Effects of Alkali and Alkaline Earth Metals on N-Containing Species Release during Rice Straw Pyrolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Pretreatments
| Sample | Proximate analysis (wt %, air-dried basis) | Ultimate analysis (wt %, air-dried basis) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Moisture | Volatile | Ash | Fixed carbon | C | H | O a | N | S | |
| RS | 7.56 | 66.63 | 10.56 | 15.25 | 38.48 | 4.96 | 37 | 1.03 | 0.27 |
| RS-H2O | 7.23 | 68.39 | 8.23 | 16.15 | 40.82 | 5.16 | 37 | 0.86 | 0.25 |
| RS-HCl | 7.41 | 68.61 | 7.74 | 16.24 | 40.86 | 5.33 | 37 | 0.62 | 0.21 |
| Sample | AAEMs (wt %, by dry weight) | Demineralization efficiency (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| K | Na | Ca | Mg | K | Na | Ca | Mg | |
| RS | 2.214 | 0.200 | 0.641 | 0.250 | - | - | - | - |
| RS-H2O | 0.030 | 0.039 | 0.561 | 0.246 | 98.63 | 80.35 | 12.57 | 1.260 |
| RS-HCl | 0.010 | 0.010 | 0.017 | 0.012 | 99.57 | 95.04 | 97.39 | 95.13 |
2.2. Pyrolysis and Product Analysis
2.3. Pyrolysis Kinetic Analysis
3. Results and Discussion
3.1. Thermal Gravity/Differential Thermal Gravity (TG/DTG) and Kinetic Analysis
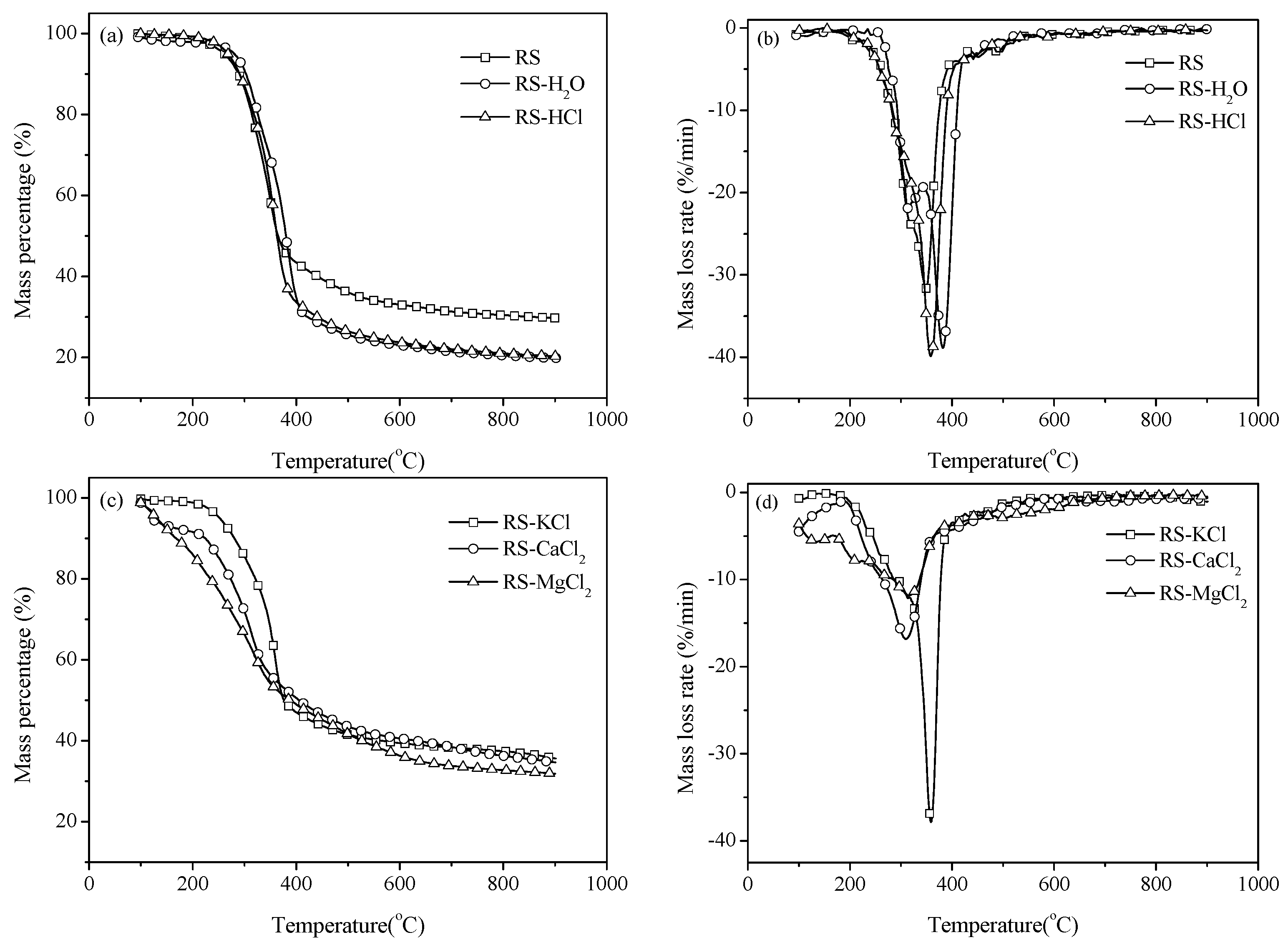
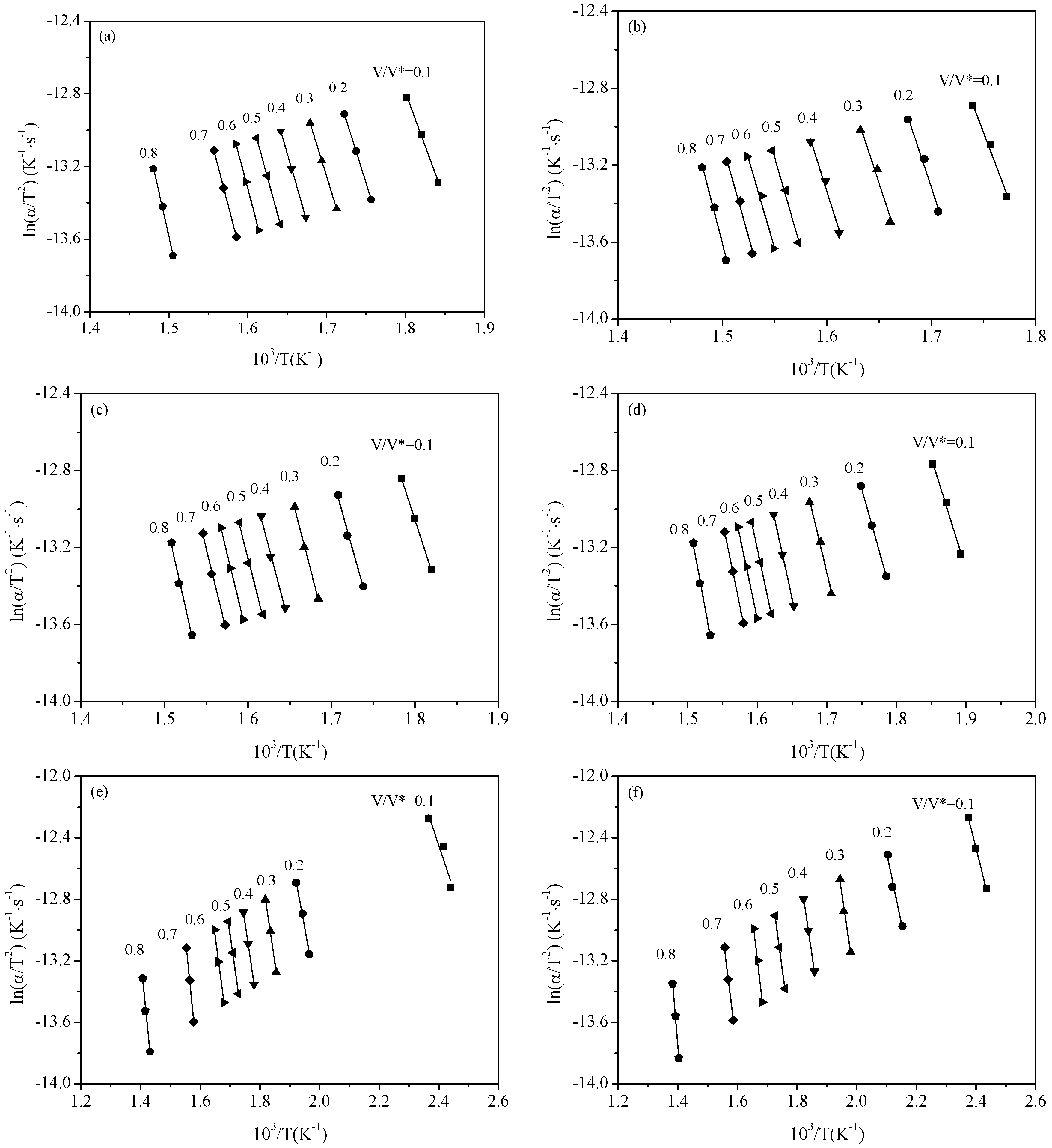
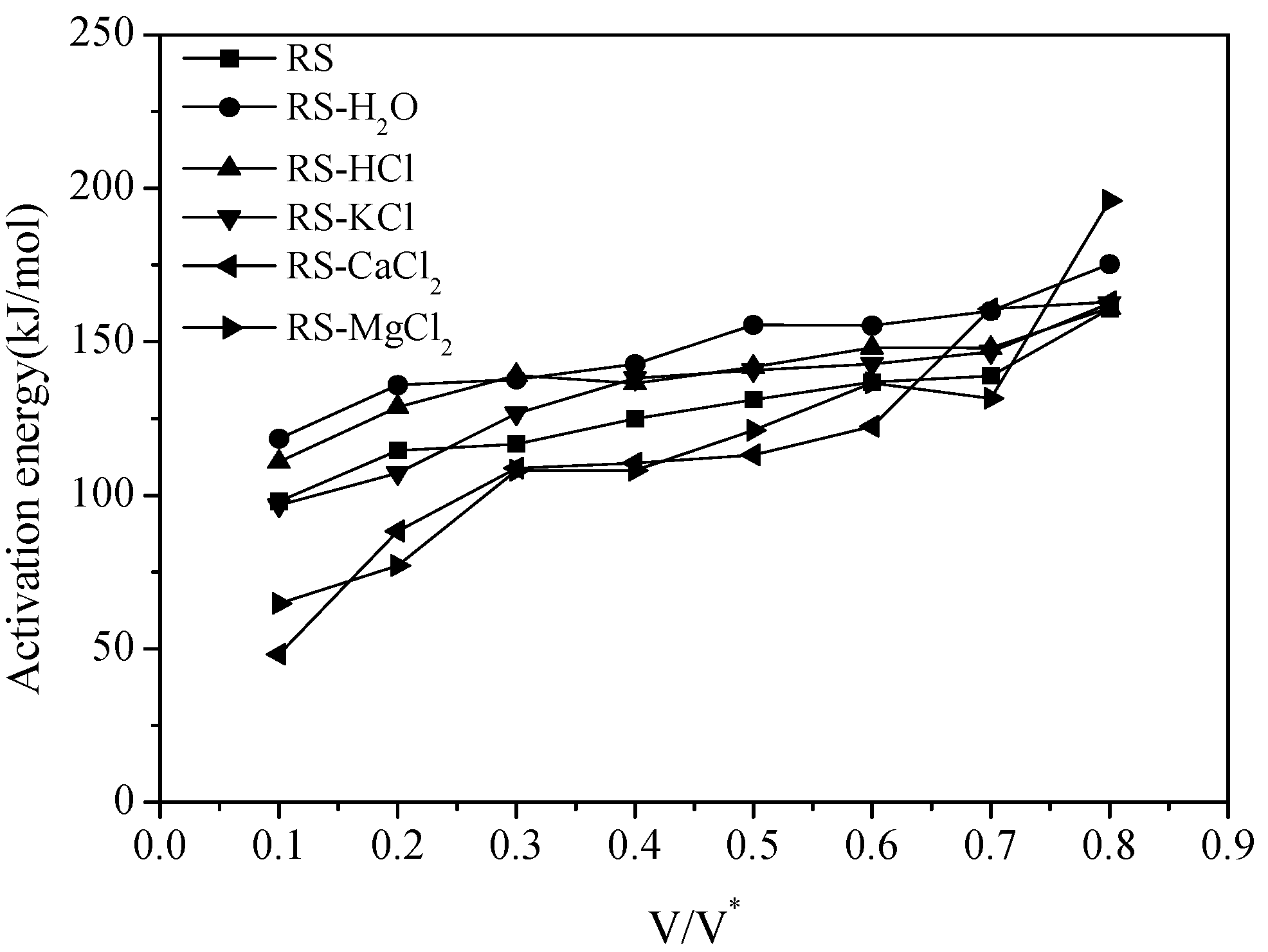
3.2. Effect of Inherent Alkaline Earth Metallic Species (AAEMs) on N-Containing Species Release
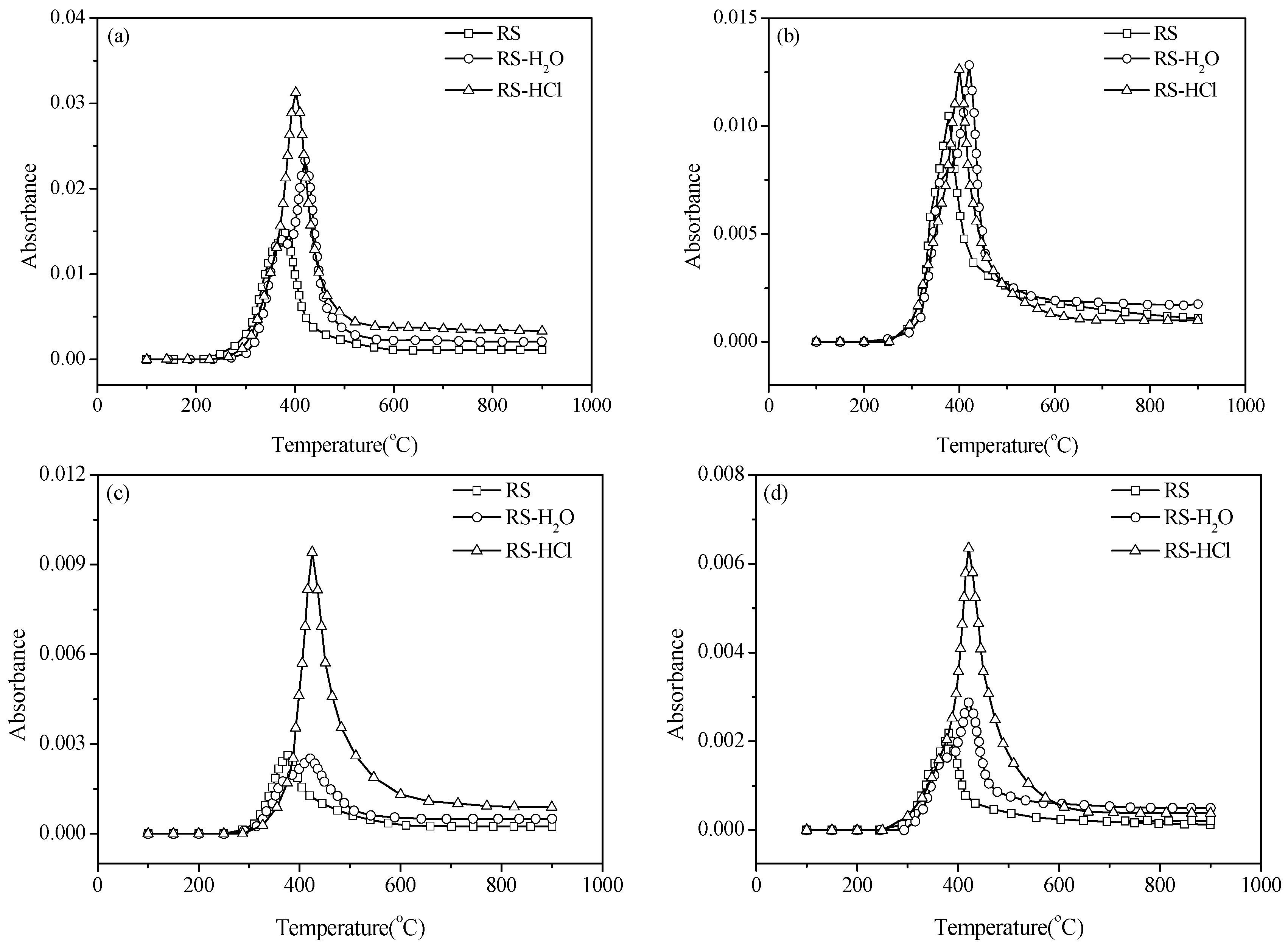
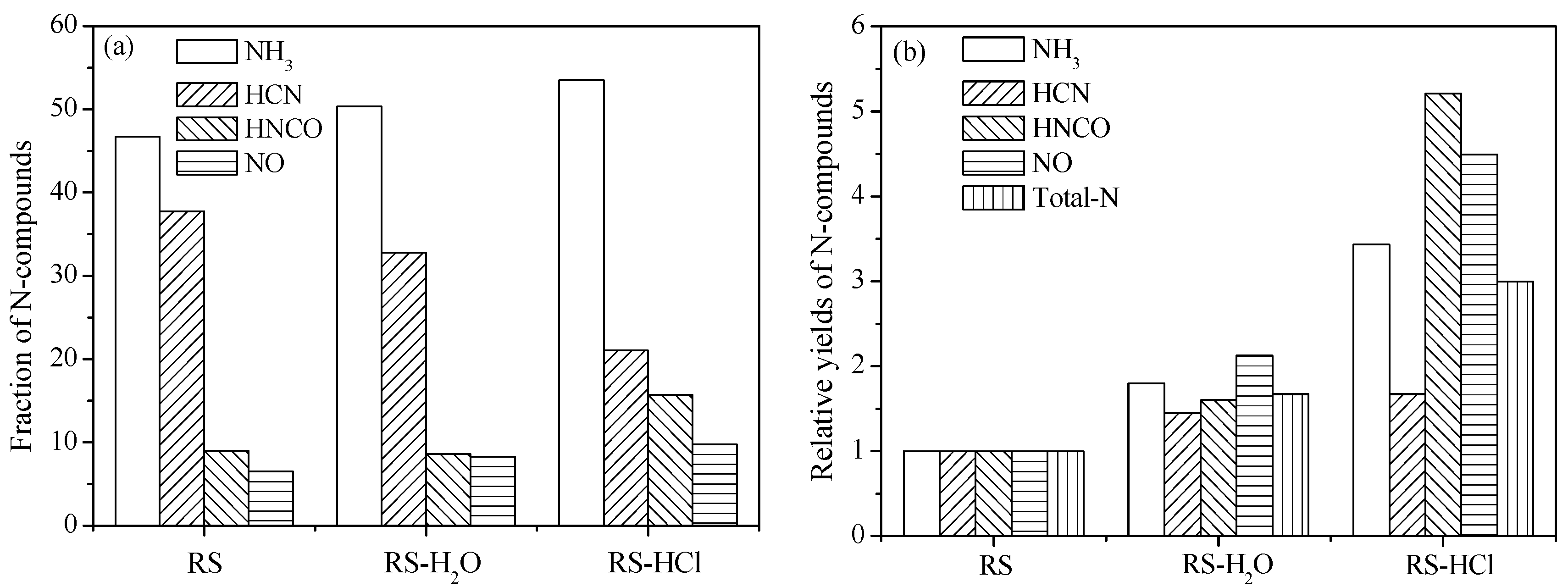
3.3. Effect of External Alkaline Earth Metallic Species on N-Containing Species Release
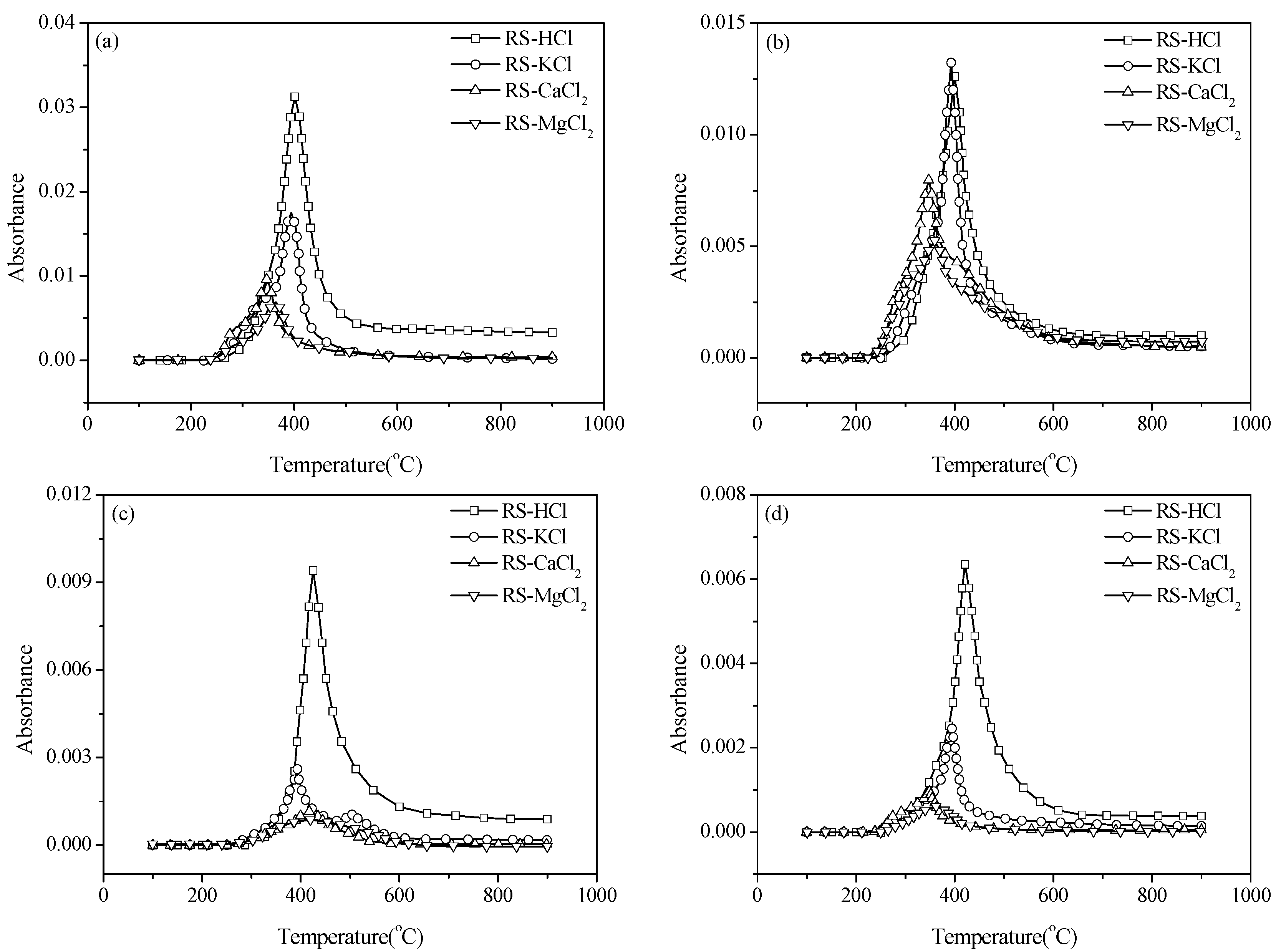
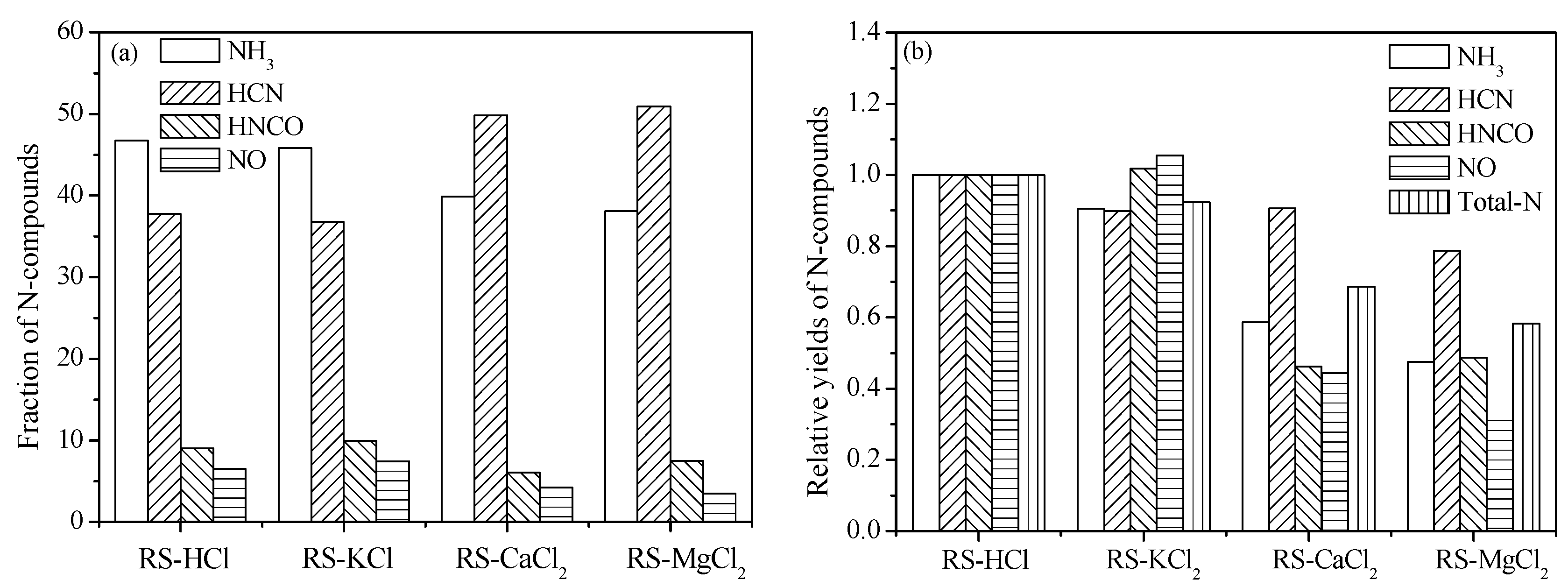
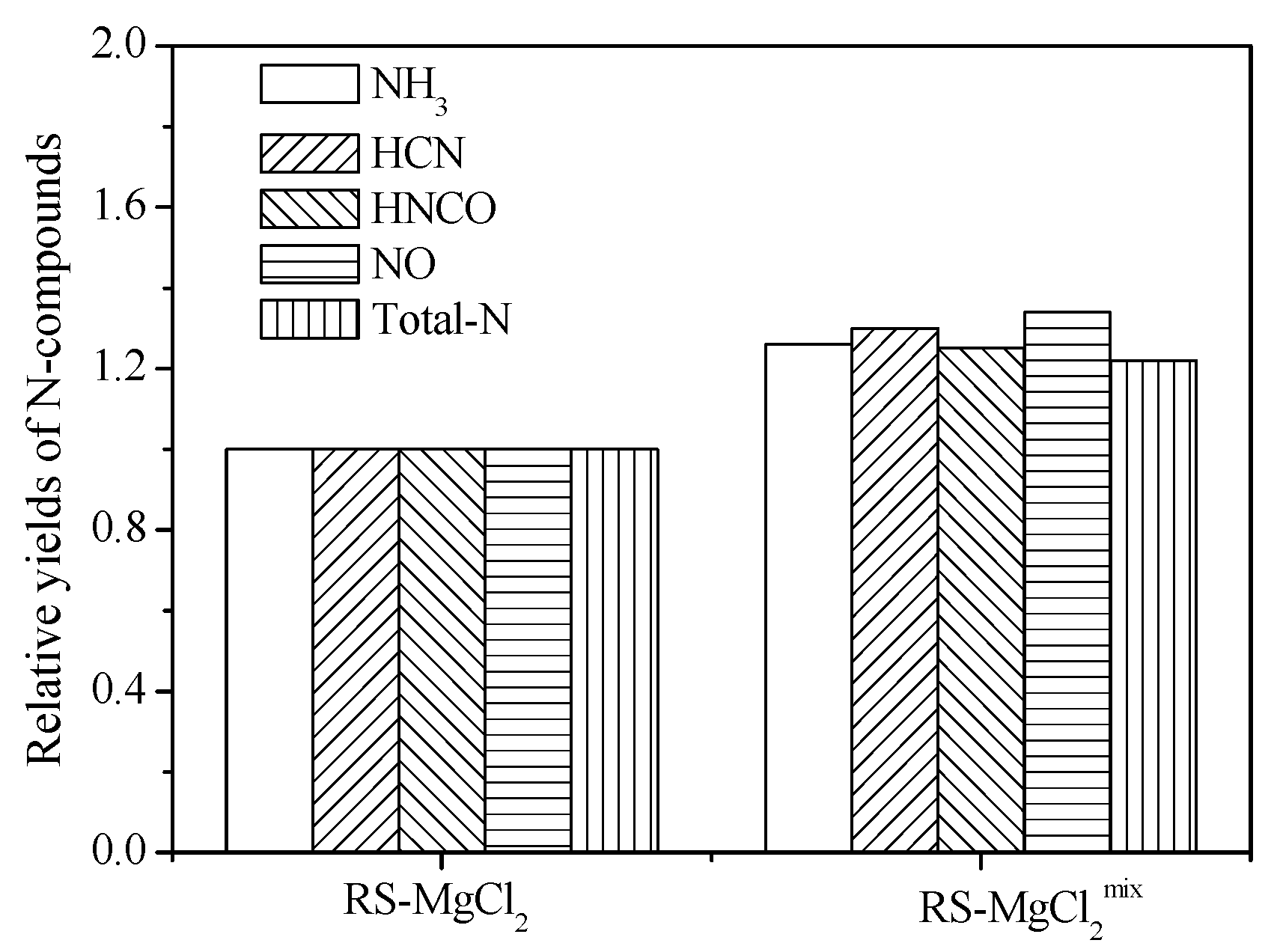
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, W.Y.; Wei, O.Y.; Hao, F.H. A supply-chain analysis framework for assessing densified biomass solid fuel utilization policies in China. Energies 2015, 8, 7122–7139. [Google Scholar] [CrossRef]
- Lu, K.M.; Lee, W.J.; Chen, W.H.; Lin, T.C. Thermogravimetric analysis and kinetics of co-pyrolysis of raw/torrefied wood and coal blends. Appl. Energy 2013, 105, 57–65. [Google Scholar] [CrossRef]
- Zhao, X.G.; Feng, T.T.; Ma, Y.; Yang, Y.S.; Pan, X.F. Analysis on investment strategies in China: The case of biomass direct combustion power generation sector. Renew. Sustain. Energy Rev. 2015, 42, 760–772. [Google Scholar] [CrossRef]
- Siva, S.R.P.; Anders, R.; Rasmus, F. Alkali resistant Cu/zeolite deNOx catalysts for flue gas cleaning in biomass fired applications. Appl. Catal. B Environ. 2011, 101, 183–188. [Google Scholar] [CrossRef]
- Li, Z.Q.; Zhao, W.; Li, R.Y.; Wang, Z.W.; Li, Y.; Zhao, G.B. Combustion characteristics and NO formation for biomass blends in a 35-ton-per-hour traveling grate utility boiler. Bioresour. Technol. 2009, 100, 2278–2283. [Google Scholar] [CrossRef] [PubMed]
- Hansson, K.M.; Samuelsson, J.; Åmand, L.E.; Tullin, C. The temperature’s influence on the selectivity between HNCO and HCN from pyrolysis of 2,5-diketopiperazine and 2-pyridone. Fuel 2003, 82, 2163–2172. [Google Scholar] [CrossRef]
- Hansson, K.M.; Samuelsson, J.; Tullin, C.; Åmand, L.E. Formation of HNCO, HCN, and NH3 from the pyrolysis of bark and nitrogen-containing model compounds. Combust. Flame 2004, 137, 265–277. [Google Scholar] [CrossRef]
- Sun, S.Z.; Tian, H.M.; Zhao, Y.J.; Sun, R.; Zhou, H. Experimental and numerical study of biomass flash pyrolysis in an entrained flow reactor. Bioresour. Technol. 2010, 101, 3678–3684. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.J.; Yu, J.L.; Mckenzie, L.J.; Hayashi, J.; Li, C.Z. Conversion of fuel-N into HCN and NH3 during the pyrolysis and gasification in steam: A comparative study of coal and biomass. Energy Fuel. 2007, 21, 517–521. [Google Scholar] [CrossRef]
- Wu, J.G.; Gao, S.; Wan, J.L.; Zeng, Y.L.; Ma, F.Y.; Zhang, X.Y. Thermogravimetric kinetics of corn stalk pretreated by oleaginous fungi Cunninghamella echinulate. Bioresour. Technol. 2011, 102, 5255–5258. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Jensen, A.D.; Glarborg, P.P.; Jensen, A.; Kavaliauskas, A. Numerical modeling of straw combustion in a fixed bed. Fuel 2005, 84, 389–403. [Google Scholar] [CrossRef]
- Ren, Q.Q.; Zhao, C.S. NOx and N2O precursors (NH3 and HCN) from biomass pyrolysis: Interaction between amino acid and mineral matter. Appl. Energy 2013, 112, 170–174. [Google Scholar] [CrossRef]
- Raveendran, K.; Ganesh, A.; Khilart, K.C. Influence of mineral matter on biomass pyrolysis characteristics. Fuel 1995, 74, 1812–1822. [Google Scholar] [CrossRef]
- Wei, X.L.; Schnell, U.; Hein, K.R.G. Behavior of gaseous chlorine and alkali metals during biomass thermal utilization. Fuel 2005, 84, 841–848. [Google Scholar] [CrossRef]
- Williams, P.; Horne, P.A. The role of metal salts in the pyrolysis of biomass. Renew. Energy 1994, 4, 1–13. [Google Scholar] [CrossRef]
- Yang, H.P.; Yan, R.; Chen, H.P.; Zheng, C.G.; Lee, D.H.; Liang, D.T. Influence of mineral matter on pyrolysis of palm oil waste. Combust. Flame 2006, 164, 605–611. [Google Scholar] [CrossRef]
- Ren, Q.Q.; Zhao, C.S.; Wu, X.; Liang, C.; Chen, X.P.; Shen, J.Z.; Tang, G.Y.; Wang, Z. Effect of mineral matter on the formation of NOx precursors during biomass pyrolysis. J. Anal. Appl. Pyrolysis 2009, 85, 447–453. [Google Scholar] [CrossRef]
- Zhu, H.M.; Jiang, X.G.; Yan, J.H.; Chi, Y.; Cen, K.F. TG-FTIR analysis of PVC thermal degradation and HCl removal. J. Anal. Appl. Pyrolysis 2008, 82, 1–9. [Google Scholar] [CrossRef]
- Gao, P.; Sun, Z.X.; Kong, Y.; Zhou, J.Q.; Dong, C.Q.; Yang, Y.P. Experimental study of nitrogen transformation in biomass pyrolysis. Chin. Sol. Energy Soc. 2014, 35, 2541–2546. (In Chinese) [Google Scholar]
- Kouichi, M.; Taisuke, M. A simple method for estimating f(E) and k0(E) in the distributed activation energy model. Energy Fuel 1998, 12, 864–869. [Google Scholar] [CrossRef]
- Kouichi, M. A new and simple method to estimate f(E) and k0(E) in the distributed activation energy model from three sets of experimental data. Energy Fuel. 1995, 9, 302–307. [Google Scholar] [CrossRef]
- Antonio, S.V.; Elke, G.; Nestor, G.H. Effect of the number of TGA curves employed on the biomass pyrolysis kinetics results obtained using the distributed activation energy model. Fuel Process. Technol. 2015, 134, 360–371. [Google Scholar] [CrossRef]
- Long, J.; Song, H.; Sun, L.S.; Su, S.; Xu, K.; He, L.M.; Xiang, J. Influence of different demineralization treatments on physicochemical structure and thermal degradation of biomass. Bioresour. Technol. 2013, 146, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Ferrante, L.; Briens, C.; Berruti, F. Flash pyrolysis of grape residues into biofuel in a bubbling fluid bed. J. Anal. Appl. Pyrolysis 2009, 86, 58–65. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, P.; Xue, L.; Lu, Q.; Dong, C. Effects of Alkali and Alkaline Earth Metals on N-Containing Species Release during Rice Straw Pyrolysis. Energies 2015, 8, 13021-13032. https://doi.org/10.3390/en81112356
Gao P, Xue L, Lu Q, Dong C. Effects of Alkali and Alkaline Earth Metals on N-Containing Species Release during Rice Straw Pyrolysis. Energies. 2015; 8(11):13021-13032. https://doi.org/10.3390/en81112356
Chicago/Turabian StyleGao, Pan, Lu Xue, Qiang Lu, and Changqing Dong. 2015. "Effects of Alkali and Alkaline Earth Metals on N-Containing Species Release during Rice Straw Pyrolysis" Energies 8, no. 11: 13021-13032. https://doi.org/10.3390/en81112356
APA StyleGao, P., Xue, L., Lu, Q., & Dong, C. (2015). Effects of Alkali and Alkaline Earth Metals on N-Containing Species Release during Rice Straw Pyrolysis. Energies, 8(11), 13021-13032. https://doi.org/10.3390/en81112356