LaNi5-Assisted Hydrogenation of MgNi2 in the Hybrid Structures of La1.09Mg1.91Ni9D9.5 and La0.91Mg2.09Ni9D9.4
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
3.1. XRD Characterization of the Initial Intermetallic Alloys La0.91Mg2.09Ni9 and La1.09Mg1.91Ni9
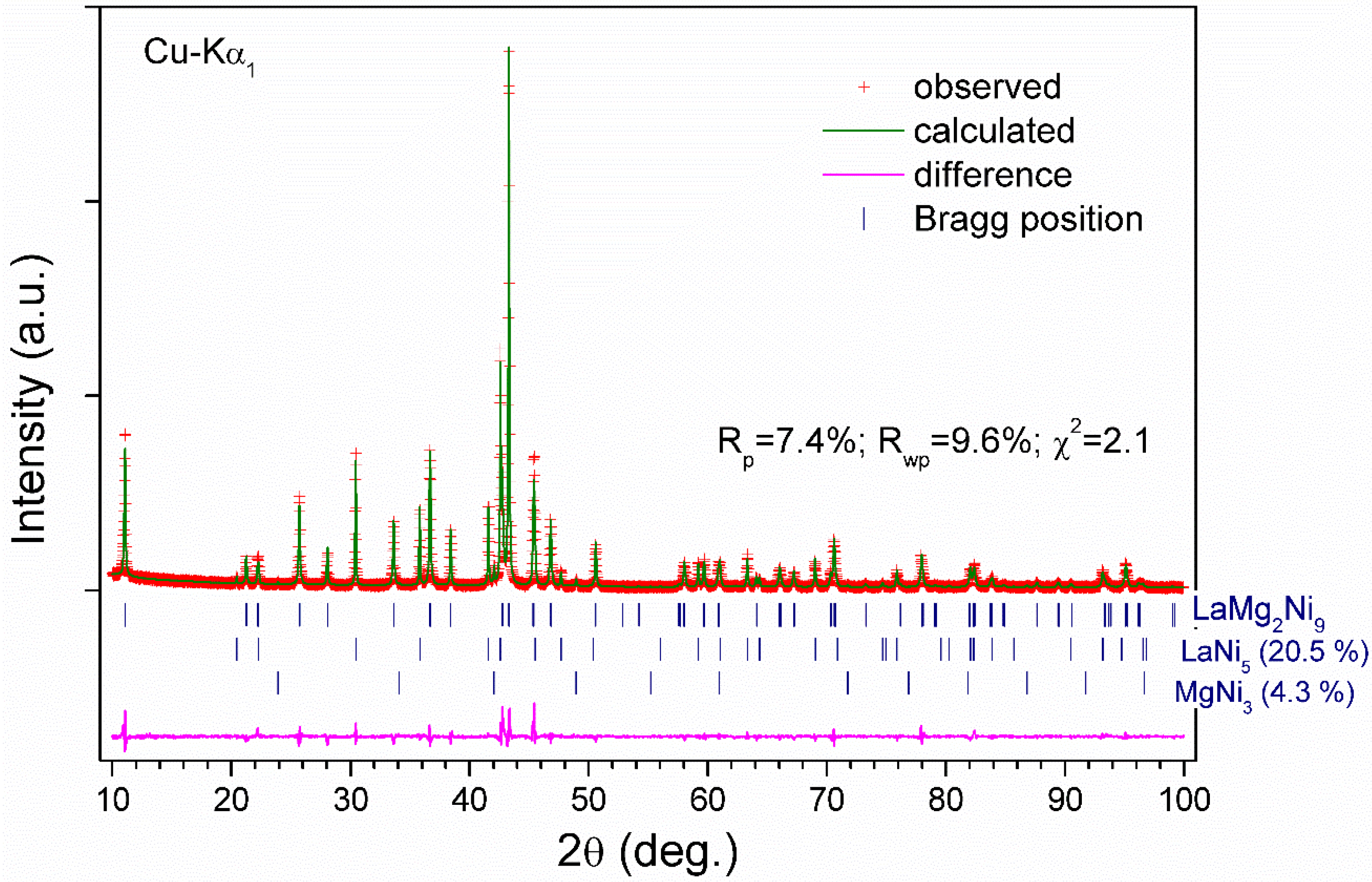
| Alloy | Sample 1 | Sample 2 |
|---|---|---|
| Source of experimental data | SR XRD collected at BM01B, SNBL using a wavelength λ = 0.5009(1) Å | Siemens D5000 diffractometer, Cu Kα1 radiation |
| Composition of AB3 phase | La1.09(1)Mg1.91(1)Ni9 | La0.91(1)Mg2.09(1)Ni9 |
| Unit cell parameters: | ||
| a (Å) | 4.94024(8) | 4.8986(1) |
| c (Å) | 23.8188(4) | 23.957(1) |
| V (Å3) | 503.44(1) | 497.86(2) |
| Atomic parameters: | ||
| La1/Mg1 in 3a (0, 0, 0) | ||
| Uiso×100 (Å2) | 0.43(5) | 2.1(2) |
| nMg, (nLa = 1–nMg) | 0.0(–) | 0.09(1) |
| La2/Mg2 in 6c (0, 0, z) | ||
| z | 0.1453(3) | 0.1471(6) |
| Uiso×100 (Å2) | 1.2(3) | 0.5(3) |
| nMg, (nRE = 1–nMg) | 0.954(5) | 1.0(–) |
| Ni1 in 3b (0, 0, ½) Uiso × 100 (Å2) | 0.7(1) | 0.8(3) |
| Ni2 in 6c (0, 0, z) | ||
| z | 0.3335(2) | 0.3334(4) |
| Uiso×100 (Å2) | 0.13(8) | 1.8(3) |
| Ni3 in 18h (x, –x, z) | ||
| x | 0.5009(3) | 0.5014(6) |
| z | 0.08529(8) | 0.0854(2) |
| Uiso × 100 (Å2) | 0.57(5) | 1.4(2) |
| R-factors of refinements | ||
| Rp | 8.9 | 7.4 |
| Rwp | 11.9 | 9.6 |
| χ2 | 2.0 | 2.1 |
| Impurity phases | LaNi5 7.8(2) wt% MgNi2 12.0(2) wt% | LaNi5 20.5(2) wt% MgNi3 4.2(3) wt% |
3.2. Thermodynamics of the (La,Mg)3Ni9—H2 systems
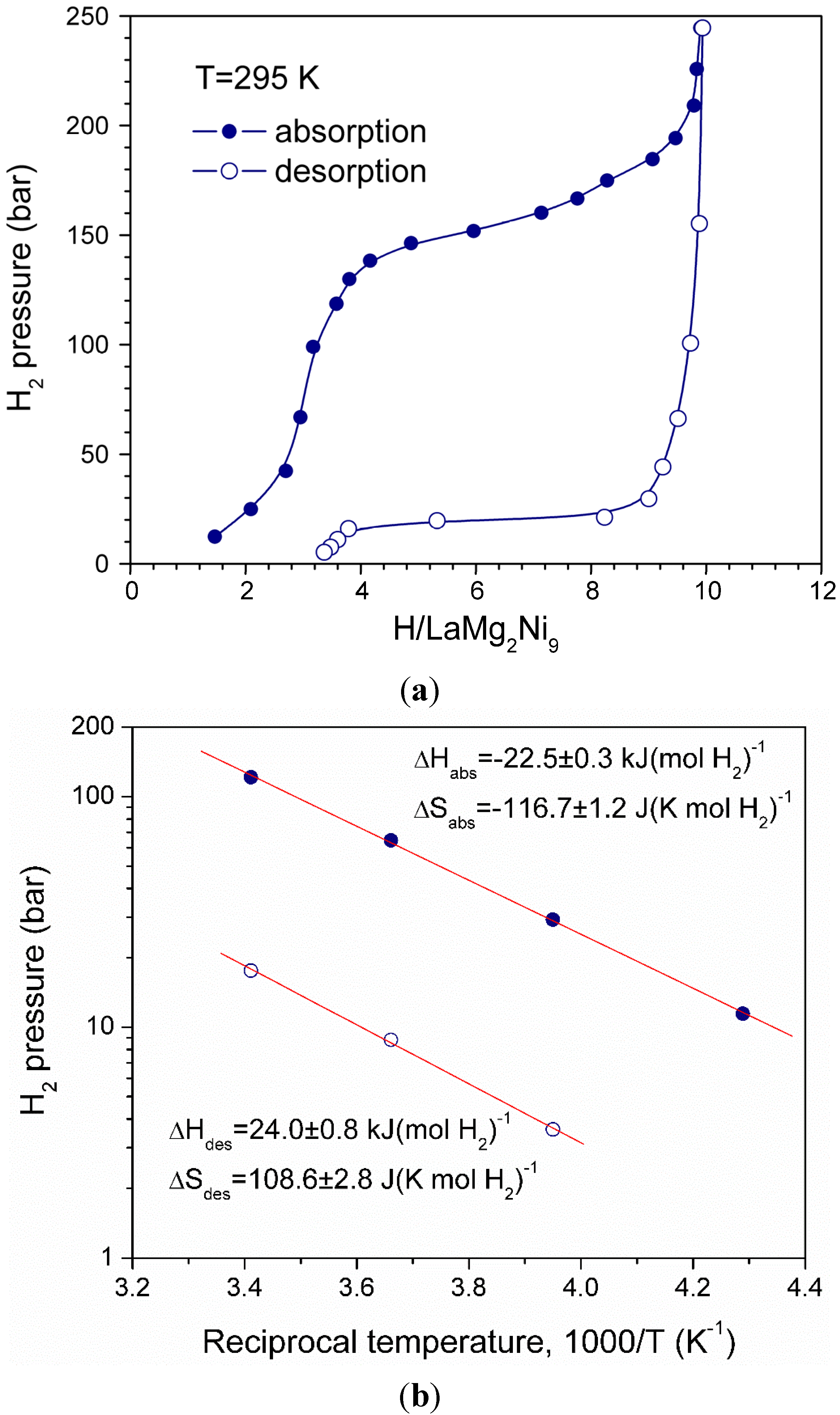
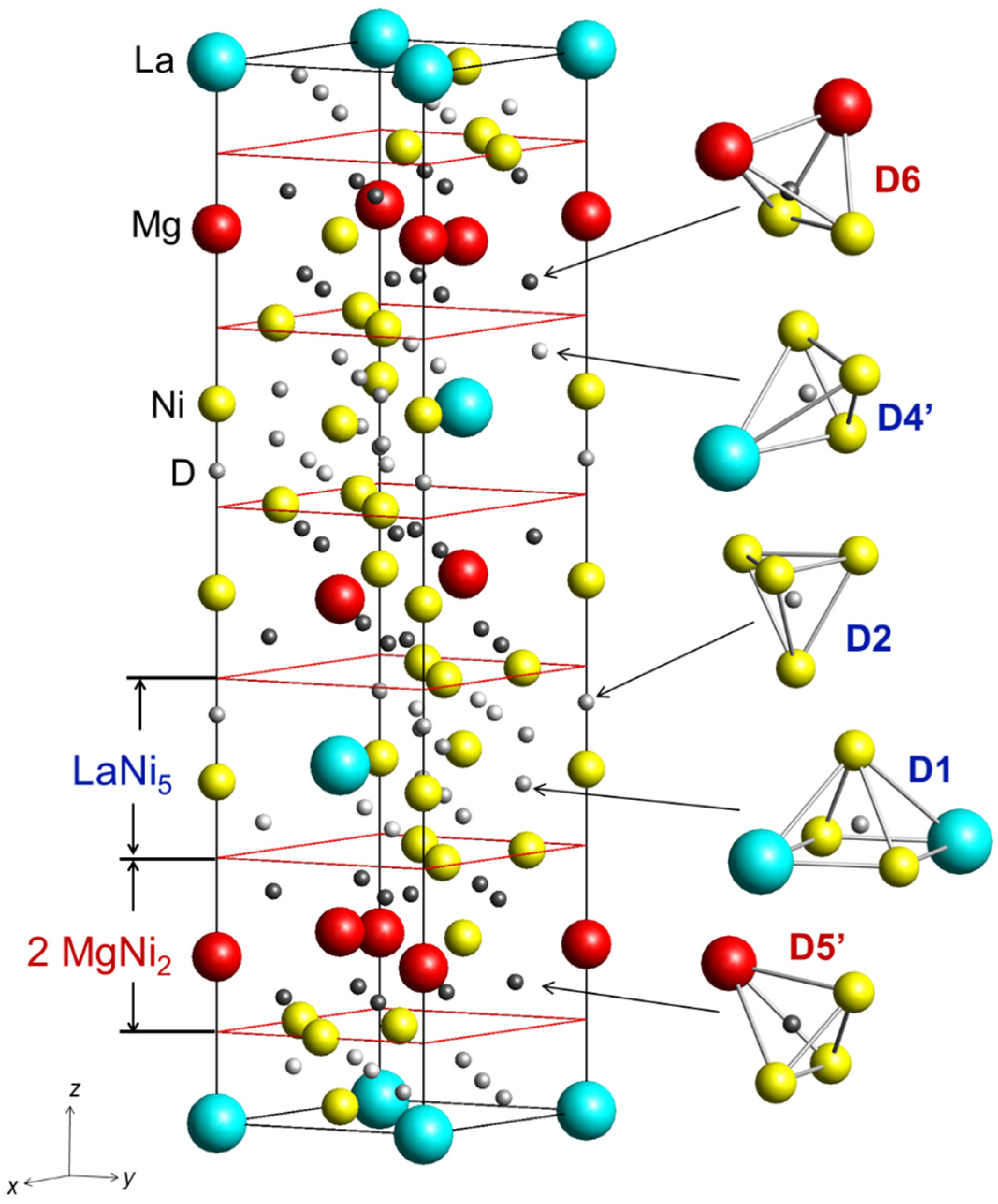
3.3. In situ NPD studies
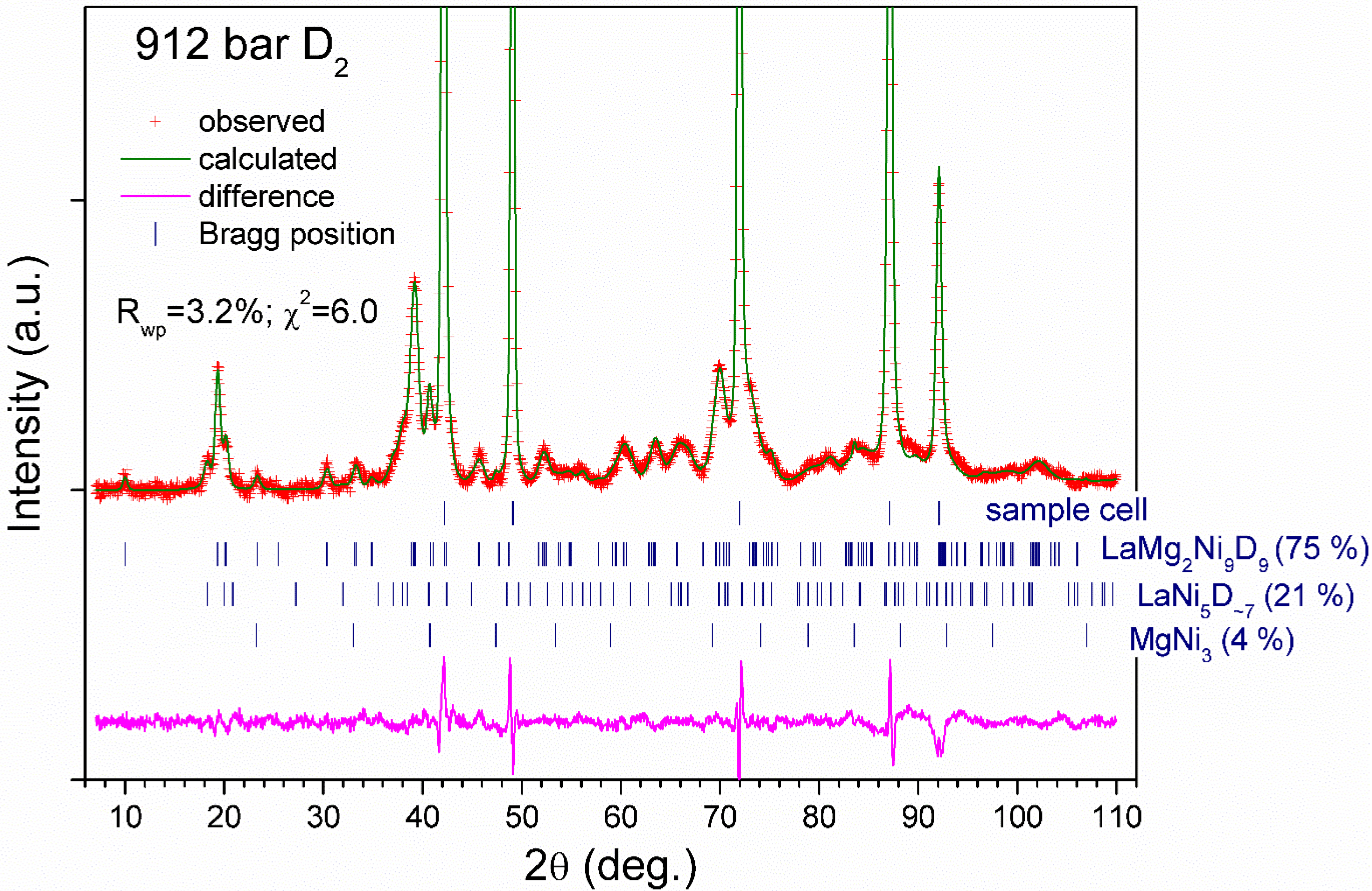
| Deuteride | La1.09Mg1.91Ni9D9.5(5) | La0.91Mg2.09Ni9D9.4(6) |
|---|---|---|
| Conditions | 25 bar at 25 °C (prepared at −30 °C) | 912 bar at 25 °C |
| Unit cell parameters: | ||
| a (Å) | 5.263(1) | 5.212(1) |
| c (Å) | 25.803(9) | 25.71(1) |
| V (Å3) | 618.9(3) | 604.8(3) |
| Unit cell parameters: | ||
| Δa/a (%) | 6.5 | 6.4 |
| Δc/c (%) | 8.3 | 7.3 |
| ΔV/V (%) | 23.0 | 21.6 |
| ΔV/V[LaNi5] (%) | 20.4 | 20.7 |
| ΔV/V[MgNi2] (%) | 25.4 | 22.2 |
| Atomic parameters: | ||
| La1/Mg1 in 3a (0, 0, 0) nMg, (nLa = 1–nMg) | 0.0(–) | 0.09(–) |
| La2/Mg2 in 6c (0, 0, z) z Uiso × 100 (Å2) nMg, (nRE = 1–nMg) | 1.0(–) 0.95(–) | 1.0(–) 1.0(–) |
| Ni1 in 3b (0, 0, ½) Uiso × 100 (Å2) | 1.0(–) | 1.0(–) |
| Ni2 in 6c (0, 0, z) z Uiso × 100 (Å2) | 0.3279(7) 1.0(–) | 0.3220(6) 1.0(–) |
| Ni3 in 18h (x, –x, z) x z Uiso × 100 (Å2) | 0.498(1) 0.0871(4) 1.0(–) | 0.506(1) 0.0859(3) 1.0(–) |
| D1 in 18h (x, –x, z) x z n | 0.484(4) 0.023(1) 0.33(1) | 0.496(3) 0.023(1) 0.31(2) |
| D2 in 6c (0, 0, z) z n | 0.390(1) 0.50(3) | 0.385(1) 0.58(3) |
| D4’ in 18h (x, –x, z) x z n | 0.814(3) 0.0626(9) 0.43(2) | 0.792(2) 0.051(1) 0.33(3) |
| D5’ in 18h (x, –x, z) x z n | 0.201(2) 0.120(1) 0.45(2) | 0.192(3) 0.123(1) 0.35(2) |
| D6 in 18h (x, –x, z) x z n | 0.819(4) 0.117(1) 0.20(2) | 0.819(4) 0.117(1) 0.39(2) |
| Uiso × 100 (Å2) for D1-D6 | 2.0(–) | 2.0(–) |
| Atomic parameters: | ||
| D distribution in the structure LaNi5 2 MgNi2 | 5.6(3) 3.9(2) | 5.0(4) 4.4(2) |
| Shortest Metal—Hydrogen distances, Å La…D Mg…D Ni…D | 2.34(3) 1.97(3) 1.56(3) | 2.29(2) 1.93(2) 1.53(2) |
| R-factors of refinements Rp Rwp χ2 | 2.7 3.4 5.0 | 2.4 3.2 6.0 |
| Secondary constituents | α-solid solution La0.9Mg2.1Ni9D0.9. Sp.gr. Rm; a = 4.9459(2); c = 23.842(2) Å; V = 505.10(4). 0.3 D in D3 18h (0.15, 0.3, 0.085) and 0.6 D in D4 18h (0.3, 0.15, 0.085); 35.7(2) wt% LaNi5D7; Sp.gr. P63mc; a = 5.438(3), c= 8.598(5) Å; V = 220.3(2) Å3; 4.6(3) wt%. Atomic structure was taken from [3]. MgNi2; MgNi2 structure type; Sp.gr. P63/mmc; a = 4.8356(4), c = 15.850(3) Å; V = 320.97(5) Å3; 12.4(2) wt%. Atomic structure was taken from [4]. Sample holder: stainless steel; Sp.gr. Fm; a = 3.598 Å. | LaNi5D7; Sp.gr. P63mc; a = 5.430(1), c = 8.606(4) Å; V = 219.8(2) Å3; 21.5(5) wt%. Atomic structure was taken from [3]. MgNi3; AuCu3 structure type; Sp.gr. Pmm; a = 3.7185 Å; 1 Mg in 1a: 0, 0, 0; 3 Ni in 3c: 1/2, 1/2, 0; 3.7(2) wt%. Sample holder: zero matrix TiZr alloy with Fe liner. The peaks from Fe liner are only observed. Sp.gr. Fm; a = 3.5949(1) Å. |
4. Conclusions
- (a)
- significant decrease of the stability of the LaNi5-type hydride;
- (b)
- much easier hydrogenation of the MgNi2 slabs compared to the parent intermetallic compound;
- (c)
- increased hysteresis.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Denys, R.V.; Yartys, V.A. Effect of magnesium on crystal structure and thermodynamics of the La3−xMgxNi9 hydrides. J. Alloys Compd. 2011, 509 (Suppl. 2), S540–S548. [Google Scholar] [CrossRef]
- Denys, R.V.; Yartys, V.A.; Webb, C.J. LaNi5-assisted hydrogenation of MgNi2 in the hybrid structure of LaMg2Ni9D9.5. In Proceedings of the International Symposium on Metal-Hydrogen Systems MH2012, Fundamental and Applications, Kyoto, Japan, 21–26 October 2012. Poster Presentation. MoP38. Collected Abstracts. P.92.
- Lartigue, C.; Percheron Guégan, A.; Achard, J.C.; Soubeyroux, J.L. Hydrogen (deuterium) ordering in the β-LaNi5Dx phases: A neutron diffraction study. J. Less-Common. Met. 1985, 113, 127–148. [Google Scholar] [CrossRef]
- Yartys, V.A.; Antonov, V.E.; Beskrovnyi, A.I.; Crivello, J.-C.; Denys, R.V.; Fedotov, V.K.; Gupta, M.; Kulakov, V.I.; Kuzovnikov, M.A.; Latroche, M.; et al. Hydrogen assisted phase transition in a trihydride MgNi2H3 synthesised at high H2 pressures: thermodynamics, crystallographic and electronic structures. Acta Mater. 2015, 82, 316–327. [Google Scholar] [CrossRef]
- Denys, R.V.; Yartys, V.A.; Webb, C.J. Hydrogen in La2MgNi9D13. The role of magnesium. Inorg. Chem. 2012, 51, 4231–4238. [Google Scholar] [CrossRef]
- Nwakwuo, C.C.; Holm, T.H.; Denys, R.V.; Hu, W.; Maehlen, J.P.; Solberg, J.K.; Yartys, V.A. Effect of magnesium content and quenching rate on the phase structure and composition of rapidly solidified La2MgNi9 metal hydride battery electrode alloy. J. Alloys Compd. 2013, 555, 201–208. [Google Scholar] [CrossRef]
- Hu, W.-K.; Denys, R.V.; Nwakwuo, C.C.; Holm, T.H.; Maehlen, J.P.; Solberg, J.K.; Yartys, V.A. Annealing effect on phase composition and electrochemical properties of the Co-free La2MgNi9 anode for Ni-Metal Hydride batteries. Electrochim. Acta 2013, 96, 27–33. [Google Scholar] [CrossRef]
- Latroche, M.; Cuevas, F.; Hu, W.-K.; Sheptyakov, D.; Denys, R.V.; Yartys, V.A. Mechanistic and kinetic study of the electrochemical charge and discharge of La2MgNi9 by in situ powder neutron diffraction. J. Phys. Chem. C 2014, 118, 12162–12169. [Google Scholar] [CrossRef]
- Gabis, I.E.; Evard, E.A.; Voyt, A.P.; Kuznetsov, V.G.; Tarasov, B.P.; Crivello, J.-C.; Latroche, M.; Denys, R.V.; Hu, W.; Yartys, V.A. Modeling of metal hydride battery anodes at high discharge current densities. Electrochim. Acta 2014, 147, 73–81. [Google Scholar] [CrossRef]
- Yartys, V.A.; Denys, R.V. Structure-properties relationship in RE3−xMgxNi9H10−13 (RE = La, Pr, Nd) hydrides for energy storage. J. Alloys Compd. 2015. [Google Scholar] [CrossRef]
- Yartys, V.A.; Denys, R.V. Thermodynamics and crystal chemistry of the RE2MgNi9H12−13 (RE=La and Nd) hydrides. Chem. Met. Alloys 2014, 7, 1–8. [Google Scholar]
- Larson, A.C.; Dreele, R.B.V. General structure analysis system (GSAS). In Los Alamos National Laboratory Report LAUR; 2000; Alamos National Lab: Los Alamos, NM, USA, 2000; pp. 86–748. [Google Scholar]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B: Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Liu, G.; Xi, S.; Ran, G.; Zuo, K.; Li, P.; Zhou, J. A new phase MgNi3 synthesized by mechanical alloying. J. Alloys Compd. 2008, 448, 206–209. [Google Scholar] [CrossRef]
- Kadir, K.; Yamamoto, H.; Sakai, T.; Uehara, I.; Kanehisa, N.; Kai, Y.; Eriksson, L. LaMg2Ni9, an example of the new AB2C9 structure type. Acta Crystallogr. 1999, C55. [Google Scholar] [CrossRef]
- De Negri, S.; Giovannini, M.; Saccone, A. Phase relationships of the La–Ni–Mg system at 500 °C from 66.7 to 100 at% Ni. J. Alloys Compd. 2005, 397, 126–134. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denys, R.V.; Yartys, V.A.; Gray, E.M.; Webb, C.J. LaNi5-Assisted Hydrogenation of MgNi2 in the Hybrid Structures of La1.09Mg1.91Ni9D9.5 and La0.91Mg2.09Ni9D9.4. Energies 2015, 8, 3198-3211. https://doi.org/10.3390/en8043198
Denys RV, Yartys VA, Gray EM, Webb CJ. LaNi5-Assisted Hydrogenation of MgNi2 in the Hybrid Structures of La1.09Mg1.91Ni9D9.5 and La0.91Mg2.09Ni9D9.4. Energies. 2015; 8(4):3198-3211. https://doi.org/10.3390/en8043198
Chicago/Turabian StyleDenys, Roman V., Volodymyr A. Yartys, Evan MacA. Gray, and Colin J. Webb. 2015. "LaNi5-Assisted Hydrogenation of MgNi2 in the Hybrid Structures of La1.09Mg1.91Ni9D9.5 and La0.91Mg2.09Ni9D9.4" Energies 8, no. 4: 3198-3211. https://doi.org/10.3390/en8043198
APA StyleDenys, R. V., Yartys, V. A., Gray, E. M., & Webb, C. J. (2015). LaNi5-Assisted Hydrogenation of MgNi2 in the Hybrid Structures of La1.09Mg1.91Ni9D9.5 and La0.91Mg2.09Ni9D9.4. Energies, 8(4), 3198-3211. https://doi.org/10.3390/en8043198






