2016).
- Gramajo de Doz, M.B.; Bonatti, C.M.; Sólimo, H.N. Water tolerance and ethanol concentration in ethanol-gasoline fuels at three temperatures. Energy Fuels 2004, 18, 334–337. [Google Scholar] [CrossRef]
- Liu, S.-H.; Shen, L.-Z.; Ye, N.-Y.; Bi, Y.-H.; Luo, X. Research on effects of E10 hydrous ethanol gasoline blend on performance and emissions of gasoline engine. Chin. Int. Combust. Engine Eng. 2012, 33, 46–51. (In Chinese) [Google Scholar]
- Kyriakides, A.; Dimas, V.; Lymperopoulou, E.; Karonis, D.; Lois, E. Evaluation of gasoline-ethanol-water ternary mixtures used as a fuel for an otto engine. Fuel 2013, 108, 208–215. [Google Scholar] [CrossRef]
- Karaosmanoglu, F.; Isigigür, A.; Aksoy, H.A. Effects of a new blending agent on ethanol-gasoline fuels. Energy Fuels 1996, 10, 816–820. [Google Scholar] [CrossRef]
- Schifter, I.; Dıaz, L.; Vera, M.; Guzmán, E.; López-Salinas, E. Fuel formulation and vehicle exhaust emissions in mexico. Fuel 2004, 83, 2065–2074. [Google Scholar] [CrossRef]
- MacKinven, R.; Hublin, M. European Programme on Emissions, Fuels and Engine Technologies-Objectives and Design; Society of Automotive Engineers Papers: Warrendale, PA, USA, 1996. [Google Scholar]
- Rajan, S.; Saniee, F.F. Water-ethanol-gasoline blends as spark ignition engine fuels. Fuel 1983, 62, 117–121. [Google Scholar] [CrossRef]
- Muzikova, Z.; Pospisil, M.; Sebor, G. Volatility and phase stability of petrol blends with ethanol. Fuel 2009, 88, 1351–1356. [Google Scholar] [CrossRef]
- Kumar, A.; Khatri, D.; Babu, M. An Investigation of Potential and Challenges with Higher Ethanol-Gasoline Blend on a Single Cylinder Spark Ignition Research Engine; Society of Automotive Engineers Papers: Warrendale, PA, USA, 2009. [Google Scholar]
- Al-Farayedhi, A.A.; Al-Dawood, A.; Gandhidasan, P. Effects of Blending Crude Ethanol with Unleaded Gasoline on Exhaust Emissions of SI Engine; Society of Automotive Engineers Papers: Warrendale, PA, USA, 2000. [Google Scholar]
- Ye, Y.-S.; Yan, W.-S.; Shen, L.-Z.; Chen, H.; Lei, J.-L.; Bi, Y.-H. Study on emulsification technology of E10 hydrous ethanol gasoline fuel. Appl. Chem. Ind. 2009, 12, 002. (In Chinese) [Google Scholar]
- Reynolds, R.E. Fuel Specifications and Fuel Property Issues and Their Potential Impact on the Use of Ethanol as a Transportation Fuel; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2002. [Google Scholar]
- Water Phase Separation in Oxygenated Gasoline. Available online: https://www.epa.gov/sites/production/files/2015-09/documents/waterphs.pdf (accessed on 17 November 2016).
- Badrana, O.; Emeishb, S.; Abu-Zaidc, M.; Abu-Rahmaa, T.; Al-Hasana, M.; Al-Ragheba, M. Impact of emulsified water/diesel mixture on engine performance and environment. Int. J. Ther. Environ. Eng. 2011, 3, 1–7. [Google Scholar] [CrossRef]
- Das, A.K.; Kumar, K.; Sung, C.J. Laminar flame speeds of moist syngas mixtures. Combust. Flame 2011, 158, 345–353. [Google Scholar] [CrossRef]
- Dryer, F. Water Addition to Practical Combustion Systems—Concepts and Applications; Elsevier: Amsterdam, The Netherlands, 1977; pp. 279–295. [Google Scholar]
- Rajan, S. Water-ethanol-gasoline blends—Physical properties, power, and pollution characteristics. J. Eng. Gas Turbines Power 1984, 106, 841–848. [Google Scholar] [CrossRef]
- Zhang, W.; Shu, G.; Chen, Z.; Shen, Y.; Weng, J. Chemical kinetics of ignition timing of diesel engine fueled with water emulsion diesel. Trans. Chin. Soc. Agric. Eng. 2012, 28, 59–66. [Google Scholar]
- Melo, T.; Machado, G.; Carvalho, L.; Belchior, C.; Colaço, M.; Barros, J.E.; Paiva, C. In Cylinder Pressure Curve and Combustion Parameters Variability with Ethanol Addition; Society of Automotive Engineers Papers: Warrendale, PA, USA, 2012. [Google Scholar]
- Venugopal, T.; Sharma, A.; Satapathy, S.; Ramesh, A.; Gajendra Babu, M. Experimental study of hydrous ethanol gasoline blend (E10) in a four stroke port fuel‑injected spark ignition engine. Int. J. Energy Res. 2013, 37, 638–644. [Google Scholar] [CrossRef]
- Augoye, A.; Aleiferis, P. Characterization of Flame Development with Hydrous and Anhydrous Ethanol Fuels in a Spark-Ignition Engine with Direct injection and Port Injection Systems; Society of Automotive Engineers Papers: Warrendale, PA, USA, 2014. [Google Scholar]
- Chen, R.-H.; Chiang, L.-B.; Wu, M.-H.; Lin, T.-H. Gasoline displacement and NOX reduction in an si engine by aqueous alcohol injection. Fuel 2010, 89, 604–610. [Google Scholar] [CrossRef]
- Amorim, R.J.; Baeta, J.G.C.; Valle, R.M.; Barros, J.; Carvalho, R. Analysis of an otto cycle engine performance regarding alcohol concentration in gasoline and CNG usage. In Proceedings of the XVIII International Congress of Mechanical Engineering, Ouro Preto, Brazil, 6–11 November 2005.
- Costa, R.C.; Sodré, J.R. Compression ratio effects on an ethanol/gasoline fuelled engine performance. Appl. Ther. Eng. 2011, 31, 278–283. [Google Scholar] [CrossRef]
- Zheng, J.; Huang, Z.; Wang, J.; Wang, B.; Ning, D.; Zhang, Y. Effect of compression ratio on cycle-by-cycle variations in a natural gas direct injection engine. Energy Fuel 2009, 23, 5357–5366. [Google Scholar] [CrossRef]
- Young, M.B. Cyclic Dispersion in the Homogeneous-Charge Spark-Ignition engine—A Literature Survey; Society of Automotive Engineers Papers: Warrendale, PA, USA, 1981. [Google Scholar]
- Heywood, J.B. Internal Combustion Engine Fundamentals; Mcgraw-Hill: New York, NY, USA, 1988; Volume 930. [Google Scholar]
- Stone, R.; Chen, L.; Hinton, N.; Leach, F.; Xu, F. GDI engine operation with ethanol/gasoline blends and aqueous ethanol. J. Autom. Saf. Energy 2012, 3, 257–264. [Google Scholar]
- Martins, M.; Lanzanova, T.; Sari, R. Low cost wet ethanol for spark-ignited engines: Further investigations. SAE Int. J. Fuel Lubr. 2015, 8, 367–373. [Google Scholar] [CrossRef]
- Ambrós, W.; Lanzanova, T.; Fagundez, J.; Sari, R.; Pinheiro, D.; Martins, M.; Salau, N. Experimental analysis and modeling of internal combustion engine operating with wet ethanol. Fuel 2015, 158, 270–278. [Google Scholar] [CrossRef]
- Lanzanova, T.D.; Vielmo, H.A.; Sari, R.L.; Dornelles, H.M.; Tatsch, G.A.; Martins, M.E.; Michels, L. Performance Analysis of a Spark Ignited Engine Running on Different Water-in-Ethanol Mixtures; Society of Automotive Engineers Papers: Warrendale, PA, USA, 2013. [Google Scholar]
- Brewster, S.; Railton, D.; Maisey, M.; Frew, R. The Effect of E100 Water Content on High Load Performance of a Spray Guide Direct Injection Boosted Engine; Society of Automotive Engineers Papers: Warrendale, PA, USA, 2007. [Google Scholar]
- Munsin, R.; Laoonual, Y.; Jugjai, S.; Imai, Y. An experimental study on performance and emissions of a small SI engine generator set fuelled by hydrous ethanol with high water contents up to 40%. Fuel 2013, 106, 586–592. [Google Scholar] [CrossRef]
- Nguyen, H.T.-H.; Takenaka, N.; Bandow, H.; Maeda, Y.; de Oliva, S.T.; Botelho, M.M.; Tavares, T.M. Atmospheric alcohols and aldehydes concentrations measured in Osaka, Japan and in Sao paulo, Brazil. Atmos. Environ. 2001, 35, 3075–3083. [Google Scholar] [CrossRef]
- Merritt, P.M.; Ulmet, V.; McCormick, R.L.; Mitchell, W.E.; Baumgard, K.J. Regulated and Unregulated Exhaust Emissions Comparison for Three Tier II Non-Road Diesel Engines Operating on Ethanol-Diesel Blends; Society of Automotive Engineers Papers: Warrendale, PA, USA, 2005. [Google Scholar]
- Jeuland, N.; Montagne, X.; Gautrot, X. Potentiality of ethanol as a fuel for dedicated engine. Oil Gas Sci. Technol. 2004, 59, 559–570. [Google Scholar] [CrossRef]
- Lanzanova, T.D.M.; Dalla Nora, M.; Zhao, H. Performance and economic analysis of a direct injection spark ignition engine fueled with wet ethanol. Appl. Energy 2016, 169, 230–239. [Google Scholar] [CrossRef]
- De Almeida, P.R.; Nakamura, A.L.; Sodré, J.R. Evaluation of catalytic converter aging for vehicle operation with ethanol. Appl. Ther. Eng. 2014, 71, 335–341. [Google Scholar] [CrossRef]
- He, B.-Q.; Wang, J.-X.; Hao, J.-M.; Yan, X.-G.; Xiao, J.-H. A study on emission characteristics of an efi engine with ethanol blended gasoline fuels. Atmos. Environ. 2003, 37, 949–957. [Google Scholar] [CrossRef]
- Peters, B.D.; Stebar, R.F. Water-Gasoline Fuels—Their Effect on Spark Ignition Engine Emissions and Performance; Society of Automotive Engineers Papers: Warrendale, PA, USA, 1976. [Google Scholar]
- Suarez-Bertoa, R.; Zardini, A.; Keuken, H.; Astorga, C. Impact of ethanol containing gasoline blends on emissions from a flex-fuel vehicle tested over the worldwide harmonized light duty test cycle (WLTC). Fuel 2015, 143, 173–182. [Google Scholar] [CrossRef]
- Hu, J.; Yan, W.-S.; Liu, L.-D.; Xu, Y.-F.; Wang, Q.-F.; Liu, X.; Zhang, W.; Liang, X.-Y. Experiment study of E10 hydrous ethanol/gasoline blends on gasoline engine. Int. Combust. Engine Power Plant 2007, 1, 001. (In Chinese) [Google Scholar]
- Zarante, P.; Costa, T.; Sodre, J. Aldehyde emissions from an ethanol-fuelled spark ignition engine: Simulation and FTIR measurements. Blucher Chem. Eng. Proc. 2015, 1, 7738–7745. [Google Scholar]
- Poulopoulos, S.; Samaras, D.; Philippopoulos, C. Regulated and unregulated emissions from an internal combustion engine operating on ethanol-containing fuels. Atmos. Environ. 2001, 35, 4399–4406. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).