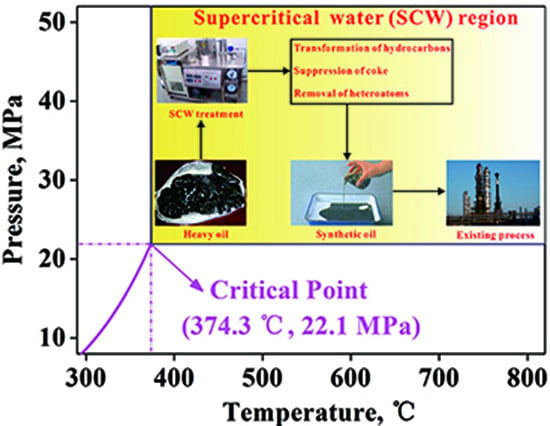
A Review of Laboratory-Scale Research on Upgrading Heavy Oil in Supercritical Water
Abstract
:1. Introduction
| Carbon Rejection | Hydrogen Addition |
|---|---|
| Deasphalting | Fixed bed hydrotreating |
| Visbreaking | Fixed bed hydrocracking |
| Thermal cracking | Ebullated bed hydrotreating |
| Coking | Ebullated bed hydrocracking |
| Catalytic cracking | - |
2. Transformations of Hydrocarbons
2.1. Extraction, Fractionation and Reaction of Hydrocarbons
| Heavy Oil | Reaction Parameters | Major Results | References | |
|---|---|---|---|---|
| Oil shale | Maoming |
|
| [37] |
| Beypazari |
|
| [38] | |
| GGyniik |
|
| [28] | |
| Timahdit |
|
| [39] | |
| Oil sand | Albert Athabaca |
|
| [40] |
| Tumuji |
|
| [29] | |
| Bitumen | From SAGD method |
|
| [11] |
| Omsk Oil Refinery |
|
| [41] | |
| From SAGD method |
|
| [42] | |
| Canadian Athabasca |
|
| [43] | |
| Residues | Shanghai Petroleum Co. |
|
| [44] |
| VR |
|
| [45] | |
| Daqing |
|
| [46] | |
| Gudao |
|
| [47] | |
| Gudao |
|
| [48] | |
| Residual oil |
|
| [49] | |
| Residual oil |
|
| [35] | |
| Asphaltene | From heavy Tatar oil |
|
| [50] |
| Coal-tar |
|
| [51] | |
| From Canadian oilsand bitumen |
|
| [52] | |
| Tahe |
|
| [53] | |
2.1.1. Oil Shale
2.1.2. Oil Sand
2.1.3. Bitumen
2.1.4. Residues
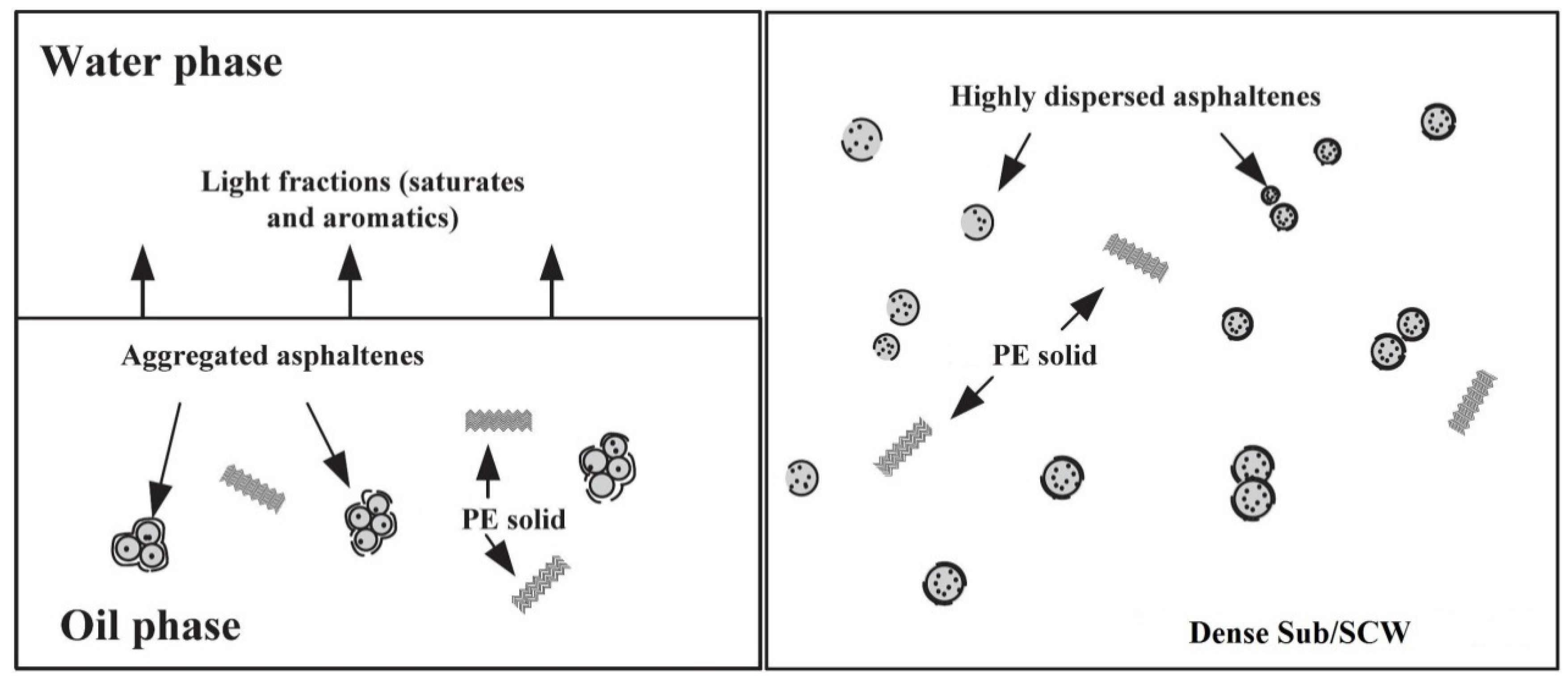
2.1.5. Asphaltene
2.2. Mechanisms of Hydrocarbons Transformation
3. Suppression of Coke
3.1. Provision of Hydrogen
3.1.1. Addition of Entrainers
3.1.2. Partial SCWO
3.1.3. Addition of Hydrogen-Rich Materials
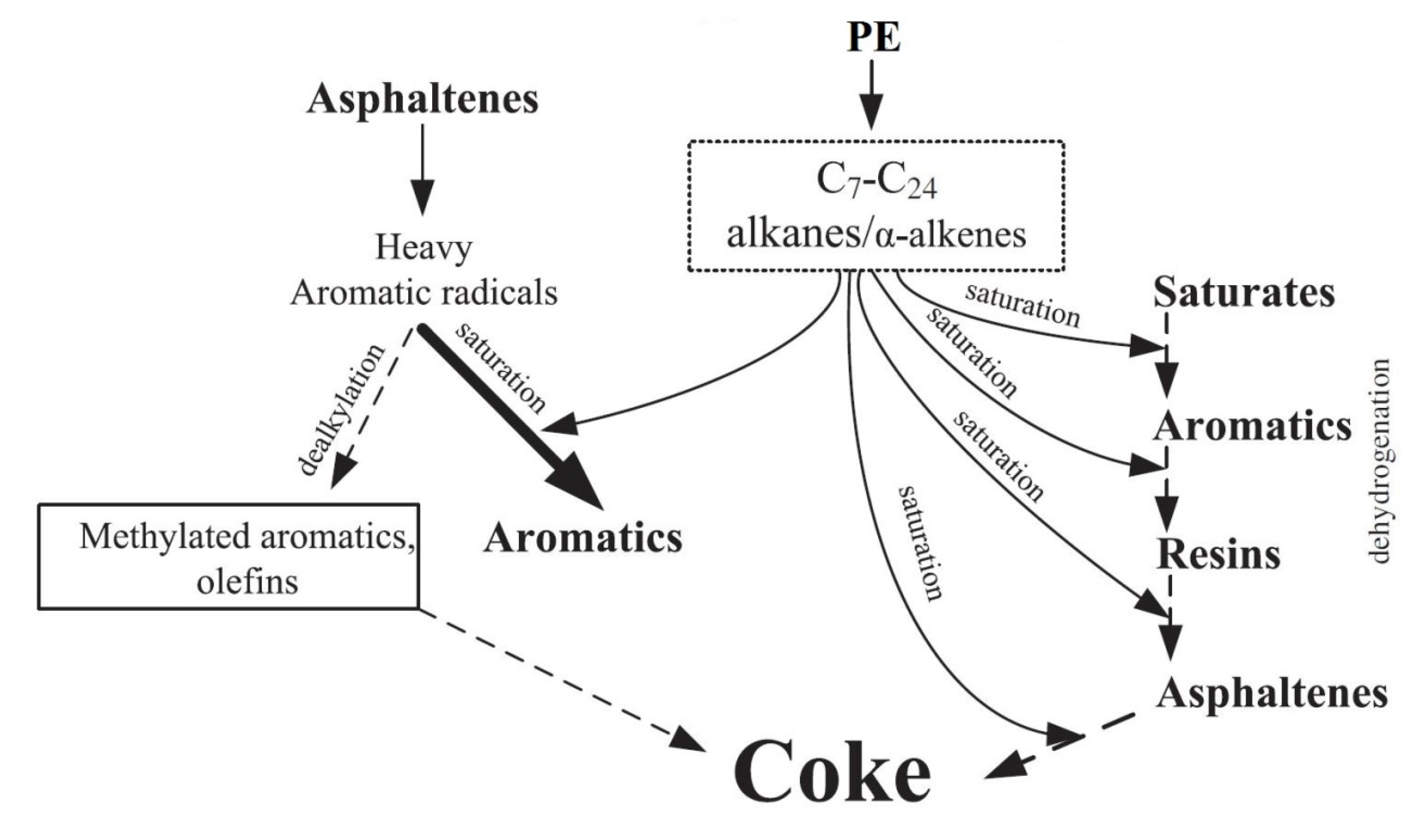
3.1.4. Addition of Catalysts
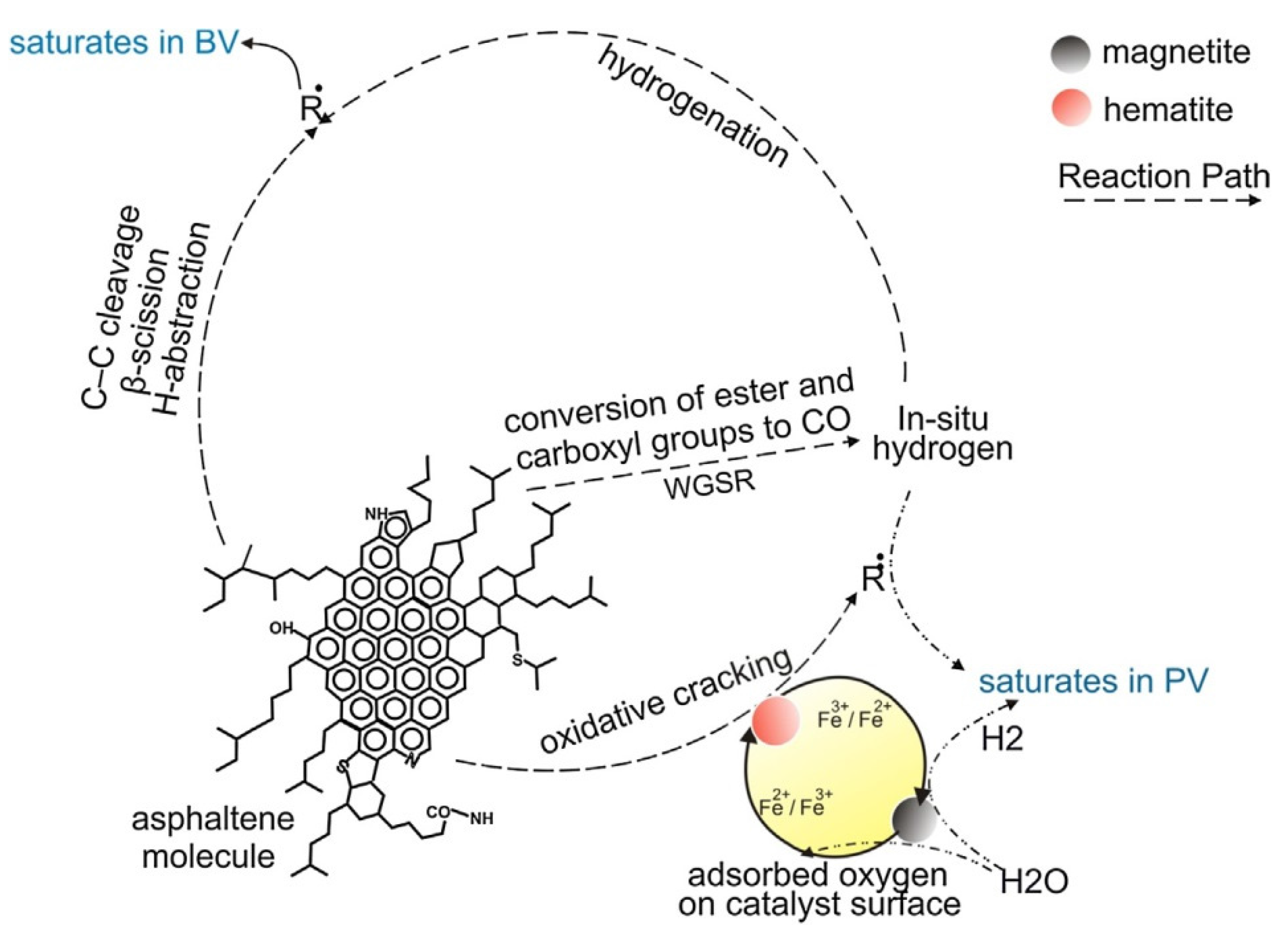
3.2. Operation Mode
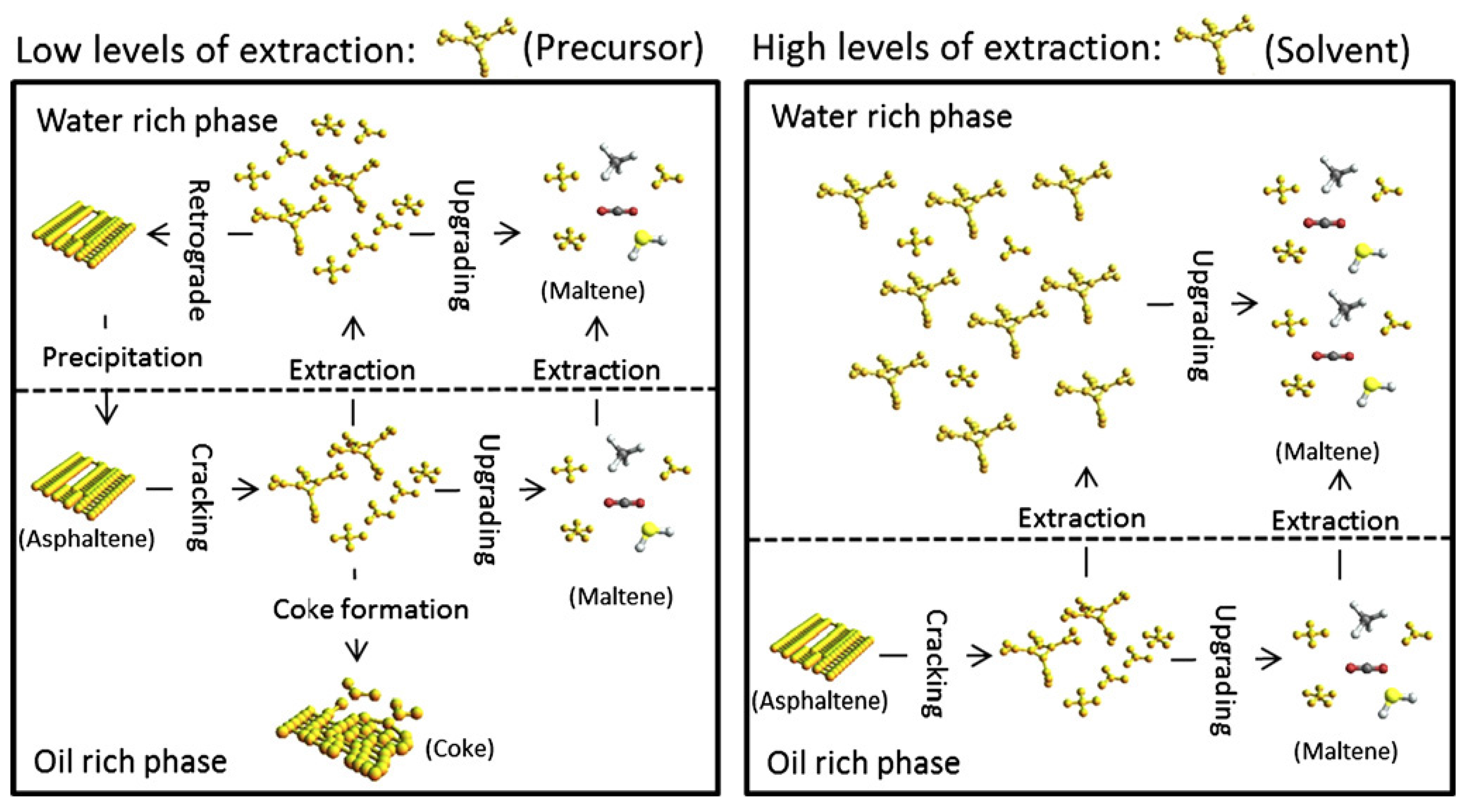
4. Removals of Heteroatoms
| Heavy Oil | S (wt %) | Ni + V (ppmw) |
|---|---|---|
| Alaska, north slope | 1.8 | 71 |
| Arabian, safaniya | 4.3 | 125 |
| Canada, Athabasca | 5.4 | 374 |
| Canada, Cold Lake | 5.0 | 333 |
| California, Hondo | 5.8 | 489 |
| Iranian | 2.6 | 197 |
| Kuwait, Export | 4.1 | 75 |
| Mexico, Maya | 4.7 | 620 |
| Venezuela Bachaqueo | 3.0 | 509 |
| Korea VR | 5.3 | 142.6 |
| Tahe Residual | 2.1 | 236.9 |
4.1. Removal of Nitrogen and Sulfur
4.1.1. Removal of Nitrogen
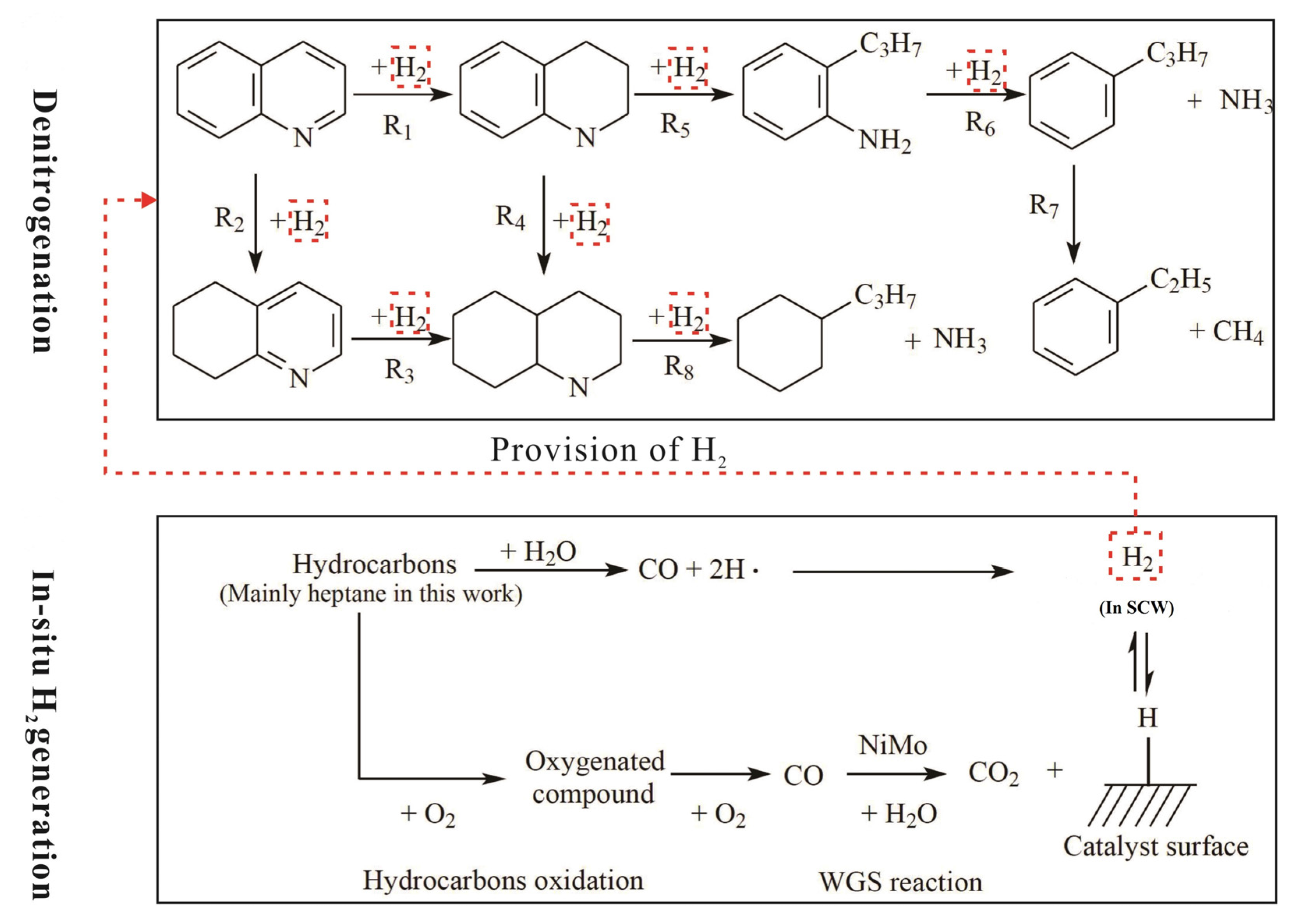
4.1.2. Removal of Sulfur
| Type of Organic Sulfur Compounds | Chemical Structure |
|---|---|
| Mercaptanes | R–S–H |
| Sulfides | R1–S–R2 |
| Disulfides | R1–S–S–R2 |
| Thiophene | 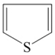 |
| Benzothiophene | 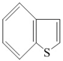 |
| Dibenzothiophene |  |
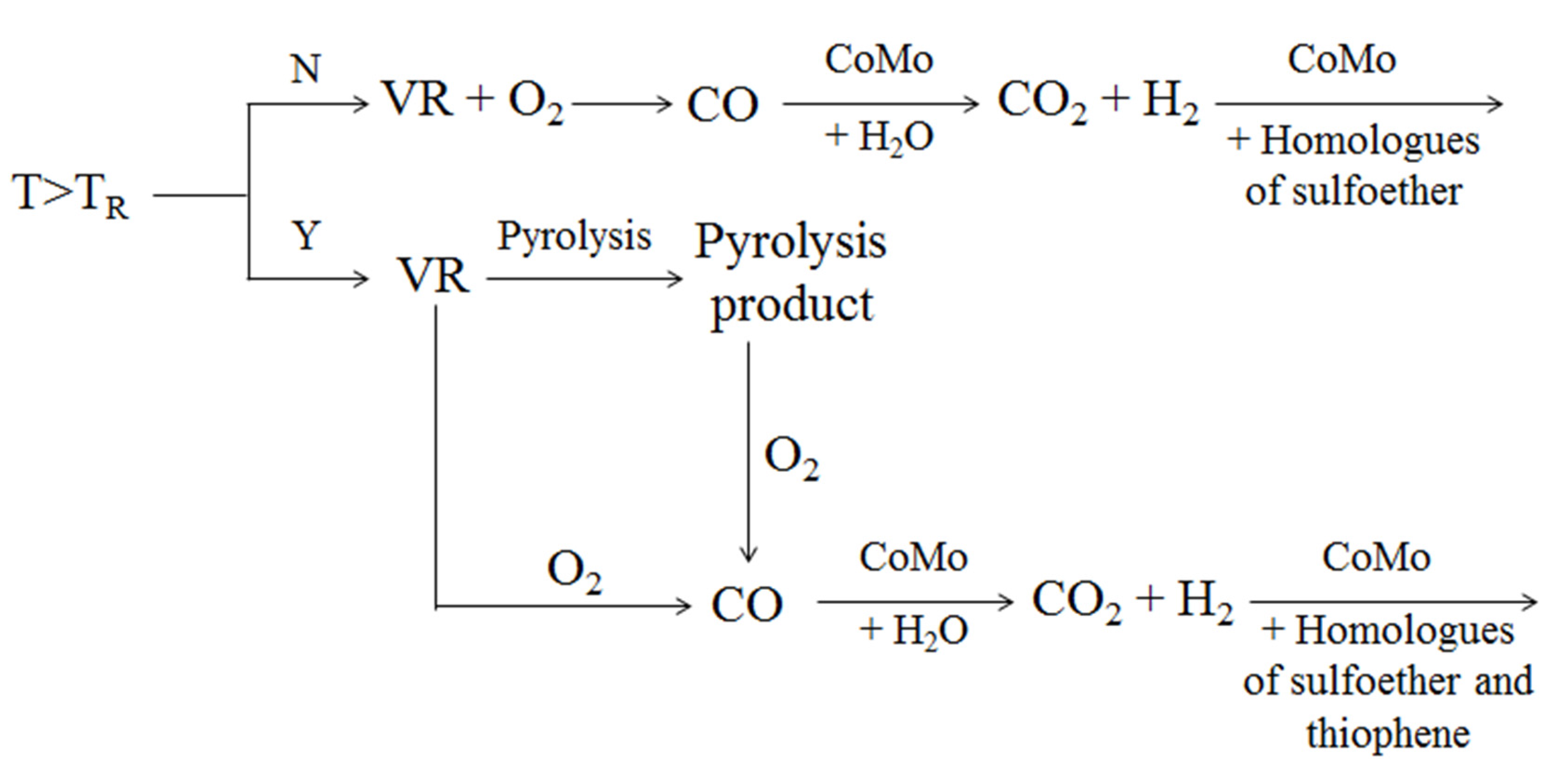
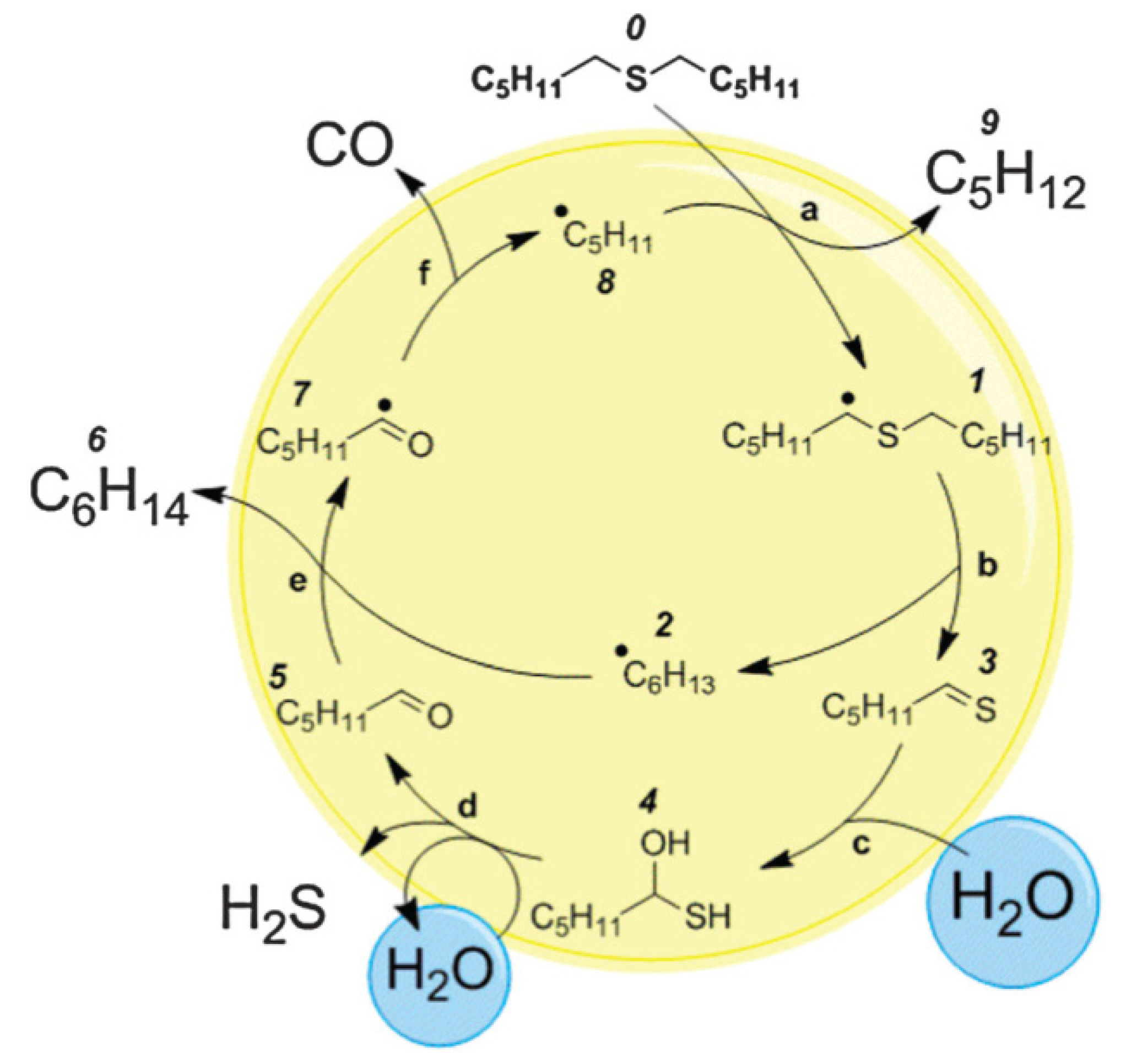
4.2. Removal of Nickel and Vanadium
5. Conclusions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Santos, R.; Loh, W.; Bannwart, A.; Trevisan, O. An overview of heavy oil properties and its recovery and transportation methods. Braz. J. Chem. Eng. 2014, 31, 571–590. [Google Scholar] [CrossRef]
- Shah, A.; Fishwick, R.; Wood, J.; Leeke, G.; Rigby, S.; Greaves, M. A review of novel techniques for heavy oil and bitumen extraction and upgrading. Energy Environ. Sci. 2010, 3, 700–714. [Google Scholar] [CrossRef]
- Rana, M.S.; Sámano, V.; Ancheyta, J.; Diaz, J.A.I. A review of recent advances on process technologies for upgrading of heavy oils and residua. Fuel 2007, 86, 1216–1231. [Google Scholar] [CrossRef]
- Canıaz, R.O.; Erkey, C. Process intensification for heavy oil upgrading using supercritical water. Chem. Eng. Res. Des. 2014, 92, 1845–1863. [Google Scholar] [CrossRef]
- Kapadia, P.R.; Kallos, M.S.; Gates, I.D. A review of pyrolysis, aquathermolysis, and oxidation of athabasca bitumen. Fuel Process. Technol. 2015, 131, 270–289. [Google Scholar] [CrossRef]
- Upreti, S.R.; Lohi, A.; Kapadia, R.A.; El-Haj, R. Vapor extraction of heavy oil and bitumen: A review. Energy Fuels 2007, 21, 1562–1574. [Google Scholar] [CrossRef]
- Bridjanian, H.; Samimi, A.K. Bottom of the barrel, an important challenge of the petroleum refining industry. Petrol. Coal 2011, 53, 13–21. [Google Scholar]
- Kiasari, H.; Nokandeh, N.; Khishvand, M.; Mansoori, A.; Firoozjaee, R. A review of SAGD-ISSLW. Petrol. Sci. Technol. 2014, 32, 753–760. [Google Scholar] [CrossRef]
- Wang, Z.J.; Deng, S.H.; Gu, Q.; Cui, X.J.; Zhang, Y.M.; Wang, H.Y. Subcritical water extraction of huadian oil shale under isothermal condition and pyrolysate analysis. Energy Fuels 2014, 28, 2305–2313. [Google Scholar] [CrossRef]
- Strausz, O.P.; Mojelsky, T.W.; Payzant, J.D.; Olah, G.A.; Prakash, G.K.S. Upgrading of alberta’s heavy oils by superacid-catalyzed hydrocracking. Energy Fuels 1999, 13, 558–569. [Google Scholar] [CrossRef]
- Sato, T.; Mori, S.; Watanabe, M.; Sasaki, M.; Itoh, N. Upgrading of bitumen with formic acid in supercritical water. J. Supercrit. Fluids 2010, 55, 232–240. [Google Scholar] [CrossRef]
- Ates, A.; Azimi, G.; Choi, K.H.; Green, W.H.; Timko, M.T. The role of catalyst in supercritical water desulfurization. Appl. Catal. B 2014, 147, 144–155. [Google Scholar] [CrossRef]
- Rudyk, S.; Spirov, P. Upgrading and extraction of bitumen from nigerian tar sand by supercritical carbon dioxide. Appl. Energy 2014, 113, 1397–1404. [Google Scholar] [CrossRef]
- Ali, M.F.; Abbas, S. A review of methods for the demetallization of residual fuel oils. Fuel Process. Technol. 2006, 87, 573–584. [Google Scholar] [CrossRef]
- Brons, G.; Yu, J.M. Solvent deasphalting effects on whole cold lake bitumen. Energy Fuels 1995, 9, 641–647. [Google Scholar] [CrossRef]
- Castaneda, L.C.; Munoz, J.A.D.; Ancheyta, J. Current situation of emerging technologies for upgrading of heavy oils. Catal. Today 2014, 220, 248–273. [Google Scholar] [CrossRef]
- Siskin, M.; Kelemen, S.R.; Eppig, C.P.; Brown, L.D.; Afeworki, M. Asphaltene molecular structure and chemical influences on the morphology of coke produced in delayed coking. Energy Fuels 2006, 20, 1227–1234. [Google Scholar] [CrossRef]
- Xu, C.; Hamilton, S.; Mallik, A.; Ghosh, M. Upgrading of athabasca vacuum tower bottoms (VTB) in supercritical hydrocarbon solvents with activated carbon-supported metallic catalysts. Energy Fuels 2007, 21, 3490–3498. [Google Scholar] [CrossRef]
- Furimsky, E. Hydroprocessing in aqueous phase. Ind. Eng. Chem. Res. 2013, 52, 17695–17713. [Google Scholar] [CrossRef]
- Xu, Y.; Yuan, M.; Zhao, S.; Xu, C. Upgrading heavy oil using syngas as the hydrogen source with dispersed catalysts. Petrol. Sci. Technol. 2009, 27, 712–732. [Google Scholar] [CrossRef]
- Reddy, S.N.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Supercritical water gasification of biomass for hydrogen production. Int. J. Hydrogen Energy 2014, 39, 6912–6926. [Google Scholar] [CrossRef]
- Muthukumaran, P.; Gupta, R.B. Sodium-carbonate-assisted supercritical water oxidation of chlorinated waste. Ind. Eng. Chem. Res. 2000, 39, 4555–4563. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, S.Z.; Xu, D.H.; Gong, Y.M.; Ma, H.H.; Tang, X.Y. Review of catalytic supercritical water gasification for hydrogen production from biomass. Renew. Sustain. Energy Rev. 2010, 14, 334–343. [Google Scholar] [CrossRef]
- Azadi, P.; Farnood, R. Review of heterogeneous catalysts for sub- and supercritical water gasification of biomass and wastes. Int. J. Hydrogen Energy 2011, 36, 9529–9541. [Google Scholar] [CrossRef]
- Marrone, P.A. Supercritical water oxidation-current status of full-scale commercial activity for waste destruction. J. Supercrit. Fluids 2013, 79, 283–288. [Google Scholar] [CrossRef]
- Vilcaez, J.; Watanabe, M.; Watanabe, N.; Kishita, A.; Adschiri, T. Hydrothermal extractive upgrading of bitumen without coke formation. Fuel 2012, 102, 379–385. [Google Scholar] [CrossRef]
- Kruse, A.; Dinjus, E. Hot compressed water as reaction medium and reactant—Properties and synthesis reactions. J. Supercrit. Fluids 2007, 39, 362–380. [Google Scholar] [CrossRef]
- Yanik, J.; Yüksel, M.; Saǧlam, M.; Olukçu, N.; Bartle, K.; Frere, B. Characterization of the oil fractions of shale oil obtained by pyrolysis and supercritical water extraction. Fuel 1995, 74, 46–50. [Google Scholar] [CrossRef]
- Meng, M.; Hu, H.Q.; Zhang, Q.M.; Ding, M. Extraction of tumuji oil sand with sub- and supercritical water. Energy Fuels 2006, 20, 1157–1160. [Google Scholar] [CrossRef]
- Hu, H.Q.; Zhang, J.; Guo, S.C.; Chen, G.H. Extraction of huadian oil shale with water in sub- and supercritical states. Fuel 1999, 78, 645–651. [Google Scholar] [CrossRef]
- Johannes, I.; Luik, H.; Palu, V.; Kruusement, K.; Gregor, A. Synergy in co-liquefaction of oil shale and willow in supercritical water. Fuel 2015, 144, 180–187. [Google Scholar] [CrossRef]
- Wahyudiono; Shiraishi, T.; Sasaki, M.; Goto, M. Non-catalytic liquefaction of bitumen with hydrothermal/solvothermal process. J. Supercrit. Fluids 2011, 60, 127–136. [Google Scholar] [CrossRef]
- Dejhosseini, M.; Aida, T.; Watanabe, M.; Takami, S.; Hojo, D.; Aoki, N.; Arita, T.; Kishita, A.; Adschiri, T. Catalytic cracking reaction of heavy oil in the presence of cerium oxide nanoparticles in supercritical water. Energy Fuels 2013, 27, 4624–4631. [Google Scholar] [CrossRef]
- Liu, Y.; Bai, F.; Zhu, C.C.; Yuan, P.Q.; Cheng, Z.M.; Yuan, W.K. Upgrading of residual oil in sub- and supercritical water: An experimental study. Fuel Process. Technol. 2013, 106, 281–288. [Google Scholar] [CrossRef]
- Tan, X.C.; Zhu, C.C.; Liu, Q.K.; Ma, T.Y.; Yuan, P.Q.; Cheng, Z.M.; Yuan, W.K. Co-pyrolysis of heavy oil and low density polyethylene in the presence of supercritical water: The suppression of coke formation. Fuel Process. Technol. 2014, 118, 49–54. [Google Scholar] [CrossRef]
- Timko, M.T.; Ghoniem, A.F.; Green, W.H. Upgrading and desulfurization of heavy oils by supercritical water. J. Supercrit. Fluids 2015, 96, 114–123. [Google Scholar] [CrossRef]
- Funazukuri, T.; Yokoi, S.; Wakao, N. Supercritical fluid extraction of chinese maoming oil shale with water and toluene. Fuel 1988, 67, 10–14. [Google Scholar] [CrossRef]
- Olukcu, N.; Yanik, J.; Saglam, M.; Yuksel, M.; Karaduman, M. Solvent effect on the extraction of beypazari oil shale. Energy Fuels 1999, 13, 895–902. [Google Scholar] [CrossRef]
- El harfi, K.; Bennouna, C.; Mokhlisse, A.; Ben chanaa, M.; Lemee, L.; Joffre, J.; Ambles, A. Supercritical fluid extraction of moroccan (timahdit) oil shale with water. J. Anal. Appl. Pyrolysis 1999, 50, 163–174. [Google Scholar] [CrossRef]
- Berkowitz, N.; Calderon, J. Extraction of oil sand bitumens with supercritical water. Fuel Process. Technol. 1990, 25, 33–44. [Google Scholar] [CrossRef]
- Fedyaeva, O.N.; Vostrikov, A.A. Hydrogenation of bitumen in situ in supercritical water flow with and without addition of zinc and aluminum. J. Supercrit. Fluids 2012, 72, 100–110. [Google Scholar] [CrossRef]
- Sato, T.; Trung, P.H.; Tomita, T.; Itoh, N. Effect of water density and air pressure on partial oxidation of bitumen in supercritical water. Fuel 2012, 95, 347–351. [Google Scholar] [CrossRef]
- Morimoto, M.; Sugimoto, Y.; Sato, S.; Takanohashi, T. Bitumen cracking in supercritical water upflow. Energy Fuels 2014, 28, 858–861. [Google Scholar] [CrossRef]
- Cheng, Z.M.; Ding, Y.; Zhao, L.Q.; Yuan, P.Q.; Yuan, W.K. Effects of supercritical water in vacuum residue upgrading. Energy Fuels 2009, 23, 3178–3183. [Google Scholar] [CrossRef]
- Zhao, L.Q.; Cheng, Z.M.; Ding, Y.; Yuan, P.Q.; Lu, S.X.; Yuan, W.K. Experimental study on vacuum residuum upgrading through pyrolysis in supercritical water. Energy Fuels 2006, 20, 2067–2071. [Google Scholar] [CrossRef]
- Fan, H.L.; Han, B.X.; Jiang, T.; Guo, J.; Wang, Q.; Cheng, Y.; Wu, S.X. Hydrocracking of anthracene to ethyl biphenyl promoted by coupling supercritical water and cracking catalysts. ChemCatChem 2011, 3, 1474–1479. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, Y.H.; Lou, Y.H.; Que, G.H. Hydrocracking of Gudao residual oil with dispersed catalysts using supercritical water-syngas as a hydrogen source. Pet. Sci. Technol. 2005, 23, 1453–1462. [Google Scholar]
- Cheng, J.; Liu, Y.; Luo, Y.; Quem, G. Hydrocracking of Gudao residual oil with dispersed catalysts using supercritical water-syngas as a hydrogen source. Part II: The comparison of residue hydrocracking in different hydrogen sources. Petrol. Sci. Technol. 2006, 24, 1339–1346. [Google Scholar]
- Bai, F.; Zhu, C.C.; Liu, Y.; Yuan, P.Q.; Cheng, Z.M.; Yuan, W.K. Co-pyrolysis of residual oil and polyethylene in sub- and supercritical water. Fuel Process. Technol. 2013, 106, 267–274. [Google Scholar] [CrossRef]
- Kozhevnikov, I.V.; Nuzhdin, A.L.; Martyanov, O.N. Transformation of petroleum asphaltenes in supercritical water. J. Supercrit. Fluids 2010, 55, 217–222. [Google Scholar] [CrossRef]
- Han, L.N.; Zhang, R.; Bi, J.C.; Cheng, L.M. Pyrolysis of coal-tar asphaltene in supercritical water. J. Anal. Appl. Pyrolysis 2011, 91, 281–287. [Google Scholar] [CrossRef]
- Morimoto, M.; Sato, S.; Takanohashi, T. Effect of water properties on the degradative extraction of asphaltene using supercritical water. J. Supercrit. Fluids 2012, 68, 113–116. [Google Scholar] [CrossRef]
- Li, N.; Yan, B.; Zhang, L.; Quan, S.X.; Hu, C.; Xiao, X.M. Effect of NaOH on asphaltene transformation in supercritical water. J. Supercrit. Fluids 2015, 97, 116–124. [Google Scholar] [CrossRef]
- Dyni, J.R. Geology and resources of some world oil-shale deposits. Oil Shale 2003, 20, 193–252. [Google Scholar]
- Luik, H.; Luik, L. Extraction of fossil fuels with sub- and supercritical water. Energy Sources 2001, 23, 449–459. [Google Scholar] [CrossRef]
- Subramanian, M.; Hanson, F.V. Supercritical fluid extraction of bitumens from utah oil sands. Fuel Process. Technol. 1998, 55, 35–53. [Google Scholar] [CrossRef]
- Fedyaeva, O.N.; Vostrikov, A.A.; Sokol, M.Y.; Fedorova, N.I. Hydrogenation of bitumen in supercritical water flow and the effect of zinc addition. Russ. J. Phys. Chem. B 2013, 7, 820–828. [Google Scholar] [CrossRef]
- Sato, T.; Tomita, T.; Trung, P.H.; Itoh, N.; Sato, S.; Takanohashi, T. Upgrading of bitumen in the presence of hydrogen and carbon dioxide in supercritical water. Energy Fuels 2013, 27, 646–653. [Google Scholar] [CrossRef]
- Sato, T.; Adschiri, T.; Arai, K.; Rempel, G.L.; Ng, F.T.T. Upgrading of asphalt with and without partial oxidation in supercritical water. Fuel 2003, 82, 1231–1239. [Google Scholar] [CrossRef]
- Canter, D.A.; Bermejo, M.D.; Cocero, M.J. Reaction engineering for process intensification of supercritical water biomass refining. J. Supercrit. Fluids 2015, 96, 21–35. [Google Scholar] [CrossRef]
- Fedyaeva, O.N.; Shatrova, A.V.; Vostrikov, A.A. Effect of temperature on bitumen conversion in a supercritical water flow. J. Supercrit. Fluids 2014, 95, 437–443. [Google Scholar] [CrossRef]
- Sun, Y.D.; Yang, C.H.; Zhao, H.; Shan, H.H.; Shen, B.X. Influence of asphaltene on the residue hydrotreating reaction. Energy Fuels 2010, 24, 5008–5011. [Google Scholar] [CrossRef]
- Yasar, M.; Trauth, D.M.; Klein, M.T. Asphaltene and resid pyrolysis. 2. The effect of reaction environment on pathways and selectivities. Energy Fuels 2001, 15, 504–509. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Wei, F.; Zhang, S.J.; Yu, Y. Kinetics and selectivity of asphaltene hydrocracking. Fuel 2011, 90, 1900–1906. [Google Scholar] [CrossRef]
- Ayala, M.; Hernandez-Lopez, E.L.; Perezgasga, L.; Vazquez-Duhalt, R. Reduced coke formation and aromaticity due to chloroperoxidase-catalyzed transformation of asphaltenes from maya crude oil. Fuel 2012, 92, 245–249. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Yu, Y. Kinetics of asphaltene thermal cracking and catalytic hydrocracking. Fuel Process. Technol. 2011, 92, 977–982. [Google Scholar] [CrossRef]
- Cheng, Y.; Fan, H.F.; Wu, S.X.; Wang, Q.A.; Guo, J.; Gao, L.; Zong, B.N.; Han, B.X. Enhancing the selectivity of the hydrogenation of naphthalene to tetralin by high temperature water. Green Chem. 2009, 11, 1061–1065. [Google Scholar] [CrossRef]
- Akiya, N.; Savage, P.E. Roles of water for chemical reactions in high-temperature water. Chem. Rev. 2002, 102, 2725–2750. [Google Scholar] [CrossRef] [PubMed]
- Savage, P.E. Organic chemical reactions in supercritical water. Chem. Rev. 1999, 99, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Ogunsola, O.M.; Berkowitz, N. Extraction of oil shales with sub-critical and near-critical water. Fuel Process. Technol. 1995, 45, 95–107. [Google Scholar] [CrossRef]
- Morimoto, M.; Sugimoto, Y.; Sato, S.; Takanohashi, T. Solvent effect of water on supercritical water treatment of heavy oil. J. Jpn. Petrol. Inst. 2014, 57, 11–17. [Google Scholar] [CrossRef]
- Vostrikov, A.A.; Dubov, D.Y.; Psarov, S.A. The effect of thermal explosion in a supercritical water. Tech. Phys. Lett. 2001, 27, 847–849. [Google Scholar] [CrossRef]
- Dutta, R.P.; McCaffrey, W.C.; Gray, M.R.; Muehlenbachs, K. Thermal cracking of athabasca bitumen: Influence of steam on reaction chemistry. Energy Fuels 2000, 14, 671–676. [Google Scholar] [CrossRef]
- Gao, L.A.; Liu, Y.D.; Wen, L.Y.; Huang, W.X.; Mu, X.H.; Zong, B.N.; Fan, H.L.; Han, B.X. The effect of supercritical water on the hydroconversion of Tahe Residue. AICHE J. 2010, 56, 3236–3242. [Google Scholar] [CrossRef]
- Kida, Y.; Class, C.A.; Concepcion, A.J.; Timko, M.T.; Green, W.H. Combining experiment and theory to elucidate the role of supercritical water in sulfide decomposition. Phys. Chem. Chem. Phys. 2014, 16, 9220–9228. [Google Scholar] [CrossRef] [PubMed]
- Tucker, S.C.; Maddox, M.W. The effect of solvent density inhomogeneities on solute dynamics in supercritical fluids: A theoretical perspective. J. Phys. Chem. B 1998, 102, 2437–2453. [Google Scholar] [CrossRef]
- Morimoto, M.; Sugimoto, Y.; Saotome, Y.; Sato, S.; Takanohashi, T. Effect of supercritical water on upgrading reaction of oil sand bitumen. J. Supercrit. Fluids 2010, 55, 223–231. [Google Scholar] [CrossRef]
- Xu, T.; Liu, Q.Y.; Liu, Z.Y.; Wu, J.F. The role of supercritical water in pyrolysis of carbonaceous compounds. Energy Fuels 2013, 27, 3148–3153. [Google Scholar] [CrossRef]
- Towfighi, J.; Sadrameli, M.; Niaei, A. Coke formation mechanisms and coke inhibiting methods in pyrolysis furnaces. J. Chem. Eng. Jpn 2002, 35, 923. [Google Scholar] [CrossRef]
- Kishita, A.; Takahashi, S.; Kamimura, H.; Miki, M.; Moriya, T.; Enomoto, H. Upgrading of bitumen by hydrothermal visbreaking in supercritical water with alkali. J. Jpn. Petrol. Inst. 2003, 46, 215–221. [Google Scholar] [CrossRef]
- Clark, P.D.; Kirk, M.J. Studies on the upgrading of bituminous oils with water and transition-metal catalysts. Energy Fuels 1994, 8, 380–387. [Google Scholar] [CrossRef]
- Guan, Q.Q.; Wei, C.H.; Shi, H.S.; Wu, C.F.; Chai, X.S. Partial oxidative gasification of phenol for hydrogen in supercritical water. Appl. Energy 2011, 88, 2612–2616. [Google Scholar] [CrossRef]
- Houser, T.J.; Zhou, Y.; Liu, X. The destruction of selected hazardous compounds using supercritical water. J. Supercrit. Fluids 1996, 9, 106–112. [Google Scholar] [CrossRef]
- Sato, T. Upgrading of heavy oil by hydrogenation through partial oxidation and water-gas shift reaction in supercritical water. J. Jpn. Petrol. Inst. 2014, 57, 1–10. [Google Scholar] [CrossRef]
- Arai, K.; Adschiri, T.; Watanabe, M. Hydrogenation of hydrocarbons through partial oxidation in supercritical water. Ind. Eng. Chem. Res. 2000, 39, 4697–4701. [Google Scholar] [CrossRef]
- Jin, F.M.; Zeng, X.; Cao, J.L.; Kawasaki, K.; Kishita, A.; Tohji, K.; Enomoto, H. Partial hydrothermal oxidation of unsaturated high molecular weight carboxylic acids for enhancing the cold flow properties of biodiesel fuel. Fuel 2010, 89, 2448–2454. [Google Scholar] [CrossRef]
- Sato, T.; Watanabe, M.; Smith, R.L.; Adschiri, T.; Arai, K. Analysis of the density effect on partial oxidation of methane in supercritical water. J. Supercrit. Fluids 2004, 28, 69–77. [Google Scholar] [CrossRef]
- Kim, Y.L.; Kim, J.D.; Lim, J.S.; Lee, Y.W.; Yi, S.C. Reaction pathway and kinetics for uncatalyzed partial oxidation of p-xylene in sub- and supercritical water. Ind. Eng. Chem. Res. 2002, 41, 5576–5583. [Google Scholar] [CrossRef]
- Watanabe, M.; Mochiduki, M.; Sawamoto, S.; Adschiri, T.; Arai, K. Partial oxidation of n-hexadecane and polyethylene in supercritical water. J. Supercrit. Fluids 2001, 20, 257–266. [Google Scholar] [CrossRef]
- Armbruster, U.; Martin, A.; Krepel, A. Partial oxidation of propane in sub- and supercritical water. J. Supercrit. Fluids 2001, 21, 233–243. [Google Scholar] [CrossRef]
- Lee, J.H.; Foster, N.R. Direct partial oxidation of methane to methanol in supercritical water. J. Supercrit. Fluids 1996, 9, 99–105. [Google Scholar] [CrossRef]
- Hosseinpour, M.; Ahmadi, S.J.; Fatemi, S. Successive co-operation of supercritical water and silica-supported iron oxide nanoparticles in upgrading of heavy petroleum residue: Suppression of coke deposition over catalyst. J. Supercrit. Fluids 2015, 100, 70–78. [Google Scholar] [CrossRef]
- Watanabe, M.; Kato, S.; Ishizeki, S.; Inomata, H.; Smith, R.L. Heavy oil upgrading in the presence of high density water: Basic study. J. Supercrit. Fluids 2010, 53, 48–52. [Google Scholar] [CrossRef]
- Patwardhan, P.R.; Timko, M.T.; Class, C.A.; Bonomi, R.E.; Kida, Y.; Hernandez, H.H.; Tester, J.W.; Green, W.H. Supercritical water desulfurization of organic sulfides is consistent with free-radical kinetics. Energy Fuels 2013, 27, 6108–6117. [Google Scholar] [CrossRef]
- Duong, A.; Chattopadhyaya, G.; Kwok, W.Y.; Smith, K.J. An experimental study of heavy oil ultrafiltration using ceramic membranes. Fuel 1997, 76, 821–828. [Google Scholar] [CrossRef]
- Dechaine, G.P.; Gray, M.R. Chemistry and association of vanadium compounds in heavy oil and bitumen, and implications for their selective removal. Energy Fuels 2010, 24, 2795–2808. [Google Scholar] [CrossRef]
- Mandal, P.C.; Wahyudiono; Sasaki, M.; Goto, M. Non-catalytic vanadium removal from vanadyl etioporphyrin (vo-ep) using a mixed solvent of supercritical water and toluene: A kinetic study. Fuel 2012, 92, 288–294. [Google Scholar] [CrossRef]
- Yuan, P.Q.; Cheng, Z.M.; Zhang, X.Y.; Yuan, W.K. Catalytic denitrogenation of hydrocarbons through partial oxidation in supercritical water. Fuel 2006, 85, 367–373. [Google Scholar] [CrossRef]
- Babich, I.V.; Moulijn, J.A. Science and technology of novel processes for deep desulfurization of oil refinery streams: A review. Fuel 2003, 82, 607–631. [Google Scholar] [CrossRef]
- Vogelaar, B.M.; Makkee, M.; Moulijn, J.A. Applicability of supercritical water as a reaction medium for desulfurisation and demetallisation of gasoil. Fuel Process. Technol. 1999, 61, 265–277. [Google Scholar] [CrossRef]
- Adschiri, T.; Shibata, R.; Sato, T.; Watanabe, M.; Arai, K. Catalytic hydrodesulfurization of dibenzothiophene through partial oxidation and a water-gas shift reaction in supercritical water. Ind. Eng. Chem. Res. 1998, 37, 2634–2638. [Google Scholar] [CrossRef]
- Yuan, P.Q.; Cheng, Z.M.; Jiang, W.L.; Zhang, R.; Yuan, W.K. Catalytic desulfurization of residual oil through partial oxidation in supercritical water. J. Supercrit. Fluids 2005, 35, 70–75. [Google Scholar] [CrossRef]
- Fedyaeva, O.N.; Antipenko, V.R.; Vostrikov, A.A. Conversion of sulfur-rich asphaltite in supercritical water and effect of metal additives. J. Supercrit. Fluids 2014, 88, 105–116. [Google Scholar] [CrossRef]
- Mandal, P.C.; Wahyudiono; Sasaki, M.; Goto, M. Nickel removal from nickel-5,10,15,20-tetraphenylporphine using supercritical water in absence of catalyst: A basic study. J. Hazard. Mater. 2011, 187, 600–603. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.C.; Sasaki, M.; Goto, M. Nickel removal from nickel etioporphyrin (Ni-EP) using supercritical water in the absence of catalyst. Fuel Process. Technol. 2012, 104, 67–72. [Google Scholar] [CrossRef]
- Mandal, P.C.; Goto, M.; Sasaki, M. Removal of nickel and vanadium from heavy oils using supercritical water. J. Jpn. Petrol. Inst. 2014, 57, 18–28. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Yan, B.; Xiao, X.-M. A Review of Laboratory-Scale Research on Upgrading Heavy Oil in Supercritical Water. Energies 2015, 8, 8962-8989. https://doi.org/10.3390/en8088962
Li N, Yan B, Xiao X-M. A Review of Laboratory-Scale Research on Upgrading Heavy Oil in Supercritical Water. Energies. 2015; 8(8):8962-8989. https://doi.org/10.3390/en8088962
Chicago/Turabian StyleLi, Ning, Bo Yan, and Xian-Ming Xiao. 2015. "A Review of Laboratory-Scale Research on Upgrading Heavy Oil in Supercritical Water" Energies 8, no. 8: 8962-8989. https://doi.org/10.3390/en8088962
APA StyleLi, N., Yan, B., & Xiao, X.-M. (2015). A Review of Laboratory-Scale Research on Upgrading Heavy Oil in Supercritical Water. Energies, 8(8), 8962-8989. https://doi.org/10.3390/en8088962