Strategies for Reducing the Start-up Operation of Microbial Electrochemical Treatments of Urban Wastewater
Abstract
:1. Introduction
2. Results and Discussion
- (a)
- Water on the cathode to produce hydrogen gas (i.e., 2H2O +2e− → H2 + 2OH−, E° = −830 mV vs. standard hydrogen electrode (SHE) by using a MEC since the reaction is not spontaneous.
- (b)
- Fe3+ on the cathode to produce Fe2+ through a spontaneous reaction (Fe3+/Fe2+, E° = 0.77 V vs. SHE by using an MFC since the reaction is spontaneous.
2.1. Microbial Conversion of Acetate into Electrical Current
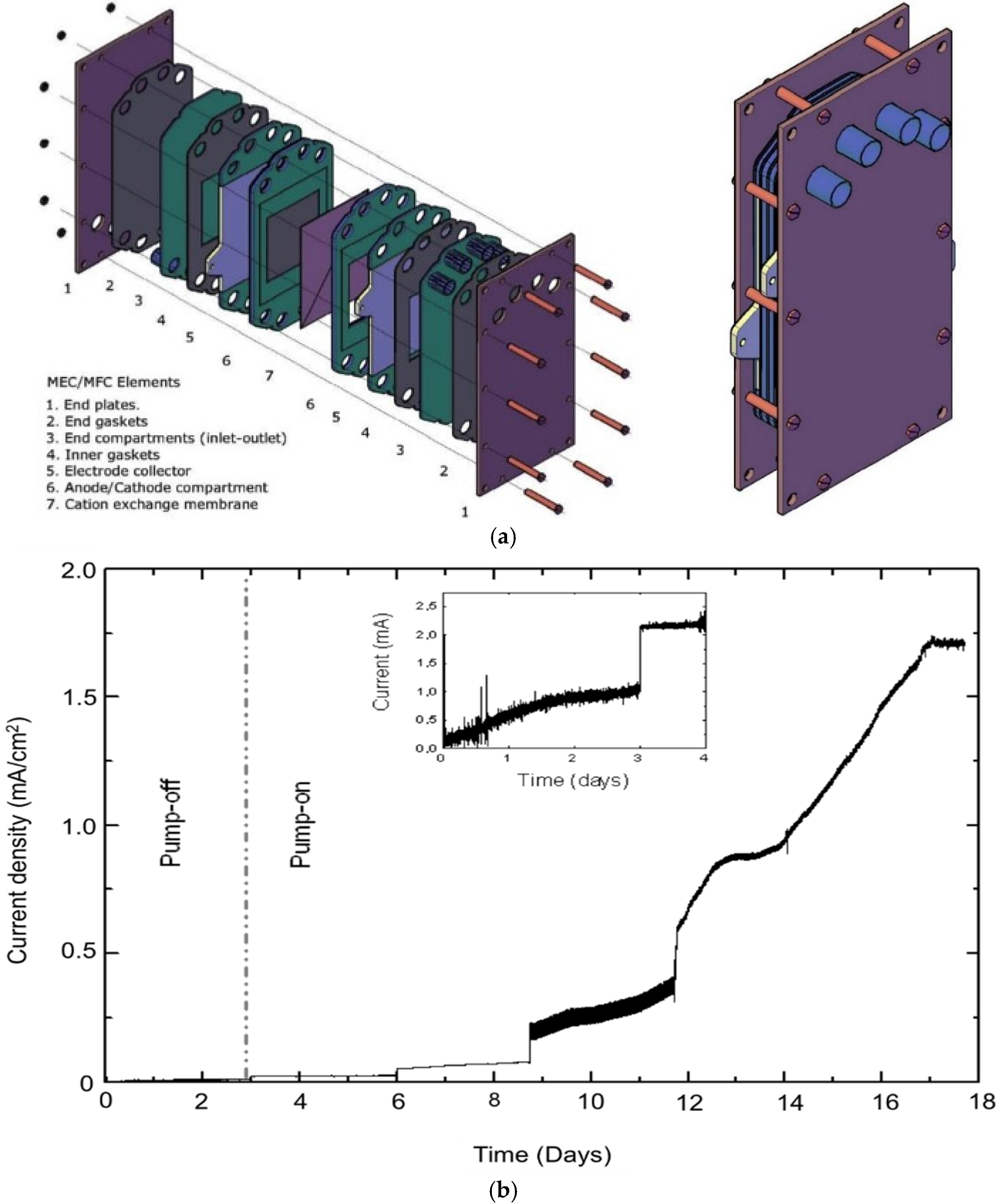
2.2. Minimizing the Start-up Operation: The Use of Plug and Play Geobacter Cells
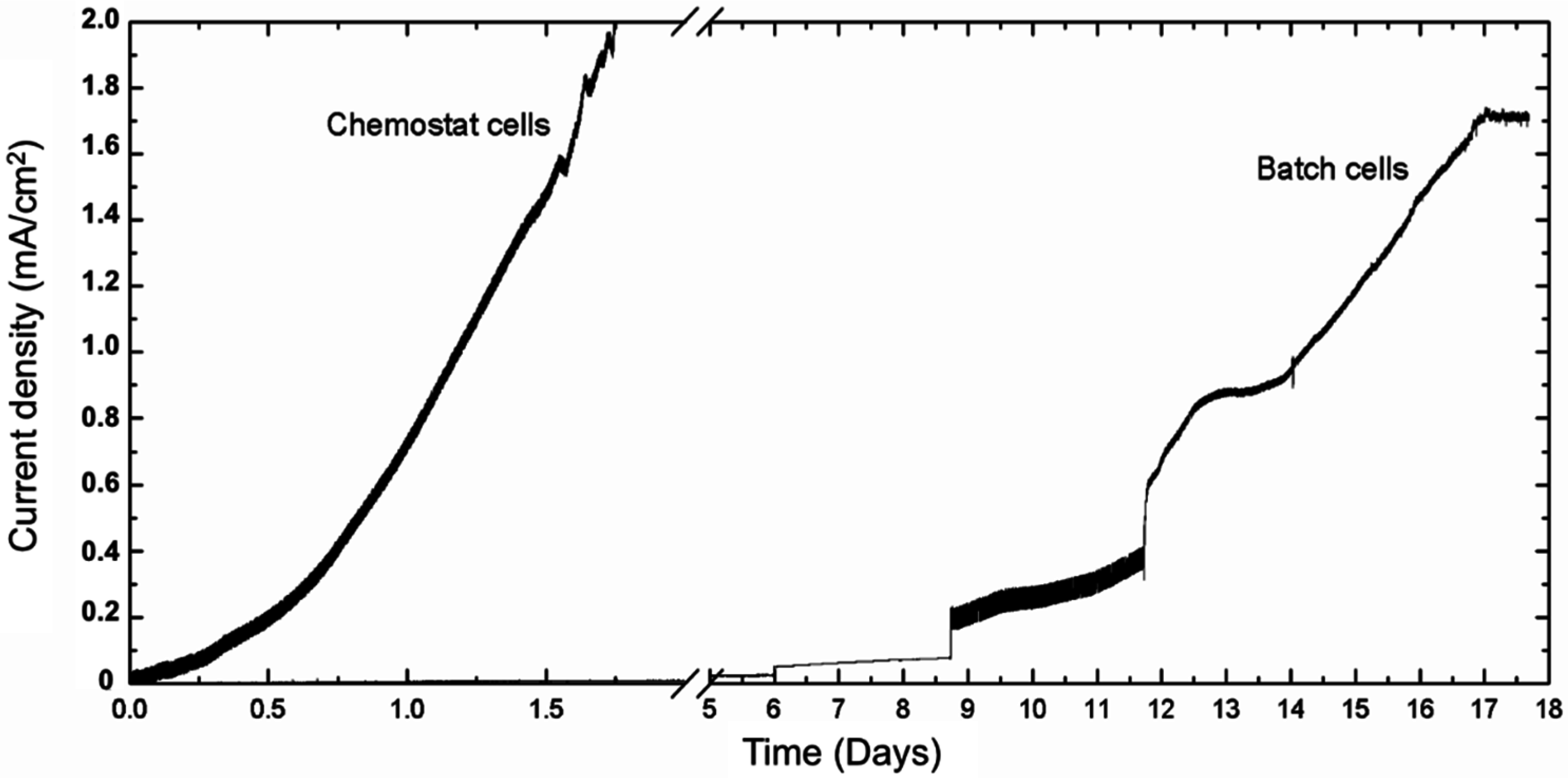
2.3. Potential Electrode Competitors and Inhibitors of Geobacter sulfurreducens

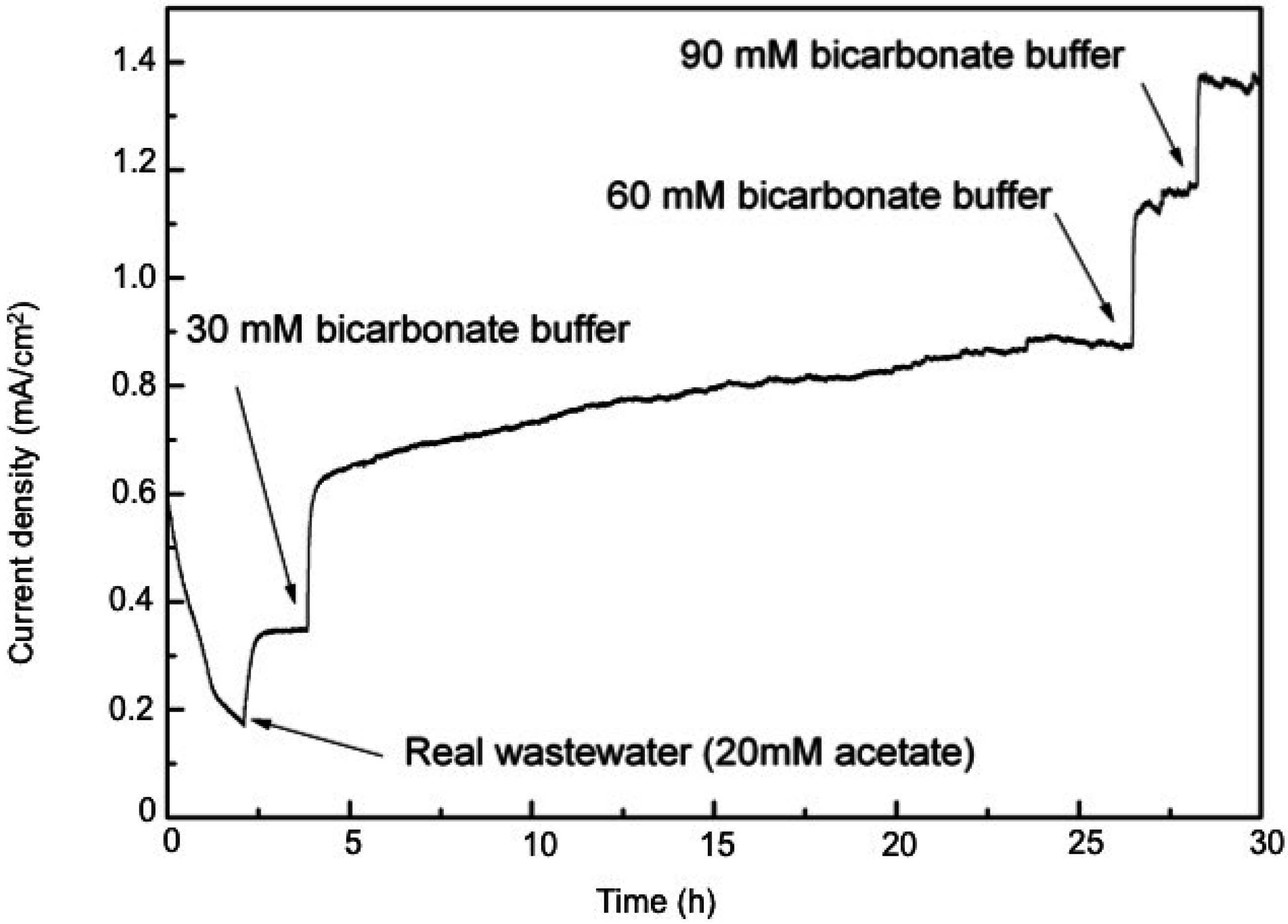
2.4. Microbial Electrolysis Cells Performance with Real Wastewater
2.5. Microbial Fuel Cell: Steady State Operation and Power Curve


3. Materials and Methods
3.1. Bacterial Strain and Culture Conditions
3.2. Microbial Electrolysis Cell Device
3.3. Start-up and Operation Procedure
3.4. Competitive Assays with Soluble Terminal Electron Acceptors
3.5. Operation as a Microbial Fuel Cell
| BES configuration | Materials/conditions | Details |
| Compartments | 1 anode compartment; 1 cathode comparment | |
| Projected area | 17 mm | |
| Compartment thickness | 100 cm2 | |
| Anode electrode | Particles 2.3–4.0 mm diameter mass: 178 g; porosity 36% | |
| Cathode electrode | Carbon felt | |
| Electric collectors | Graphite plate | |
| Membrane | Nafion 324 (DuPont) | |
| Reference electrodes | Ag/AgCl 3.5 M KCl reference electrodes units located in the geometrical center of each compartment (2 units) | |
| Flow rate | 6.4 L·h−1 (both streams) | |
| Anolyte solution | Acetate 20 mM + FWM (pH = 6.9, EC = 12.4 mS·cm−1) | |
| Catholyte solution | MEC operation: Na2SO4 0.25 M (16.0 mS·cm−1) MFC operation: FeCl3 0.20 M pH = 1 HCl (15.6 mS·cm−1) | |
| Anolyte tank | 5 L | |
| Catholyte tank | 2 L |
3.6. Assays with Real Urban Wastewater
| Real wastewater | |||||
|---|---|---|---|---|---|
| Parameter | pH | Conductivity (mS·cm−1) | BOD (mg·L−1) | Total nitrogen (mg·L−1) | Acetate (mM) |
| Value | 7.0 | 1.5 | 280 | 0.5 | 1.0 |
3.7. Analytical Methods
3.8. Electrochemical Assays
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contribution
Conflicts of Interest
References
- Huggins, T.; Fallgren, P.H.; Song, J.; Ren, Z.J. Energy and Performance Comparison of Microbial Fuel Cell and Conventional Aeration Treating of Wastewater. J. Microb. Biochem. Technol. 2013. [Google Scholar] [CrossRef]
- Heidrich, E.S.; Curtis, T.P.; Dolfing, J. Determination of the internal chemical energy of wastewater. Environ. Sci. Technol. 2011, 45, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial Fuel Cells: Methodology and Technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Nasir, I.M.; Ghazi, T.I.M.; Omar, R. Production of biogas from solid organic wastes through anaerobic digestion: A review. Appl. Microbiol. Biotechnol. 2012, 95, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Schröder, U.; Harnisch, F.; Angenent, L.T. Microbial electrochemistry and technology: Terminology and classification. Energy Environ. Sci. 2015, 8, 513–519. [Google Scholar] [CrossRef]
- Du, Z.; Li, H.; Gu, T. A state of the art review on microbial fuel cells: A promising technology for wastewater treatment and bioenergy. Biotechnol. Adv. 2007, 25, 464–482. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E. Exoelectrogenic bacteria that power microbial fuel cells. Nat. Rev. Microbiol. 2009, 7, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K. Bioelectrochemical Systems: From Extracellular Electron Transfer to Biotechnological Application; Integrated Environmental Technology Series; International Water Association Publishing: London, UK; New York, NY, USA, 2010. [Google Scholar]
- Zhu, G.; Onodera, T.; Tandukar, M.; Pavlostathis, S.G. Simultaneous carbon removal, denitrification and power generation in a membrane-less microbial fuel cell. Bioresour. Technol. 2013, 146, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ramnarayanan, R.; Logan, B.E. Production of Electricity during Wastewater Treatment Using a Single Chamber Microbial Fuel Cell. Environ. Sci. Technol. 2004, 38, 2281–2285. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Verstraete, W. Microbial fuel cells: Novel biotechnology for energy generation. Trends Biotechnol. 2005, 23, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Logan, B.E. Electricity Generation Using an Air-Cathode Single Chamber Microbial Fuel Cell in the Presence and Absence of a Proton Exchange Membrane. Environ. Sci. Technol. 2004, 38, 4040–4046. [Google Scholar] [CrossRef] [PubMed]
- Capodaglio, A.G.; Molognoni, D.; Dallago, E.; Liberale, A.; Cella, R.; Longoni, P.; Pantaleoni, L. Microbial Fuel Cells for Direct Electrical Energy Recovery from Urban Wastewaters. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Call, D.; Cheng, S.; Hamelers, H.V.M.; Sleutels, T.H.J.A.; Jeremiasse, A.W.; Rozendal, R.A. Microbial Electrolysis Cells for High Yield Hydrogen Gas Production from Organic Matter. Environ. Sci. Technol. 2008, 42, 8630–8640. [Google Scholar] [CrossRef] [PubMed]
- Call, D.; Logan, B.E. Hydrogen Production in a Single Chamber Microbial Electrolysis Cell Lacking a Membrane. Environ. Sci. Technol. 2008, 42, 3401–3406. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Girguis, P.; Nielsen, L.K. Metabolic and practical considerations on microbial electrosynthesis. Curr. Opin. Biotechnol. 2011, 22, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Rozendal, R.A. Microbial electrosynthesis—Revisiting the electrical route for microbial production. Nat. Rev. Microbiol. 2010, 8, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Huang, X.; Liang, P.; Xiao, K.; Zhou, Y.; Zhang, X.; Logan, B.E. A New Method for Water Desalination Using Microbial Desalination Cells. Environ. Sci. Technol. 2009, 43, 7148–7152. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.S.; Drew, D.M.; He, Z. Efficient salt removal in a continuously operated upflow microbial desalination cell with an air cathode. Bioresour. Technol. 2011, 102, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, J.; Boltes, K.; Esteve-Nuñez, A. Microbial-electrochemical bioremediation and detoxification of dibenzothiophene-polluted soil. Chemosphere 2014, 101, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ren, Z.J. A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnol. Adv. 2013, 31, 1796–1807. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Rabaey, K. Conversion of Wastes into Bioelectricity and Chemicals by Using Microbial Electrochemical Technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Rismani-Yazdi, H.; Carver, S.M.; Christy, A.D.; Yu, Z.; Bibby, K.; Peccia, J.; Tuovinen, O.H. Suppression of methanogenesis in cellulose-fed microbial fuel cells in relation to performance, metabolite formation, and microbial population. Bioresour. Technol. 2013, 129, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Cercado-Quezada, B.; Delia, M.-L.; Bergel, A. Testing various food-industry wastes for electricity production in microbial fuel cell. Bioresour. Technol. 2010, 101, 2748–2754. [Google Scholar] [CrossRef] [PubMed]
- Çetinkaya, A.Y.; Köroğlu, E.O.; Demir, N.M.; Baysoy, D.Y.; Özkaya, B.; Çakmakçı, M. Electricity production by a microbial fuel cell fueled by brewery wastewater and the factors in its membrane deterioration. Chin. J. Catal. 2015, 36, 1068–1076. [Google Scholar] [CrossRef]
- Kelly, P.T.; He, Z. Understanding the application niche of microbial fuel cells in a cheese wastewater treatment process. Bioresour. Technol. 2014, 157, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Kouzuma, A.; Kaku, N.; Watanabe, K. Microbial electricity generation in rice paddy fields: Recent advances and perspectives in rhizosphere microbial fuel cells. Appl. Microbiol. Biotechnol. 2014, 98, 9521–9526. [Google Scholar] [CrossRef] [PubMed]
- Bond, D.R.; Lovley, D.R. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl. Environ. Microbiol. 2003, 69, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Hu, J.; Fitzgerald, L.A.; Biffinger, J.C.; Xie, P.; Ringeisen, B.R.; Lieber, C.M. Probing electron transfer mechanisms in Shewanella oneidensis MR-1 using a nanoelectrode platform and single-cell imaging. Proc. Natl. Acad. Sci. USA 2010, 107, 16806–16810. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Park, H.S.; Kim, H.J.; Kim, G.T.; Chang, I.S.; Lee, J.; Phung, N.T. Enrichment of microbial community generating electricity using a fuel-cell-type electrochemical cell. Appl. Microbiol. Biotechnol. 2004, 63, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Richter, H.; Nevin, K.P.; Jia, H.; Lowy, D.A.; Lovley, D.R.; Tender, L.M. Cyclic voltammetry of biofilms of wild type and mutant Geobacter sulfurreducens on fuel cell anodes indicates possible roles of OmcB, OmcZ, type IV pili, and protons in extracellular electron transfer. Energy Environ. Sci. 2009, 2, 506–516. [Google Scholar] [CrossRef]
- Sangeetha, T.; Muthukumar, M. Influence of electrode material and electrode distance on bioelectricity production from sago-processing wastewater using microbial fuel cell. Environ. Prog. Sustain. Energy 2013, 32, 390–395. [Google Scholar] [CrossRef]
- Dewan, A.; Beyenal, H.; Lewandowski, Z. Scaling up microbial fuel cells. Environ. Sci. Technol. 2008, 42, 7643–7648. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Feng, C.; Huang, L.; Lv, Z.; Xie, D.; Wei, C. Anode-biofilm electron transfer behavior and wastewater treatment under different operational modes of bioelectrochemical system. Bioresour. Technol. 2014, 157, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Erable, B.; Etcheverry, L.; Bergel, A. From microbial fuel cell (MFC) to microbial electrochemical snorkel (MES): Maximizing chemical oxygen demand (COD) removal from wastewater. Biofouling 2011, 27, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Santoro, C.; Guilizzoni, M.; Correa Baena, J.P.; Pasaogullari, U.; Casalegno, A.; Li, B.; Babanova, S.; Artyushkova, K.; Atanassov, P. The effects of carbon electrode surface properties on bacteria attachment and start up time of microbial fuel cells. Carbon 2014, 67, 128–139. [Google Scholar] [CrossRef]
- Boghani, H.C.; Kim, J.R.; Dinsdale, R.M.; Guwy, A.J.; Premier, G.C. Control of power sourced from a microbial fuel cell reduces its start-up time and increases bioelectrochemical activity. Bioresour. Technol. 2013, 140, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Molognoni, D.; Puig, S.; Balaguer, M.D.; Liberale, A.; Capodaglio, A.G.; Callegari, A.; Colprim, J. Reducing start-up time and minimizing energy losses of Microbial Fuel Cells using Maximum Power Point Tracking strategy. J. Power Sources 2014, 269, 403–411. [Google Scholar] [CrossRef]
- Katuri, K.P.; Enright, A.-M.; O’Flaherty, V.; Leech, D. Microbial analysis of anodic biofilm in a microbial fuel cell using slaughterhouse wastewater. Bioelectrochemistry 2012, 87, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Ueki, T.; Zhang, T.; Malvankar, N.S.; Shrestha, P.M.; Flanagan, K.A.; Aklujkar, M.; Butler, J.E.; Giloteaux, L.; Rotaru, A.-E.; et al. Geobacter: The microbe electric’s physiology, ecology, and practical applications. Adv. Microb. Physiol. 2011, 59. [Google Scholar] [CrossRef]
- Lovley, D.R. Electromicrobiology. Annu. Rev. Microbiol. 2012, 66, 391–409. [Google Scholar] [CrossRef] [PubMed]
- Mehta, T.; Coppi, M.V.; Childers, S.E.; Lovley, D.R. Outer membrane c-type cytochromes required for Fe(III) and Mn(IV) oxide reduction in Geobacter sulfurreducens. Appl. Environ. Microbiol. 2005, 71, 8634–8641. [Google Scholar] [CrossRef] [PubMed]
- Busalmen, J.P.; Esteve-Núñez, A.; Berná, A.; Feliu, J.M. C-type cytochromes wire electricity-producing bacteria to electrodes. Angew. Chem. 2008, 120, 4952–4955. [Google Scholar] [CrossRef]
- Esteve-Núñez, A.; Busalmen, J.P.; Berná, A.; Gutiérrez-Garrán, C.; Feliu, J.M. Opportunities behind the unusual ability of geobacter sulfurreducens for exocellular respiration and electricity production. Energy Environ. Sci. 2011, 4, 2066–2069. [Google Scholar] [CrossRef]
- Robuschi, L.; Tomba, J.P.; Schrott, G.D.; Bonanni, P.S.; Desimone, P.M.; Busalmen, J.P. Spectroscopic Slicing to Reveal Internal Redox Gradients in Electricity-Producing Biofilms. Angew. Chem. Int. Ed. 2013, 52, 925–928. [Google Scholar] [CrossRef] [PubMed]
- Estevez-Canales, M.; Kuzume, A.; Borjas, Z.; Füeg, M.; Lovley, D.; Wandlowski, T.; Esteve-Núñez, A. A severe reduction in the cytochrome C content of Geobacter sulfurreducens eliminates its capacity for extracellular electron transfer. Environ. Microbiol. Rep. 2015, 7, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Malvankar, N.S.; Lovley, D.R. Microbial nanowires for bioenergy applications. Curr. Opin. Biotechnol. 2014, 27, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Malvankar, N.S.; Vargas, M.; Nevin, K.; Tremblay, P.-L.; Evans-Lutterodt, K.; Nykypanchuk, D.; Martz, E.; Tuominen, M.T.; Lovley, D.R. Structural Basis for Metallic-Like Conductivity in Microbial Nanowires. mBio 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Xing, D.; Call, D.F.; Logan, B.E. Direct biological conversion of electrical current into methane by electromethanogenesis. Environ. Sci. Technol. 2009, 43, 3953–3958. [Google Scholar] [CrossRef] [PubMed]
- Geelhoed, J.S.; Stams, A.J.M. Electricity-Assisted Biological Hydrogen Production from Acetate by Geobacter sulfurreducens. Environ. Sci. Technol. 2011, 45, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Esteve-Nunez, A.; Rothermich, M.; Sharma, M.; Lovley, D. Growth of Geobacter sulfurreducens under nutrient-limiting conditions in continuous culture. Environ. Microbiol. 2005, 7, 641–648. [Google Scholar] [CrossRef] [PubMed]
- De-Bashan, L.E.; Hernandez, J.-P.; Morey, T.; Bashan, Y. Microalgae growth-promoting bacteria as “helpers” for microalgae: A novel approach for removing ammonium and phosphorus from municipal wastewater. Water Res. 2004, 38, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, R.; Bond, D.R.; Butler, J.E.; Esteve-Nunez, A.; Coppi, M.V.; Palsson, B.O.; Schilling, C.H.; Lovley, D.R. Characterization of Metabolism in the Fe(III)-Reducing Organism Geobacter sulfurreducens by Constraint-Based Modeling. Appl. Environ. Microbiol. 2006, 72, 1558–1568. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Coppi, M.V.; Lovley, D.R. Geobacter sulfurreducens Can Grow with Oxygen as a Terminal Electron Acceptor. Appl. Environ. Microbiol. 2004, 70, 2525–2528. [Google Scholar] [CrossRef] [PubMed]
- Nunez, C.; Adams, L.; Childers, S.; Lovley, D.R. The RpoS Sigma Factor in the Dissimilatory Fe(III)-Reducing Bacterium Geobacter sulfurreducens. J. Bacteriol. 2004, 186, 5543–5546. [Google Scholar] [CrossRef] [PubMed]
- Nakada, N.; Shinohara, H.; Murata, A.; Kiri, K.; Managaki, S.; Sato, N.; Takada, H. Removal of selected pharmaceuticals and personal care products (PPCPs) and endocrine-disrupting chemicals (EDCs) during sand filtration and ozonation at a municipal sewage treatment plant. Water Res. 2007, 41, 4373–4382. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.I.; Kato Marcus, A.; Rittmann, B.E. Proton transport inside the biofilm limits electrical current generation by anode-respiring bacteria. Biotechnol. Bioeng. 2008, 100, 872–881. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borjas, Z.; Ortiz, J.M.; Aldaz, A.; Feliu, J.; Esteve-Núñez, A. Strategies for Reducing the Start-up Operation of Microbial Electrochemical Treatments of Urban Wastewater. Energies 2015, 8, 14064-14077. https://doi.org/10.3390/en81212416
Borjas Z, Ortiz JM, Aldaz A, Feliu J, Esteve-Núñez A. Strategies for Reducing the Start-up Operation of Microbial Electrochemical Treatments of Urban Wastewater. Energies. 2015; 8(12):14064-14077. https://doi.org/10.3390/en81212416
Chicago/Turabian StyleBorjas, Zulema, Juan Manuel Ortiz, Antonio Aldaz, Juan Feliu, and Abraham Esteve-Núñez. 2015. "Strategies for Reducing the Start-up Operation of Microbial Electrochemical Treatments of Urban Wastewater" Energies 8, no. 12: 14064-14077. https://doi.org/10.3390/en81212416
APA StyleBorjas, Z., Ortiz, J. M., Aldaz, A., Feliu, J., & Esteve-Núñez, A. (2015). Strategies for Reducing the Start-up Operation of Microbial Electrochemical Treatments of Urban Wastewater. Energies, 8(12), 14064-14077. https://doi.org/10.3390/en81212416






