Template-Free Fabrication of Bi2WO6 Hierarchical Hollow Microspheres with Visible-Light-Driven Photocatalytic Activity
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis
2.2. Characterization
2.3. Photocatalytic Activity Test
3. Results and Discussion
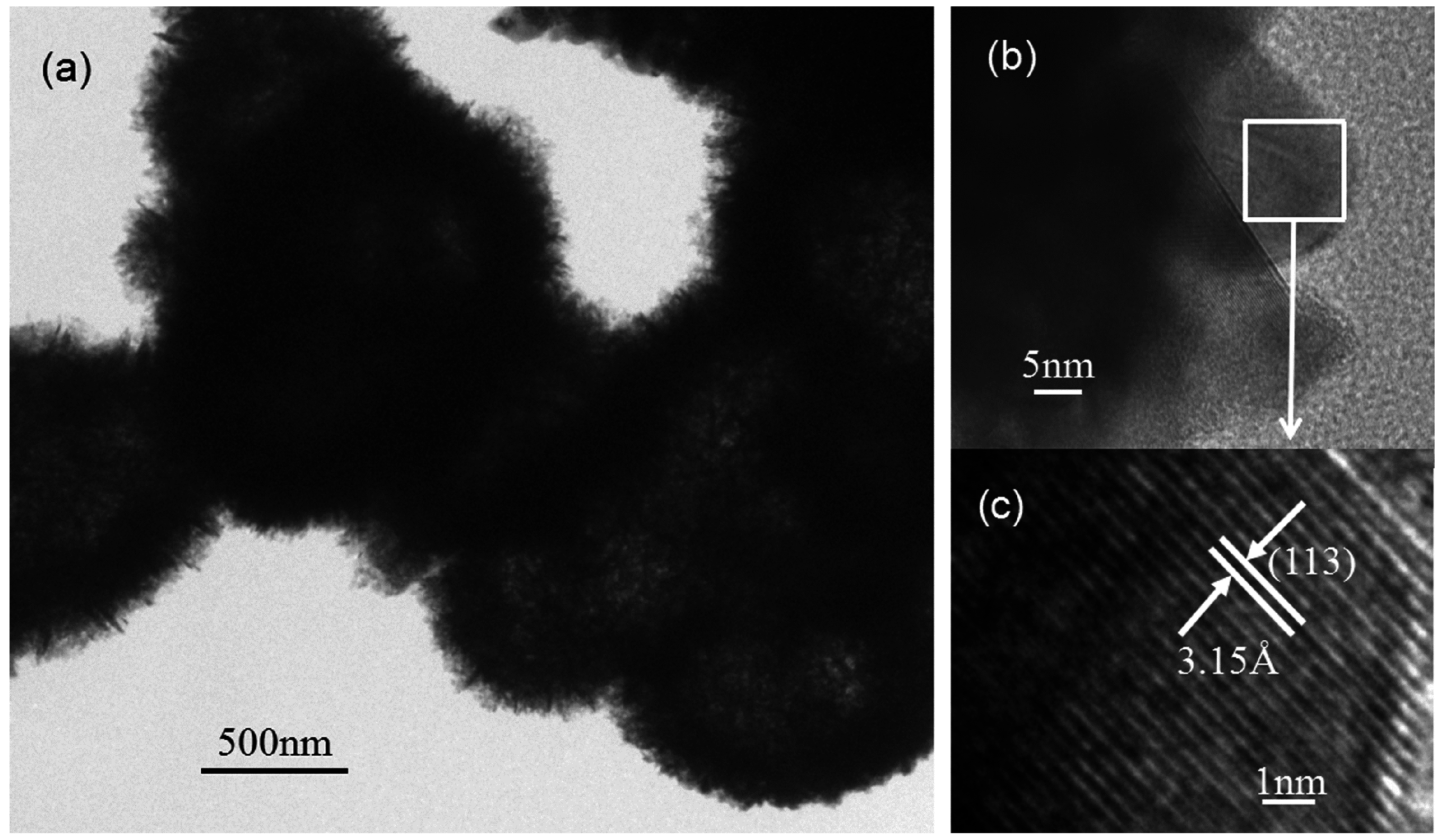
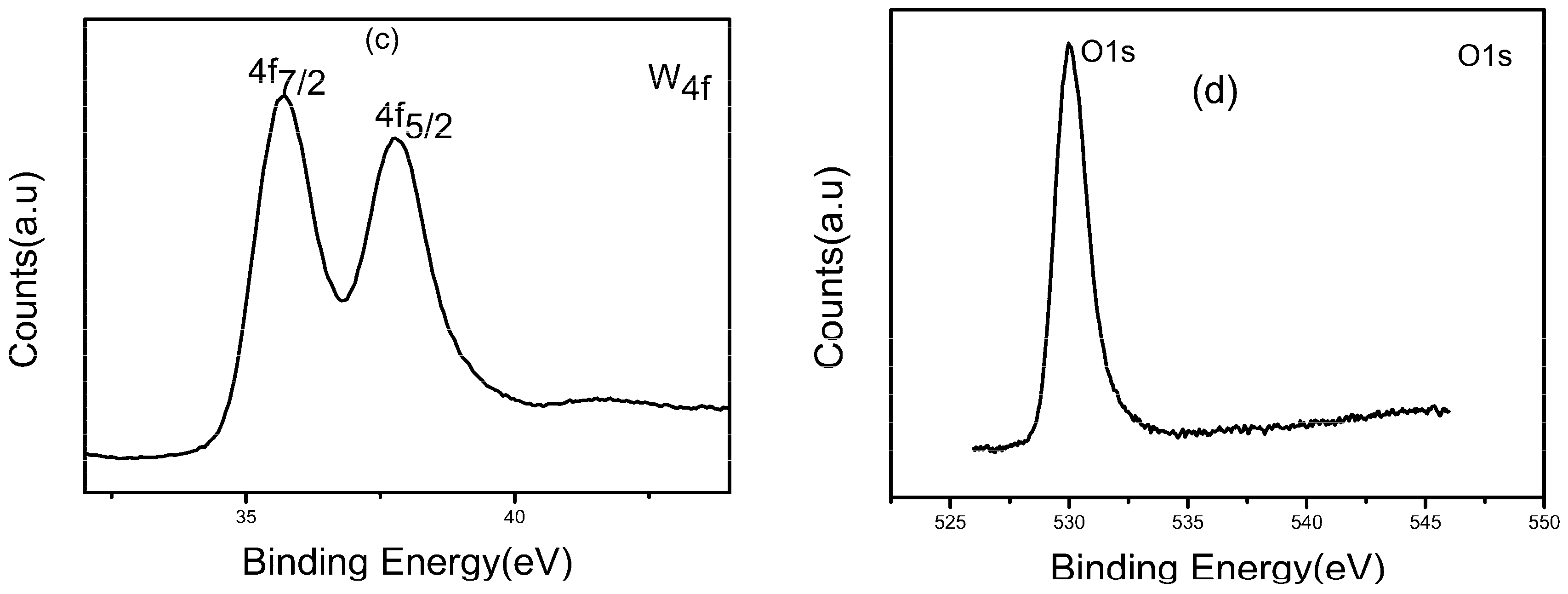
3.1. Structure and Morphology
3.2. Ultraviolet-Visible Diffuse Reflectance Spectra Analysis
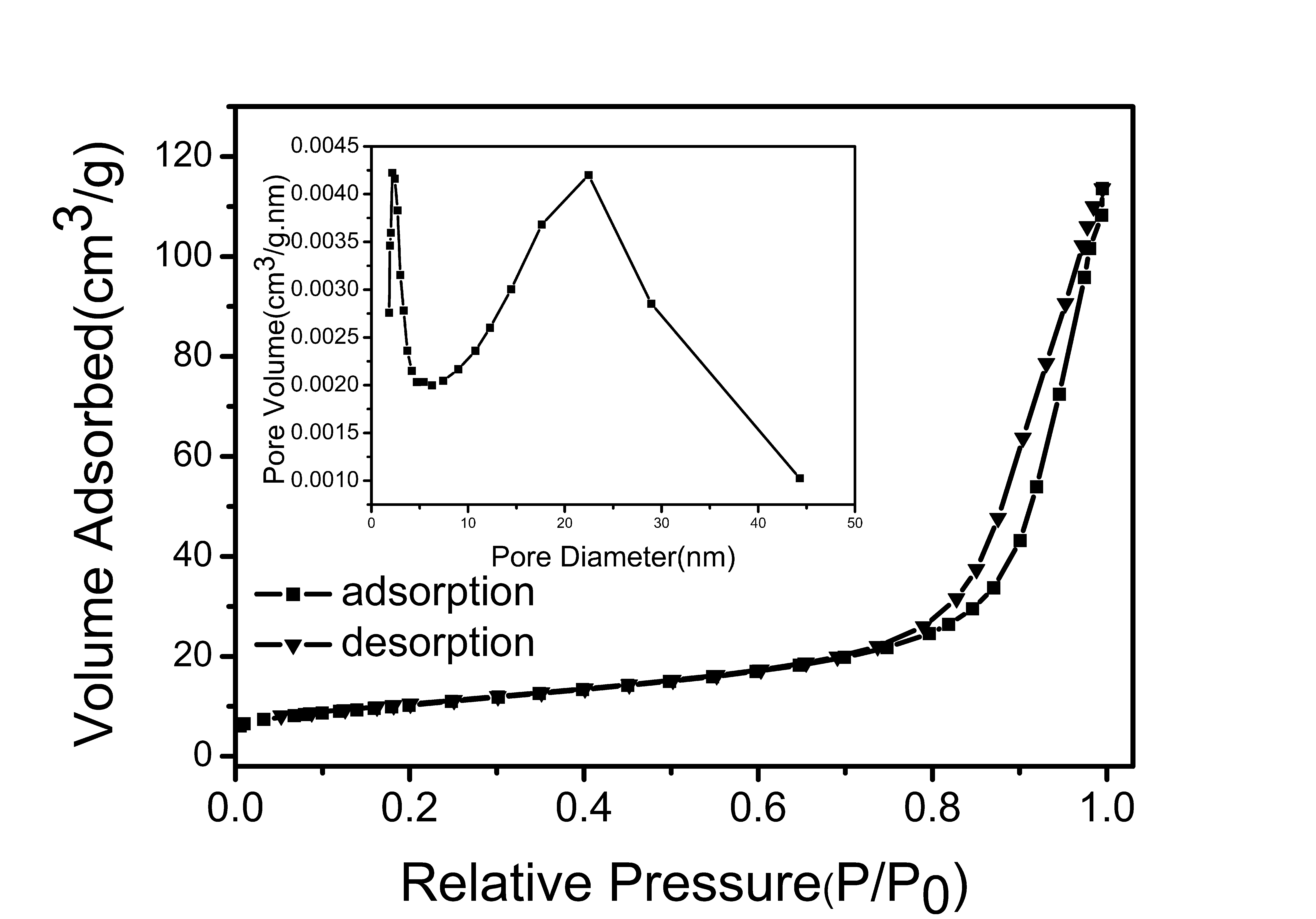
3.3. Brunauer–Emmett–Teller Surface Area Analysis
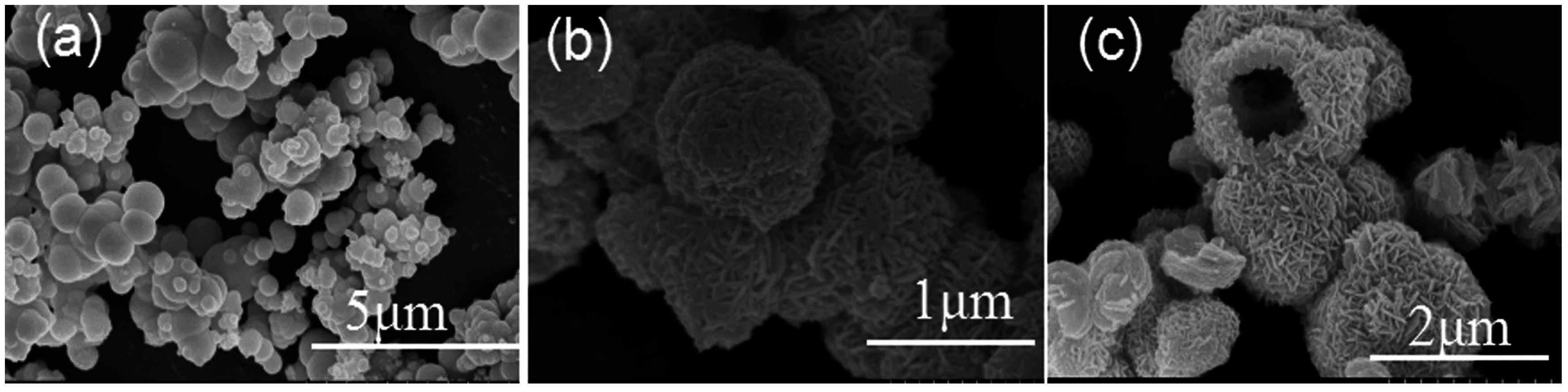
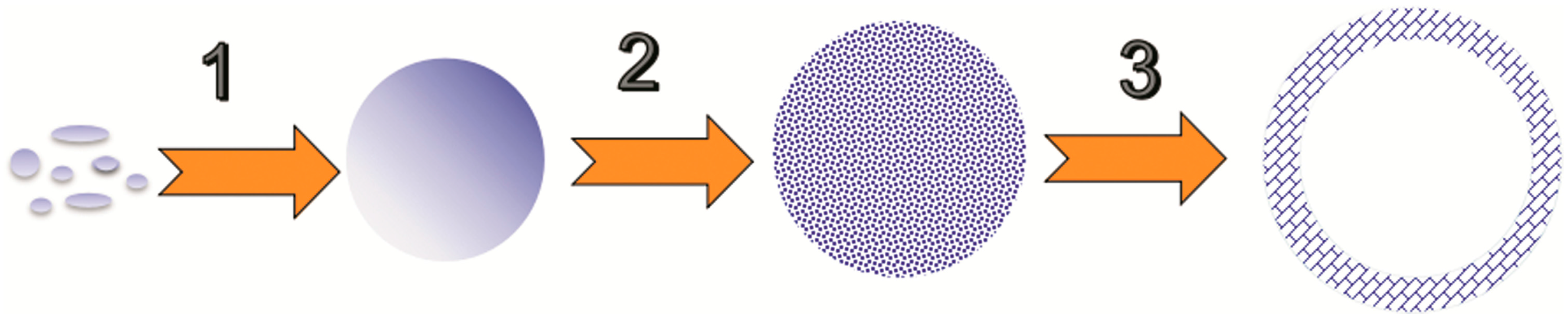
3.4. Formation Mechanism
3.5. Photocatalytic Activity
4. Conclusions
Supplementary Materials
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Cheng, X.L.; Jiang, J.S.; Hu, M.; Mao, G.Y.; Liu, Z.W.; Zeng, Y.; Zhang, Q.H. Liquid–liquid interface-assisted solvothermal synthesis of durian-like α-Fe2O3 hollow spheres constructed by nano-polyhedrons. Cryst. Eng. Comm. 2012, 14, 3056–3062. [Google Scholar] [CrossRef]
- Lin, Q.Y.; Li, L.; Liang, S.J.; Liu, M.H.; Bi, J.H.; Wu, L. Efficient synthesis of monolayer carbon nitride 2D nanosheet with tunable concentration and enhanced visible-light photocatalytic activities. Appl. Catal. B Environ. 2015, 163, 135–142. [Google Scholar] [CrossRef]
- Zhang, N.; Ciriminna, R.; Pagliaro, M.; Xu, Y.J. Nanochemistry-derived Bi2WO6 nanostructures: Towards production of sustainable chemicals and fuels induced by visible light. Chem. Soc. Rev. 2014, 43, 5276–5287. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.G.; Zhang, Y.F.; Lin, M.S.; Long, J.L.; Zhang, Z.Z.; Lin, H.X.; Wu, J.C.-S.; Wang, X.X. Monolayered Bi2WO6 nanosheets mimicking heterojunction interface with open surfaces for photocatalysis. Nat. Commun. 2015, 6, 8340–8347. [Google Scholar] [CrossRef] [PubMed]
- Feteira, A.; Sinclair, D.C. Microwave dielectric properties of low firing temperature Bi2W2O9 Ceramics. J. Am. Ceram. Soc. 2008, 91, 1338–1341. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, L.Z.; Zhang, J.L. A direct route for the synthesis of nanometer-sized Bi2WO6 particles loaded on a spherical MCM-48 mesoporous molecular sieve. Chem. Commun. 2010, 46, 8067–8069. [Google Scholar] [CrossRef] [PubMed]
- Shang, M.; Wang, W.Z.; Sun, S.M.; Zhou, L.; Zhang, L. Bi2WO6 nanocrystals with high photocatalytic activities under visible light. J. Phys. Chem. C 2008, 112, 10407–10411. [Google Scholar] [CrossRef]
- Chen, Z.; Qian, L.W.; Zhu, J.; Yuan, Y.P.; Qian, X.F. Controlled synthesis of hierarchical Bi2WO6 microspheres with improved visible-light-driven photocatalytic activity. Cryst. Eng. Comm. 2010, 12, 2100–2106. [Google Scholar] [CrossRef]
- Zhang, L.S.; Wang, H.L.; Chen, Z.G.; Wong, P.K.; Liu, J.S. Bi2WO6 micro/nano-structures: Synthesis, modifications and visible-light-driven photocatalytic applications. Appl. Catal. B Environ. 2011, 106, 1–13. [Google Scholar] [CrossRef]
- Ma, D.K.; Huang, S.M.; Chen, W.X.; Hu, S.W.; Shi, F.F.; Fan, K. Self-assembled three-dimensional hierarchical umbilicate Bi2WO6 microspheres from nanoplates: Controlled synthesis, photocatalytic activities, and wettability. J. Phys. Chem. C 2009, 113, 4369–4374. [Google Scholar] [CrossRef]
- Nguyen, C.C.; Vu, N.N.; Do, T.-O. Recent advances in the development of sunlight-driven hollow structure photocatalysts and their applications. J. Mater. Chem. A 2015, 3, 18345–18359. [Google Scholar] [CrossRef]
- Dai, X.J.; Luo, Y.S.; Zhang, W.D.; Fu, S.Y. Facile hydrothermal synthesis and photocatalytic activity of bismuth tungstate hierarchical hollow spheres with an ultrahigh surface area. Dalton Trans. 2010, 39, 3426–3432. [Google Scholar] [CrossRef] [PubMed]
- Li, X.N.; Huang, R.K.; Hu, Y.H.; Chen, Y.J.; Liu, W.J.; Yuan, R.S.; Li, Z.H. A templated method to Bi2WO6 hollow microspheres and their conversion to double-shell B2O3/Bi2WO6 hollow microspheres with improved photocatalytic performance. Inorg. Chem. 2012, 51, 6245–6250. [Google Scholar] [CrossRef] [PubMed]
- Shang, M.; Wang, W.Z.; Xu, H.L. New Bi2WO6 nanocages with high visible-light-driven photocatalytic activities prepared in refluxing EG. Cryst. Growth Des. 2009, 9, 991–996. [Google Scholar] [CrossRef]
- Wu, J.; Duan, F.; Zheng, Y.; Xie, Y. Synthesis of Bi2WO6 nanoplate-built hierarchical nest-like structures with visible-light-induced photocatalytic activity. J. Phys. Chem. C 2007, 111, 12866–12971. [Google Scholar] [CrossRef]
- Wang, D.J.; Guo, L.; Zhen, Y.Z.; Yue, L.L.; Xue, G.L.; Fu, F. AgBr quantum dots decorated mesoporous Bi2WO6 architectures with enhanced photocatalytic activities for methylene blue. J. Mater. Chem. A 2014, 2, 11716–11727. [Google Scholar] [CrossRef]
- Kudo, A.; Tsuji, I.; Kato, H. AgInZn7S9 solid solution photocatalyst for H2 evolution from aqueous solutions under visible light irradiation. Chem. Commun. 2002, 17, 1958–1959. [Google Scholar] [CrossRef]
- Fu, H.B.; Yao, W.Q.; Zhang, L.W.; Zhu, Y.F. The enhanced photoactivity of nanosized Bi2WO6 catalyst for the degradation of 4-chlorophenol. Mater. Res. Bull. 2008, 43, 2617–2625. [Google Scholar] [CrossRef]
- Tang, J.W.; Zou, Z.G.; Ye, J.H. Photocatalytic decomposition of organic contaminants by Bi2WO6 under visible light irradiation. Catal. Lett. 2004, 92, 53–56. [Google Scholar] [CrossRef]
- Liu, S.J.; Hou, Y.F.; Zheng, S.L.; Zhang, Y.; Wang, Y. One-dimensional hierarchical Bi2WO6 hollow tubes with porous walls: Synthesis and photocatalytic property. Cryst. Eng. Comm. 2013, 15, 4124–4130. [Google Scholar] [CrossRef]
- Dong, F.; Zheng, A.M.; Sun, Y.J.; Fu, M.; Jiang, B.Q.; Ho, W.K.; Lee, S.C.; Wu, Z.B. One-pot template-free synthesis, growth mechanism and enhanced photocatalytic activity of monodisperse (BiO)2CO3 hierarchical hollow microspheres self-assembled with single-crystalline nanosheets. Cryst. Eng. Comm. 2012, 14, 3534–3544. [Google Scholar] [CrossRef]
- Li, H.X.; Bian, Z.F.; Zhu, J.; Zhang, D.Q.; Li, G.S.; Huo, Y.N.; Li, H.; Lu, Y.F. Mesoporous titania spheres with tunable chamber structure and enhanced photocatalytic activity. J. Am. Chem. Soc. 2007, 129, 8406–8407. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.G.; Zeng, H.C. Preparation of hollow anatase TiO2 nanospheres via Ostwald Ripening. J. Phys. Chem. B 2004, 108, 3492–3495. [Google Scholar] [CrossRef]
- Hu, J.; Chen, M.; Fang, X.S.; Wu, L.M. Fabrication and application of inorganic hollow spheres. Chem. Soc. Rev. 2011, 40, 5472–5491. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.H.; Chen, Y.J.; Zhou, W.; Pan, K.; Dong, Y.Z.; Tian, C.G.; Fu, H.G. Facile solvothermal synthesis of hierarchical flower-like Bi2MoO6 hollow spheres as high performance visible-light driven photocatalysts. J. Mater. Chem. 2011, 21, 887–892. [Google Scholar] [CrossRef]
- Xu, Y.M.; Zhang, Y.W.; Zhou, Y.M.; Xiang, S.M.; Wang, Q.L.; Zhang, C.; Sheng, X.L. CeO2 hollow nanospheres synthesized by a one pot template-free hydrothermal method and their application as catalyst support. RSC Adv. 2015, 5, 58237–58245. [Google Scholar] [CrossRef]
- Zhou, X.; Li, X.W.; Sun, H.B.; Sun, P.; Liang, X.S.; Liu, F.M.; Hu, X.L.; Lu, G.Y. Nanosheet-assembled ZnFe2O4 hollow microspheres for high-sensitive acetone sensor. ACS Appl. Mater. Interfaces 2015, 7, 15414–15421. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.C.; Wang, L.M.; Chu, D.Q.; Sun, H.M.; Wang, A.X. Template-free synthesis of complicated double-wall Cu2O hollow spheres with enhanced visible photocatalytic activities. RSC Adv. 2015, 5, 8223–8227. [Google Scholar] [CrossRef]
- Zheng, Z.K.; Huang, B.B.; Qin, X.Y.; Zhang, X.Y.; Dai, Y. Facile synthesis of SrTiO3 hollow microspheres built as assembly of nanocubes and their associated photocatalytic activity. J. Colloid Interface Sci. 2011, 358, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, G.K.; Yu, J.C.; Guo, Y.D. In situ synthesis of Zn2GeO4 hollow spheres and their enhanced photocatalytic activity for the degradation of antibiotic metronidazole. Dalton Trans. 2013, 42, 5092–5099. [Google Scholar] [CrossRef] [PubMed]
- Dai, K.; Lu, L.H.; Dong, J.; Ji, Z.Y.; Zhu, G.P.; Liu, Q.Z.; Liu, Z.L.; Zhang, Y.X.; Li, D.P.; Liang, C.H. Facile synthesis of a surface plasmon resonance enhanced Ag/AgBr heterostructure and its photocatalytic performance with 450 nm LED illumination. Dalton Trans. 2013, 42, 4657–4662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Y.; Feng, Y.; Wu, Q.S.; Xu, Y.Y.; Gao, D.Z. Facile fabrication of flower-shaped Bi2WO6 superstructures and visible-light-driven photocatalytic performance. Mater. Res. Bull. 2012, 47, 1919–1924. [Google Scholar] [CrossRef]
- Merka, O.; Yarovyi, V.; Bahnemann, D.W.; Wark, M. pH-control of the photocatalytic degradation mechanism of Rhodamine B over Pb3Nb4O13. J. Phys. Chem. C 2011, 115, 8014–8023. [Google Scholar] [CrossRef]
- Fu, H.B.; Pan, C.S.; Yao, W.Q.; Zhu, Y.F. Visible-light-induced degradation of Rhodamine B by nanosized Bi2WO6. J. Phys. Chem. B 2005, 109, 22432–22439. [Google Scholar] [CrossRef] [PubMed]




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Meng, X.; Tong, L.; Zeng, X.; Chen, X. Template-Free Fabrication of Bi2WO6 Hierarchical Hollow Microspheres with Visible-Light-Driven Photocatalytic Activity. Energies 2016, 9, 764. https://doi.org/10.3390/en9100764
Zhou Y, Meng X, Tong L, Zeng X, Chen X. Template-Free Fabrication of Bi2WO6 Hierarchical Hollow Microspheres with Visible-Light-Driven Photocatalytic Activity. Energies. 2016; 9(10):764. https://doi.org/10.3390/en9100764
Chicago/Turabian StyleZhou, Yuxue, Xiangdong Meng, Ling Tong, Xianghua Zeng, and Xiaobing Chen. 2016. "Template-Free Fabrication of Bi2WO6 Hierarchical Hollow Microspheres with Visible-Light-Driven Photocatalytic Activity" Energies 9, no. 10: 764. https://doi.org/10.3390/en9100764
APA StyleZhou, Y., Meng, X., Tong, L., Zeng, X., & Chen, X. (2016). Template-Free Fabrication of Bi2WO6 Hierarchical Hollow Microspheres with Visible-Light-Driven Photocatalytic Activity. Energies, 9(10), 764. https://doi.org/10.3390/en9100764





