Heat Recovery from High Temperature Slags: A Review of Chemical Methods
Abstract
:1. Introduction
2. Challenges for Heat Recovery
2.1. Low Thermal Conductivity
| Constraints | Details | Solutions | References |
|---|---|---|---|
| Low thermal conductivity | 1 W/(m·K) for solid slags; 0.1 W/(m·K) for liquid slags | Dry granulation into small particles/droplets | [7,8,21,22,23,24,25,26] |
| Easy crystallization trend | Large temperature difference → Easy inside crystallization trend | Small particles; Water quenching | [7,8,29,30] |
| Discontinuous availability | Temperature discontinuity; Production discontinuity | Chemical methods; Phase change materials | [7,8,20,31,32] |
2.2. Crystallization Behaviors
2.3. Discontinuous Availability
3. Chemical Reactions in Detail
3.1. Fuel Gases Production
3.1.1. Methane Decomposing Reactions
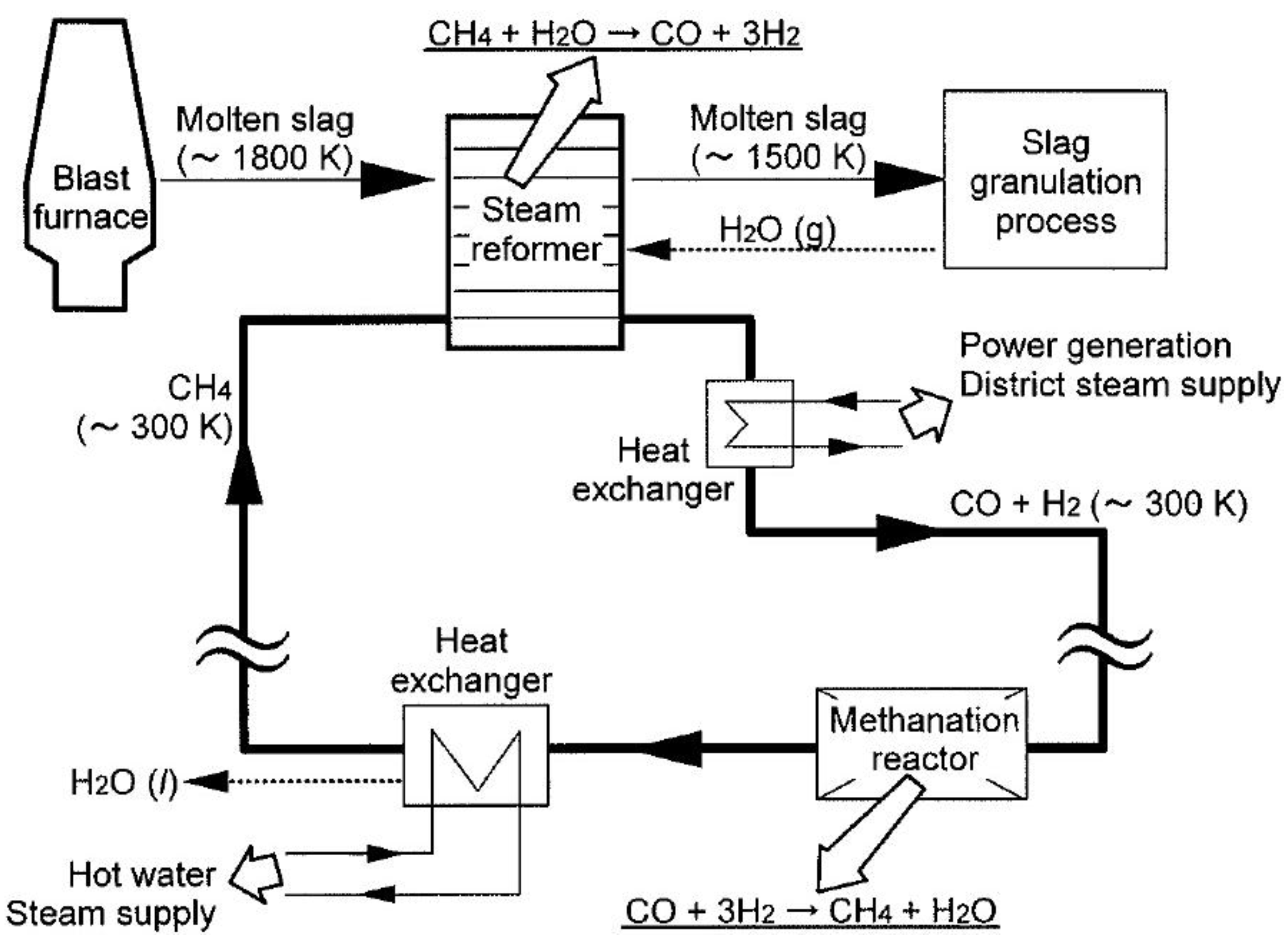
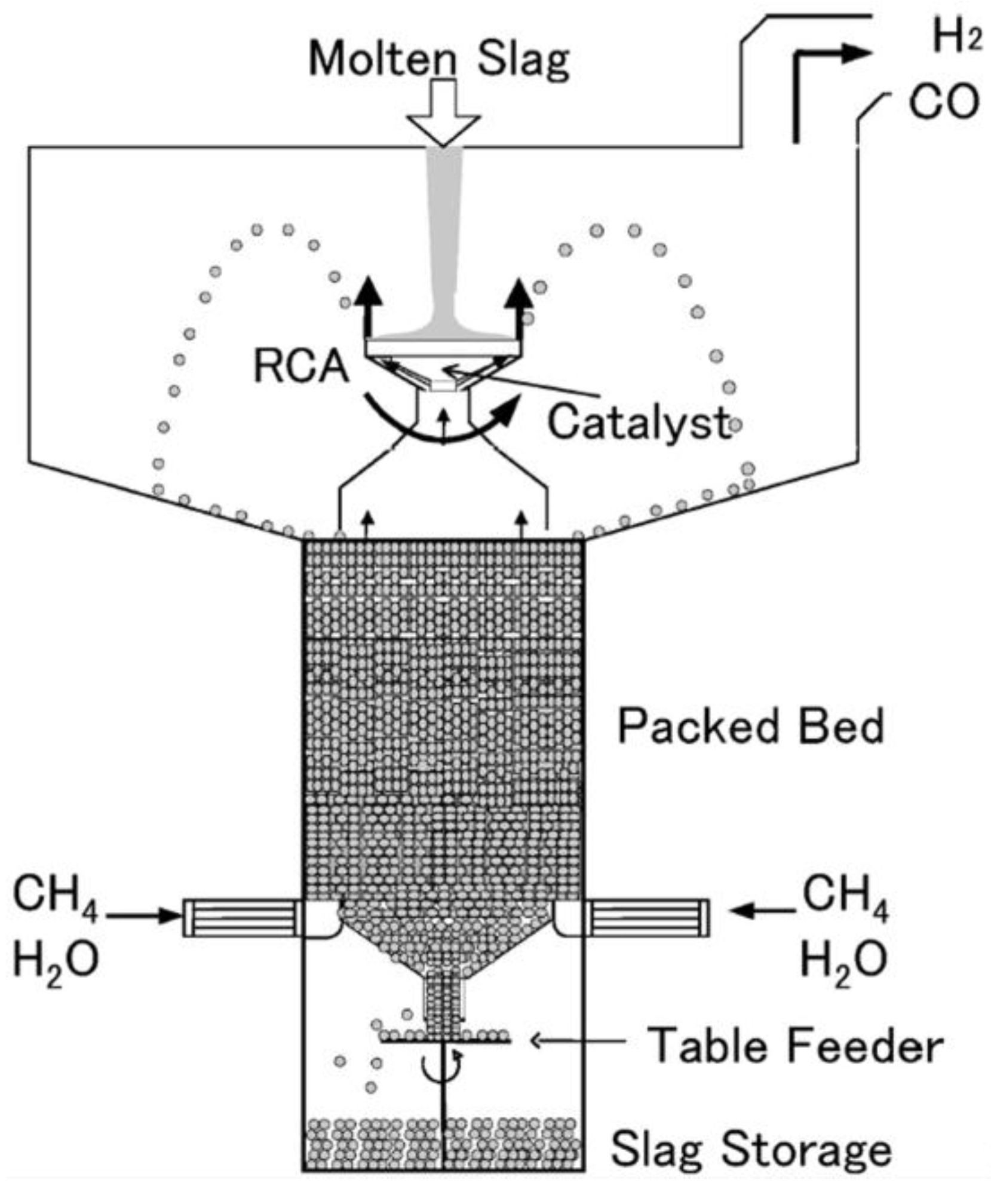
3.1.2. Pyrolysis and Gasification of Coal
3.1.3. Pyrolysis and Gasification of Solid Wastes

3.1.4. Pyrolysis and Gasification of Biomass
3.1.5. Reduction of the Slag Compositions
3.2. Other Chemical Methods
3.2.1. Production of Slag Wool
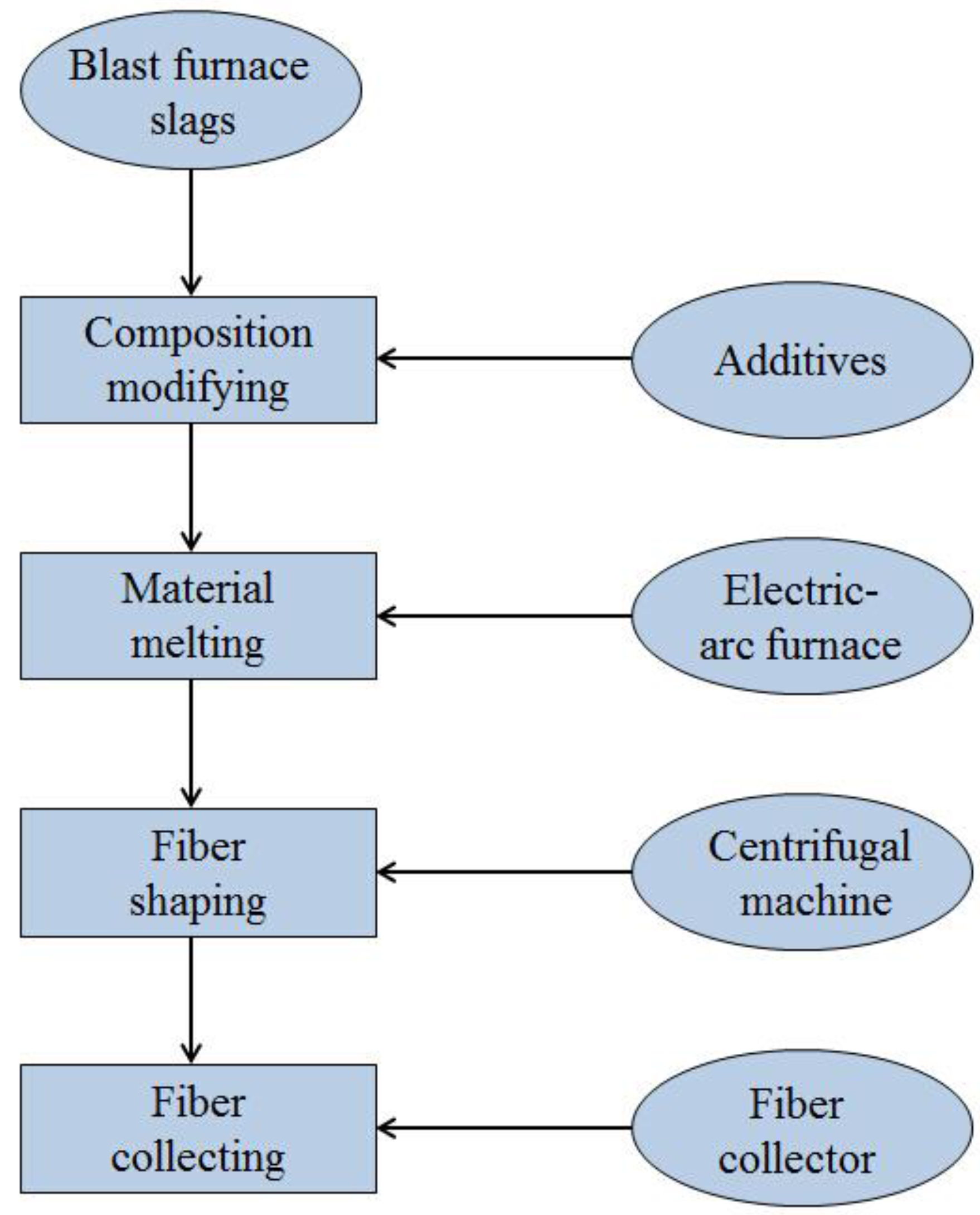
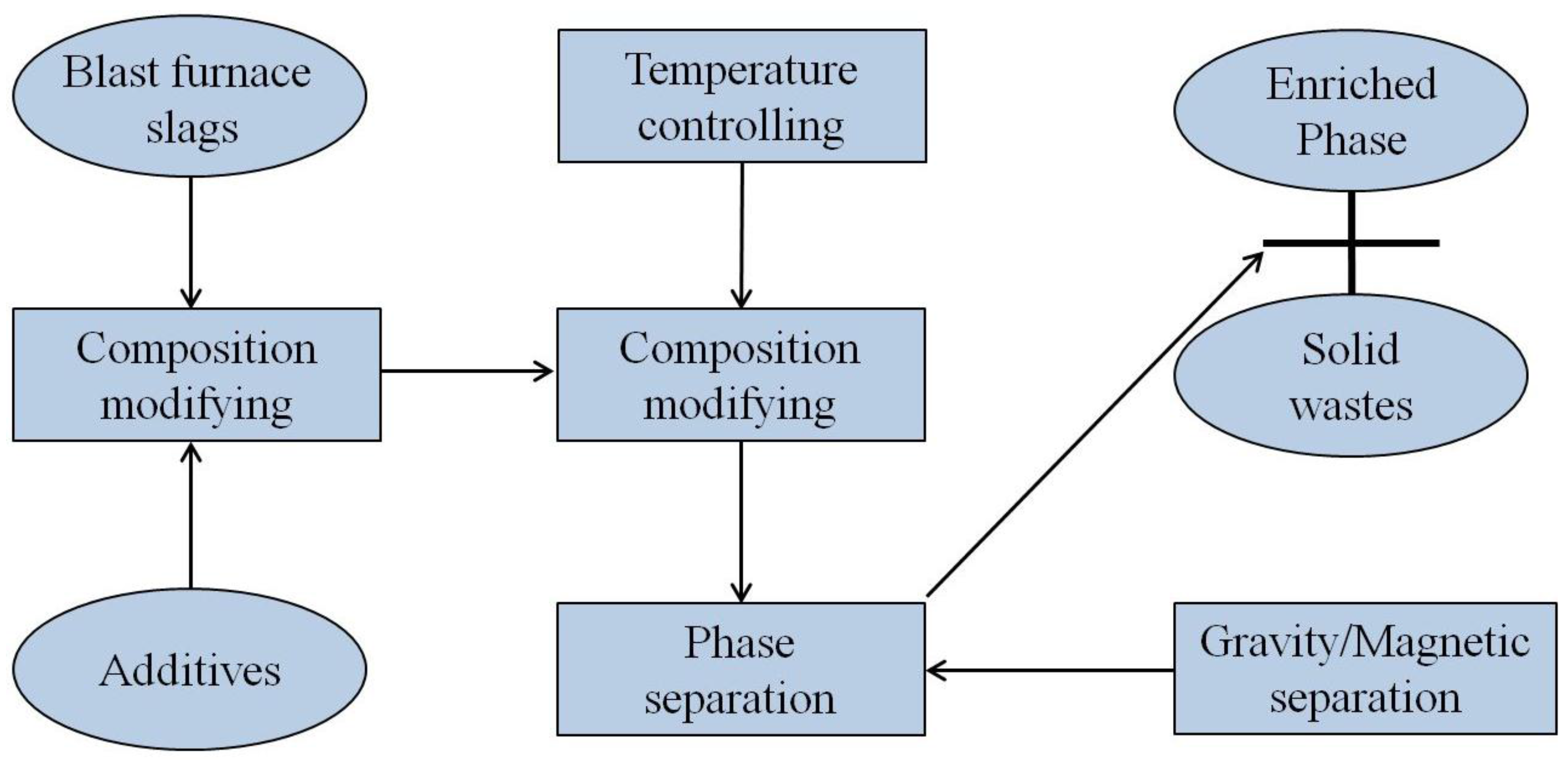
3.2.2. Selective Crystallization and Phase Separation
4. Analysis of These Chemical Methods
| Reaction Type | Reaction Equation | Temperature (K) | Agent | Slag Type | Reaction Role of Slag | References |
|---|---|---|---|---|---|---|
| Methane-steam gasification | CH4 + H2O = CO + 3H2 | 1473–1823 | H2O(g) | Blast furnace slag | Heat carrier, catalyst | [33,36,37,41] |
| Methane-CO2 decomposition | CH4 + CO2 = 2CO + 2H2 | 973–1273 | CO2 | Blast furnace slag | Heat carrier, catalyst | [44] |
| Coal pyrolysis | Tar→H2 + CO + CO2 + other light hydrocarbon + C | 773–1173 | Blast furnace slag, steel slag | Heat carrier, catalyst | [54,57] | |
| Coal-steam gasification | C + H2O = CO + H2 | 1048 | H2O(g) | Blast furnace slag | Heat carrier | [53] |
| Coal-CO2 gasification | C + CO2 = 2CO | 1223–1423, 1573–1773 | CO2 | Blast furnace slag | Heat carrier, catalyst | [47,48,49,50,51] |
| Gasification of municipal solid waste | - | 873–1173 | N2, air, steam | Blast furnace slag | Heat carrier, catalyst | [60] |
| Pyrolysis of printed circuit boards | CmHnO → CaHbOc + CO + H2 + CH4 + CO2 | 873–1173 | - | Blast furnace slag | Heat carrier | [61] |
| Biomass pyrolysis | CmHnOx → aCO2 + bH2O + cCO + dCH4 + fC2+ | 773–1023 | - | Blast furnace slag | Heat carrier | [65] |
| Biomass gasification | CmHnOx + H2O → aCO2 + bH2O + cCO + dCH4 + fC2+ | 1073–1473, 523–773 | H2O(g), H2O(g)-O2 | Blast furnace slag | Heat carrier, catalyst | [64,66] |
| FeO reduction | 2FeO + H2O = Fe2O3 + H2 3FeO + H2O = Fe3O4 + H2 | 1723, 1873–1973, 473–673 | H2O(g) | Steel slag | Heat carrier, reactant | [68,69,70] |
| V2O3 reduction | 3CaO + V2O3 + 2CO2 = (CaO)3(V2O5) + 2CO 3CaO + V2O3 + 2H2O = (CaO)3(V2O5) + 2H2 | 1678–1733 | CO2, H2O(g) | CaO-rich metallurgical slag; V2O3-rich gasifier slag | Heat carrier, reactant | [71] |
5. Conclusions
- (1)
- The basic challenges that restrict the waste heat recovery from molten slags are the low thermal conductivity, the inside crystallization, and the discontinuous time-temperature availability;
- (2)
- Many chemical reactions have been utilized in the waste heat recovery, including fuel gas production and direct modification of slag compositions to generate high value products;
- (3)
- Fuel gas production is the main R&D trend because of the strong demand for alternative and renewable fuels in modern society, such as the production of combustible gas by coal gasification, biomass gasification and methane reforming;
- (4)
- The role of slags in these chemical reactions can be that of heat carrier, catalyst and reactant, and especially that the latter two roles expand the field of utilization of molten slags;
- (5)
- Direct utilization of the waste heat and material resources of the slags has also been developed in the past decades, such as the slag wool production by compositional modification and mineral extraction by the SCPS method;
- (6)
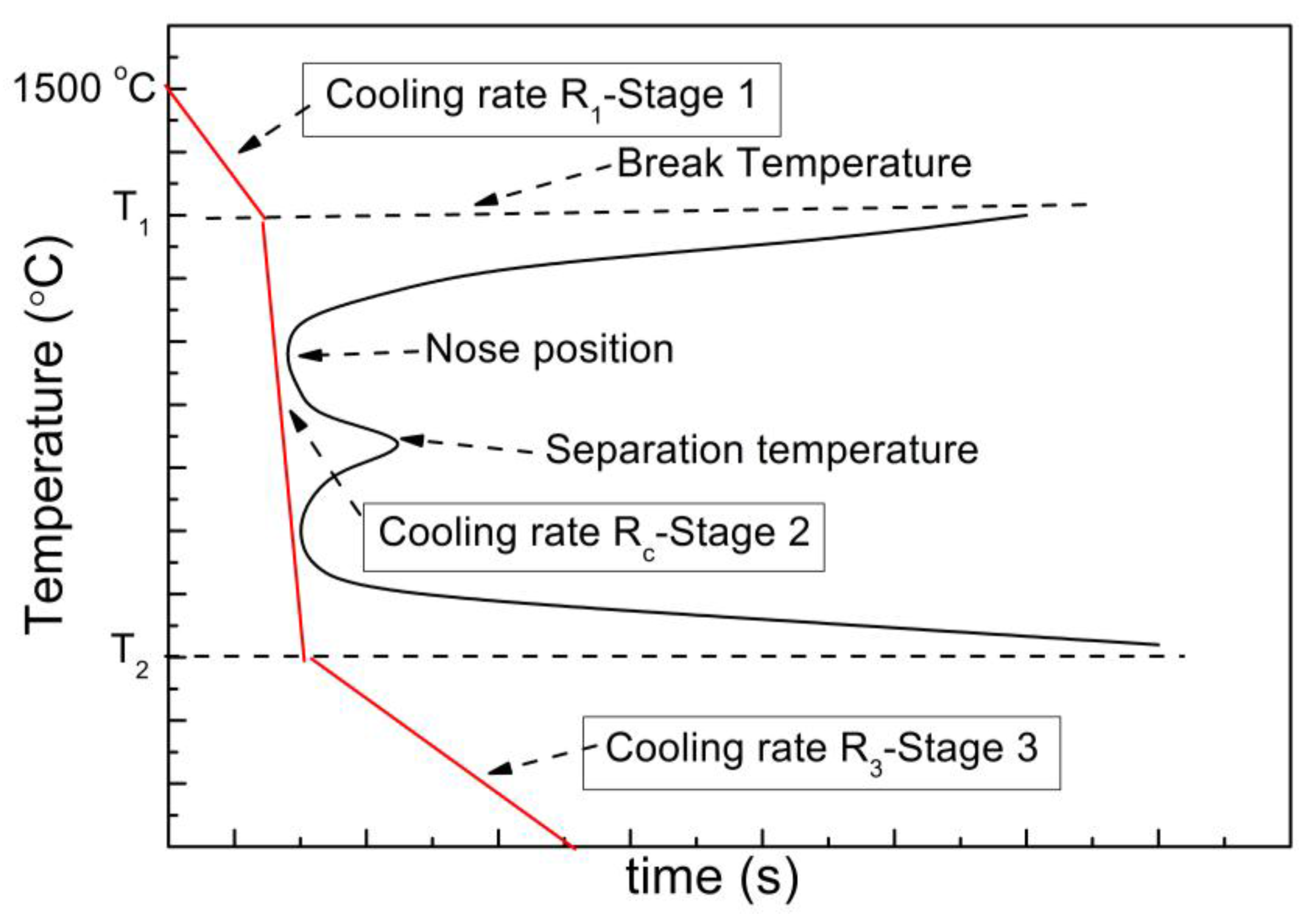
- Dry granulation of slags into small droplets can effectively address the issue of the low thermal conductivity of slags and avoid crystallization inside slags; while chemical methods can address the problem of the discontinuous availability and create high quality fuel gas; therefore a combination of dry granulation and chemical reactions to make up an integrated system is the main direction of heat recovery from slags, as summarized in Figure 7.

Acknowledgments
Author Contributions
Conflicts of Interest
References
- Allwood, J.M.; Cullen, J.M.; Milford, R.L. Options for achieving a 50% cut in industrial carbon emissions by 2050. Environ. Sci. Technol. 2010, 44, 1888–1894. [Google Scholar] [CrossRef] [PubMed]
- Cullen, J.M.; Allwood, J.M.; Bambach, M.D. Mapping the global flow of steel: From steelmaking to end-use goods. Environ. Sci. Technol. 2012, 46, 13048–13055. [Google Scholar] [CrossRef] [PubMed]
- Milford, R.L.; Pauliuk, S.; Allwood, J.M.; Müller, D.B. The roles of energy and material efficiency in meeting steel industry CO2 targets. Environ. Sci. Technol. 2013, 47, 3455–3462. [Google Scholar] [CrossRef] [PubMed]
- Barati, M. Energy intensity and greenhouse gases footprint of metallurgical processes: A continuous steelmaking case study. Energy 2010, 35, 3731–3737. [Google Scholar] [CrossRef]
- Yellishetty, M.; Ranjith, P.G.; Tharumarajah, A. Iron ore and steel production trends and material flows in the world: Is this really sustainable? Resour. Conserv. Recycl. 2010, 54, 1084–1094. [Google Scholar] [CrossRef]
- Fruehan, R.J.; Fortini, O.; Paxton, H.W.; Brindle, R. Theoretical Minimum Energies to Produce Steel for Selected Conditions; U.S. Department of Energy: Washington, DC, USA, 2000. [Google Scholar]
- Bisio, G. Energy recovery from molten slag and exploitation of the recovered energy. Energy 1997, 22, 501–509. [Google Scholar] [CrossRef]
- Barati, M.; Esfahani, S.; Utigard, T.A. Energy recovery from high temperature slags. Energy 2011, 36, 5440–5449. [Google Scholar] [CrossRef]
- Steel Statistical Yearbook 2013. Worldsteel Association. Available online: http://www.worldsteel.org/publications/bookshop/product-details.~Steel-Statistical-Yearbook-2013~PRODUCT~SSY2013~.html (accessed on 5 December 2014).
- Hendrik, G.V.O. Slag, Iron and Steel. US Geological Survey Minerals Yearbook, 2010. Available online: http://minerals.usgs.gov/minerals/pubs/commodity/iron_&_steel_slag/index.html#myb (accessed on 5 December 2014).
- McDavid, R.M.; Thomas, B.G. Flow and thermal behavior of the top surface flux/powder layers in continuous casting molds. Metall. Mater. Trans. B 1996, 27, 672–685. [Google Scholar] [CrossRef]
- Cai, J.J.; Wang, J.J.; Chen, C.X.; Lu, Z.W. Recovery of residual heat integrated steelworks. Iron Steel 2007, 42, 1–6. [Google Scholar]
- Pickering, S.J.; Hay, N.; Roylance, T.F.; Thomas, G.H. New process for dry granulation and heat recovery from molten blast furnace slag. Ironmak. Steelmak. 1985, 12, 14–21. [Google Scholar]
- Mizuochi, T.; Akiyama, T.; Shimada, T.; Kasai, E.; Yagi, J.I. Feasibility of rotary cup atomizer for slag granulation. ISIJ Int. 2001, 41, 1423–1428. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Q.; Li, P.; Du, W. Cold experiments on ligament formation for blast furnace slag granulation. Appl. Therm. Eng. 2012, 40, 351–357. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Q.; Duan, W.; Qin, Q. Experimental investigation on ligament formation for molten slag granulation. Appl. Therm. Eng. 2014, 73, 886–891. [Google Scholar]
- Kashiwaya, Y.; In-Nami, Y.; Akiyama, T. Development of a rotary cylinder atomizing method of slag for the production of amorphous slag particles. ISIJ Int. 2010, 50, 1245–1251. [Google Scholar] [CrossRef]
- Kashiwaya, Y.; In-Nami, Y.; Akiyama, T. Mechanism of the formation of slag particles by the rotary cylinder atomization. ISIJ Int. 2010, 50, 1252–1258. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; Zhu, X.; Qiu, Y.J.; Li, K.; Chen, R.; Liao, Q. A review of waste heat recovery technologies towards molten slag in steel industry. Appl. Energy 2013, 112, 956–966. [Google Scholar] [CrossRef]
- Nomura, T.; Okinaka, N.; Akiyama, T. Technology of latent heat storage for high temperature application: A review. ISIJ Int. 2010, 50, 1229–1239. [Google Scholar] [CrossRef]
- Nishioka, K.; Maeda, T.; Shimizu, M. Application of square-wave pulse heat method to thermal properties measurement of CaO–SiO2–Al2O3 system fluxes. ISIJ Int. 2006, 46, 427–433. [Google Scholar] [CrossRef]
- Li, F.; Susa, M.; Nagata, K. Temperature and composition dependence of thermal conductivity, thermal diffusivity and specific heat of the PbO-SiO2 system. J. Jpn. Inst. Met. 1991, 55, 194–203. [Google Scholar]
- Kang, Y.; Morita, K. Thermal conductivity of the CaO–Al2O3–SiO2 system. ISIJ Int. 2006, 46, 420–426. [Google Scholar] [CrossRef]
- Nagata, K.; Susa, M.; Goto, K.S. Thermal conductivities of slags for ironmaking and steelmaking. Tetsu-to-Hagané 1983, 69, 1417–1424. [Google Scholar]
- Yoshinaga, M.; Fujii, K.; Shigematsu, T.; Nakata, T. Method of dry granulation and solidification of molten blast furnace slag. Tetsu-to-Hagané 1981, 67, 917–924. [Google Scholar]
- Yoshinaga, M.; Fujii, K.; Shigematsu, T.; Nakata, T. Dry granulation and solidification of molten blast furnace slag. Tetsu-to-Hagané 1982, 22, 823–829. [Google Scholar]
- Meng, Y.; Thomas, B.G. Heat-transfer and solidification model of continuous slab casting: CON1D. Metall. Mater. Trans. B 2003, 34, 685–705. [Google Scholar] [CrossRef]
- Sun, Y.Q.; Shen, H.W.; Wang, H.; Wang, X.D.; Zhang, Z.T. Experimental investigation and modeling of cooling processes of high temperature slags. Energy 2014, 76, 761–767. [Google Scholar] [CrossRef]
- Ryu, H.G.; Zhang, Z.T.; Cho, J.W.; Wen, G.H.; Sridhar, S. Crystallization behaviors of slags through a heat flux simulator. ISIJ Int. 2010, 50, 1142–1150. [Google Scholar] [CrossRef]
- Kashiwaya, Y.; Nakauchi, T.; Pham, K.S.; Akiyama, S.; Ishii, K. Crystallization behaviors concerned with TTT and CCT diagrams of blast furnace slag using hot thermocouple technique. ISIJ Int. 2007, 47, 44–52. [Google Scholar] [CrossRef]
- Allibert, M.; Gaye, H.; Geiseler, J.; Janke, D.; Keene, B.J.; Kirner, D.; Kowalski, M.; Lehmann, J.; Mills, K.C.; Neuschutz, D.; et al. Slag Atlas; Verlag Stahleisen GmbH: Düsseldorf, Germany, 1995. [Google Scholar]
- Sun, Y.Q.; Zhang, Z.T.; Liu, L.L.; Wang, X.D. Multi-Stage control of waste heat recovery from high temperature slags based on time temperature transformation curves. Energies 2014, 7, 1673–1684. [Google Scholar] [CrossRef]
- Akiyama, T.; Oikawa, K.; Shimada, T.; Kasai, E.; Yagi, J.I. Thermodynamic analysis of thermochemical recovery of high temperature wastes. ISIJ Int. 2000, 40, 288–291. [Google Scholar] [CrossRef]
- Andrade, M.L.; Almeida, L.; do Carmo Rangel, M.; Pompeo, F.; Nichio, N. Ni-catalysts supported on Gd-doped ceria for solid oxide fuel cells in methane steam reforming. Chem. Eng. Technol. 2014, 37, 343–348. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Liu, W.; Liu, H.; Qiu, J.; Yeung, K.L. A new alkali-resistant Ni/Al2O3-MSU-1 core-shell catalyst for methane steam reforming in a direct internal reforming molten carbonate fuel cell. J. Power Sources 2014, 246, 74–83. [Google Scholar] [CrossRef]
- Kasai, E.; Kitajima, T.; Akiyama, T.; Yagi, J.I.; Saito, F. Rate of methane-steam reforming reaction on the surface of molten BF slag: For heat recovery from molten slag by using a chemical reaction. ISIJ Int. 1997, 37, 1031–1036. [Google Scholar] [CrossRef]
- Shimada, T.; Kochura, V.; Akiyama, T.; Kasai, E.; Yagi, J.I. Effects of slag compositions on the rate of methane-steam reaction. ISIJ Int. 2000, 41, 111–115. [Google Scholar] [CrossRef]
- Bodrov, N.M.; Apel'Baum, L.O.; Temkin, M.I. Kinetics of the reaction of methane with steam on the surface of nickel. Kinet. Katal. 1964, 5, 696–702. [Google Scholar]
- Dippenaar, R. Industrial uses of slag (the use and re-use of iron and steelmaking slags). Ironmak. Steelmak. 2005, 32, 35–46. [Google Scholar] [CrossRef]
- Aranda Usón, A.; López-Sabirón, A.M.; Ferreira, G.; Llera Sastresa, E. Uses of alternative fuels and raw materials in the cement industry as sustainable waste management options. Renew. Sustain. Energy. Rev. 2013, 23, 242–260. [Google Scholar] [CrossRef]
- Maruoka, N.; Mizuochi, T.; Purwanto, H.; Akiyama, T. Feasibility study for recovering waste heat in the steelmaking industry using a chemical recuperator. ISIJ Int. 2004, 44, 257–262. [Google Scholar] [CrossRef]
- Liu, C.J.; Ye, J.; Jiang, J.; Pan, Y. Progresses in the preparation of coke resistant Ni-Based catalyst for steam and CO2 reforming of methane. ChemCatChem 2011, 3, 529–541. [Google Scholar] [CrossRef]
- Wang, S.; Lu, G.Q.; Millar, G.J. Carbon reforming of methane to produce synthesis gas over metal-supported catalysts: State of art. Energy Fuels 1996, 10, 896–904. [Google Scholar] [CrossRef]
- Purwanto, H.; Akiyama, T. Hydrogen production from biogas using hot slag. Int. J. Hydrog. Energy 2006, 31, 491–495. [Google Scholar] [CrossRef]
- Bhutto, A.W.; Bazmi, A.A.; Zahedi, G. Underground coal gasification: From fundamentals to applications. Prog. Energy Combust. Sci. 2013, 39, 189–214. [Google Scholar] [CrossRef]
- Shabbar, S.; Janajreh, I. Thermodynamic equilibrium analysis of coal gasification using Gibbs energy minimization method. Energy Convers. Manag. 2013, 65, 755–763. [Google Scholar] [CrossRef]
- Liu, H.X. Investigation of coal gasification using blast furnace molten slag as heat carrier. Energy Conserv. 2004, 6, 41–43. [Google Scholar]
- Li, P.; Yu, Q.; Qin, Q.; Lei, W. Kinetics of CO2/Coal gasification in molten blast furnace slag. Ind. Eng. Chem. Res. 2012, 51, 15872–15883. [Google Scholar] [CrossRef]
- Li, P.; Yu, Q.; Qin, Q.; Liu, J. Adaptability of coal gasification in molten blast furnace slag on coal samples and granularities. Energy Fuels 2011, 25, 5678–5682. [Google Scholar] [CrossRef]
- Li, P.; Yu, Q.; Xie, H.; Qin, Q.; Wang, K. CO2 gasification rate analysis of Datong coal using slag granules as heat carrier for heat recovery from blast furnace slag by using a chemical reaction. Energy Fuels 2013, 27, 4810–4817. [Google Scholar] [CrossRef]
- Duan, W.; Yu, Q.; Zuo, Z.; Qin, Q.; Li, P.; Liu, J. The technological calculation for synergistic system of BF slag waste heat recovery and carbon resources reduction. Energy Convers. Manag. 2014, 87, 185–190. [Google Scholar] [CrossRef]
- Kajitani, S.; Tay, H.L.; Zhang, S.; Li, C.Z. Mechanisms and kinetic modelling of steam gasification of brown coal in the presence of volatile-char interactions. Fuel 2013, 103, 7–13. [Google Scholar] [CrossRef]
- Duan, W.; Yu, Q.; Xie, H.; Qin, Q.; Zuo, Z. Thermodynamic analysis of hydrogen-rich gas generation from coal/steam gasification using blast furnace slag as heat carrier. Int. J. Hydrog. Energy 2014, 39, 11611–11619. [Google Scholar] [CrossRef]
- Shatokha, V.I.; Sokolovskaya, I.V. Study on effect of coal treatment with blast furnace slag on char reactivity in air. Ironmak. Steelmak. 2012, 39, 439–445. [Google Scholar] [CrossRef]
- Chen, T.; Wu, J.; Zhang, J.; Wu, J.; Sun, L. Gasification kinetic analysis of the three pseudocomponents of biomass-cellulose, semicellulose and lignin. Bioresour. Technol. 2014, 153, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Magdziarz, A.; Werle, S. Analysis of the combustion and pyrolysis of dried sewage sludge by TGA and MS. Waste Manag. 2014, 34, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Cahyono, R.B.; Rozhan, A.N.; Yasuda, N.; Nomura, T.; Hosokai, S.; Kashiwaya, Y.; Akiyama, T. Integrated coal-pyrolysis tar reforming using steelmaking slag for carbon composite and hydrogen production. Fuel 2013, 109, 439–444. [Google Scholar] [CrossRef]
- Guerrero, L.A.; Maas, G.; Hogland, W. Solid waste management challenges for cities in developing countries. Waste Manag. 2013, 33, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Marshall, R.E.; Farahbakhsh, K. Systems approaches to integrated solid waste management in developing countries. Waste Manag. 2013, 33, 988–1003. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, H.; Qing, S.; Liu, H. Characteristics of gaseous product from municipal solid waste gasification with hot blast furnace slag. J. Nat. Gas Chem. 2010, 19, 403–408. [Google Scholar] [CrossRef]
- Qin, Y.L.; Lv, X.W.; Bai, C.G.; Qiu, G.B.; Chen, P. Waste heat recovery from blast furnace slag by chemical reactions. JOM 2012, 64, 997–1001. [Google Scholar] [CrossRef]
- Bridgwater, A.V. The technical and economic feasibility of biomass gasification for power generation. Fuel 1995, 74, 631–653. [Google Scholar] [CrossRef]
- Devi, L.; Ptasinski, K.J.; Janssen, F.J. A review of the primary measures for tar elimination in biomass gasification processes. Biomass Bioenergy 2003, 24, 125–140. [Google Scholar] [CrossRef]
- Luo, S.; Zhou, Y.; Yi, C. Hydrogen-rich gas production from biomass catalytic gasification using hot blast furnace slag as heat carrier and catalyst in moving-bed reactor. Int. J. Hydrog. Energy 2012, 37, 15081–15085. [Google Scholar] [CrossRef]
- Luo, S.; Yi, C.; Zhou, Y. Bio-oil production by pyrolysis of biomass using hot blast furnace slag. Renew. Energy 2013, 50, 373–377. [Google Scholar] [CrossRef]
- Sun, Y.Q.; Zhang, Z.T.; Seetharaman, S.; Liu, L.L.; Wang, X.D. Characteristics of low temperature biomass gasification and syngas release behavior using hot slags. RSC Adv. 2014, 4, 62105–62114. [Google Scholar] [CrossRef]
- Hacker, V.; Fankhauser, R.; Faleschini, G.; Fuchs, H.; Friedrich, K.; Muhr, M.; Kordesch, K. Hydrogen production by steam-iron process. J. Power Sources 2000, 86, 531–535. [Google Scholar] [CrossRef]
- Matsuura, H.; Tsukihashi, F. Thermodynamic calculation of generation of H2 gas by reaction between FeO in steelmaking slag and water vapor. ISIJ Int. 2012, 52, 1503–1512. [Google Scholar] [CrossRef]
- Sato, M.; Matsuura, H.; Tsukihashif, F. Generation behavior of H2 gas by reaction between FeO-containing slag and H2O-Ar gas. ISIJ Int. 2012, 52, 1500–1502. [Google Scholar] [CrossRef]
- Malvoisin, B.; Brunet, F.; Carlut, J.; Hernandez, G.M.; Findling, N.; Lanson, M.; Vidal, O.; Bottero, J.Y.; Bruno, G. High-purity hydrogen gas from the reaction between BOF steel slag and water in the 473–673 K range. Int. J. Hydrog. Energy 2013, 38, 7382–7393. [Google Scholar] [CrossRef]
- Nakano, J.; Bennett, J. CO2 and H2O gas conversion into CO and H2 using highly exothermic reactions induced by mixed industrial slags. Int. J. Hydrog. Energy 2014, 39, 4954–4958. [Google Scholar] [CrossRef]
- Yang, H. A New One-Step Technology of Mineral Wool Production by Using the Sensible Heat of Industrial BF Slag High-Efficiently. Patent No. 02152584, 21 May 2003. [Google Scholar]
- Ji, R.; Zhang, Z.T.; Liu, L.L.; Wang, X.D. Numerical modeling and experimental study of heat transfer in ceramic fiberboard. Text. Res. J. 2014, 84, 411–421. [Google Scholar] [CrossRef]
- Zhao, D.W.; Zhang, Z.T.; Tang, X.L.; Liu, L.L.; Wang, X.D. Preparation of slag wool by integrated waste-heat recovery and resource recycling of molten blast furnace slags: From fundamental to industrial application. Energies 2014, 7, 3121–3135. [Google Scholar] [CrossRef]
- Li, J.; Liu, W.; Zhang, Y.; Yang, A.; Zhao, K. Research on modifying blast furnace slag as a raw of slag fiber. Mater. Manuf. Process. 2014. [Google Scholar] [CrossRef]
- Wu, X.R.; Li, L.S.; Dong, Y.C. Enrichment and crystallization of vanadium in factory steel slag. Metallurgist 2011, 55, 401–409. [Google Scholar] [CrossRef]
- Diao, J.; Xie, B.; Wang, Y.H.; Guo, X. Recovery of phosphorus from dephosphorization slag produced by duplex high phosphorus hot metal refining. ISIJ Int. 2012, 52, 955–959. [Google Scholar] [CrossRef]
- Sui, Z.T.; Zhang, P.X. Selective precipitating behavior of the boron components from the boron slag. Acta Metall. Sin. 1997, 33, 943–951. [Google Scholar]
- Li, J.; Zhang, Z.T.; Zhang, M.; Guo, M.; Wang, X.D. The Influence of SiO2 on the extraction of Ti element from Ti-bearing blast furnace slag. Steel Res. Int. 2011, 82, 607–614. [Google Scholar] [CrossRef]
- Zhang, Z.T.; Li, J.; Liu, P. Crystallization behavior in fluoride-free mold fluxes containing TiO2/ZrO2. J. Iron Steel Res. Int. 2011, 18, 31–37. [Google Scholar] [CrossRef]
- Liu, H.; Luo, C.H.; Toyota, M.; Kato, S.; Uemiya, S.; Kojima, T.; Tominaga, H. Mineral reaction and morphology change during gasification of coal in CO2 at elevated temperatures. Fuel 2003, 82, 523–530. [Google Scholar] [CrossRef]
- Ma, Z.; Bai, J.; Bai, Z.; Kong, L.; Guo, Z.; Yan, J.; Li, W. Mineral transformation in char and its effect on coal char gasification reactivity at high temperatures, part 2: Char gasification. Energy Fuels 2014, 28, 1846–1853. [Google Scholar] [CrossRef]
- Jim, C.Y.; Chen, S.S. Comprehensive greenspace planning based on landscape ecology principles in compact Nanjing city, China. Landsc. Urban Plan. 2003, 65, 95–116. [Google Scholar] [CrossRef]
- City Plan of Beijing (2004–2020). Available online: http://www.china.com.cn/aboutchina/zhuanti/09dfgl/2009-03/04/content_17371797.htm (accessed on 22 January 2015).
- Dong, L.; Fujita, T.; Zhang, H.; Dai, M.; Fujii, M.; Ohnishi, S.; Gneg, Y.; Liu, Z. Promoting low-carbon city through industrial symbiosis: A case in China by applying HPIMO model. Energy Policy 2013, 61, 864–873. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Zhang, Z.; Liu, L.; Wang, X. Heat Recovery from High Temperature Slags: A Review of Chemical Methods. Energies 2015, 8, 1917-1935. https://doi.org/10.3390/en8031917
Sun Y, Zhang Z, Liu L, Wang X. Heat Recovery from High Temperature Slags: A Review of Chemical Methods. Energies. 2015; 8(3):1917-1935. https://doi.org/10.3390/en8031917
Chicago/Turabian StyleSun, Yongqi, Zuotai Zhang, Lili Liu, and Xidong Wang. 2015. "Heat Recovery from High Temperature Slags: A Review of Chemical Methods" Energies 8, no. 3: 1917-1935. https://doi.org/10.3390/en8031917
APA StyleSun, Y., Zhang, Z., Liu, L., & Wang, X. (2015). Heat Recovery from High Temperature Slags: A Review of Chemical Methods. Energies, 8(3), 1917-1935. https://doi.org/10.3390/en8031917





