Eicosapentaenoic Acid from Porphyridium Cruentum: Increasing Growth and Productivity of Microalgae for Pharmaceutical Products
Abstract
:1. Introduction
2. Experimental
2.1. Strains and Growth Conditions
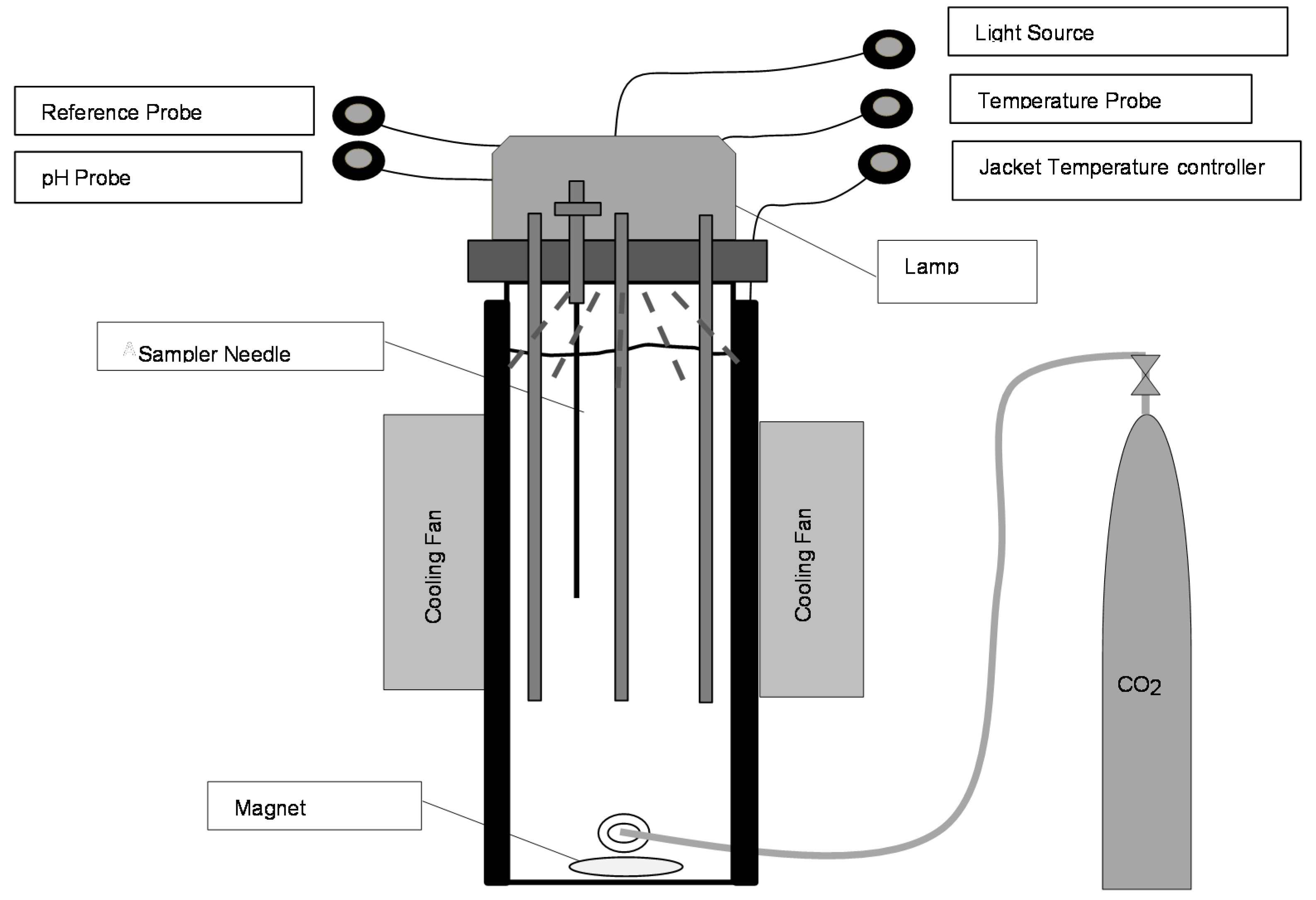
2.2. Lipid Extraction
2.3. Lipid Transmethylations
2.4. Gas Chromatography Analysis
3. Results and Discussion
3.1. Light Intensity, Temperature, and CO2 Supplementation
3.1.1. Cell Densities and Growth Rates
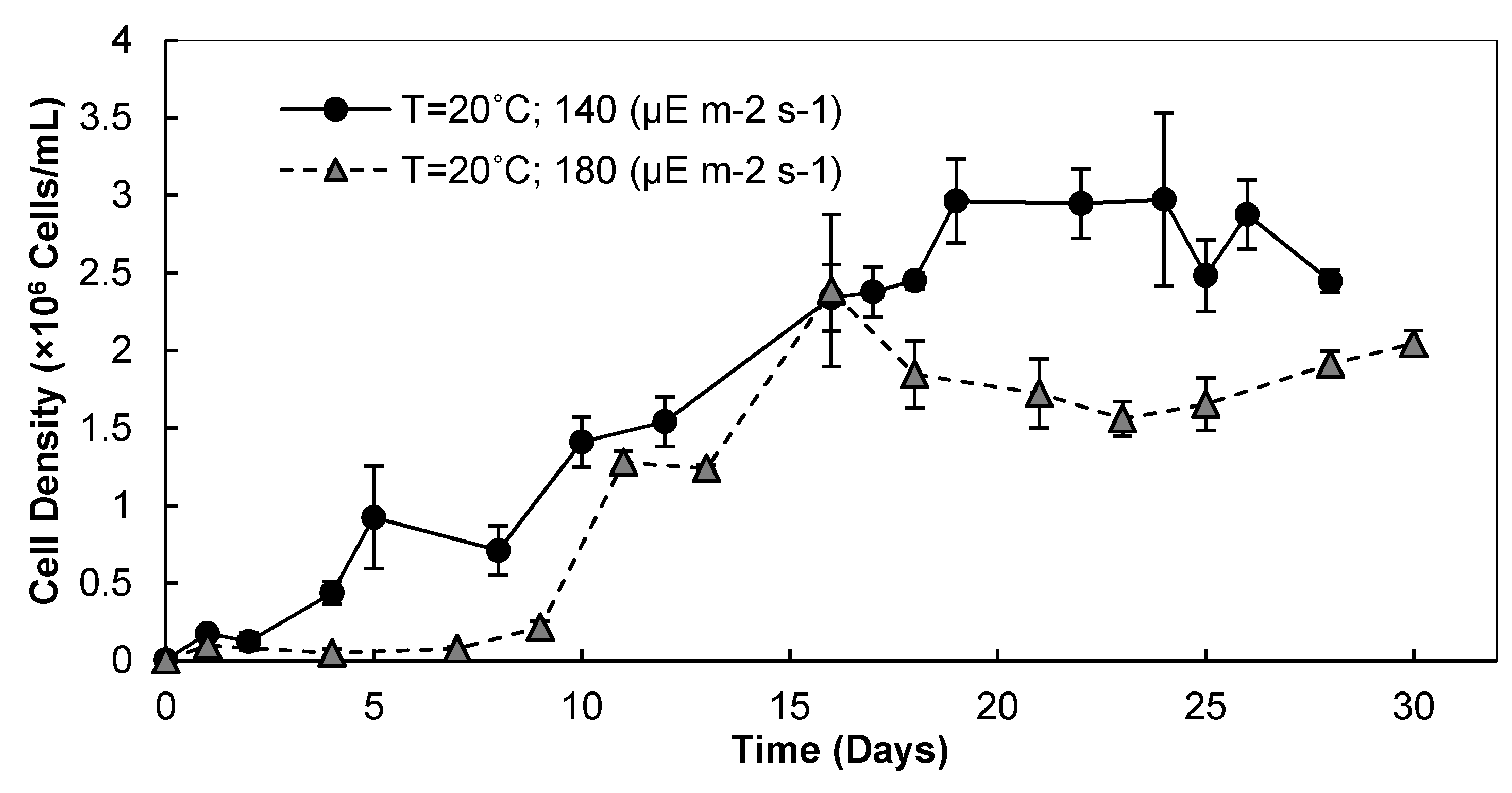
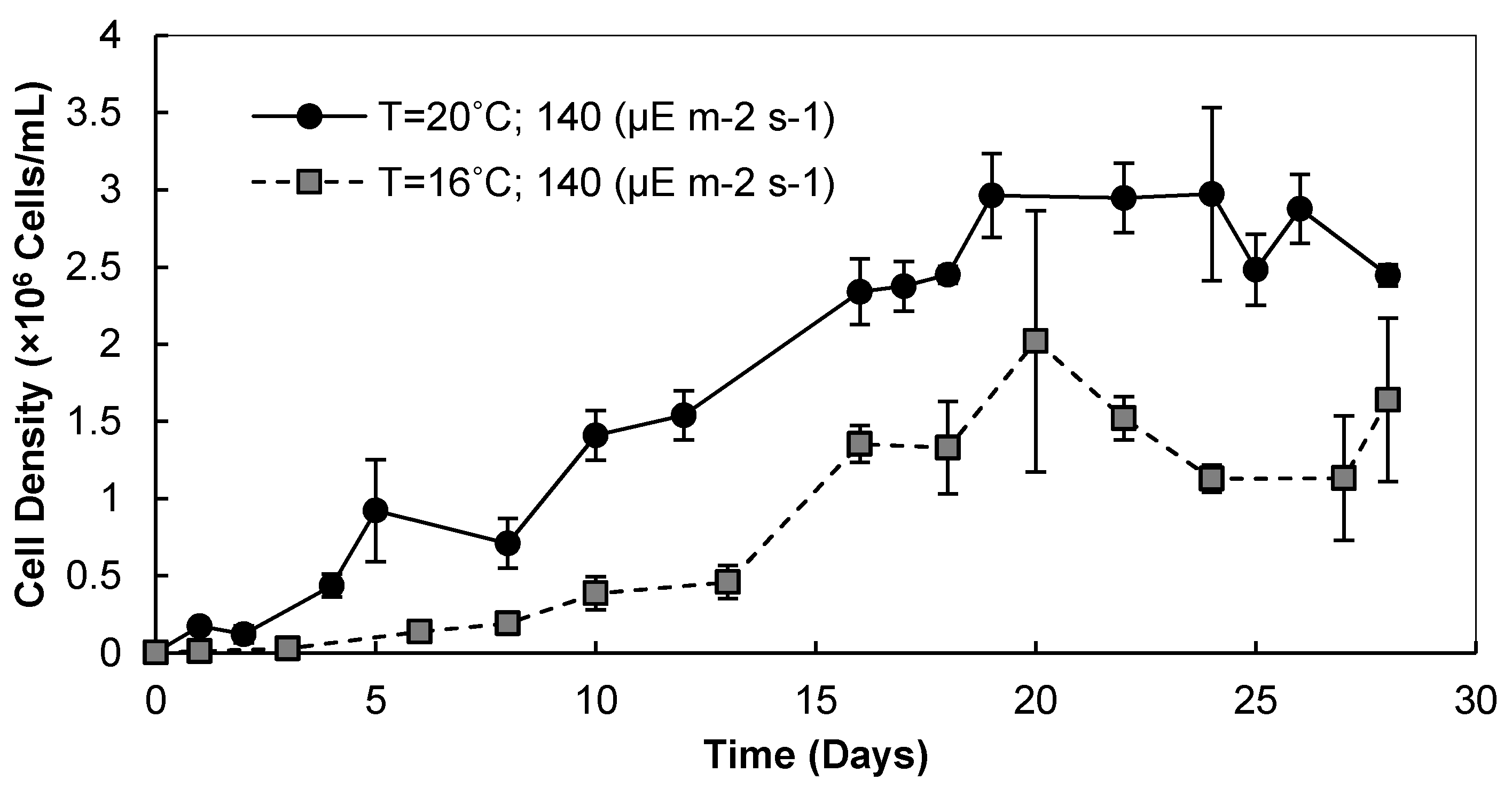
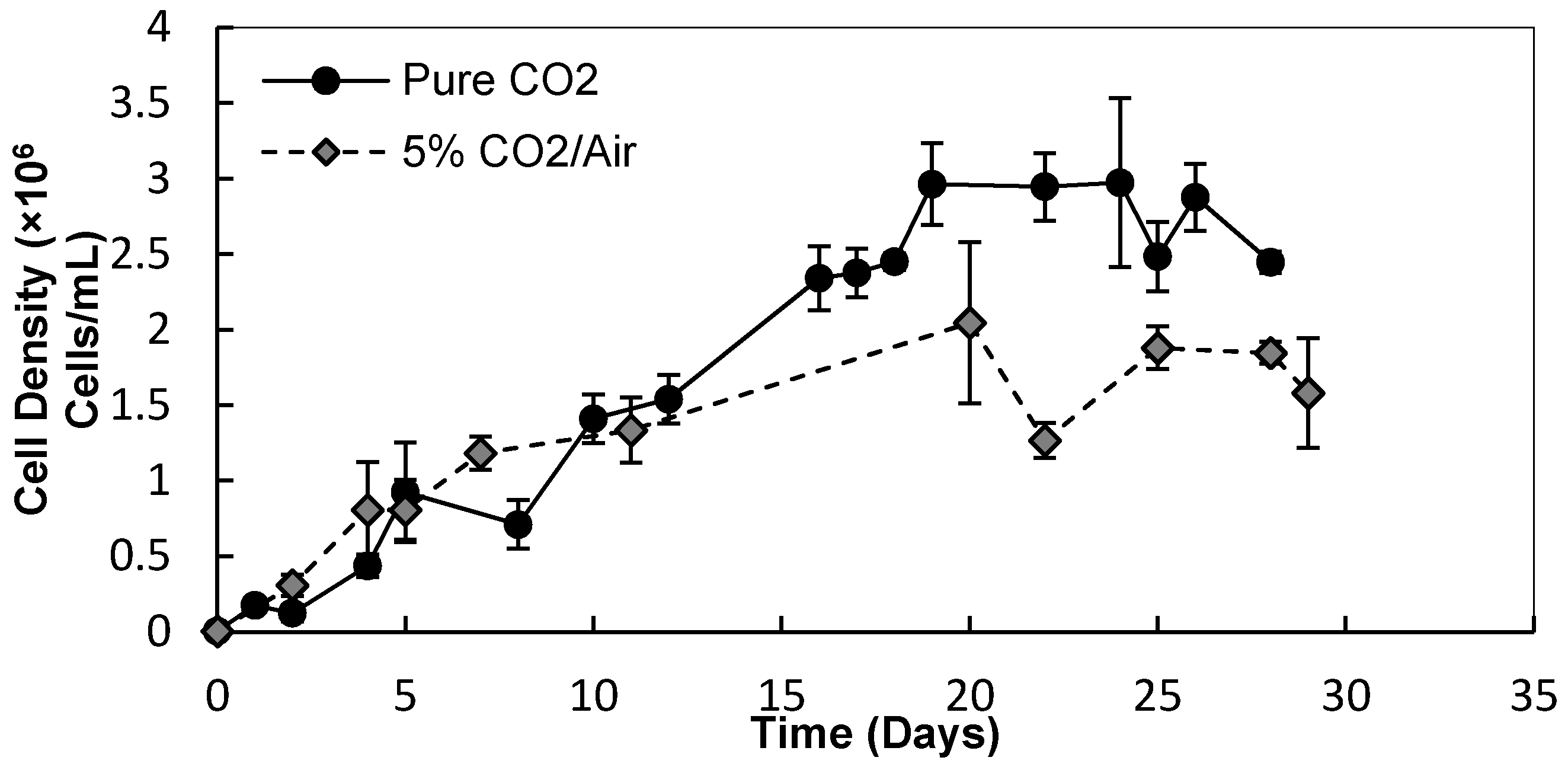
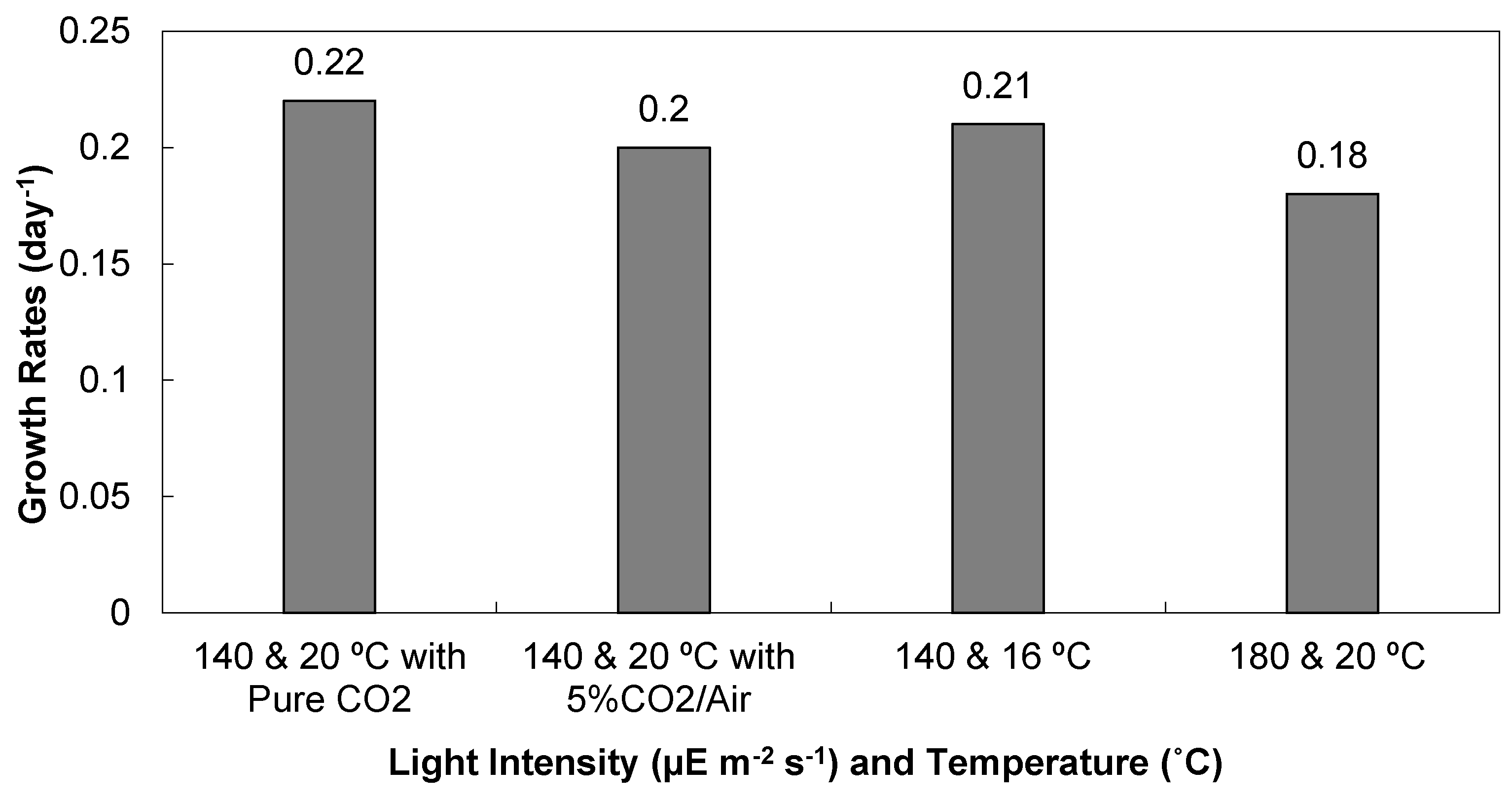
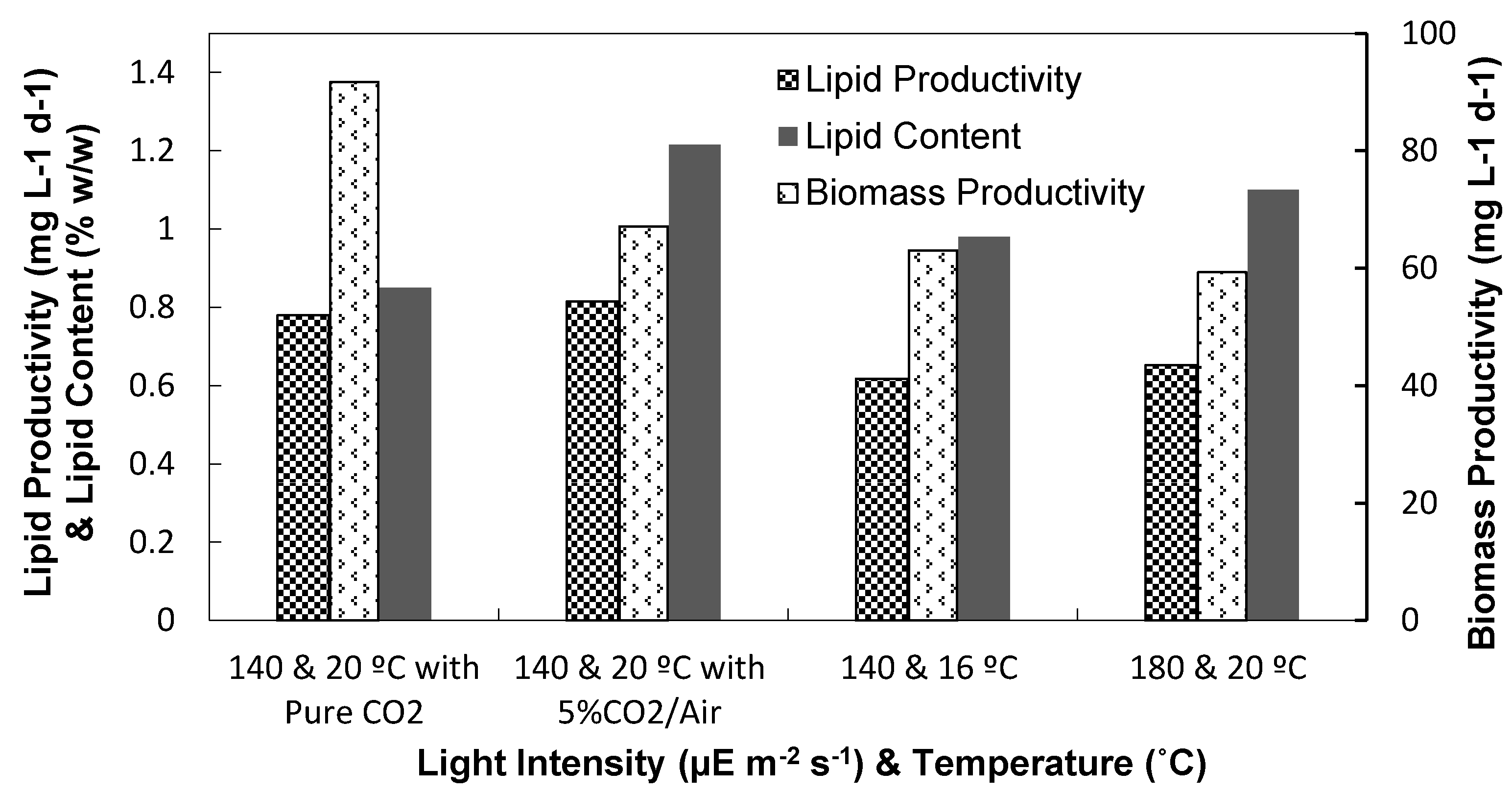
3.1.2. Biomass and Lipid Contents
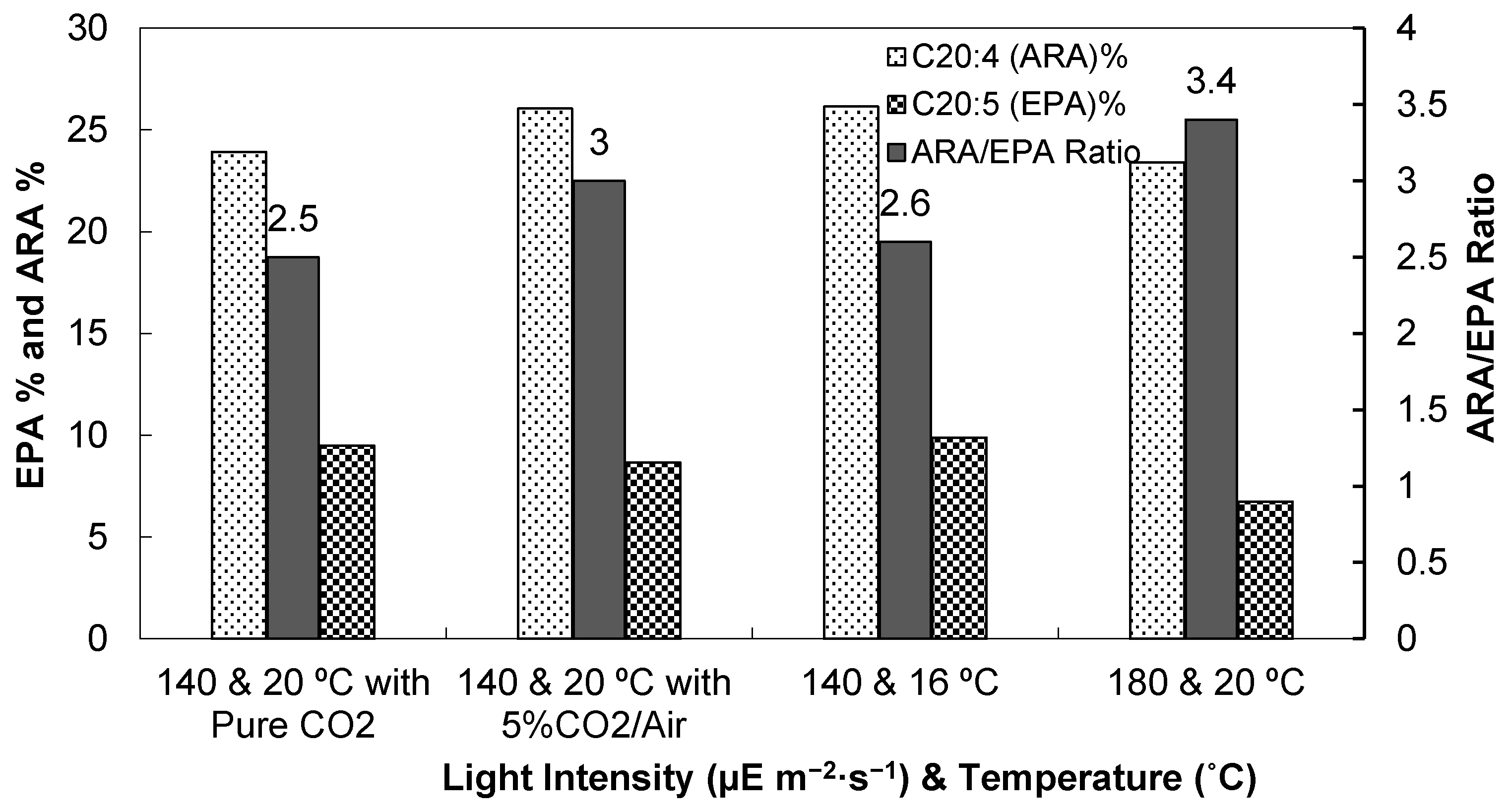
3.1.3. Fatty Acid Compositions
| T (°C) | 20 | 20 | 16 | 20 | 20 | 20 | 20 | |
|---|---|---|---|---|---|---|---|---|
| Light intensity (µE m−2 s−1) | 140 | 140 | 140 | 180 | 140 | 140 | 140 | |
| Nitrate concentrations | 0.075 | 0.075 (5% CO2/Air) | 0.075 | 0.075 | 0.3 | 0.5 | 0.7 | |
| Saturated | C14:0 | - | 0.2% | - | - | 0.2% | 0.3% | 0.3% |
| C16:0 | 30.5% | 25.0% | 27.7% | 26.6% | 29.6% | 27.4% | 30.6% | |
| C18:0 | 2.5% | 3.1% | 3.0% | 3.5% | 1.5% | 2.3% | 2.9% | |
| C20:0 | - | 0.1% | - | - | 0.1% | - | - | |
| C22:0 | - | - | - | - | - | - | - | |
| C24:0 | - | - | - | - | - | - | - | |
| Total | 33.0% | 28.3% | 30.7% | 30.1% | 31.3% | 29.9% | 33.7% | |
| Mono-unsaturated | C14:1 | - | - | - | - | - | 0.1% | 0.4% |
| C16:1 | 1.0% | 0.2% | 1.0% | 1.0% | 0.2% | 2.0% | 2.8% | |
| C18:1 (cis-9) | 3.2% | 3.9% | 3.8% | 4.3% | 1.3% | 2.3% | 1.6% | |
| C18:1 (trans) | - | 0.3% | - | - | 0.7% | 0.4% | 0.8% | |
| C20:1 | - | 0.2% | - | - | 0.1% | 0.1% | - | |
| C22:1 | 1.2% | - | - | - | - | - | - | |
| C24:1 | - | - | - | - | - | - | - | |
| Total | 5.4% | 4.5% | 4.8% | 5.3% | 2.3% | 5.0% | 5.5% | |
| Poly-unsaturated | C18:2 | 21.0% | 24.6% | 22.4% | 27.6% | 12.8% | 19.5% | 12.4% |
| C18:3 | - | 0.1% | - | - | 0.1% | 0.4% | - | |
| C20:2 | 0.8% | 1.9% | 1.1% | 1.3% | 2.1% | 2.5% | 1.7% | |
| C20:4 (ARA) | 23.9% | 26.1% | 26.2% | 23.4% | 30.5% | 25.1% | 29.1% | |
| C20:3 | - | 0.1% | - | - | 0.1% | 0.1% | - | |
| C20:5 (EPA) | 9.5% | 8.7% | 9.9% | 6.7% | 13.1% | 11.5% | 13.1% | |
| C22:6 (DHA) | - | - | - | - | - | - | - | |
| Total | 55.2% | 61.3% | 59.5% | 59.0% | 58.8% | 59.1% | 56.3% | |
| Unknown FAs | 6.4% | 5.8% | 5.1% | 5.6% | 7.7% | 6.1% | 4.6% | |
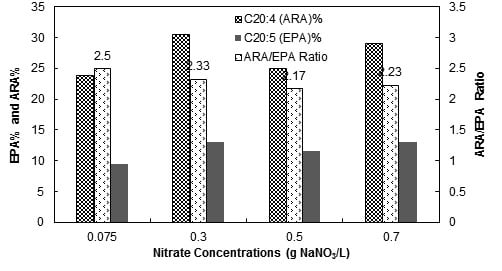
3.2. Nitrate Concentration
3.2.1. Cell Densities and Growth Rates
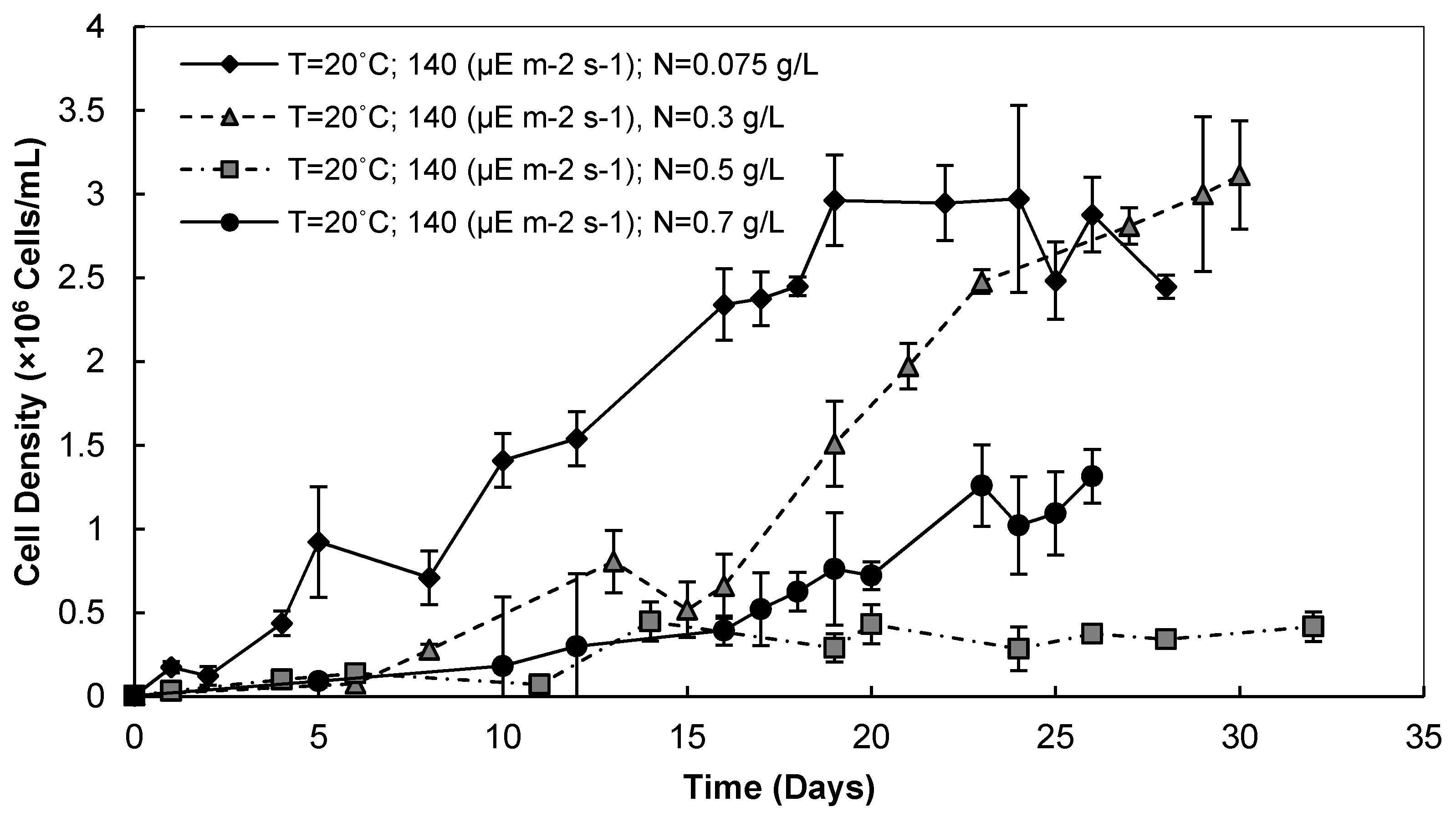
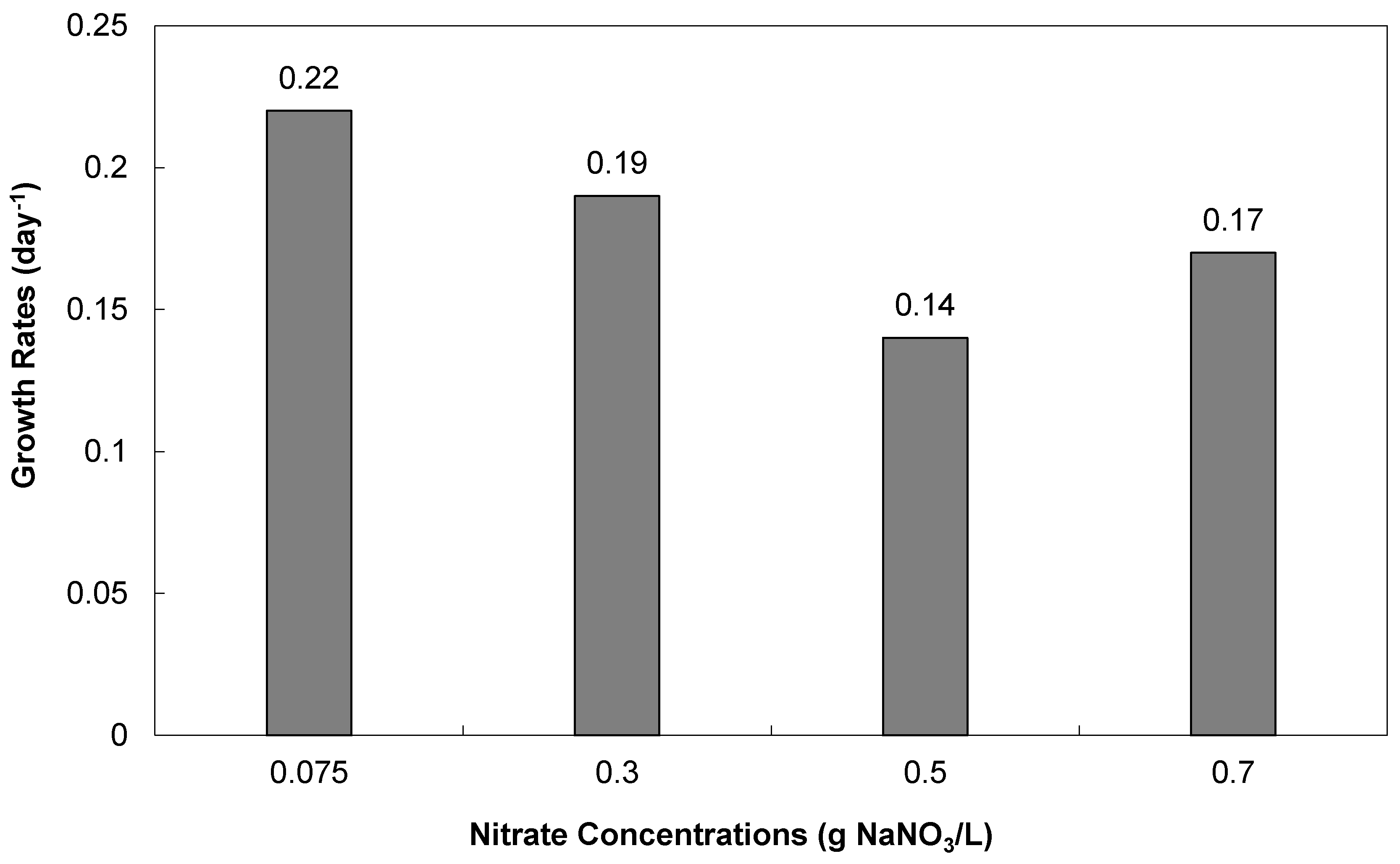
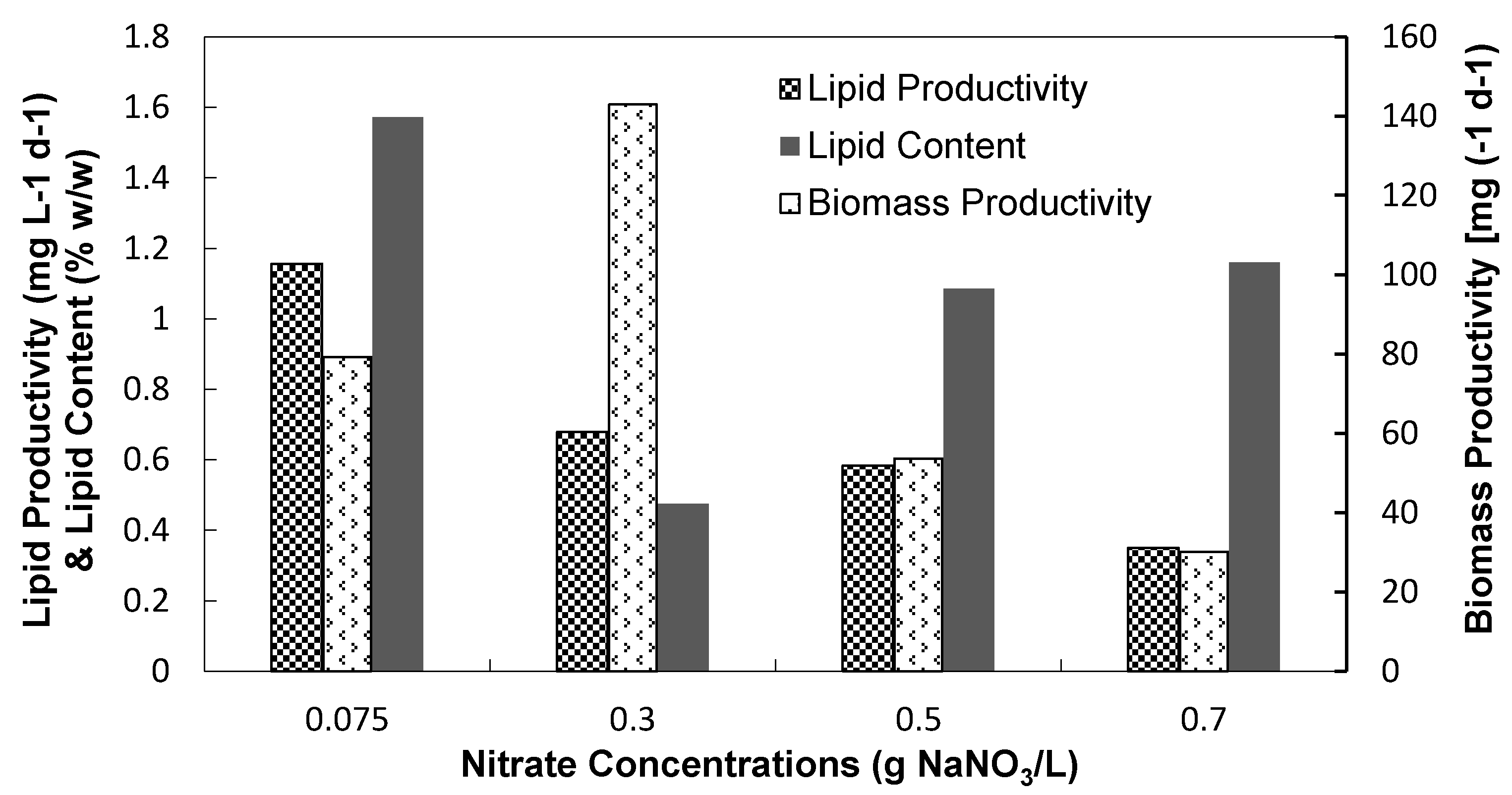
3.2.2. Biomass and Lipid Contents
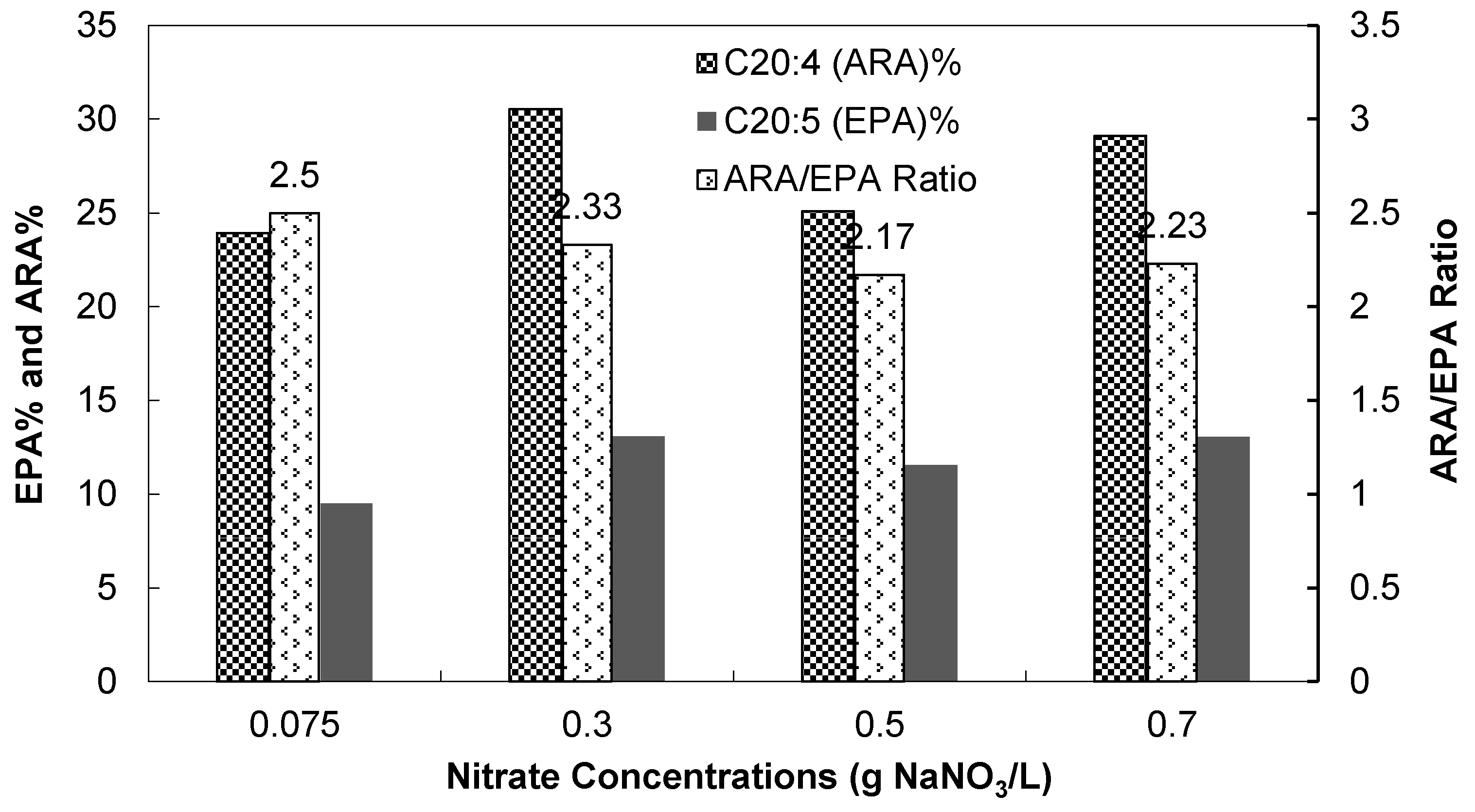
3.2.3. Fatty Acid Compositions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.A.; Davey, M.P.; Dennis, J.S.; Horst, I.; Howe, C.J.; Lea-Smith, D.J.; Smith, A.G. Biodiesel from algae: Challenges and prospects. Curr. Opin. Biotechnol. 2010, 21, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Rodolfi, L.; Zittelli, G.C.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for Oil: Strain Selection, Induction of Lipid Synthesis and Outdoor Mass Cultivation in a Low-Cost Photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Drevon, C.A.; Baksaas, I.; Krokan, H.E. Omega-3 Fatty Acids: Metabolism and Biological Effects; Birkhauser Verlag: Basel, Switzerland, 1993. [Google Scholar]
- Vazhappilly, R.; Chen, F. Eicosapentaenoic Acid and Docosahexaenoic Acid Production Potential of Microalgae and Their Heterotrophic Growth. J. Am. Oil Chem. Soc. 1998, 75, 393–397. [Google Scholar] [CrossRef]
- Agostoni, C.; Marangoni, F.; Giovannini, M.; Riva, E.; Galli, C.M. Long-chain polyunsaturated fatty acids, infant formula, and breastfeeding. Lancet 1998, 352, 1703–1704. [Google Scholar] [CrossRef]
- Khozin-Goldberg, I.; Iskandarov, U.; Cohen, K. LC-PUFA from photosynthetic microalgae: Occurrence, biosynthesis, and prospects in biotechnology. Appl. Microbiol. Biotechnol. 2011, 91, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Cao, Y.; Zhao, M. Biotechnological production of eicosapentaenoic acid: From a metabolic engineering point of view. Process Biochem. 2012, 47, 1320–1326. [Google Scholar] [CrossRef]
- Nomura, S.; Kanazawa, S.; Fukuhara, S. Effects of eicosapentaenoic acid on platelet activation markers and cell adhesion molecules in hyperlipidemic patients with type 2 diabetes mellitus. J. Diabetes Complicat. 2003, 17, 153–159. [Google Scholar] [CrossRef]
- Ward, O.P.; Singh, A. Omega-3/6 fatty acids: Alternative sources of production. Process Biochem. 2005, 40, 3627–3652. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; El-Hassan, B.; Fuentes, M.M.R. Eicosapentaenoic and arachidonic acids purification from the red microalga Porphyridium cruentum. Bioseparation 2001, 9, 299–306. [Google Scholar] [CrossRef]
- Roche, H.M. Unsaturated fatty acids. Proc. Nutr. Soc. 1999, 58, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Z.; Cohen, S. Preparation of eicosapentaenoic acid (EPA) concentrate from Porphyridium cruentum. J. Am. Oil Chem. Soc. 1991, 68, 16–19. [Google Scholar] [CrossRef]
- Ozogul, Y.; Simsek, A.; Balikci, E.; Kenar, M. The effects of extraction methods on the contents of fatty acids, especially EPA and DHA in marine lipids. Int. J. Food Sci. Nutr. 2012, 63, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Abomohra, A.E.F.; Wagner, M.; El-Sheekh, M.; Hanelt, D. Lipid and total fatty acid productivity in photoautotrophic fresh water microalgae: Screening studies towards biodiesel production. J. Appl. Phycol. 2013, 25, 931–936. [Google Scholar] [CrossRef]
- Seto, A.; Wang, H.L.; Hesseltine, C.W. Culture conditions affect eicosapentaenoic acid content of Chlorella minutissima. J. Am. Oil Chem. Soc. 1984, 61, 892–894. [Google Scholar] [CrossRef]
- Cohen, Z. The production potential of eicosapentaenoic and arachidonic acids by the red algae Porphyridium cruentum. J. Am. Oil Chem. Soc. 1990, 67, 916–920. [Google Scholar] [CrossRef]
- Nichols, B.W.; Appleby, R.S. The distribution and biosynthesis of arachidonic acid in algae. Phytochemistry 1969, 8, 1907–1915. [Google Scholar] [CrossRef]
- Yongmanitchai, W.; Ward, O.P. Screening of algae for potential alternative sources of eicosapentaenoic acid. Phytochemistry 1991, 30, 2963–2967. [Google Scholar] [CrossRef]
- Iwamoto, H.; Sato, S. Production of EPA by freshwater unicellular algae. J. Am. Oil Chem. Soc. 1986, 63, 434–438. [Google Scholar]
- Renaud, S.M.; Zhou, H.C.; Parry, D.L.; Thinh, L.V.; Woo, K.C. Effect of temperature on the growth, total lipid content and fatty acid composition of recently isolated tropical microalgae Isochrysis sp., Nitzschia closterium, Nitzschia paleacea, and commercial species Isochrysis sp. (clone T. ISO). J. Appl. Phycol. 1995, 7, 595–602. [Google Scholar] [CrossRef]
- Stinson, E.E.; Kwoczak, R.; Kurantz, M.J. Effect of Cultural Conditions on Production of Eicosapentaenoic Acid by Pythium irregular. J. Ind. Microbiol. 1991, 8, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, Y.; Monteiro, M.; Bandarra, N.; Gökpinar, Ş.; Işik, O. The effect of low temperature on fatty acid composition and tocopherols of the red microalga, Porphyridium cruentum. J. Appl. Phycol. 2007, 19, 223–227. [Google Scholar] [CrossRef]
- Thompson, P.A.; Guo, M.; Harrison, P.J.; Whyte, J.N. Effects of variation in temperature. I. On the fatty acid composition of eight species of marine phytoplankton. J. Phycol. 1992, 28, 488–497. [Google Scholar] [CrossRef]
- Cohen, Z. Porphyridium cruentum . In Chemicals from Microalgae; Taylor & Francis: London, UK, 1999; pp. 41–56. [Google Scholar]
- Brennan, L.; Owende, P. Biofuels from microalgae-A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sust. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Sicko-Goad, L.; Simmons, M.S.; Lazinskey, D.; Hall, J. Effect of light cycle on diatom fatty acid composition and quantitative morphology. J. Phycol. 1988, 24, 1–7. [Google Scholar] [CrossRef]
- Erwin, J.; Bloch, K. Biosynthesis of unsaturated fatty acids in microorganisms. Science 1964, 143, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Contantopoulos, G.; Bloch, K. Effect of light intensity on the lipid composition of Euglena gracilis. J. Biol. Chem. 1967, 242, 3538–3542. [Google Scholar]
- Rezanka, T.; Doucha, J.; Mares, P.; Podojil, M. Effect of cultivation temperature and light intensity on fatty acid production in the red algae Porphyridium cruentum. J. Basic Microbiol. 1987, 27, 275–278. [Google Scholar] [CrossRef]
- Qin, J.G. Bio-Hydrocarbons from Algae-Impacts of Temperature, Light and Salinity on Algae Growth; Rural Industries Research and Development Corporation Report (RIRDC): Barton, Australia, 2005. [Google Scholar]
- Becker, E.W. Measurement of algal growth. In Microalgae Biotechnology and Microbiology; Cambridge University Press: Cambridge, UK, 1994; pp. 56–62. [Google Scholar]
- Miyamoto, K. Renewable Biological Systems for Alternative Sustainable Energy Production; Food & Agriculture Organization (FAO), United Nations: Osaka, Japan, 1997. [Google Scholar]
- Roessler, P.G. Environmental control of glycerolipid metabolism in microalgae: Commercial implications and future research directions. J. Phycol. 1990, 26, 393–399. [Google Scholar] [CrossRef]
- Cohen, Z. Products from microalgae. In Handbook for Microalgal Mass Culture; Richmond, A., Ed.; CRC Press: Boca Raton, FL, USA, 1986; pp. 421–454. [Google Scholar]
- Guillard, R.R.L.; Hargraves, P.E. Stichochrysis immobilis is a diatom, not a chrysophyte. Phycologia 1993, 32, 234–236. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method for total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, L.U.; Mutie, A.M.; Kenya, E.U.; Muhoho, A. Characterization of algae oil (oilgae) and its potential as biofuel in Kenya. J. Appl. Phytotechnol. Environ. Sanit. 2012, 1, 147–153. [Google Scholar]
- Li, Y.; Horsman, M.; Wang, B.; Wu, N.; Lan, C.Q. Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl. Microbiol. Biotechnol. 2008, 81, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Z.; Vonshak, A.; Richmond, A. Effect of environmental conditions on fatty acid composition of the red alga Porphyridium cruentum: Correlation to growth rate. J. Phycol. 1988, 24, 328–332. [Google Scholar] [CrossRef]
- Oh, S.H.; Han, J.G.; Kim, Y.; Ha, J.H.; Kim, S.S.; Jeong, M.H.; Jeong, H.S.; Kim, N.Y.; Cho, J.S.; Yoon, W.B.; et al. Lipid production in Porphyridium cruentum grown under different culture conditions. J. Biosci. Bioeng. 2009, 108, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, M.; Shirai, A.; Ohtaguchi, K.; Koide, K. Carbon dioxide fixation and polyunsaturated fatty acid production by the red alga Porphyridium cruentum. Appl. Biochem. Biotechnol. 1998, 73, 269–278. [Google Scholar] [CrossRef]
- Cohen, Z.; Norman, H.A.; Heimer, Y.M. Microalgae as a source of omega-3 fatty acids. World Rev. Nutr. Diet. 1995, 77, 1–31. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asgharpour, M.; Rodgers, B.; Hestekin, J.A. Eicosapentaenoic Acid from Porphyridium Cruentum: Increasing Growth and Productivity of Microalgae for Pharmaceutical Products. Energies 2015, 8, 10487-10503. https://doi.org/10.3390/en80910487
Asgharpour M, Rodgers B, Hestekin JA. Eicosapentaenoic Acid from Porphyridium Cruentum: Increasing Growth and Productivity of Microalgae for Pharmaceutical Products. Energies. 2015; 8(9):10487-10503. https://doi.org/10.3390/en80910487
Chicago/Turabian StyleAsgharpour, Maryam, Brigitte Rodgers, and Jamie A. Hestekin. 2015. "Eicosapentaenoic Acid from Porphyridium Cruentum: Increasing Growth and Productivity of Microalgae for Pharmaceutical Products" Energies 8, no. 9: 10487-10503. https://doi.org/10.3390/en80910487
APA StyleAsgharpour, M., Rodgers, B., & Hestekin, J. A. (2015). Eicosapentaenoic Acid from Porphyridium Cruentum: Increasing Growth and Productivity of Microalgae for Pharmaceutical Products. Energies, 8(9), 10487-10503. https://doi.org/10.3390/en80910487