A Comparative Study on EB-Radiation Deterioration of Nafion Membrane in Water and Isopropanol Solvents
Abstract
:1. Introduction

2. Experimental Section
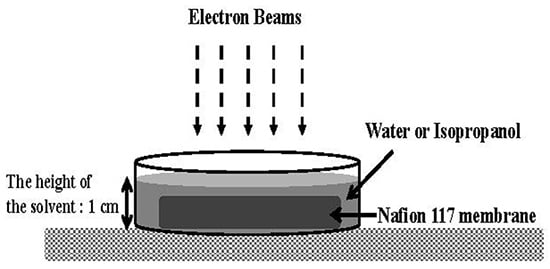
2.1. Materials and Sample Preparation
2.2. Irradiation

2.3. Mechanical Property
2.4. Thermal Analysis
2.5. Ion Exchange Capacity
2.6. Solvent Uptakes and Thickness Measurement
3. Results and Discussion
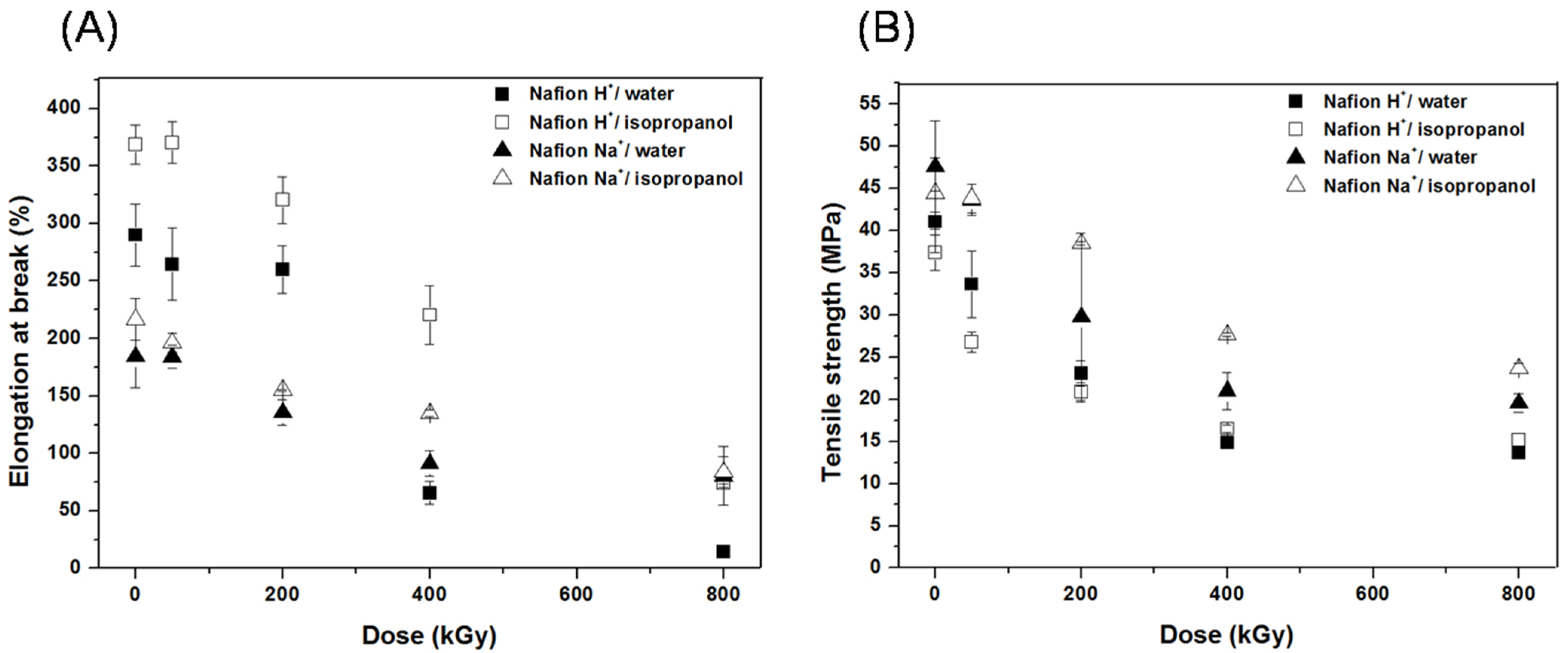
3.1. Mechanical Properties
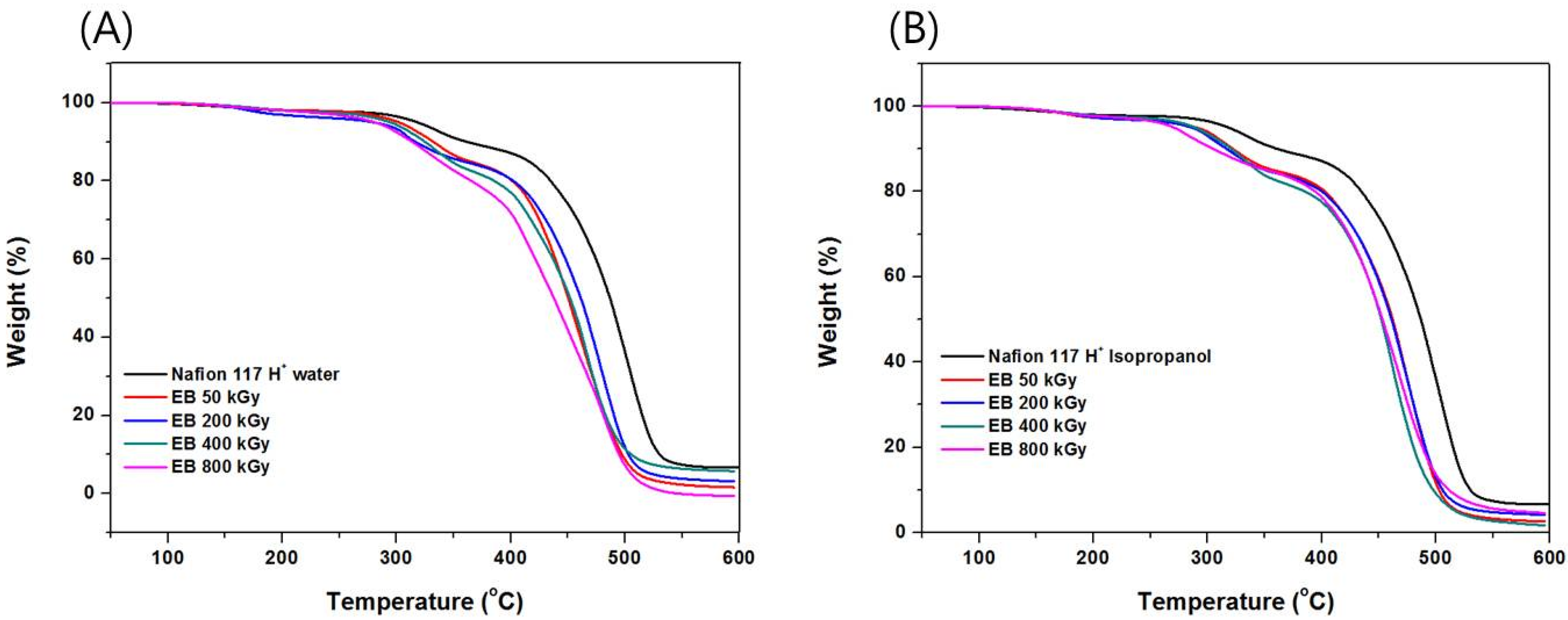
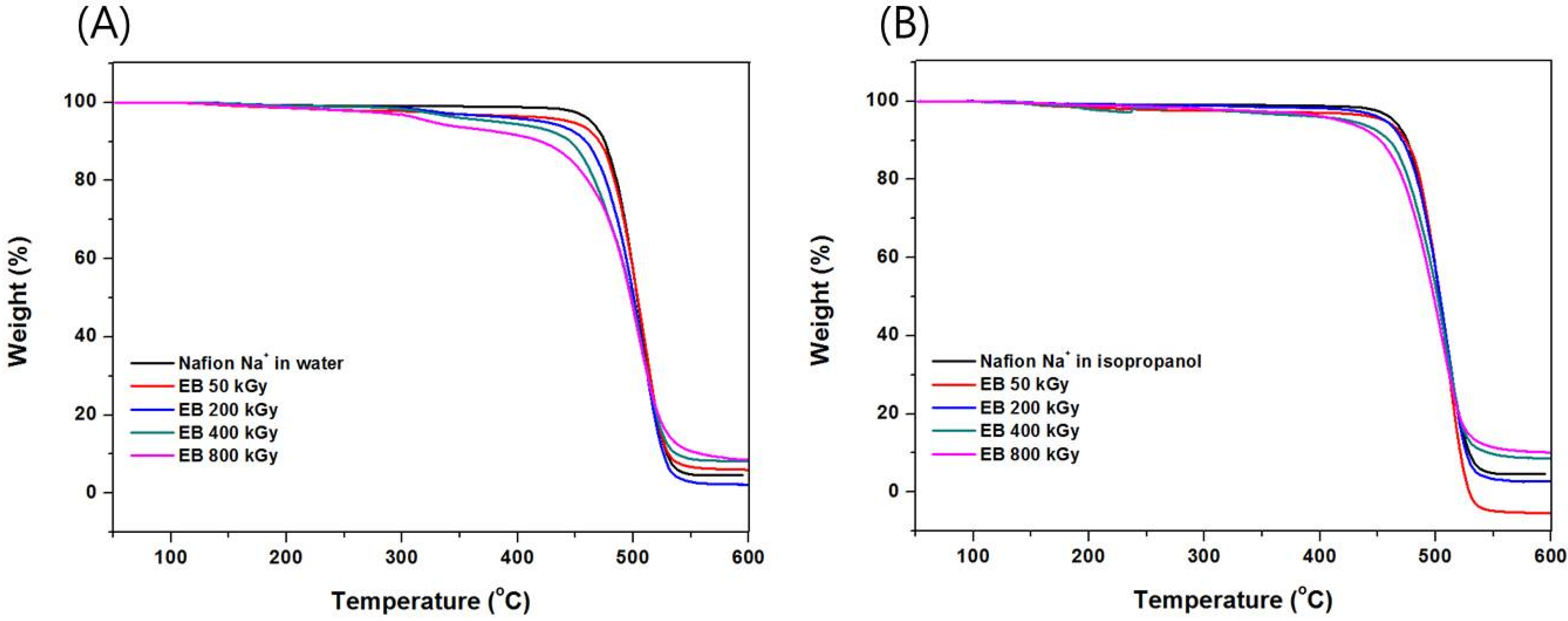
3.2. Thermal Analysis
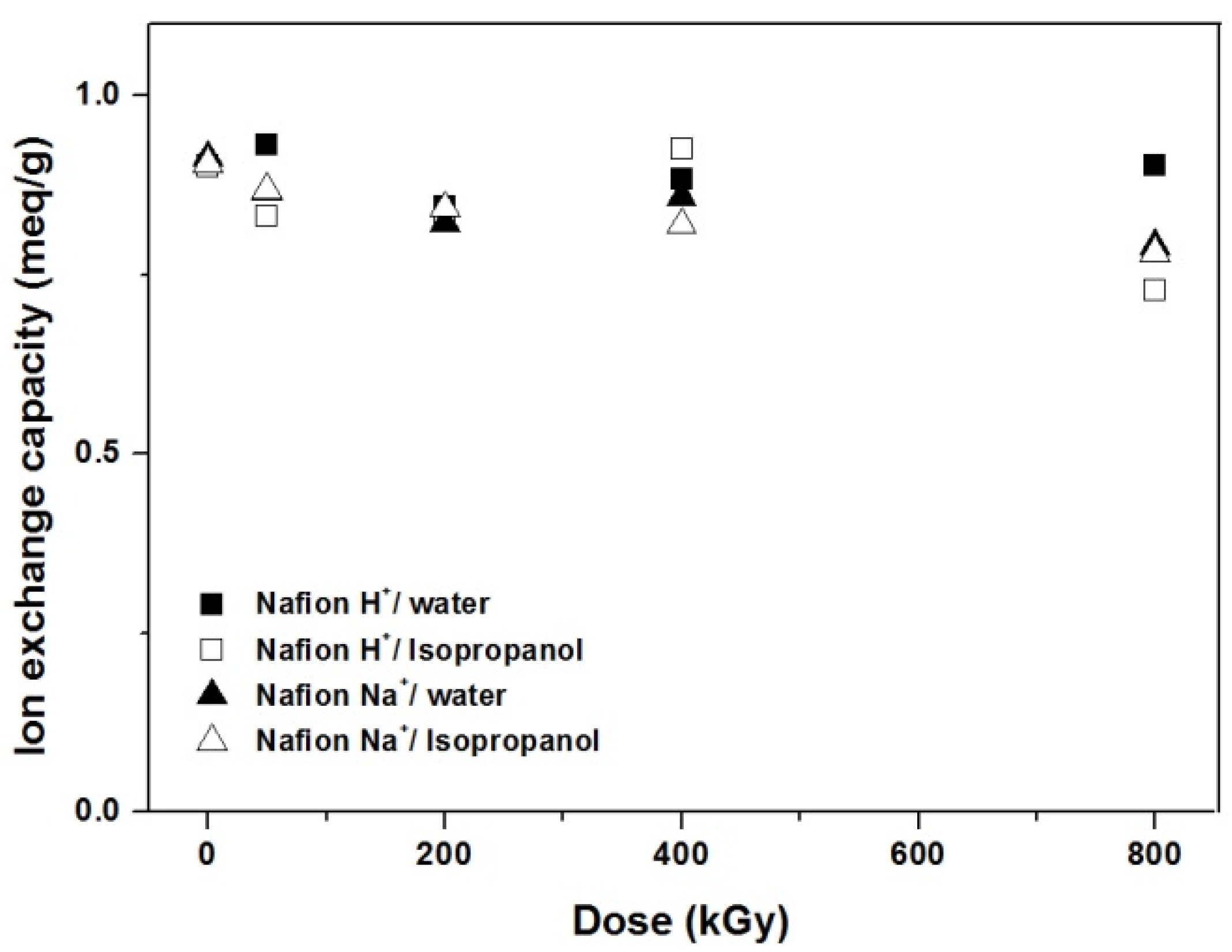
3.3. Ion Exchange Capacity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mauritz, K.A.; Moore, R.B. State of understanding of Nafion. Chem. Rev. 2004, 104, 4535–4585. [Google Scholar] [CrossRef] [PubMed]
- Yeo, R.S.; McBreen, J.; Kissel, G.; Srinivasan, S. Perfluorosulphonic acid (Nafion) membrane as a separator for an advanced alkaline water electrolyser. J. Appl. Electrochem. 1980, 10, 741–747. [Google Scholar] [CrossRef]
- Kujawski, W.; Nguyen, Q.T.; Neel, J. Infrared investigations of sulfonated ionomer membranes. I. Water-alcohol compositions and counterions effects. J. Appl. Polym. Sci. 1992, 44, 951–958. [Google Scholar] [CrossRef]
- Santhosh Kumar, K.S.; Vijayalakshim, K.P.; Sivanath, S.; Jayalatha, T.; Mohanty, S.; Shaneeth, M. Interaction of Nafion ionomers toward various solvents. J. Appl. Polym. Sci. 2012, 128, 3710–3719. [Google Scholar] [CrossRef]
- Yeo, R.S. Swelling studies of perfluorinated ionomer membranes. J. Appl. Polym. Sci. 1986, 32, 5733–5741. [Google Scholar] [CrossRef]
- Balko, E.N.; Chaklos, J.T. Effects of ionizing radiation on perfluorosulfonic acid ion-exchange polymer. J. Appl. Polym. Sci. 1981, 26, 1519–1531. [Google Scholar] [CrossRef]
- Hobso, L.J.; Ozu, H.; Yamaguchi, M.; Muramatsu, M.; Hayase, S. Nafion 117 modified by low dose EB irradiation: Surface structure and physical properties. J. Mater. Chem. 2002, 12, 1650–1656. [Google Scholar] [CrossRef]
- Iwai, Y.; Hiroki, A.; Tamada, M.; Yamanishi, T. Radiation deterioration in mechanical properties and ion exchange capacity of Nafion N117 swelling in water. J. Membr. Sci. 2008, 322, 249–255. [Google Scholar] [CrossRef]
- Ghassemzadeh, L.; Peckham, T.J.; Weissbach, T.; Luo, X.; Holdcroft, S. Selective Formation of Hydrogen and hydroxyl radicals by electron beam irradiation and their reactivity with perfluorosulfonated acid ionomer. J. Am. Chem. Soc. 2013, 135, 15923–15932. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.F.; Ingram, D.J.E.; Symons, M.C.R.; Townsend, M.G. Electron resonance studies of different radical species formed in rigid solutions of hydrogen peroxide after UV irradiation. Trans. Faraday Soc. 1956, 53, 914–920. [Google Scholar] [CrossRef]
- Russell, J.C.; Freeman, G.R. Reactions of the primary reducing species in the radiolysis of liquid 2-propanol. J. Phys. Chem. 1968, 72, 808–815. [Google Scholar] [CrossRef]
- Mincher, B.J.; Arbon, R.E.; Knighton, W.B.; Meikrantz, D.H. Gamma-ray-induced degradation of PCBs in neutral isopropanol using spent reactor fuel. Appl. Radiat. Isot. 1994, 45, 879–887. [Google Scholar] [CrossRef]
- Belloni, J.; Mostafavi, M.; Remita, H.; Marignier, J.-L.; Delcourt, M.-O. Radiation-induced synthesis of mono- and multi-metallic clusters and nanocolloids. New J. Chem. 1998, 22, 1239–1255. [Google Scholar] [CrossRef]
- Connolly, D.J.; Gresham, W.F. Fluorocarbon Vinyl Ether Polymers. U.S. Patent 3,282,875, 1 November 1966. [Google Scholar]
- Bro, M.I.; Lovejoy, E.R.; McKay, G.R. Reactions of irradiated polytetrafluoroethylene resin. J. Appl. Polym. Sci. 1963, 7, 2121–2133. [Google Scholar] [CrossRef]
- Yeo, R.S. Dual cohesive energy densities of perfluorosulphonic acid (Nafion) membrane. Polymer 1979, 21, 432–435. [Google Scholar] [CrossRef]
- Gebel, G.; Aldebert, P.; Pineri, M. Swelling study of perfluorosulphonated ionomer membranes. Polymer 1992, 34, 333–339. [Google Scholar] [CrossRef]
- Eisenberg, A. Clustering of ions in organic polymers. Theor. Approach. 1970, 3, 147–154. [Google Scholar]
- Kawano, Y.; Wang, Y.; Palmer, R.A.; Aubuchon, S.R. Stress-strain curves of Nafion membranes in acid and salt forms. Polimeros 2002, 12, 96–101. [Google Scholar] [CrossRef]
- Iwai, Y.; Yamanishi, T.; Nishi, M.; Yagi, T.; Tamada, M. Durability of irradiated polymers in solid-polymer-electrolyte water electrolyzer. J. Nucl. Sci. Technol. 2005, 42, 636–642. [Google Scholar] [CrossRef]
- Iwai, Y.; Yamanishi, T.; Isobe, K.; Nishi, M.; Yagi, T.; Tamada, M. Distinctive radiation durability of an ion exchange membrane in the SPE water electrolyzer for the ITER water detritiation system. Fusion Eng. Des. 2006, 81, 815–820. [Google Scholar] [CrossRef]
- Stefanithis, I.D.; Mauritz, K.A. Microstructural evolution of a silicon oxide phase in a perfluorosulfonic acid ionomer by an in situ sol-gel reaction. 3. Thermal analysis studies. Macromolecules 1990, 23, 2397–2402. [Google Scholar] [CrossRef]
- Almeida, S.H.; Kawano, Y. Thermal behavior of Nafion membranes. J. Therm. Anal. Cal. 1999, 58, 569–577. [Google Scholar] [CrossRef]
- Wilkie, C.A.; Thomsen, J.R.; Mittleman, M.L. Interaction of poly(methyl methacrylate) and Nafions. J. Appl. Polym. Sci. 1991, 42, 901–909. [Google Scholar] [CrossRef]
- Feldheim, D.L.; Lawson, R.; Martin, C.R. Influence of the sulfonate counteraction on the thermal stability of Nafion perfluorosulfonate membranes. J. Polym. Sci. Polym. Phys. Ed. 1993, 31, 953–957. [Google Scholar] [CrossRef]
- Iwai, Y.; Yamanishi, T. Thermal stability of ion-exchange Nafion N117CS membranes. Polym. Degrad. Stabil. 2009, 94, 679–687. [Google Scholar] [CrossRef]
- Lage, L.G.; Delgado, P.; Kawano, Y. Thermal stability and decomposition of Nafion membranes with different cations using high-resolution thermogravimetry. J. Therm. Anal. Calorim. 2004, 75, 521–530. [Google Scholar] [CrossRef]
- Iwai, Y.; Hiroki, A.; Tamada, M. Radiation-induced crosslinking of Nafion N117CS membranes. J. Membr. Sci. 2011, 369, 397–403. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.S.; Sohn, J.-Y.; Shin, J. A Comparative Study on EB-Radiation Deterioration of Nafion Membrane in Water and Isopropanol Solvents. Energies 2015, 8, 5370-5380. https://doi.org/10.3390/en8065370
Choi JS, Sohn J-Y, Shin J. A Comparative Study on EB-Radiation Deterioration of Nafion Membrane in Water and Isopropanol Solvents. Energies. 2015; 8(6):5370-5380. https://doi.org/10.3390/en8065370
Chicago/Turabian StyleChoi, Ji Sun, Joon-Yong Sohn, and Junhwa Shin. 2015. "A Comparative Study on EB-Radiation Deterioration of Nafion Membrane in Water and Isopropanol Solvents" Energies 8, no. 6: 5370-5380. https://doi.org/10.3390/en8065370
APA StyleChoi, J. S., Sohn, J.-Y., & Shin, J. (2015). A Comparative Study on EB-Radiation Deterioration of Nafion Membrane in Water and Isopropanol Solvents. Energies, 8(6), 5370-5380. https://doi.org/10.3390/en8065370