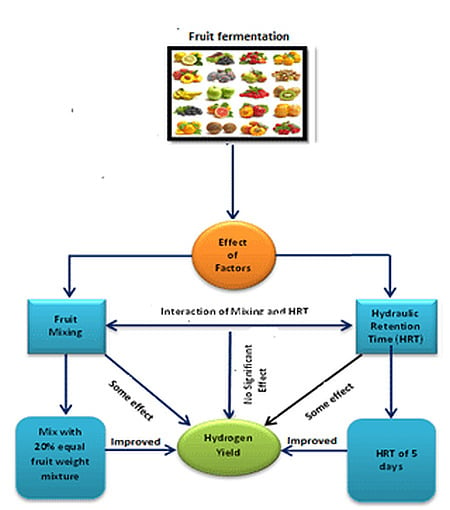
Evaluation of Fermentative Hydrogen Production from Single and Mixed Fruit Wastes
Abstract
:1. Introduction
| Fruit | Fruit Flavor | References | |
|---|---|---|---|
| Flavor compound | Flavor group | ||
| Orange | Hexanal and nonanal | Aldehydes | [10] |
| Octanol, 3-methyl butanol | Alcohols | [10,23] | |
| α-pinene, car-3-ene, myrcene and limonene | Terpenoid | [10,11] | |
| Banana | Pentanone, heptanone, undecanone | Ketones | [12,13] |
| Butanal, hexanal and E-2-hexanal | Aldehydes | [12,13] | |
| 1-butanol, 2-pentanol, 3-methyl-1-butanol, 1-hexanol and eugenol | Alcohol | [12,13] | |
| Ethyl acetate, butyl acetate, 2-methyl propyl acetate, hexyl acetate, hexyl butanoate and butyl butanoate | Esters | [12,13] | |
| Apple | n-Hexanal, E-2-hexenal, nonanal, acetaldehyde | Aldehydes | [10,14] |
| Hexanol and butanol | Alcohols | [14] | |
| Car-3-ene | Terpenoid | [10] | |
| Ethyl butanoate, ethyl -2-methylbutanoate, hexyl acetate, etc. | Ester | [14,15,23] | |
| Epicatechin | Polyphenol | [16,17] | |
| Grape | Hexanal | Aldehyde | [10] |
| Octanol and hexanol | Alcohols | [14,18] | |
| Hexyl acetate, ethyl acetate and ethyl hexanoate | Esters | [10,18,19] | |
| Quercetin and epicatechin | Polyphenol | [16] | |
| Melon | Nonanal, benzaldehyde and E-2-nonenal | Aldehydes | [20,21,22] |
| Ethyl 2-methyl propyl acetate and 2-methyl butyl acetate | Esters | [20,21,22] | |
2. Results and Discussion
2.1. Hydrogen Production Yields from Singly-Digested Fruits
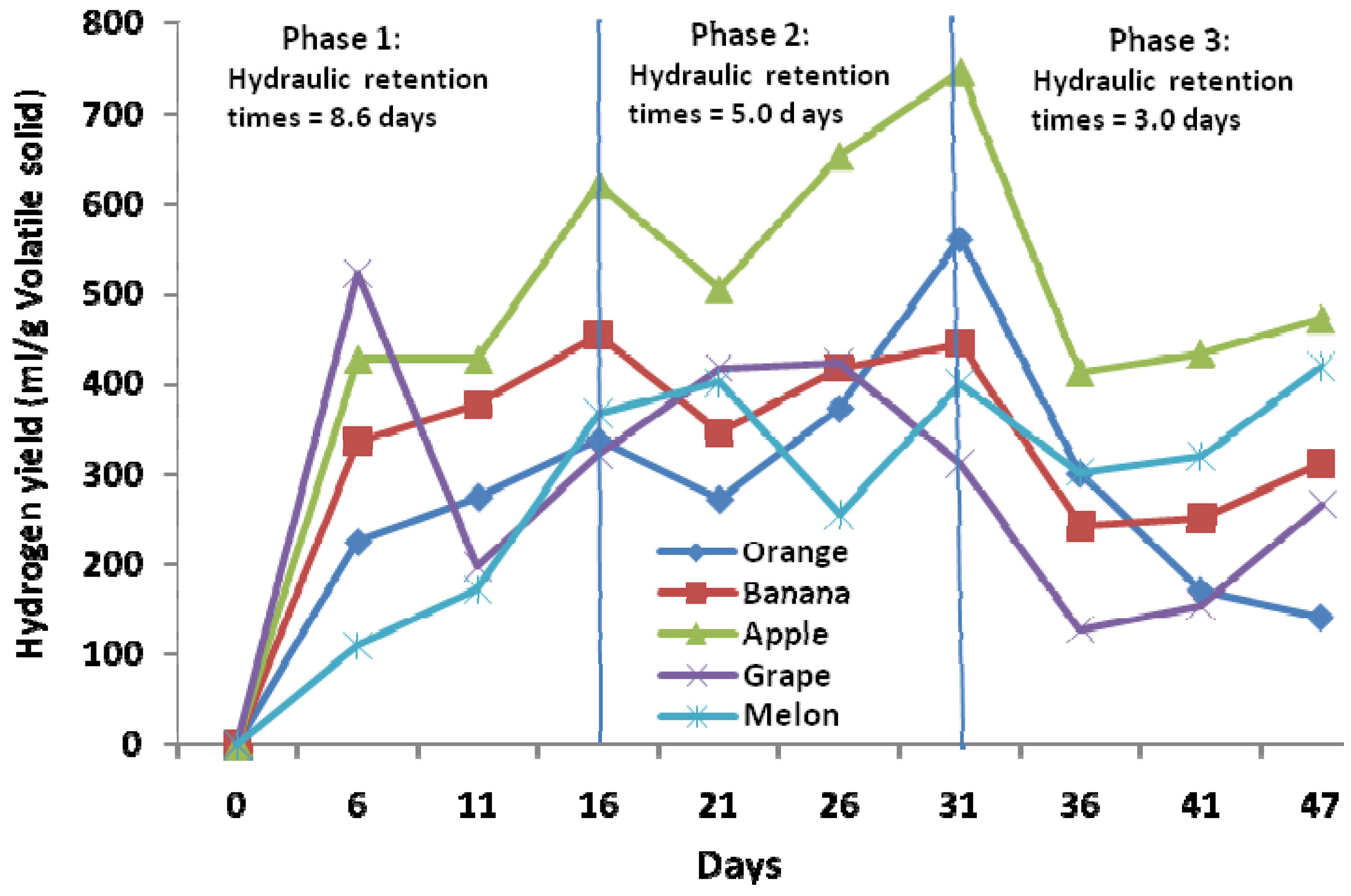
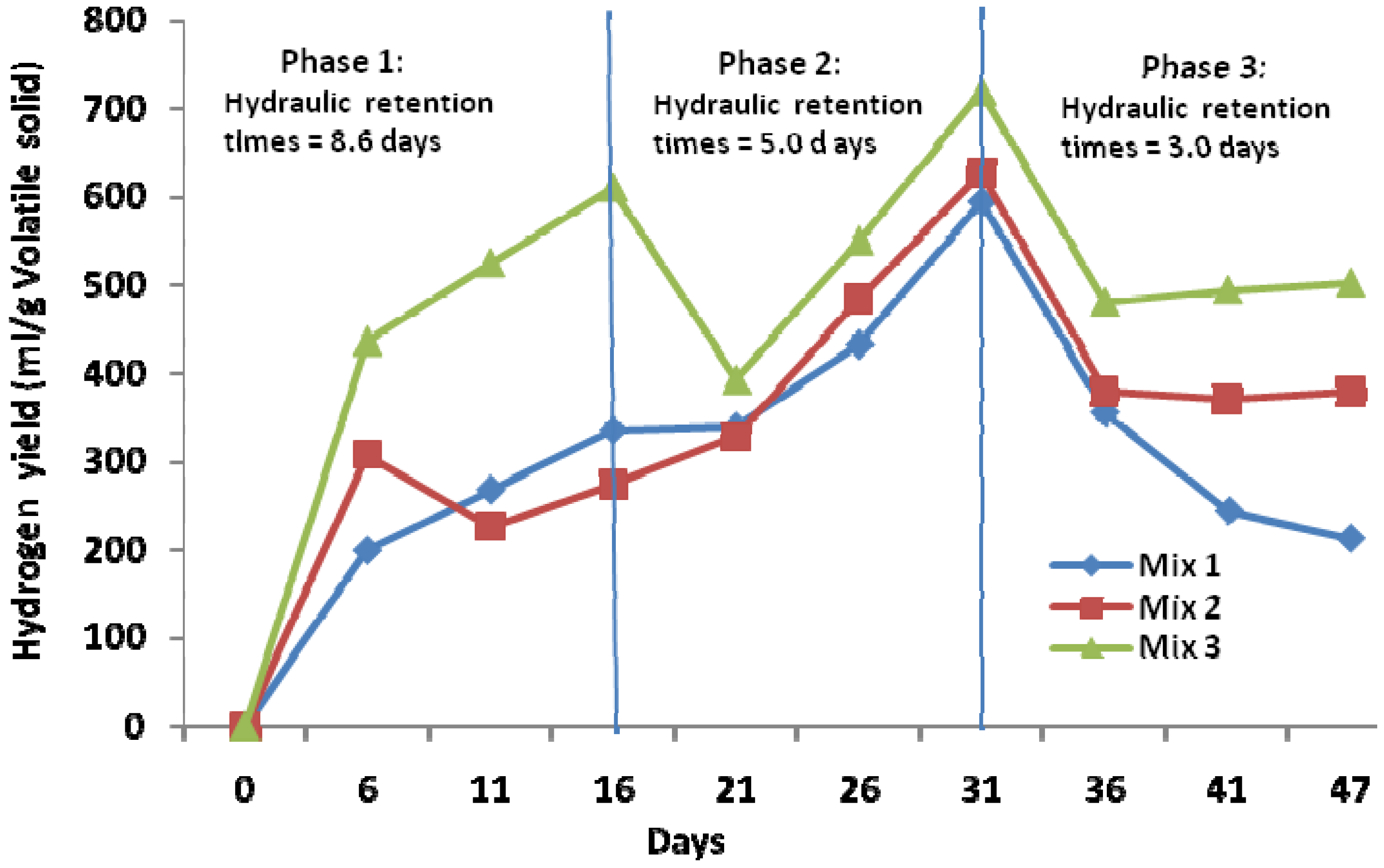
2.2. Hydrogen Production Yields from Mixed Fruits
2.3. VFAs Production

2.4. Comparison of Hydrogen Yields and Acetic Acid Productions with Theoretical Values
2.4.1. Relative Yield of Hydrogen Production
| Parameter | Average hydrogen yields | Average acetic acid production | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Period (d) | 1–15 | 16–30 | 31–47 | 1–15 | 16–30 | 31–47 | ||||||
| HRT (d) | 8.6 | 5.0 | 3.0 | 8.6 | 5.0 | 3.0 | ||||||
| Fruit | AY (mL/gVS) | RY (%) | AY (mL/gVS) | RY (%) | AY (mL/gVS) | RY (%) | AY (g/L) | RY (%) | AY (g/L) | RY (%) | AY (g/L) | RY (%) |
| Orange | 279 | 16.1 | 403 | 23.3 | 204 | 11.8 | 0.37 | 55 | 0.71 | 118 | 1.21 | 181 |
| Banana | 389 | 22.7 | 403 | 23.5 | 268 | 15.7 | 0.62 | 93 | 1.05 | 157 | 1.04 | 155 |
| Apple | 493 | 28.9 | 635 | 37.3 | 440 | 25.8 | 0.73 | 109 | 0.91 | 136 | 1.30 | 194 |
| Grape | 347 | 20.5 | 384 | 22.6 | 182 | 10.7 | 0.75 | 112 | 0.71 | 106 | 1.07 | 160 |
| Melon | 216 | 12.6 | 352 | 20.5 | 347 | 20.2 | 0.48 | 72 | 0.37 | 55 | 0.86 | 128 |
| Mix 1 | 268 | 15.5 | 456 | 26.4 | 271 | 15.7 | 0.56 | 84 | 1.03 | 154 | 1.20 | 179 |
| Mix 2 | 270 | 15.7 | 479 | 28.0 | 377 | 22.0 | 0.74 | 110 | 1.03 | 154 | 1.18 | 176 |
| Mix 3 | 523 | 30.5 | 553 | 32.3 | 491 | 28.6 | 1.05 | 157 | 1.15 | 172 | 1.62 | 242 |
2.4.2. Relative Yield of Volatile Fatty Acids
2.5. Significant Effects of Varying Hydraulic Retention Times, Fruit Mixing and Their Interaction on Hydrogen Yield and Acetic Acid Production
| Response | Factor | Factor Type | Factor Levels | Factor Values | dF | Adj SS | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Hydrogen yield | HRT | Fixed | 3 | 3.0; 5.0; 8.6 | 2 | 3,8951 | 3.28 | 3.28 | 0.061 |
| Mix | Fixed | 2 | N; Y | 1 | 16,187 | 1.36 | 1.36 | 0.259 | |
| HRT and mix interaction | 2 | 3,266 | 0.27 | 0.27 | 0.763 | ||||
| Error | 18 | 11,890 | |||||||
| Total | 23 | ||||||||
| Acetic acid production | HRT | Fixed | 3 | 3.0; 5.0: 8.6 | 2 | 1.05371 | 0.526854 | 12.82 | 0.000 |
| Mix | Fixed | 2 | N, Y | 1 | 0.35219 | 0.352188 | 8.57 | 0.009 | |
| HRT and mix interaction | 2 | 0.01551 | 0.007754 | 0.19 | 0.830 | ||||
| Error | 18 | 0.73985 | 0.041103 | ||||||
| Total | 23 |
| Response | Factor | Difference of factor levels | Difference of means | SE of difference | Simultaneous 95% CI | t-value | Adjusted p-value |
|---|---|---|---|---|---|---|---|
| H2 yield | HRT | 5.0–3.0 | 131.8 | 56.3 | (−12.0; 275.5) | 2.34 | 0.076 |
| 8.6–3.0 | 15.3 | 56.3 | (−128.4; 159.0) | 0.27 | 0.960 | ||
| 8.6–5.0 | −116.5 | 56.3 | (−260.2; 27.3) | −2.07 | 0.125 | ||
| Mix | YES–NO | 53.6 | 46.0 | (−42.9; 150.2) | 1.17 | 0.259 | |
| Mix and HRT interaction | (NO 5.0)–(NO 3.0) | 147.2 | 69.0 | (−71.8; 366.2) | 2.13 | 0.314 | |
| (NO 8.6)–(NO 3.0) | 56.6 | 69.0 | (−162.4; 275.6) | 0.82 | 0.960 | ||
| (YES 3.0)–(NO 3.0) | 91.5 | 79.6 | (−161.4; 344.3) | 1.15 | 0.855 | ||
| (YES 5.0)–(NO 3.0) | 207.8 | 79.6 | (−45.0; 460.6) | 2.61 | 0.145 | ||
| (YES 8.6)–(NO 3.0) | 65.5 | 79.6 | (−187.4; 318.3) | 0.82 | 0.960 | ||
| (NO 8.6)–(NO 5.0) | −90.6 | 69.0 | (−309.6; 128.4) | −1.31 | 0.774 | ||
| (YES 3.0)–(NO 5.0) | −55.7 | 79.6 | (−308.6; 197.1) | −0.70 | 0.980 | ||
| (YES 5.0)–(NO 5.0) | 60.6 | 79.6 | (−192.2; 313.4) | 0.76 | 0.971 | ||
| (YES 8.6)–(NO 5.0) | −81.7 | 79.6 | (−334.6; 171.1) | −1.03 | 0.903 | ||
| (YES 3.0)–(NO 8.6) | 34.9 | 79.6 | (−218.0; 287.7) | 0.44 | 0.998 | ||
| (YES 5.0)–(NO 8.6) | 151.2 | 79.6 | (−101.6; 404.0) | 1.90 | 0.434 | ||
| (YES 8.6)–(NO 8.6) | 8.9 | 79.6 | (−244.0; 261.7) | 0.11 | 1.000 | ||
| (YES 5.0)–(YES 3.0) | 116.3 | 89.0 | (−166.3; 399.0) | 1.31 | 0.778 | ||
| (YES 8.6)–(YES 3.0) | −26.0 | 89.0 | (−308.7; 256.7) | −0.29 | 1.000 | ||
| (YES 8.6)–(YES 5.0) | −142.3 | 89.0 | (−425.0; 140.3) | −1.60 | 0.610 | ||
| Acetic acid | HRT | 5.0–3.0 | −0.305 | 0.105 | (−0.572; −0.037) | −2.91 | 0.024 |
| 8.6–3.0 | −0.528 | 0.105 | (−0.795; −0.261) | −5.04 | 0.000 | ||
| 8.6–5.0 | −0.223 | 0.105 | (−0.491; 0.044) | −2.13 | 0.111 | ||
| Mix | YES–NO | 0.2502 | 0.0855 | (0.0706; 0.4298) | 2.93 | 0.124 | |
| Mix and HRT interaction | (NO 5.0)–(NO 3.0) | −0.346 | 0.128 | (−0.753; 0.061) | −2.70 | 0.124 | |
| (NO 8.6)–(NO 3.0) | −0.506 | 0.128 | (−0.913; −0.099) | −3.95 | 0.010 | ||
| (YES 3.0)–(NO 3.0) | 0.237 | 0.148 | (−0.233; 0.707) | 1.60 | 0.607 | ||
| (YES 5.0)–(NO 3.0) | −0.026 | 0.148 | (−0.496; 0.444) | −0.18 | 1.000 | ||
| (YES 8.6)–(NO 3.0) | −0.313 | 0.148 | (−0.783; 0.157) | −2.11 | 0.325 | ||
| (NO 8.6)–(NO 5.0) | −0.160 | 0.128 | (−0.567; 0.247) | −1.25 | 0.808 | ||
| (YES 3.0)–(NO 5.0) | 0.583 | 0.148 | (0.113; 1.053) | 3.94 | 0.010 | ||
| (YES 5.0)–(NO 5.0) | 0.320 | 0.148 | (−0.150; 0.790) | 2.16 | 0.302 | ||
| (YES 8.6)–(NO 5.0) | 0.033 | 0.148 | (−0.437; 0.503) | 0.23 | 1.000 | ||
| (YES 3.0)–(NO 8.6) | 0.743 | 0.148 | (0.273; 1.213) | 5.02 | 0.001 | ||
| (YES 5.0)–(NO 8.6) | 0.480 | 0.148 | (0.010; 0.950) | 3.24 | 0.044 | ||
| (YES 8.6)–(NO 8.6) | 0.193 | 0.148 | (−0.227; 0.663) | 1.31 | 0.778 | ||
| (YES 5.0)–(YES 3.0) | −0.263 | 0.166 | (−0.789; 0.262) | −1.59 | 0.614 | ||
| (YES 8.6)–(YES 3.0) | −0.550 | 0.166 | (−1.076; −0.024) | −3.32 | 0.037 | ||
| (YES 8.6)–(YES 5.0) | −0.287 | 0.166 | (−0.812; 0.239) | −1.73 | 0.530 |
| Response | Factor | Factor Type | Factor Levels | Factor Values | dF | Adj SS | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Hydrogen yield | HRT | Fixed | 3 | 3.0; 5.0; 8.6 | 2 | 83,069 | 41,534 | 11.52 | 0.001 |
| Substrate | Fixed | 8 | Apple; Banana; Grape; Melon; Mix1; Mix 2; Mix 3; Orange | 7 | 186,264 | 26,609 | 7.38 | 0.001 | |
| Error | 14 | 50,472 | 3,605 | ||||||
| Total | 23 | 319,805 | |||||||
| Acetic acid production | HRT | Fixed | 3 | 3.0; 5.0; 8.6 | 2 | 1.1074 | 0.55372 | 30.39 | 0.000 |
| Mix | Fixed | 8 | Apple; Banana; Grape; Melon; Mix1; Mix 2; Mix 3; Orange | 7 | 0.8524 | 0.12178 | 6.68 | 0.001 | |
| Error | 14 | 0.2551 | 0.01822 | ||||||
| Total | 23 |
| Response | Factor | Difference of Factor Levels | Difference of Means | SE of Difference | Simultaneous 95% CI | t-Value | Adjusted p-Value |
|---|---|---|---|---|---|---|---|
| H2 yield | HRT | 5.0–3.0 | 135.6 | 30.0 | (57.1; 214.2) | 4.52 | 0.001 |
| 8.6–3.0 | 25.6 | 30.0 | (−52.9; 104.2) | 0.85 | 0.677 | ||
| 8.6–5.0 | −110.0 | 30.0 | (−188.5; −31.5) | −3.66 | 0.007 | ||
| Substrate | Banana-Apple | −169.3 | 49.0 | (−342.3; 3.6) | −3.45 | 0.057 | |
| Grape-apple | −218.3 | 49.0 | (−391.3; −45.4) | −4.45 | 0.010 | ||
| Melon-Apple | −217.7 | 49.0 | (−390.6; −44.7) | −4.44 | 0.010 | ||
| Mix 1-Apple | −191.0 | 49.0 | (−364.0; −18.0) | −3.90 | 0.026 | ||
| Mix 2-Apple | −147.3 | 49.0 | (−320.3; 25.6) | −3.01 | 0.123 | ||
| Mix 3-Apple | −0.3 | 49.0 | (−173.3; 172.6) | −0.01 | 1.000 | ||
| Orange-Apple | −227.3 | 49.0 | (−400.3; −54.4) | −4.64 | 0.007 | ||
| Grape-Banana | −49.0 | 49.0 | (−222.0; 124.6) | −1.00 | 0.967 | ||
| Melon-Banana | −48.3 | 49.0 | (−221.3; 124.6) | −0.99 | 0.969 | ||
| Mix 1-Banana | −21.7 | 49.0 | (−194.6; 151.3) | −0.44 | 1.000 | ||
| Mix 2-Banana | 22.0 | 49.0 | (−151.0; 195.0) | 0.45 | 1.000 | ||
| Mix 3-Banana | 169.0 | 49.0 | (−4.0; 342.0) | 3.45 | 0.058 | ||
| Orange-Banana | −58.0 | 49.0 | (−231.0; 115.0) | −1.18 | 0.924 | ||
| Melon-Grape | 0.7 | 49.0 | (−172.3; 173.6) | 0.01 | 1.000 | ||
| Mix 1-Grape | 27.3 | 49.0 | (−145.6; 200.3) | 0.56 | 0.999 | ||
| Mix 2-Grape | 71.0 | 49.0 | (−102.0; 244.0) | 1.45 | 0.821 | ||
| Mix 3-Grape | 218.0 | 49.0 | (45.0; 391.0) | 4.45 | 0.010 | ||
| Orange− Grape | −9.0 | 49.0 | (−182.0; 164.0) | −0.18 | 1.000 | ||
| Mix 1-Melon | 26.7 | 49.0 | (−146.3; 199.6) | 0.54 | 0.999 | ||
| Mix 2-Melon | 70.3 | 49.0 | (−102.6; 243.3) | 1.43 | 0.827 | ||
| Mix 3-Melon | 217.3 | 49.0 | (44.4; 390.3) | 4.43 | 0.010 | ||
| Orange-Melon | −9.7 | 49.0 | (−182.6; 163.3) | −0.20 | 1.000 | ||
| Mix 2-Mix 1 | 43.7 | 49.0 | (−129.3; 216.6) | 0.89 | 0.982 | ||
| Mix 3-Mix 1 | 190.7 | 49.0 | (17.7; 363.6) | 3.89 | 0.026 | ||
| Orange-Mix 1 | −36.3 | 49.0 | (−209.3; 136.6) | −0.74 | 0.994 | ||
| Mix 3-Mix 2 | 147.0 | 49.0 | (−26.0; 320.0) | 3.00 | 0.124 | ||
| Orange-Mix 2 | −80.0 | 49.0 | (−253.0; 93.0) | −1.63 | 0.726 | ||
| Orange-Mix 3 | −227.0 | 49.0 | (−400.0; −54.0) | −4.63 | 0.007 | ||
| Acetic acid | HRT | 5.0–3.0 | −0.3150 | 0.0675 | (−0.4916; −0.1384) | −4.67 | 0.001 |
| 8.6–3.0 | −0.5225 | 0.0675 | (−0.6991; −0.3459) | −7.74 | 0.002 | ||
| 8.6–5.0 | −0.2075 | 0.0675 | (−0.3841; −0.0309) | −3.07 | 0.021 | ||
| Substrate | Banana-Apple | −0.077 | 0.110 | (−0.466; 0.312) | −0.70 | 0.996 | |
| Grape-apple | −0.137 | 0.110 | (−0.526; 0.252) | −1.24 | 0.906 | ||
| Melon-Apple | −0.410 | 0.110 | (−0.799; −0.021) | −3.72 | 0.036 | ||
| Mix 1-Apple | −0.050 | 0.110 | (−0.439; 0.339) | −0.45 | 1.000 | ||
| Mix 2-Apple | 0.003 | 0.110 | (−0.386; 0.392) | 0.03 | 1.000 | ||
| Mix 3-Apple | 0.293 | 0.110 | (−0.096; 0.682) | 2.66 | 0.213 | ||
| Orange-Apple | −0.217 | 0.110 | (−0.606; 0.172) | −1.97 | 0.534 | ||
| Grape-Banana | −0.060 | 0.110 | (−0.449; 0.329) | −0.54 | 0.999 | ||
| Melon-Banana | −0.333 | 0.110 | (−0.722; 0.056) | −3.02 | 0.119 | ||
| Mix 1-Banana | 0.027 | 0.110 | (−0.362; 0.416) | 0.24 | 1.000 | ||
| Mix 2-Banana | 0.080 | 0.110 | (−0.309; 0.469) | 0.73 | 0.995 | ||
| Mix 3-Banana | 0.370 | 0.110 | (−0.019; 0.759) | 3.36 | 0.068 | ||
| Orange-Banana | −0.140 | 0.110 | (−0.529; 0.249) | −1.27 | 0.896 | ||
| Melon-Grape | −0.273 | 0.110 | (−0.662; 0.116) | −2.48 | 0.278 | ||
| Mix 1-Grape | 0.087 | 0.110 | (−0.302; 0.476) | 0.79 | 0.991 | ||
| Mix 2-Grape | 0.140 | 0.110 | (−0.249; 0.529) | 1.27 | 0.896 | ||
| Mix 3-Grape | 0.430 | 0.110 | (0.041; 0.819) | 3.90 | 0.026 | ||
| Orange− Grape | −0.080 | 0.110 | (−0.469; 0.309) | −0.73 | 0.995 | ||
| Mix 1-Melon | 0.360 | 0.110 | (−0.029; 0.749) | 3.27 | 0.079 | ||
| Mix 2-Melon | 0.413 | 0.110 | (0.024; 0.802) | 3.75 | 0.034 | ||
| Mix 3-Melon | 0.703 | 0.110 | (0.314; 1.092) | 6.38 | 0.000 | ||
| Orange-Melon | 0.193 | 0.110 | (−0.196; 0.582) | 1.75 | 0.657 | ||
| Mix 2-Mix 1 | 0.053 | 0.110 | (−0.336; 0.422) | 0.48 | 1.000 | ||
| Mix 3-Mix 1 | 0.343 | 0.110 | (−0.046; 0.732) | 3.12 | 0.102 | ||
| Orange-Mix 1 | −0.167 | 0.110 | (−0.556; 0.222) | −1.51 | 0.790 | ||
| Mix 3-Mix 2 | 0.290 | 0.110 | (−0.099; 0.679) | 2.63 | 0.223 | ||
| Orange-Mix 2 | −0.220 | 0.110 | (−0.609; 0.169) | −2.00 | 0.516 | ||
| Orange-Mix 3 | −0.510 | 0.110 | (−0.899; −0.121) | −4.63 | 0.007 |
3. Experimental Section
3.1. Experimental Materials (Feedstock, Seed Sludge and Nutrient)
3.2. Experimental Setup and Procedures
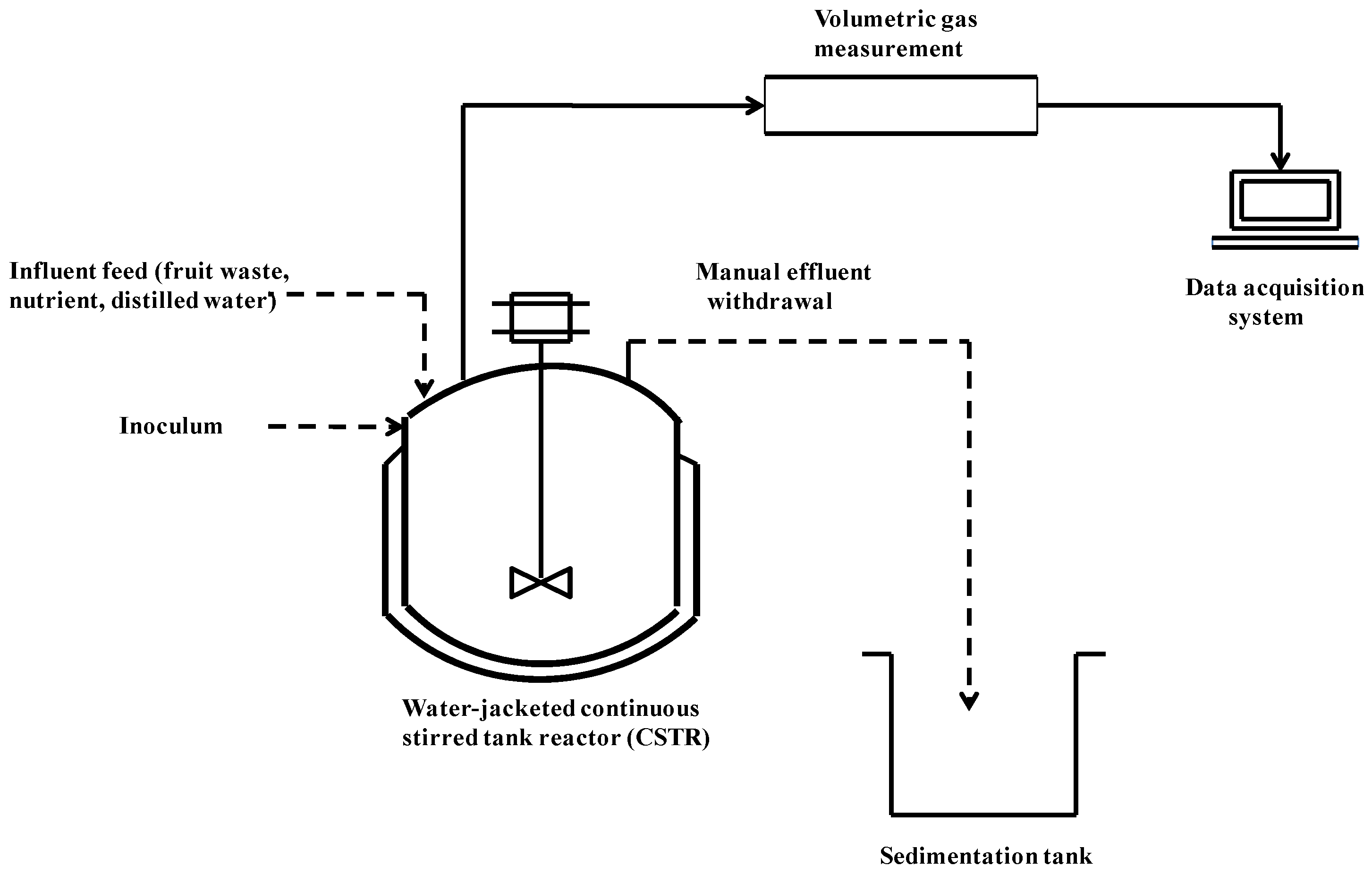
3.3. Analytical Procedures
| Parameters | Apple | Banana | Grape | Melon | Orange |
|---|---|---|---|---|---|
| TS (%) | 11.72 ± 0.02 | 16.36 ± 0.24 | 19.32 ± 0.24 | 8.93 ± 0.26 | 16.85 ± 0.30 |
| VS (%) | 11.53 ± 0.35 | 15.5 ± 0.05 | 18.68 ± 0.06 | 8.39 ± 0.16 | 16.26 ± 0.20 |
| pH | 3.90 ± 0.01 | 5.06 ± 0.02 | 3.67 ± 0.02 | 4.88 ± 0.01 | 4.04 ± 0.02 |
| Fruit | Carbohydrate | Protein | Lipid | C:N Ratio * | |||
|---|---|---|---|---|---|---|---|
| g | % | g | % | g | % | ||
| Apple | 14.06 | 97 | 0.27 | 2 | 0.20 | 1 | 52:1 |
| Melon | 9.09 | 93 | 0.54 | 6 | 0.14 | 1 | 17:1 |
| Banana | 22.84 | 94 | 1.09 | 5 | 0.33 | 1 | 21:1 |
| Orange | 15.50 | 90 | 1.30 | 8 | 0.30 | 2 | 12:1 |
| Grape | 18.10 | 95 | 0.72 | 4 | 0.16 | 1 | 25:1 |
| Mix 1 * | 15.71 | 92 | 1.10 | 6 | 0.28 | 2 | 14:1 |
| Mix 2 * | 15.77 | 95 | 0.59 | 3 | 0.26 | 2 | 27:1 |
| Mix 3 * | 15.92 | 94 | 0.78 | 5 | 0.23 | 1 | 20:1 |
4. Conclusions
Acknowledgments
Acronyms
| AY | Actual yield |
| CIs | Confidence intervals |
| COD | Chemical oxygen demand |
| dF | degree of freedom |
| HPM | Hydrogen producing microorganism |
| HRT | Hydraulic retention time |
| MS | Mean square |
| Mix | Mixing |
| Mix 1 | 70% orange mixed fruit |
| Mix 2 | 50% orange mixed fruit |
| Mix 3 | 20% orange mixed fruit |
| OLR | Organic loading rate |
| PY | Percent yield |
| SS | Sum of squares |
| SV | Source of variation |
| TS | Total solid |
| TVFA | Total volatile fatty acids |
| VFA | Volatile fatty acids |
| VS | Volatile solid |
Conflicts of Interest
References
- Benemann, J. Hydrogen biotechnology: Progress and prospects. Nat. Biotechnol. 1996, 14, 1101–1103. [Google Scholar] [CrossRef] [PubMed]
- Bockris, J.O.M. The economics of hydrogen as a fuel. Int. J. Hydrog. Energy 1981, 6, 223–241. [Google Scholar] [CrossRef]
- Mormirlan, M.; Veziroglu, T.N. Current status of hydrogen energy. Renew. Sustain. Energy Rev. 2002, 6, 141–179. [Google Scholar] [CrossRef]
- Ros, M.; Franke-Whittle, I.H.; Morales, A.B.; Insam, H.; Ayuso, M.; Pascual, J.A. Archael community dynamics and abiotic characteristics in a mesophilic anaerobic co-digestion process treating fruit and vegetable processing waste sludge with chopped fresh artichoke waste. Bioresour. Technol. 2013, 136, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.; Haruta, S.; Ishii, M.; Igarashi, Y. Microbial community in anaerobic hydrogen-producing microflora enriched from sludge compost. Appl. Microbiol. Biotechnol. 2001, 57, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Kapdan, I.K.; Kargi, F. Biohydrogen production from waste materialk. Enzyme Microb. Technol. 2006, 38, 569–582. [Google Scholar] [CrossRef]
- Levin, D.B.; Chahine, R. Challenges for renewable hydrogen production processes. Int. J. Hydrog. Energy 2008, 33, 279–286. [Google Scholar] [CrossRef]
- Westermann, P.; Jørgensen, B.; Lange, L.; Ahring, B.K.; Christensen, C.H. Maximizing renewable hydrogen production from biomass in a bio/catalytic refinery. Int. J. Hydrog. Energy 2007, 32, 4135–4141. [Google Scholar] [CrossRef]
- Nandi, R.; Sengupta, S. Microbial production of hydrogen: An Overview. Crit. Rev. Microbiol. 1998, 24, 61–84. [Google Scholar] [CrossRef] [PubMed]
- Nursten, H.E.; Williams, A.A. Fruit aromas: A survey of compounds identified. Chem. Ind. 1967, 486–497. [Google Scholar]
- Winniczuk, P.P.; Parish, M.E. Minimum inhibitory concentrations of antimicrobials against micro-organisms related to citrus juice. Food Microbiol. 1997, 14, 373–381. [Google Scholar]
- Jordan, M.J.; Tandon, K.; Shaw, P.E.; Goodner, K.I. Aromatic profile aqueous banana essence and banana fruit by gas-chromatography-mass spectrometry (GC-MS) and gas-chromatography-olfactometry. J. Agric. Food Chem. 2001, 49, 4813–4817. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, J.M.F.; Fernandes, P.J.P.; Nascimento, A.M.D.C. Composition of volatiles of banana cultivars from Madeira island. Phytochem. Anal. 2003, 14, 82–90. [Google Scholar] [CrossRef]
- Rizzolo, J.; Polesello, A.; Teleky-Vamossy, G. CGC/Sensory analysis of volatile compounds developed from ripening apple fruit. J. High Resolut. Chrom. 1989, 12, 824–827. [Google Scholar] [CrossRef]
- Holland, D.; Larkov, O.; Bar-Yaákov, I.; Bar, E.; Zax, A.; Brandeis, E. Developmental and varietal differences in volatile ester formation and acetyl-CoA: Alcohol acetyl transferase activities in apple (Malus domestica Borkh.) fruit. J. Agric. Food Chem. 2005, 53, 7198–7203. [Google Scholar] [CrossRef] [PubMed]
- Tsanova-Savova, S.; Fany, R.; Maria, G. (+)- Catechin and (-) Epicatechin in Bulgarian Fruits. J. Food Comp. Anal. 2005, 18, 691–698. [Google Scholar] [CrossRef]
- Schieber, A.; Petra, K.; Reinhold, C. Determination of phenolic acids and flavonoids of apple and pear by high-performance liquid chromatography. J. Chromatogr. 2000, 910, 265–273. [Google Scholar] [CrossRef]
- Dieguez, S.C.; Lois, L.C.; Gomez, E.F.; de Ia Pena, M.L.G. Aromatic composition of the Vitis vinifera grape Albariño. Lebensm. Wiss. Und. Technol. 2003, 36, 585–590. [Google Scholar] [CrossRef]
- Aubert, C.; Baumann, S.; Arguel, H. Optimisation of the analysis of flavour volatile compounds by liquid-liquid microextraction (LLME). Apllication to the aroma analysis of melons, peaches, grapes, strawberries, and tomatoes. J. Agric. Food Chem. 2005, 53, 8881–8895. [Google Scholar] [CrossRef] [PubMed]
- Perry, P.L.; Wang, Y.; Lin, J.M. Analysis of honeydew melon (Cucumis melo var. Inodorus) flavor and GC/MS identification of (E,Z)-2,6-nonadienyl acetate. Flav. Frag. J. 2009, 24, 341–347. [Google Scholar] [CrossRef]
- Portnoy, V.; Benyamini, Y.; Bar, E. The molecular and biochemical basis for varietal variation in sesquiterpene content in melon (Cucumis melo L.) rinds. Plant Mol. Biol. 2008, 66, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Aubert, C.; Pitrat, M. Volatile compounds in the skin and pulp of Queen Anne’s pocket melon. J. Agric. Food Chem. 2006, 54, 8177–8182. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.H. Handbook of Fruit and Vegetable Flavours; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Utama, I.; Made, S.; Wills, R.B.H.; Ben-yehoshua, S.; Kuek, C. In vitro efficacy of plant volatiles for inhibiting the growth of fruit and vegetable decay microorganisms. J. Agric. Food Chem. 2002, 50, 6371–6377. [Google Scholar] [CrossRef]
- Grohmann, K.; Baldwin, E.; Buslig, B. Production of ethanol from enzymatically hydrolyzed orange peel by the yeast Saccharomyces cerevisiae. Appl. Biochem. Biotechnol. 1994, 45–46, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Mizuki, E.; Akao, T.; Saruwatari, T. Inhibitory effect of citrus Unshu peel om anaerobic digestion. Biol. Wastes 1990, 33, 161–168. [Google Scholar] [CrossRef]
- Martin, M.A.; Siles, J.A.; China, A.F.; Martin, A. Biomethanization of orange peel waste. Bioresour. Technol. 2010, 101, 8993–8999. [Google Scholar] [CrossRef] [PubMed]
- Youngsukkasem, S.; Akinbomi, J.; Rakshit, S.; Taherzadeh, M.J. Biogas production by encased bacteria in synthetic membranes: Protective effects in toxic media and high loading rates. Environ. Technol. 2013, 34, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Wikandari, R.; Youngsukkasem, S.; Millati, R.; Taherzadeh, M.J. Performance of semi-continuous membrane bioreactors in biogas production from toxic feedstock containing D-limonene. Bioresour. Technol. 2014, 170, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhang, R.; el-Mashad, H.M.; Sun, H.; Yimg, Y. Effect of food to microorganism ratio on biohydrogen production from food waste via anaerobic fermentation. Int. J. Hydrog. Energy 2008, 33, 6968–6975. [Google Scholar] [CrossRef]
- Liu, D.; Zeng, R.J.; Angelidaki, I. Hydrogen and methane production from household solid waste in the two-stage fermentation process. Water Res. 2006, 40, 2230–2236. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Han, S.K.; Shin, H.S. Optimization of continuous hydrogen fermentation of food waste as a function of solids retention time independent of hydraulic retention time. Process Biochem. 2008, 43, 213–218. [Google Scholar] [CrossRef]
- Chu, C.F.; Xu, K.Q.; Li, Y.Y.; Inamori, Y. Hydrogen and methane potential based on the nature of food waste materials in a two-stage thermophilic fermentation process. Int. J. Hydrog. Energy 2012, 37, 10611–10618. [Google Scholar] [CrossRef]
- Lin, C.; Lay, C. A nutrient formulation for fermentative hydrogen production using anaerobic sewage sludge microflora. Int. J. Hydrog. Energy 2005, 30, 285–292. [Google Scholar] [CrossRef]
- Demirel, B.; Scherer, P. Trace element requirements of agricultural biogas digesters during biological conversion of renewable biomass to methane. Biomass Bioenergy 2011, 35, 992–998. [Google Scholar] [CrossRef]
- Demirel, B.; Scherer, P. Production of methane from sugar beet sludge without manure addition by a single stage anaerobic digestion process. Biomass Bioenergy 2008, 32, 203–209. [Google Scholar] [CrossRef]
- Pobeheim, H.; Munk, B.; Johansson, J.; Guebitz, G.M. Influence of trace elements on methane formation from a synthetic model substrate for maize silage. Bioresour. Technol. 2010, 101, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Thauer, R.K.; Jungermann, K.; Decker, K. Energy conservation in chemotrophic anaerobic bacteria. Microbiol.Mol. Biol. Rev. 1977, 41, 100–180. [Google Scholar]
- Dohanyos, M.; Kosova, B.; Zabranska, J.; Grau, P. Production and utilization of volatile fatty acids in various types of anaerobic reactors. Water Sci. Technol. 1985, 17, 191–205. [Google Scholar]
- Vardar, S.G.; Maeda, T.; Wood, T.K. Metabolically engineered bacteria for producing hydrogen via fermentation. Microb. Biotechnol. 2008, 1, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Thauer, R.K. Limitation of microbial H2-formation via fermentation. In Microbial Energy Conversion; Schlegel, H.G., Barnea, J., Eds.; Pergamon Press: New York, NY, USA, 1977. [Google Scholar]
- Hawkes, F.R.; Hussy, I.; Kyazze, G.; Dinsdale, R.; Hawkes, D.L. Continuous dark fermentative hydrogen production by mesophilic microflora: Principles and progress. Int. J. Hydrog. Energy 2007, 32, 172–184. [Google Scholar] [CrossRef]
- Hawkes, F.R.; Dinsdale, R.; Hawkes, D.L.; Hussy, I. Sustainable fermentative hydrogen production: Challenges for process optimisation. Int. J. Hydrog. Energy 2002, 27, 1339–1347. [Google Scholar] [CrossRef]
- Ren, N.; Li, J.; Li, B.; Wang, Y.; Liu, S. Biohydrogen production from molasses by anaerobic fermentation with a pilot-scale bioreactor system. Int. J. Hydrog. Energy 2006, 31, 2147–2157. [Google Scholar] [CrossRef]
- Buyukkamaci, N.; Filibeli, A. Volatile fatty acid formation in an anaerobic hybrid reactor. Process Biochem. 2004, 39, 1491–1494. [Google Scholar] [CrossRef]
- Hallenbeck, P.C.; Benemann, J.R. Biological hydrogen production: Fundamentals and limiting processes. Int. J. Hydrog. Energy 2002, 27, 1185–1193. [Google Scholar] [CrossRef]
- Levin, D.B.; Pitt, L.; Love, M. Biohydrogen production: Prospects and limitations to practical application. Int. J. Hydrog. Energy 2004, 29, 173–185. [Google Scholar] [CrossRef]
- Polprasert, C. Organic Waste Recycling: Technology and Management; John Wiley & Sons: Chichester, UK, 1996. [Google Scholar]
- American Public Health Association Inc. (APHA). Standard Methods for the Examination of Water and Wastewater, 20th ed.; APHA: Washington, DC, USA, 1998. [Google Scholar]
- USDA National Nutrient Database for Standard Reference, Release 27; US Department of Agriculture, Agricultural Research Service, Nutrient Data Laboratory: Beltsville, MD, USA, August 2014.
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akinbomi, J.; Taherzadeh, M.J. Evaluation of Fermentative Hydrogen Production from Single and Mixed Fruit Wastes. Energies 2015, 8, 4253-4272. https://doi.org/10.3390/en8054253
Akinbomi J, Taherzadeh MJ. Evaluation of Fermentative Hydrogen Production from Single and Mixed Fruit Wastes. Energies. 2015; 8(5):4253-4272. https://doi.org/10.3390/en8054253
Chicago/Turabian StyleAkinbomi, Julius, and Mohammad J. Taherzadeh. 2015. "Evaluation of Fermentative Hydrogen Production from Single and Mixed Fruit Wastes" Energies 8, no. 5: 4253-4272. https://doi.org/10.3390/en8054253
APA StyleAkinbomi, J., & Taherzadeh, M. J. (2015). Evaluation of Fermentative Hydrogen Production from Single and Mixed Fruit Wastes. Energies, 8(5), 4253-4272. https://doi.org/10.3390/en8054253