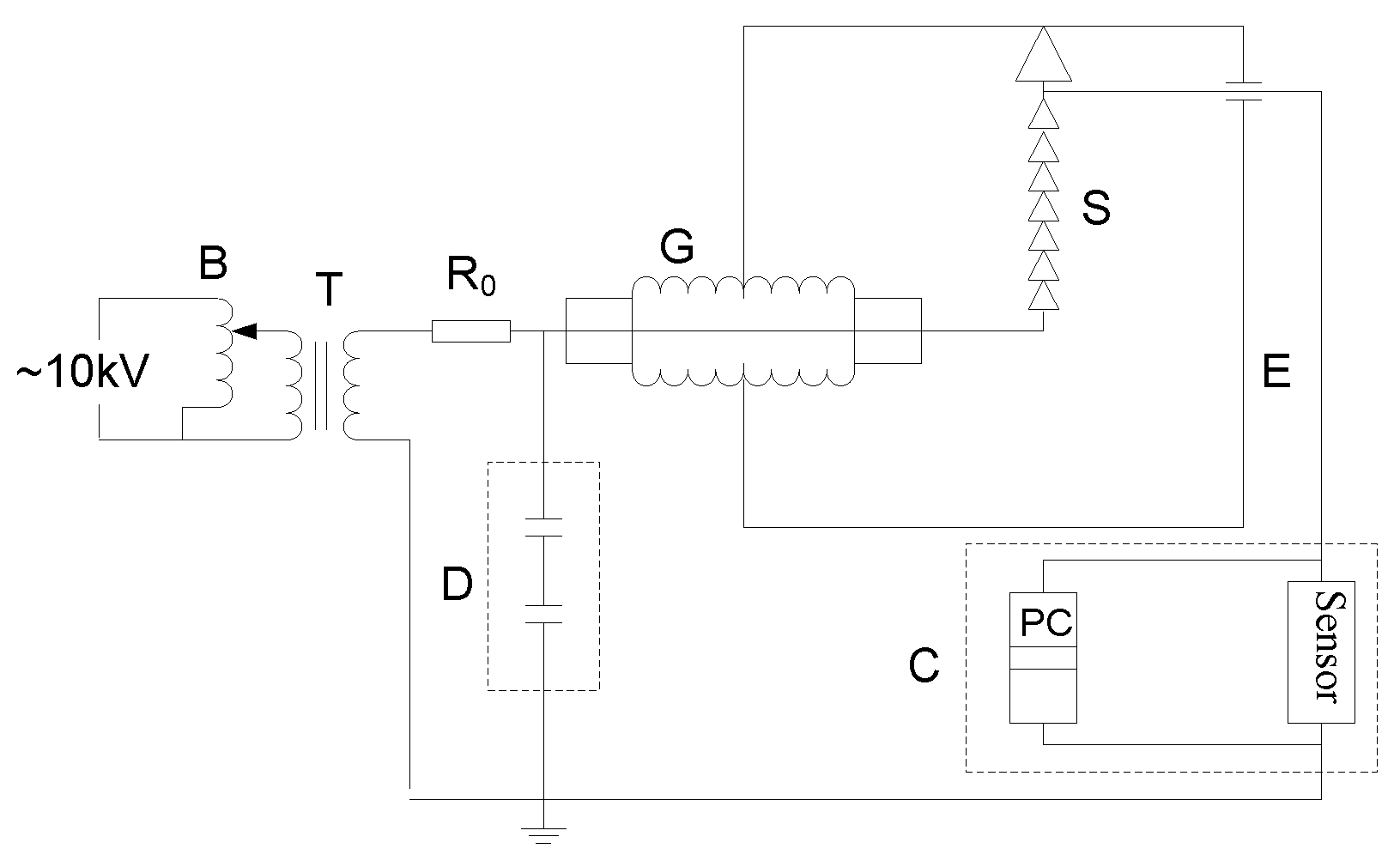
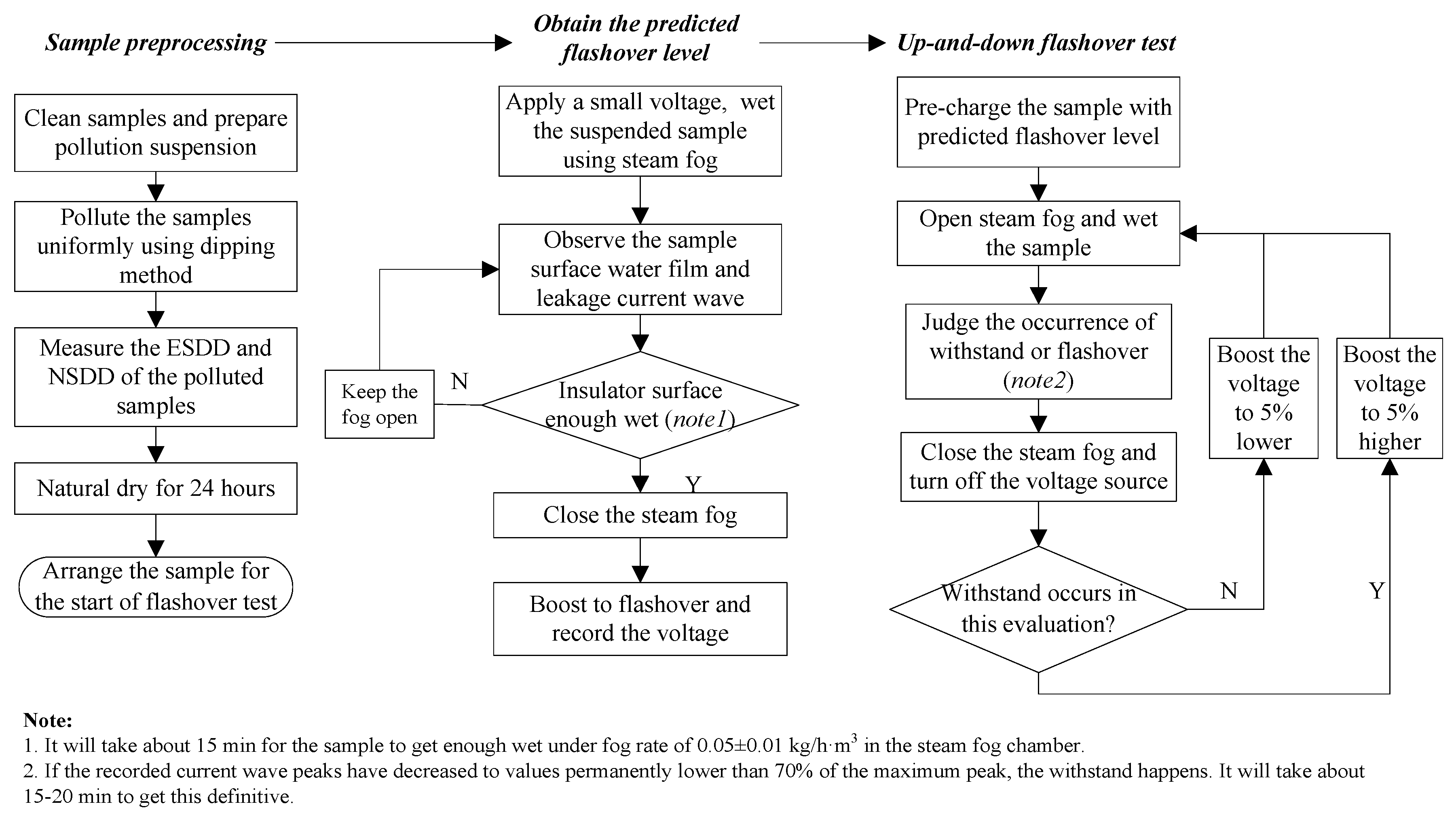
1. Introduction
External insulation equipment on transmission lines or in substations are frequently subjected to various kinds of contamination such as industry emissions, natural salt, dust, bird droppings, and other contaminants [
1]. Due to pollution source features, geographical environment, and weather conditions, there is a great diversity of chemical constituents deposited on insulator surfaces during contamination [
2].
Plenty of researches have been carried out so far to obtain the main constituents of insulator surface pollution. To sum up, insulator surface pollution is mainly made up of several conductive materials including NaCl, NaNO
3, KCl, KNO
3, Mg(NO
3)
2, MgSO
4, NH
4NO
3, NH
4Cl, (NH
4)
2SO
4, Ca(NO
3)
2, CaSO
4, and other contaminants, according to the research data in [
3,
4,
5].
All these constituents must affect an insulator’s electrical properties differentially [
6,
7], which will probably bring errors to the flashover voltage calculation. Currently, insulator flashover voltage is commonly calculated using an empirical negative exponent formula as follows [
8,
9,
10]:
The main symbols of this paper were described in
Table 1. In this formula, equivalent salt deposit density (
ESDD) is derived from converting the pollution solution conductivity to the weight of NaCl, and
A and
n are obtained through laboratory tests using NaCl as the soluble pollution. So, the formula neglects the influence of other soluble constituents on flashover voltage, which causes deficiency.
In light of this, researches concerning different soluble constituents were carried out to further reveal insulator flashover voltage characteristics.
In literature [
6], researchers studied the flashover performance of insulator polluted by different soluble materials, and proposed that, according to kinetic theory and permeation theory, a lower flashover voltage may be a function of the type of salt.
Researchers in [
7] studied the dependence of flashover voltages on the chemical composition of the insulator surface contaminants, and put forward that the limiting flashover voltage of a contaminant containing several salt types may be computed from that of each salt type measured independently:
In literature [
11], researchers recommended that when calculating the critical flashover voltage and critical current, appropriate arc constants (
A and
n) should be selected for different chemical compositions of the pollutants.
Researchers in literature [
12] showed the influence of pollution under 12 types of chemical constituents. It is proposed by the authors that in the case of simple salts and salts mixtures, the critical voltages are insensitive to the chemical nature of pollution. The critical currents are influenced by the chemical constitution of pollution for both polarities.
In literature [
13], researchers studied the flashover performance of insulator string polluted by CaSO
4 and NaCl, and reduced the flashover voltage calculation error through considering the slight solubility of CaSO
4. It is concluded by the authors of the paper that the current
ESDD method may fail to reflect specific effects of insulator contamination on its electrical property.
The above studies contributed towards the knowledge of pollution flashover principles and provide a theoretical basis for contamination constituents’ effects on the insulation property. However, during laboratory artificial tests, pollution degree measurements, as well as field insulation design, NaCl is still used as the only representation of a conductive constituent. Moreover, few of the previous works proposed a procedure for flashover voltage gradient correction that considers various characteristics of soluble constituents.
This paper presented a systematical research on insulator flashover voltage gradient correction considering soluble pollution constituents. Firstly, flashover tests of typical type insulator string polluted by NaCl, NaNO3, KNO3, NH4NO3, MgSO4, Ca(NO3)2, and CaSO4 were carried out, respectively. Then, with the flashover test data, the flashover gradient corrections basing on ESDD contribution of seven types of constituents and the saturation performance of CaSO4 were analyzed. The corrected flashover gradients agreed with insulator flashover test results under three types of soluble mixtures. This research is intended for contributing to the better design of outdoor insulation in field transmission lines.
3. Test Results and Analysis
The insulator flashover voltage gradient can be expressed by:
Test results are shown in
Table 4, where it can be seen that:
- (1)
The relative errors were lower than 7%, so the dispersion degree of the data acquired by the test procedure is very small. Besides, NSDD was controlled narrowly within 0.08–0.12 mg/cm2 through the dipping method, which means that the effects of NSDD on flashover voltage can be neglected.
- (2)
Insulator flashover performance is quite different under different soluble constituents. For example, when the constituent is NaNO3 and ESDD is 0.031 mg/cm2, insulator EL is 55.5 kV/m; however, when the soluble constituent is MgSO4 and ESDD increases to 0.049 mg/cm2, the EL increases to 57.3 kV/m. A higher ESDD value does not always mean a lower flashover voltage when the soluble constituent is different.
- (3)
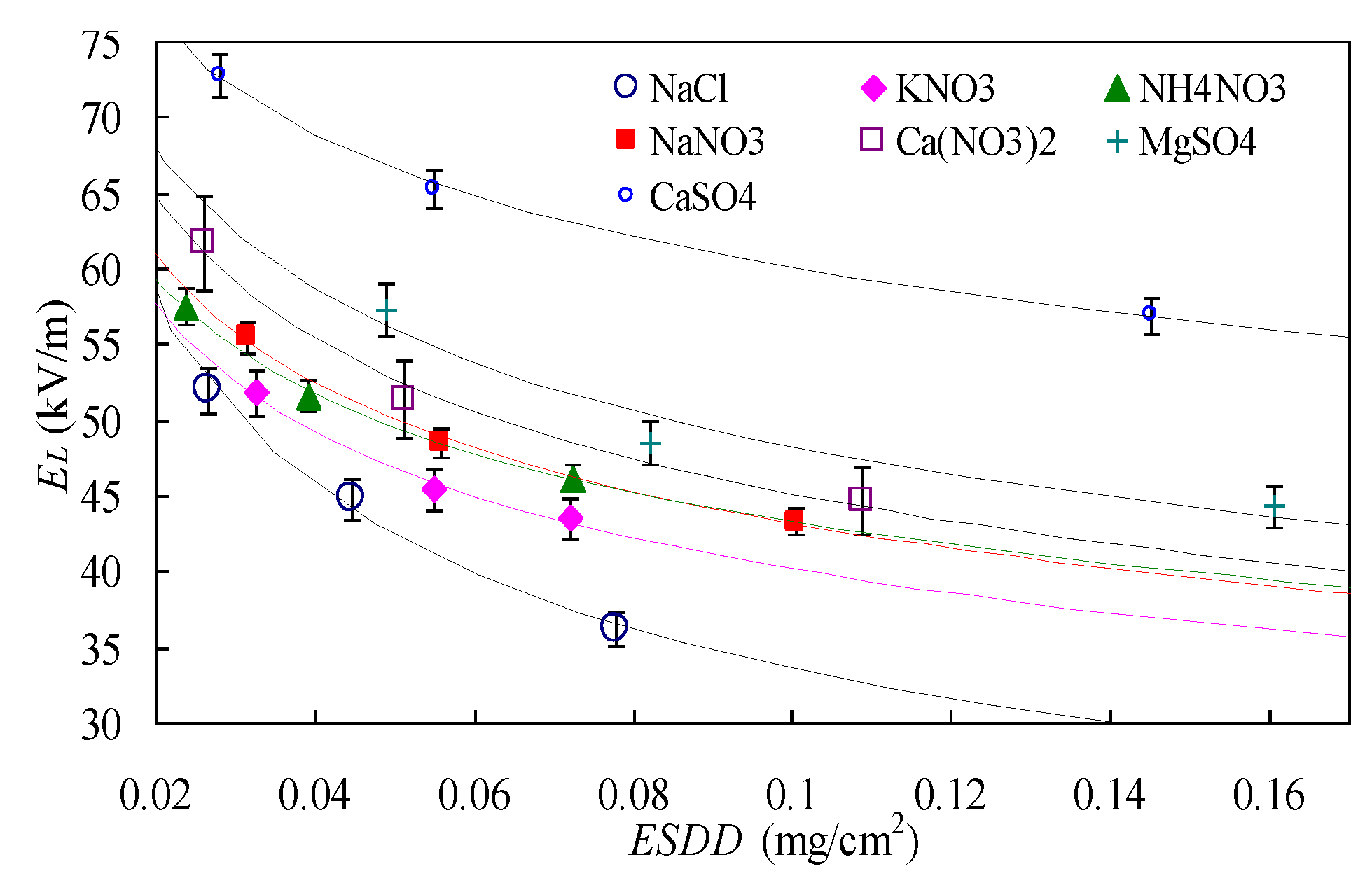
The negative exponent function in Equation (1) was used to fit the data in
Table 2; the results are shown in
Figure 3.
The curves fall into the 3% error bars of the data points, meaning the fit is good. It can be seen from the figure that different soluble constituent corresponds to different EL–ESDD variation trends. Given the same ESDD, the flashover gradient of slightly soluble salt CaSO4 is always the highest, and it decreases the most slowly with the increase of ESDD. The flashover gradient of NaCl is always the lowest, and it decreases the fastest with the increase of ESDD.
Fitting results of coefficients
A and
n are shown in
Table 5.
For the seven kinds soluble constituents, the A value of the insulator sample varies within 15.5–36.2, n varies within 0.19–0.34. It indicates that different soluble constituent corresponds to very different EL-ESDD relationship, and A is also associated with pollution chemical type. In summary, consideration of chemical compositions is necessary when calculating insulator pollution flashover voltage.
Flashover tests of the insulator sample polluted by soluble constituents’ mixture were also carried out. The components and their weight percentage of the pollution mixtures were determined basically according to the data in [
16,
17,
18]. In this study, three types of pollution mixtures were simulated, which represented a highway area, chemical plan area, and seaside, respectively. Test results are shown in
Table 6.
It can be seen from
Table 6 that under different types of pollution mixtures, the flashover performance varies. For example, when ESDD was about 0.081 mg/cm
2, the
EL under Type I pollution was 50.6 kV/cm, while that under Type II was 47.8 kV, a decrease of 6%.
Using the traditional method, which is only based on insulator flashover performance under NaCl (shown in Equation (1)), the flashover voltage gradient under pollution mixture was calculated. Calculation results and relative errors are shown in
Table 7.
The relative errors are very high, meaning that the traditional flashover voltage calculation method leads to serious deviation in guiding external insulation design of field operating lines which are inevitably polluted by various kinds of constituents. A correction procedure should be proposed to consider the factors of soluble constituents and to optimize the flashover voltage calculation results.
4. Correction of Flashover Gradient Considering Soluble Pollution
4.1. Correction Based on Equivalent Salt Density Contribution Ratio
According to Equation (2) which was provided by literature [
7], flashover voltage gradient under a salt mixture can be expressed by:
where
ELM is the flashover voltage gradient under a pollution mixture composed of
m kinds soluble constituents, kV/m;
ESDDM stands for the measured equivalent salt deposit density (
ESDD) of the mixture pollution solution;
SDDi stands for equivalent salt deposit density contributed by soluble constituent
i, mg/cm
2; and
ELi is the flashover voltage gradient corresponding to single soluble constituent
i under
ESDDM.
ELi can be obtained based on the fitting results in
Table 5:
So, if
ESDDM and
SDDi are determined, Equation (6) can be solved. For a certain pollution mixture, its
ESDDM is directly the measured equivalent salt deposit density of the mixture solution. However,
SDDi of each soluble constituent in the given pollution mixture is uncertain, because the existing chemical test techniques can only determine the ion component and weight of each chemical constituent in the soluble mixture [
16,
17,
18].
Suppose
βi is the weight percentage of soluble constituent
i in the pollution mixture,
αi is the contribution ratio of a certain weight of constituent
i to equivalent salt deposit density, and
WM is the total weight of the soluble mixture:
With Equation (8), Equation (6) can be simplified and rewritten as:
where
ηi stands for the
ESDD percentage of soluble constituent
i. It can be seen from Equation (9) that if
αi and
βi corresponding to each soluble constituent are determined, the flashover voltage gradient can be solved.
Equivalent salt density is basically derived from the conductivity of a certain solution, thus, soluble constituent conductivity tests were carried out. During the tests, the volume conductivity of each electrolyte solution under different concentrations was measured, and then the results were converted to the standard temperature (20 °C), as shown in
Figure 4:
It can be seen that the volume conductivity presents an almost linear relationship with the increment of electrolyte concentration. It is known that
ESDD is calculated based on the NaCl conductivity-weight relationship:
Sa can be calculated by volume conductivity, expressed as:
It can be seen that
ESDD is almost linear with the volume conductivity. So, the contribution ratio of soluble constituents to
ESDD can be obtained through comparing the conductivity curves’ slopes to the NaCl conductivity curve slope, as shown in
Table 8.
With
Table 8 and the weight percentage of each constituent given in 6, Equation (9) can be solved, as is shown in
Table 9.
It can be seen from the table that when considering the equivalent salt density of each soluble constituent, the relative errors between calculated and tested values decreased a lot. Two of the errors were already within 7%, which is acceptable for the prospective of practical engineering.
However, when it comes to a Type III pollution mixture, the relative error is still not acceptable. For Type III pollution, the amount of CaSO4 makes up to 60%, and the ESDD reaches 0.251 mg/cm2. In this circumstance, a large amount of CaSO4 cannot be dissolved during the insulator wetting process, which means the ESDD contribution ratio of CaSO4 needs to be reconsidered, given its easy saturation feature.
4.2. Correction Based on Calcium Sulfate’s Slight Solubility
The contribution of CaSO4 to ESDD is different from other soluble constituents, of which the solubilities are much higher, and the volume conductivities are linear with concentration. CaSO4 is slightly soluble, with a solubility of about 2 g/L at 20 °C. The CaSO4 on the surface of an insulator cannot dissolve sufficiently during the actual insulator wetting process in a steam chamber.
Under normal circumstances, one standard suspension insulator unit can only be coated by approximately 20 mL water [
13], and this volume is much smaller than the 300 mL water used when measuring the
ESDD. This fact will not affect the contribution of good diffluent constituents, but does bring obvious error when hard-to-dissolve salt CaSO
4 makes up a large part of the pollution mixture, because in this case a greater portion of the CaSO
4 does not act as conductive electrolyte if the insulator surface is wetted by just 20 mL water.
Considering this, the conductivity performance of high concentration CaSO
4 solution was measured, and then a comparison was made between the
ESDD contribution of CaSO
4 in 20 mL solution and in 300 mL solution, as shown in
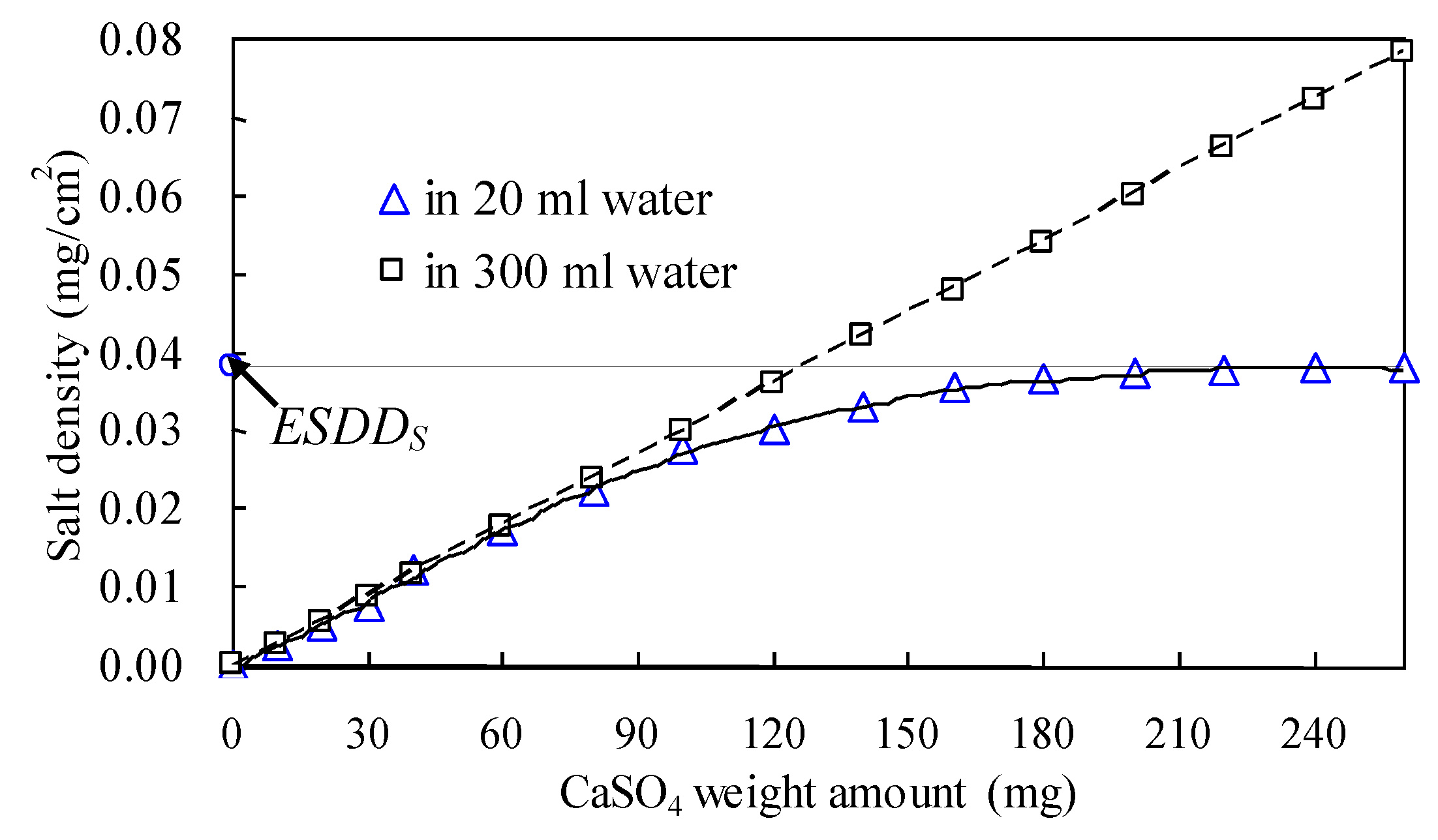
Figure 5.
It can be seen from
Figure 4 that the
ESDD contribution of CaSO
4 is overrated, considering the limited volume of insulator surface water absorbed. The saturated
ESDD contribution (
ESDDS, as shown
Figure 5) of CaSO
4 is only equal to 0.038 mg/cm
2 if the water volume is 20 mL, which is much lower than the
ESDD contribution for a volume of 300 mL.
The
ESDD contribution of CaSO
4 under 300 mL solution (
ESDDi300) can be calculated using the measured
ESDDM, based on Equation (9):
Then, according to
Figure 5, the corrected CaSO
4 ESDD contribution, considering slight solubility and limited wetting amount, can be expressed as follows:
where
ESDDi20 is the corrected value, assuming the largest surface-coated volume of water is 20 mL.
The measured
ESDD value of the pollution mixture should also be corrected to the 20 mL situation:
Then, the
ESDD percentage of each constituent should be modified:
Considering the slight solubility of CaSO
4, and using Equations (14) and (15) to correct the measured equivalent salt deposit density (
ESDDM) and
ESDD percentage (
ηi) of each constituent, the results are as shown in
Table 10.
It can be seen from the table that for the Type III pollution mixture, its actual equivalent salt deposit density and constituent ESDD percentage are changed obviously after correction. This is because the weight of CaSO4 in the Type III pollution mixture is much greater than those in the other two types.
As the table shows, for the Type III pollution mixture, its actual equivalent salt deposit density should be 0.164 mg/cm2, decreased by 34.6% compared to the measured value using 300 mL water; the ESDD percentage of CaSO4 should be 0.232, decreased by 54% compared to the measured value using 300 mL; and for other soluble constituents, their ESDD percentage increased a bit. This indicates that the traditional method of measuring ESDD will overrate the influence of slightly soluble constituents on flashover voltage.
Using the corrected data of
Table 10 to correct the
ELM value:
The corrected flashover voltage gradient values and corresponding relative errors are as shown in
Table 11.
It can be seen obviously that the relative error for the Type III pollution mixture decreases from −20.4% to −4.4%, which means the correction, when considering the slight solubility of CaSO4, does lower the calculated error, making the flashover voltage gradient calculation more accurate and scientific.
5. Discussion
The flashover tests in a laboratory climate chamber cannot actually reflect the real flashover of a natural polluted insulator, making the voltage calculating error inevitable. However, through the correction process proposed in this paper, the error can be significantly reduced. It is important to calculate the ESDD contribution ratio of each soluble constituent when predicting flashover voltage of natural polluted insulator, because different soluble constituents on an insulator surface correspond to different flashover performances. Also, consideration of CaSO4 saturation performance is a necessary step due to CaSO4 always occupies the largest proportion of natural contaminants.
Currently, the effects of pollution constituents have been brought to the attention of relative research institutes. They conducted plenty of measurements on field insulator surface chemical components, and the most commonly used method is IC (ion chromatograph) analysis [
4,
16,
17,
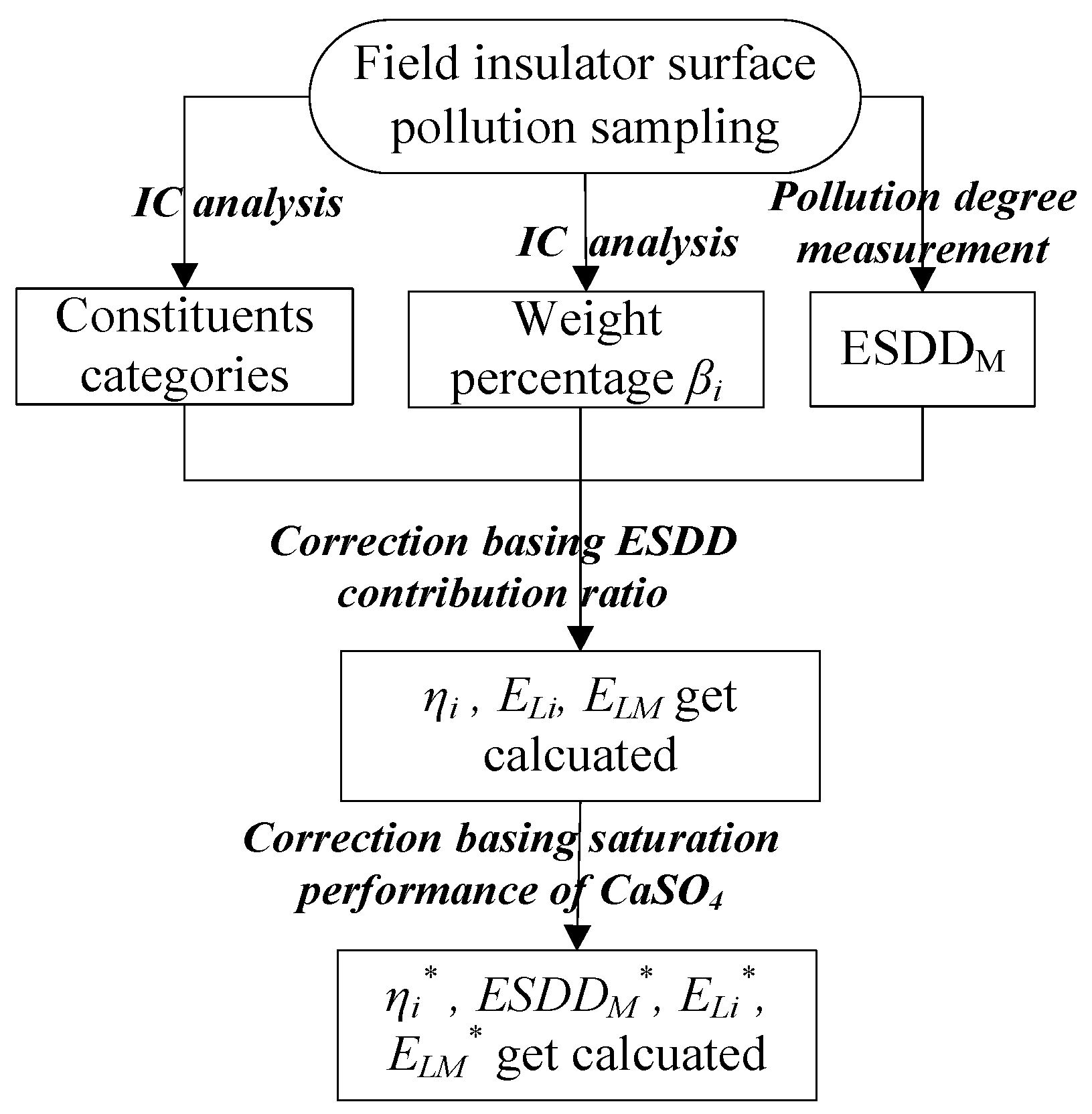
18]. This method provides accurate categorization of negative and positive ions, as well as their mass percentage in the pollution solution. Then, the soluble constituents and their weights in the pollution solution can be easily determined by pairing the ions. Based on this, the flashover gradient of an insulator under natural pollution conditions can be better determined through a systematical correction procedure shown in
Figure 6.
This procedure remedies the deficiency of the traditional ESDD method by considering the soluble constituents of insulator surface contaminants, and it is easy to implement in engineering practice.