Thermal Decomposition of Anhydrous Alkali Metal Dodecaborates M2B12H12 (M = Li, Na, K)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Decomposition of Anhydrous Li2B12H12
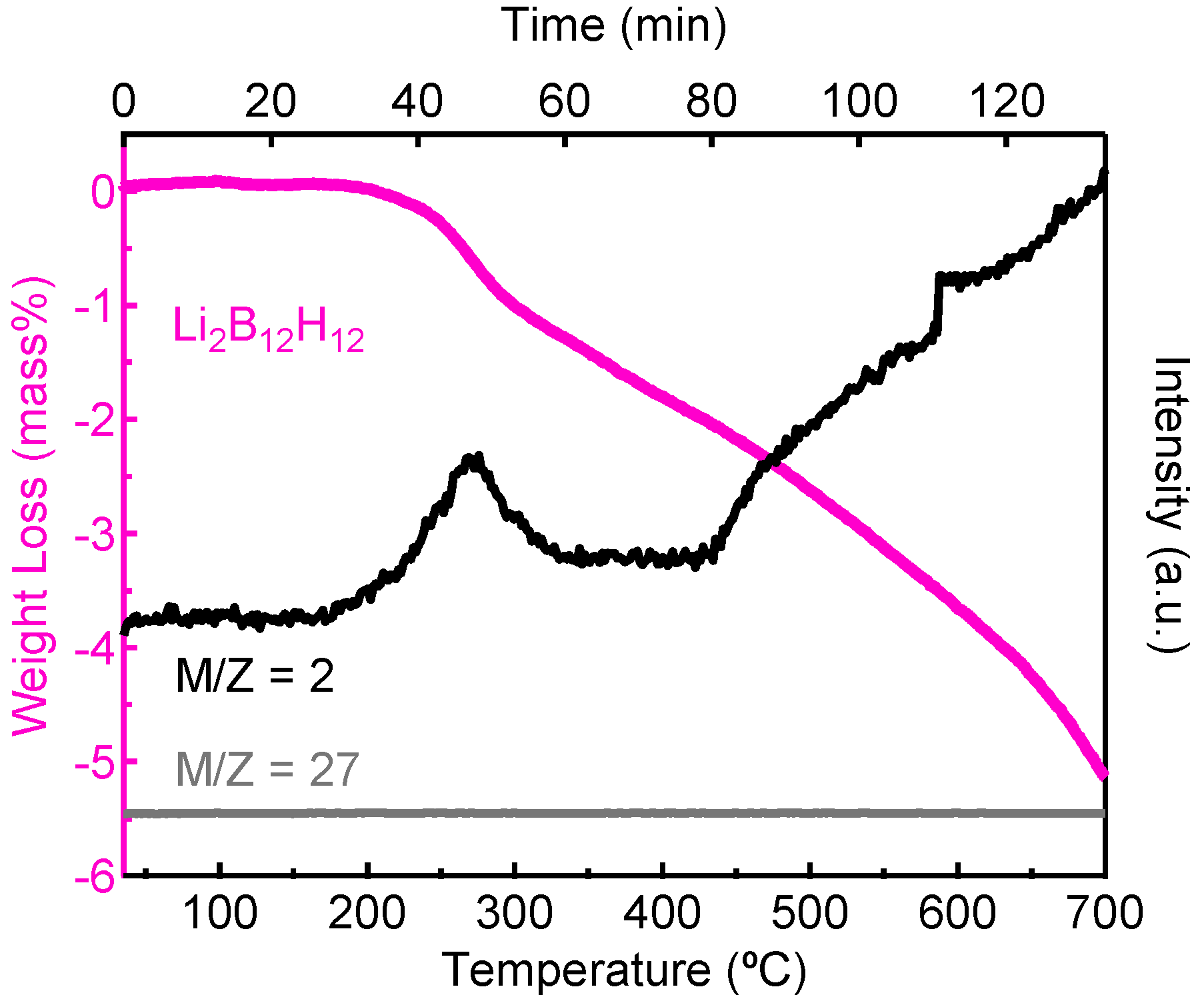
| Temperature, °C | [B12H12]2−, % | [B11H11]2−, % | [B10H10]2−, % | [BH4]−, % | Unknown, % |
|---|---|---|---|---|---|
| 200 | 60.93 | 9.03 | 18.76 | 5.14 | 6.14 |
| 225 | 65.82 | 9.50 | 12.16 | 3.76 | 8.76 |
| 250 | 70.17 | 10.00 | 17.21 | 2.62 | 0 |
| 300 | 71.62 | 11.70 | 16.20 | 0.48 | 0 |
| 400 | 86.52 | 0 | 13.48 | 0 | 0 |
| 500 | 100 | 0 | 0 | 0 | 0 |


2.2. Decomposition of Anhydrous Na2B12H12
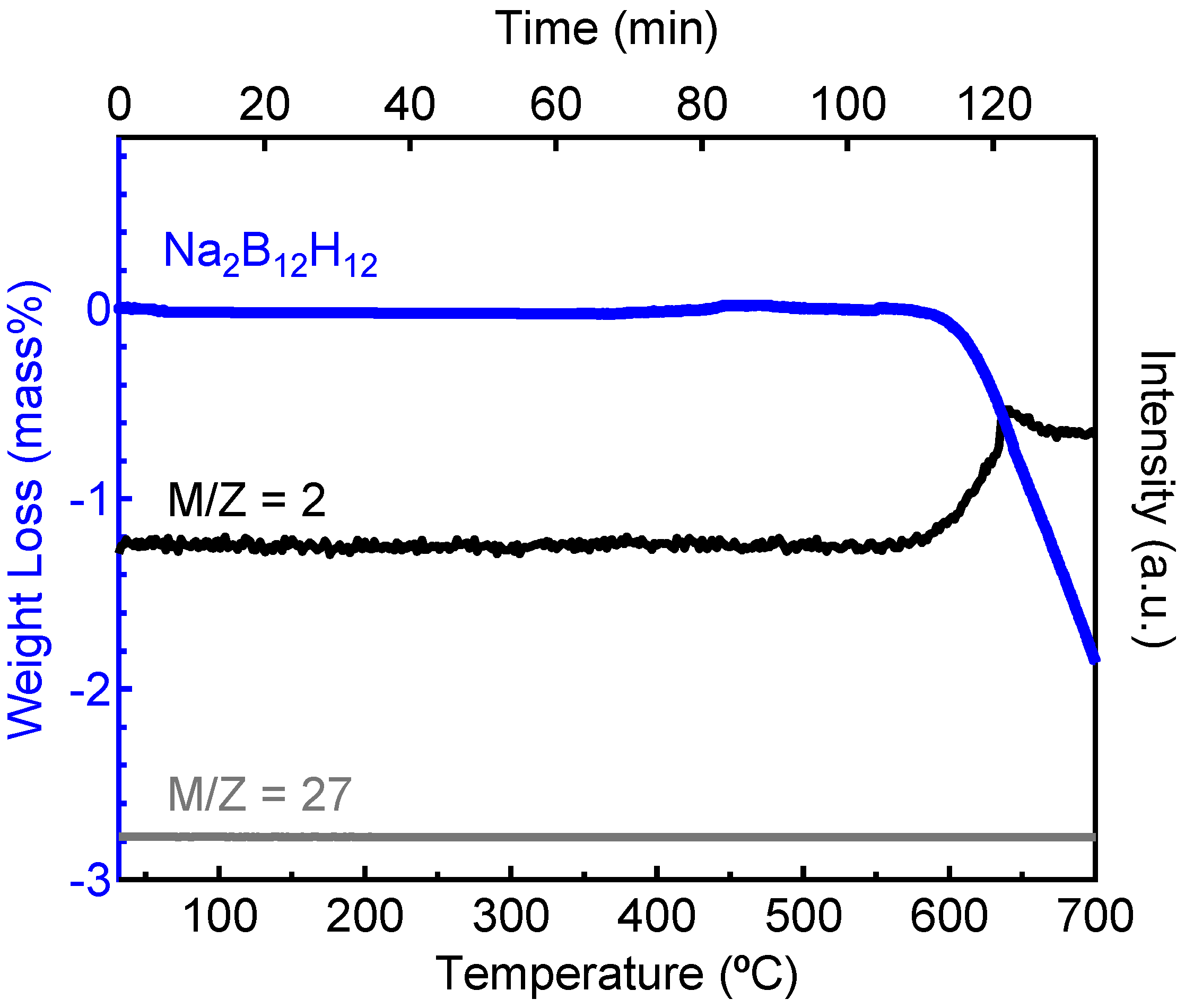
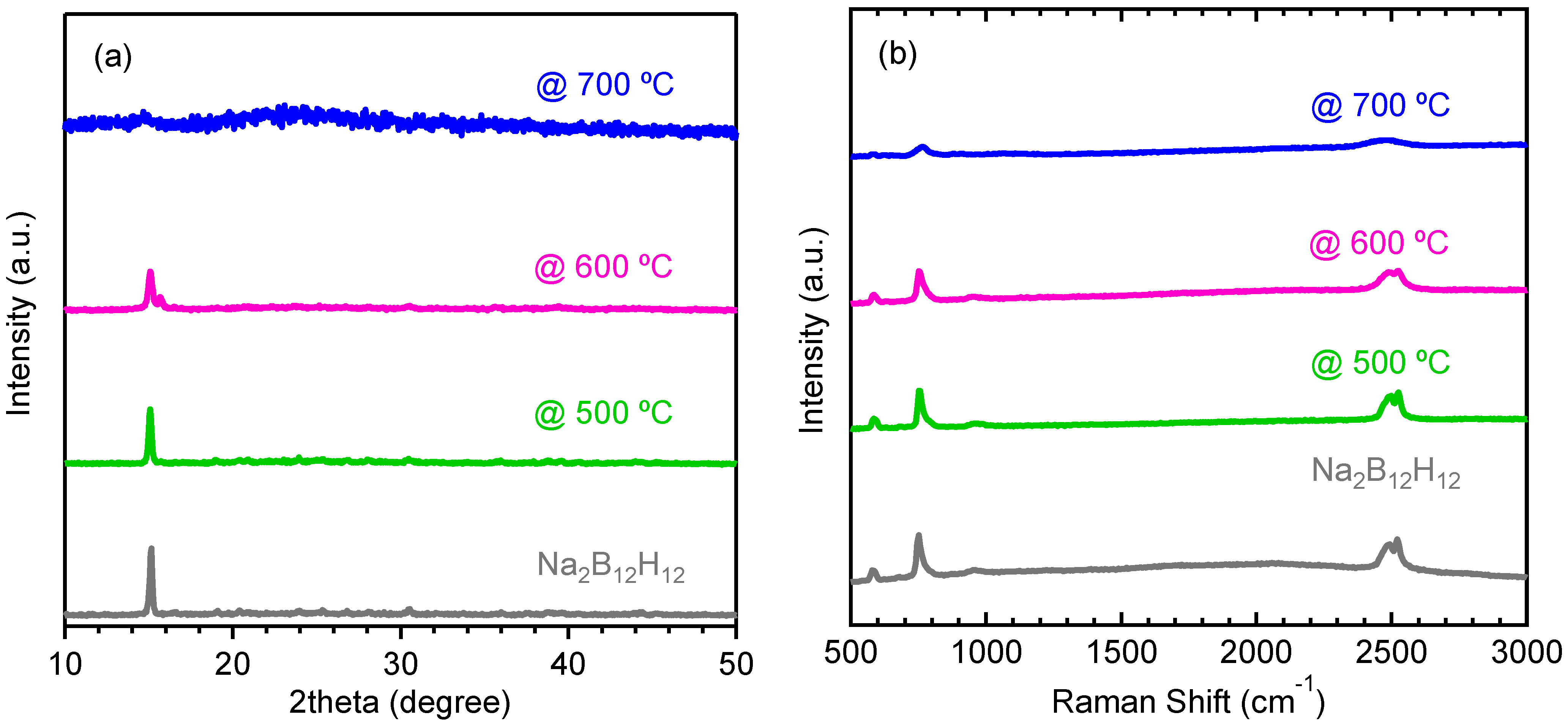
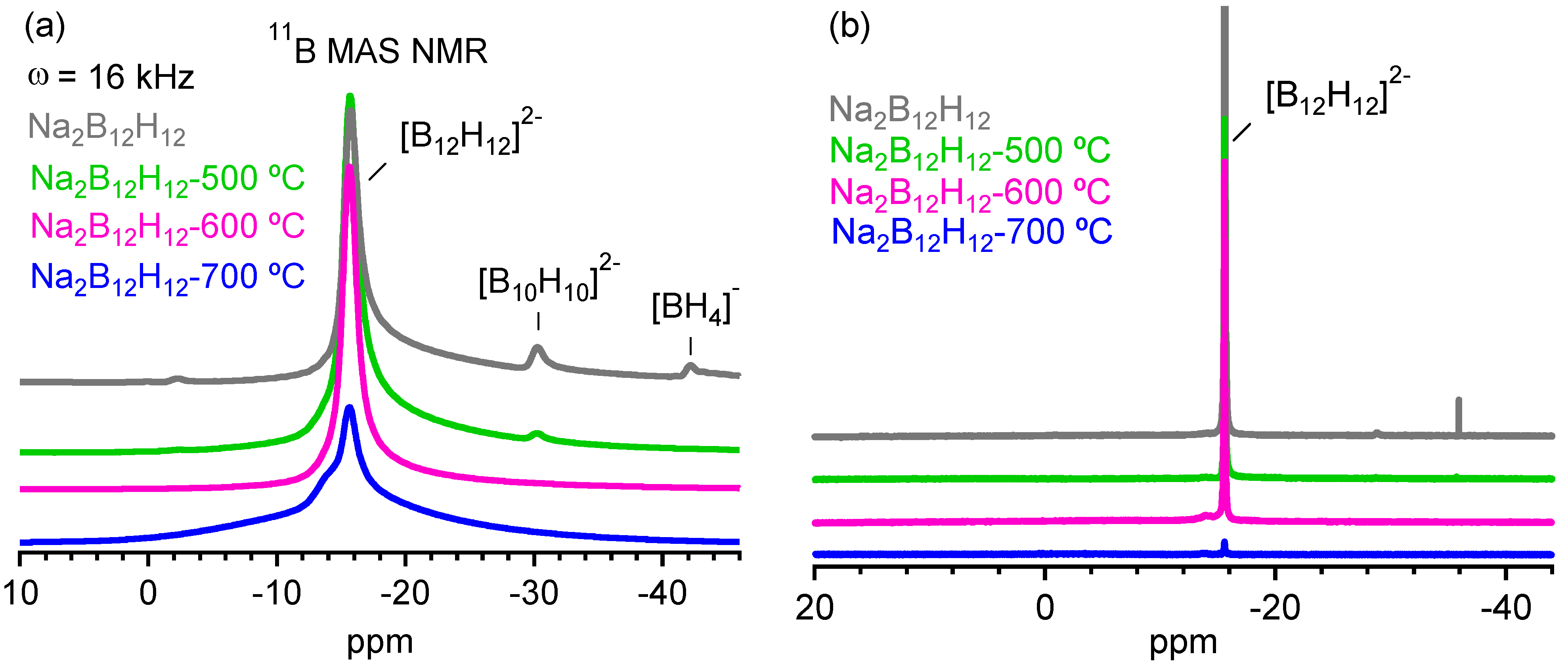
2.3. Decomposition of Anhydrous K2B12H12
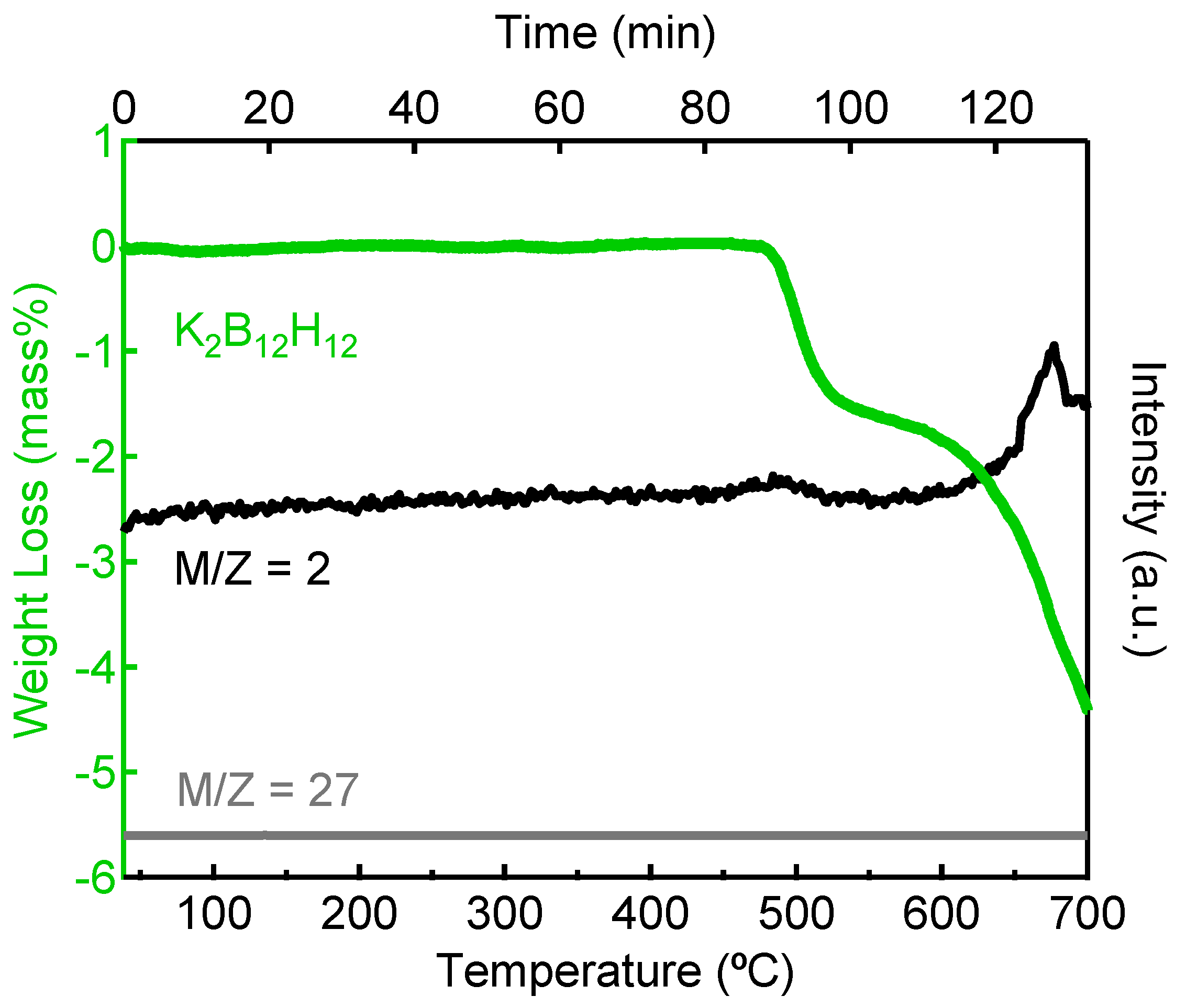
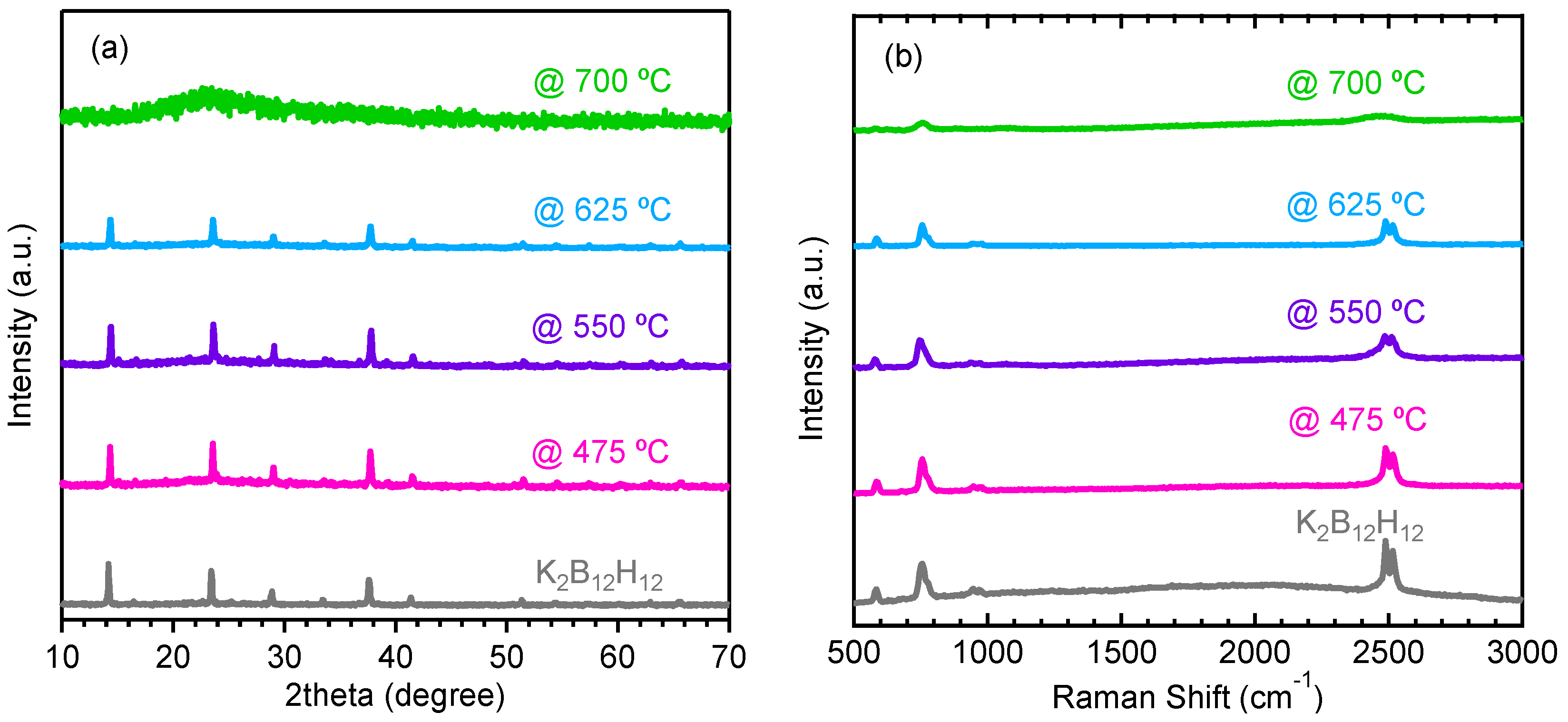
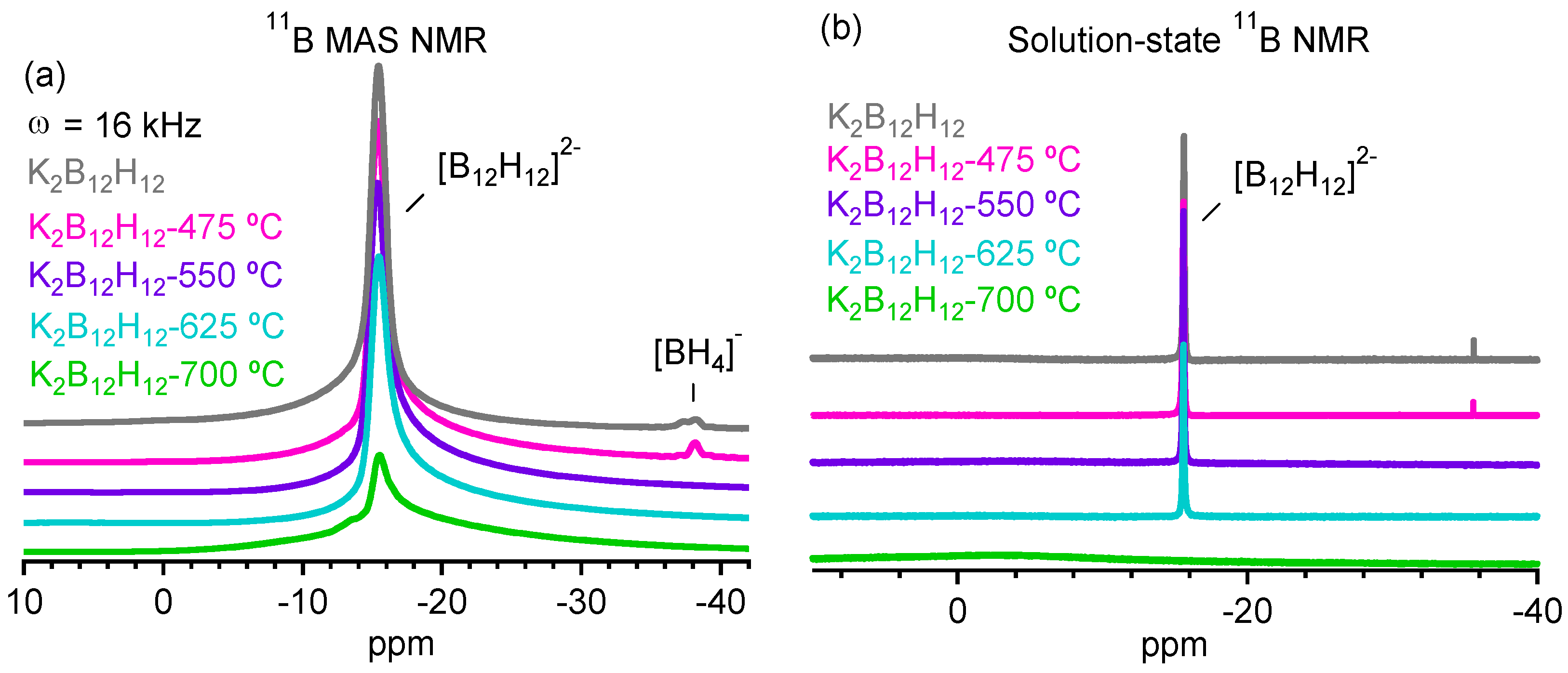
3. Experimental Section
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, H.-W.; Yan, Y.; Orimo, S.-I.; Züttel, A.; Jensen, C.M. Recent progress in metal borohydrides for hydrogen storage. Energies 2011, 4, 185–214. [Google Scholar] [CrossRef]
- Rude, L.H.; Nielsen, T.K.; Ravnsbaek, D.B.; Bösenberg, U.; Ley, M.B.; Richter, B.; Arnbjerg, L.M.; Dornheim, M.; Filinchuk, Y.; Besenbacher, F.; et al. Tailoring properties of borohydrides for hydrogen storage: A review. Phys. Status Solidi A 2011, 208, 1754–1773. [Google Scholar] [CrossRef]
- Orimo, S.-I.; Nakamori, Y.; Ohba, N.; Miwa, K.; Aoki, M.; Towata, S.-I.; Zuttel, A. Experimental studies on intermediate compound of LiBH4. Appl. Phys. Lett. 2006, 89. [Google Scholar] [CrossRef]
- Ohba, N.; Miwa, K.; Aoki, M.; Noritake, T.; Towata, S.-I.; Nakamori, Y.; Orimo, S.-I.; Züttel, A. First-principles study on the stability of intermediate compounds of LiBH4. Phys. Rev. B 2006, 74. [Google Scholar] [CrossRef]
- Li, H.-W.; Kikuchi, K.; Nakamori, Y.; Ohba, N.; Miwa, K.; Towata, S.; Orimo, S. Dehydriding and rehydriding processes of well-crystallized Mg(BH4)2 accompanying with formation of intermediate compounds. Acta Mater. 2008, 56, 1342–1347. [Google Scholar] [CrossRef]
- Hwang, S.-J.; Bowman, R.C.; Reiter, J.W.; Rijssenbeek, J.; Soloveichik, G.L.; Zhao, J.-C.; Kabbour, H.; Ahn, C.C. NMR confirmation for formation of [B12H12]2− complexes during hydrogen desorption from metal borohydrides. J. Phys. Chem. C 2008, 112, 3164–3169. [Google Scholar] [CrossRef]
- Her, J.-H.; Yousufuddin, M.; Zhou, W.; Jalisatgi, S.S.; Kulleck, J.G.; Zan, J.A.; Hwang, S.-J.; Bowman, R.C., Jr.; Udovic, T.J. Crystal structure of Li2B12H12: A possible intermediate species in the decomposition of LiBH4. Inorg. Chem. 2008, 47, 9757–9759. [Google Scholar] [CrossRef] [PubMed]
- Ozolins, V.; Majzoub, E.; Wolverton, C. First-Principles Prediction of Thermodynamically reversible hydrogen storage reactions in the Li-Mg-Ca-B-H system. J. Am. Chem. Soc. 2009, 131, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-W.; Miwa, K.; Ohba, N.; Fujita, T.; Sato, T.; Yan, Y.; Towata, S.; Chen, M.; Orimo, S. Formation of an intermediate compound with a B12H12 cluster: Experimental and theoretical studies on magnesium borohydride Mg(BH4)2. Nanotechnology 2009, 20. [Google Scholar] [CrossRef] [PubMed]
- Stavila, V.; Her, J.-H.; Zhou, W.; Hwang, S.-J.; Kim, C.; Ottley, L.A.M.; Udovic, T.J. Probing the structure, stability and hydrogen storage properties of calcium dodecahydro-closo-dodecaborate. J. Solid State Chem. 2010, 183, 1133–1140. [Google Scholar] [CrossRef]
- Yan, Y.; Li, H.-W.; Maekawa, H.; Aoki, M.; Noritake, T.; Matsumoto, M.; Miwa, K.; Towata, S.-I.; Orimo, S.-I. Formation Process of [B12H12]2− from [BH4]− during the Dehydrogenation Reaction of Mg(BH4)2. Mater. Trans. 2011, 52, 1443–1446. [Google Scholar] [CrossRef]
- Bonatto Minella, C.; Garroni, S.; Olid, D.; Teixidor, F.; Pistidda, C.; Lindemann, I.; Gutfleisch, O.; Baró, M.D.; Bormann, R.; Klassen, T.; Dornheim, M. Experimental evidence of Ca[B12H12] formation during decomposition of a Ca(BH4)2 + MgH2 based reactive hydride composite. J. Phys. Chem. C 2011, 115, 18010–18014. [Google Scholar] [CrossRef]
- Garroni, S.; Milanese, C.; Pottmaier, D.; Mulas, G.; Nolis, P.; Girella, A.; Caputo, R.; Olid, D.; Teixdor, F.; Baricco, M. Experimental evidence of Na2[B12H12] and Na formation in the desorption pathway of the 2NaBH4 + MgH2 system. J. Phys. Chem.C 2011, 115, 16664–16671. [Google Scholar] [CrossRef]
- Yan, Y.; Li, H.-W.; Maekawa, H.; Miwa, K.; Towata, S.; Orimo, S. Formation of intermediate compound Li2B12H12 during the dehydrogenation process of the LiBH4-MgH2 system. J. Phys. Chem. C 2011, 115, 19419–19423. [Google Scholar] [CrossRef]
- Yan, Y.; Remhof, A.; Hwang, S.-J.; Li, H.-W.; Mauron, P.; Orimo, S.-I.; Züttel, A. Pressure and temperature dependence of the decomposition pathway of LiBH4. Phys. Chem. Chem. Phys. 2012, 14, 6514–6519. [Google Scholar] [CrossRef] [PubMed]
- Pitt, M.P.; Paskevicius, M.; Brown, D.H.; Sheppard, D.A.; Buckley, C.E. Thermal Stability of Li2B12H12 and Its Role in the Decomposition of LiBH4. J. Am. Chem. Soc. 2013, 135, 6930–6941. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Remhof, A.; Rentsch, D.; Züttel, A. The role of MgB12H12 in the hydrogen desorption process of Mg(BH4)2. Chem. Commun. 2015, 51, 700–702. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Meng, Q.; Guo, Z.; Gu, Q.; Liu, H.; Liu, Z.; Yu, X. Nanoconfinement significantly improves the thermodynamics and kinetics of co-infiltrated 2LiBH4-LiAlH4 composites: Stable reversibility of hydrogen absorption/resorption. Acta Mater. 2013, 61, 6882–6893. [Google Scholar] [CrossRef]
- Borgschulte, A.; Callini, E.; Probst, B.; Jain, A.; Kato, S.; Friedrichs, O.; Remhof, A.; Bielmann, M.; Ramirez-Cuesta, A.; Züttel, A. Impurity gas analysis of the decomposition of complex hydrides. J. Phys. Chem. C 2011, 115, 17220–17226. [Google Scholar] [CrossRef]
- Stadie, N.P.; Callini, E.; Richter, B.; Jensen, T.R.; Borgschulte, A.; Züttel, A. Supercritical N2 Processing as a Route to the Clean Dehydrogenation of Porous Mg(BH4)2. J. Am. Chem. Soc. 2014, 136, 8181–8184. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Li, H.-W.; Tumanov, N.; Filinchuk, Y.; Akiba, E. Facile synthesis of anhydrous alkaline earth metal dodecaborates MB12H12 (M = Mg, Ca) from M(BH4)2. Dalton Trans. 2015, 44, 15882–15887. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Hwang, S.-J.; Shim, J.-H.; Lee, Y.-S.; Han, H.N.; Cho, Y.W. Investigation of the Dehydrogenation Reaction Pathway of Ca(BH4)2 and Reversibility of Intermediate Phases. J. Phys. Chem. C 2012, 116, 4330–4334. [Google Scholar] [CrossRef]
- Li, H.-W.; Akiba, E.; Orimo, S.-I. Comparative study on the reversibility of pure metal borohydrides. J. Alloy. Compd. 2013, 580, S292–S295. [Google Scholar] [CrossRef]
- Minella, C.B.; Pistidda, C.; Garroni, S.; Nolis, P.; BaroÓ, M.D.; Gutfleisch, O.; Klassen, T.; Bormann, R.; Dornheim, M. Ca(BH4)2 + MgH2: Desorption reaction and role of Mg on its reversibility. J. Phys. Chem. C 2013, 117, 3846–3852. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.I.; Sjöberg, S. Chemistry of closo-Dodecaborate Anion [B12H12]2−: A Review. Collect. Czechoslov. Chem. Commun. 2002, 67, 679–727. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.-H.; Alexander, A.-M.; Gallucci, J.C.; Hwang, S.-J.; Lingam, H.K.; Huang, Z.; Wang, C.; Li, H.; Zhao, Q.; et al. Desolvation and dehydrogenation of solvated magnesium salts of dodecahydrododecaborate: Relationship between structure and thermal decomposition. Chem. A Eur. J. 2014, 20, 7325–7333. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Li, H.-W.; Hwang, S.-J.; Akiba, E. Facile Solvent-Free Synthesis of Anhydrous Alkali Metal Dodecaborate M2B12H12 (M= Li, Na, K). J. Phys. Chem. C 2014, 118, 6084–6089. [Google Scholar] [CrossRef]
- He, L.; Li, H.-W.; Nakajima, H.; Tumanov, N.; Filinchuk, Y.; Hwang, S.-J.; Sharma, M.; Hagemann, H.; Akiba, E. Synthesis of a bimetallic dodecaborate LiNaB12H12 with outstanding superionic conductivity. Chem. Mater. 2015, 27, 5483–5486. [Google Scholar] [CrossRef]
- Hwang, S.-J.; Bowman Jr, R.C.; Kim, C.; Zan, J.A.; Reiter, J.W. Solid State NMR Characterization of Complex Metal Hydrides systems for Hydrogen Storage Applications. J. Anal. Sci. Technol. 2011, 2, A159–A162. [Google Scholar] [CrossRef]
- Heřmánek, S. B NMR spectra of boranes, main-group heteroboranes, and substituted derivatives: Factors influencing chemical shifts of skeletal atoms. Chem. Rev. 1992, 92, 325–362. [Google Scholar] [CrossRef]
- Verdal, N.; Her, J.-H.; Stavila, V.; Soloninin, A.V.; Babanova, O.A.; Skripov, A.V.; Udovic, T.J.; Rush, J.J. Complex high-temperature phase transitions in Li2B12H12 and Na2B12H12. J. Solid State Chem. 2014, 212, 81–91. [Google Scholar] [CrossRef]
- Remhof, A.; Yan, Y.; Rentsch, D.; Borgschulte, A.; Jensen, C.M.; Züttel, A. Solvent-free synthesis and stability of MgB12H12. J. Mater. Chem. A 2014, 2, 7244–7249. [Google Scholar] [CrossRef]
- Nakamori, Y.; Miwa, K.; Ninomiya, A.; Li, H.; Ohba, N.; Towata, S.-I.; Züttel, A.; Orimo, S.-I. Correlation between thermodynamical stabilities of metal borohydrides and cation electronegativites: First-principles calculations and experiments. Phys. Rev. B 2006, 74. [Google Scholar] [CrossRef]
- Guo, Y.; Jia, J.; Wang, X.-H.; Ren, Y.; Wu, H. Prediction of thermodynamically reversible hydrogen storage reactions in the KBH4/M (M= Li, Na, Ca)(BH4)n (n = 1, 2) system from first-principles calculation. Chem. Phys. 2013, 418, 22–27. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, L.; Li, H.-W.; Akiba, E. Thermal Decomposition of Anhydrous Alkali Metal Dodecaborates M2B12H12 (M = Li, Na, K). Energies 2015, 8, 12429-12438. https://doi.org/10.3390/en81112326
He L, Li H-W, Akiba E. Thermal Decomposition of Anhydrous Alkali Metal Dodecaborates M2B12H12 (M = Li, Na, K). Energies. 2015; 8(11):12429-12438. https://doi.org/10.3390/en81112326
Chicago/Turabian StyleHe, Liqing, Hai-Wen Li, and Etsuo Akiba. 2015. "Thermal Decomposition of Anhydrous Alkali Metal Dodecaborates M2B12H12 (M = Li, Na, K)" Energies 8, no. 11: 12429-12438. https://doi.org/10.3390/en81112326
APA StyleHe, L., Li, H.-W., & Akiba, E. (2015). Thermal Decomposition of Anhydrous Alkali Metal Dodecaborates M2B12H12 (M = Li, Na, K). Energies, 8(11), 12429-12438. https://doi.org/10.3390/en81112326







