Molecular Simulation Studies of Flue Gas Purification by Bio-MOF
Abstract
:1. Introduction
2. Results and Discussion
2.1. Pure Gases
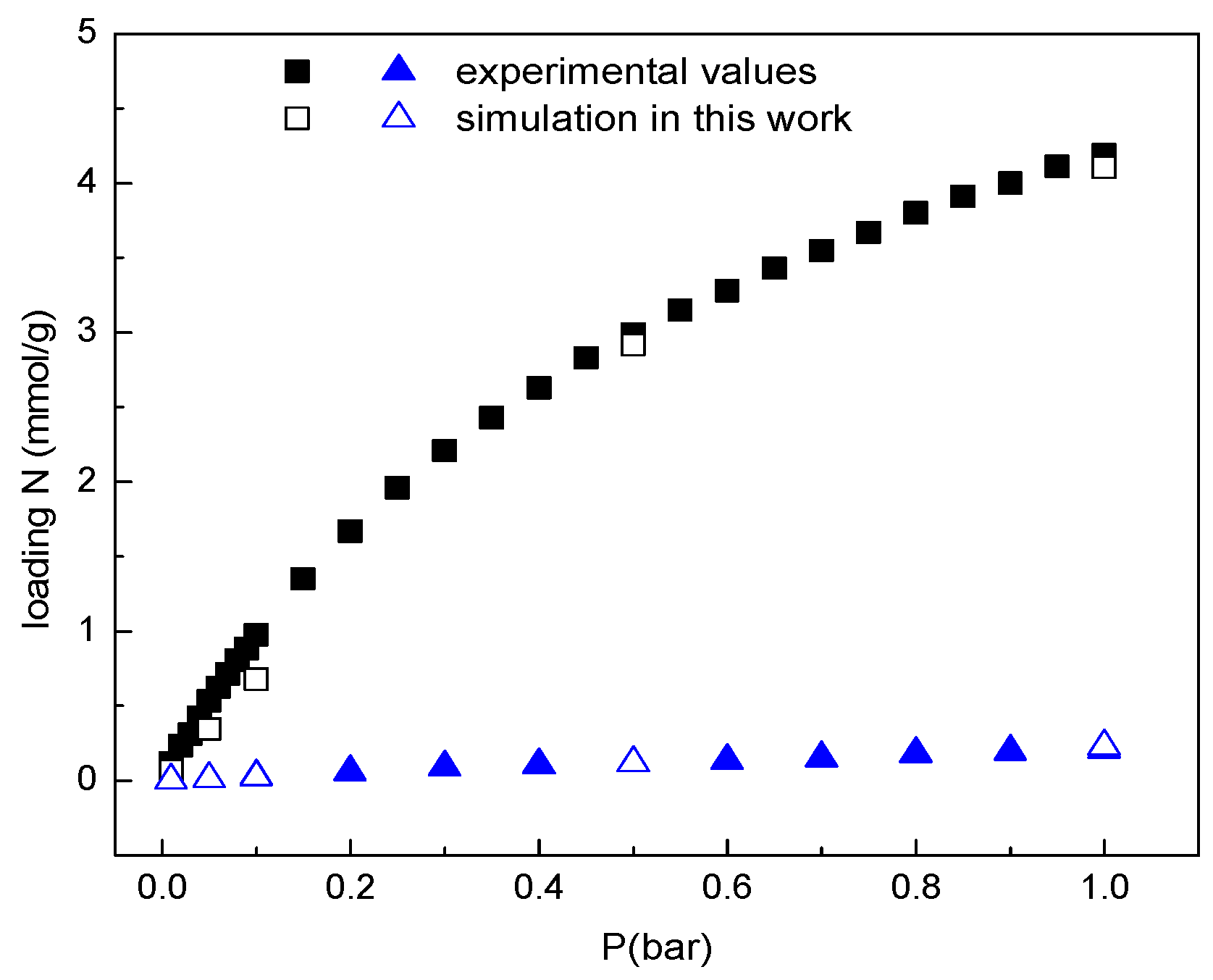
2.2. The Adsorption Selectivity of CO2/N2 Gas Mixture
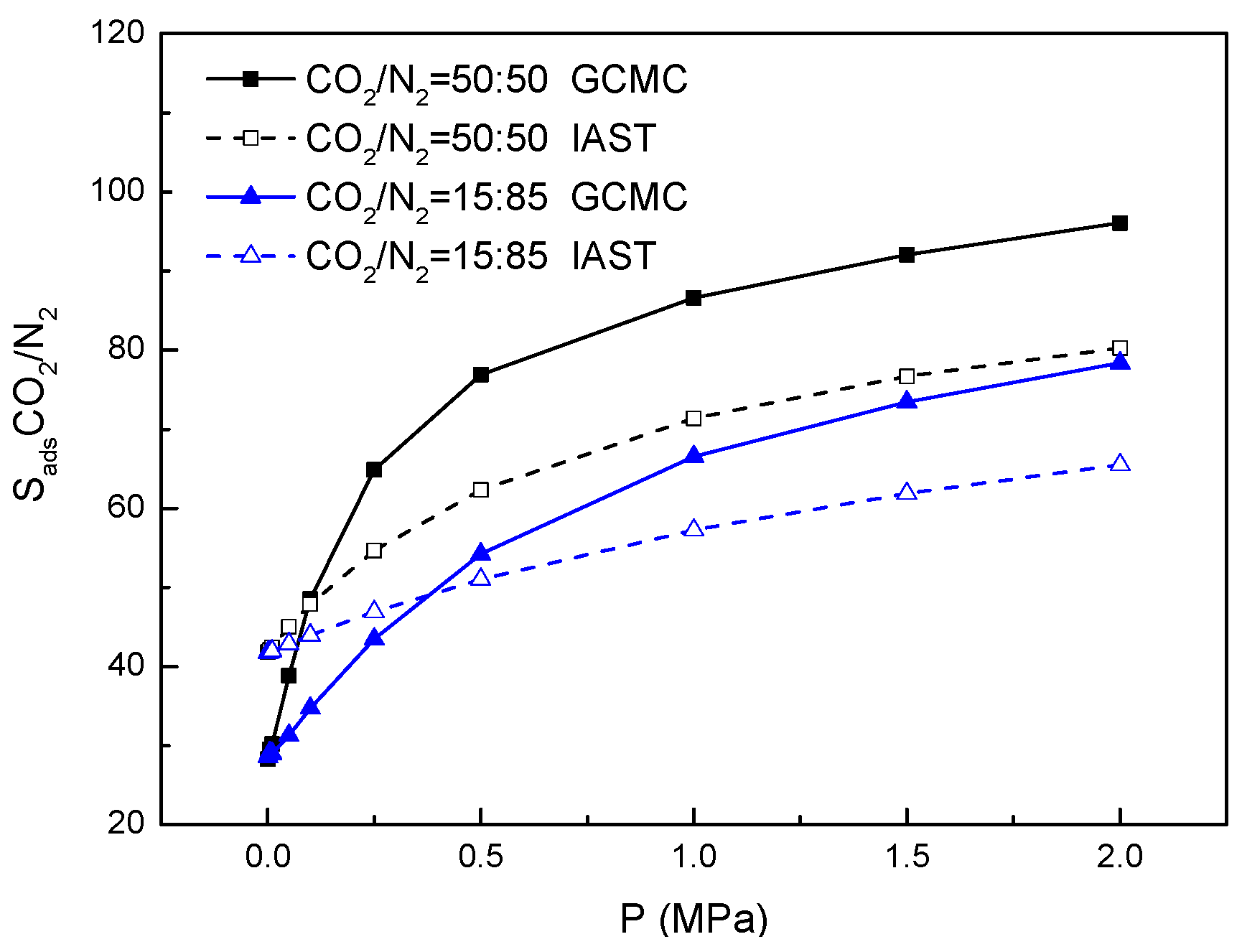

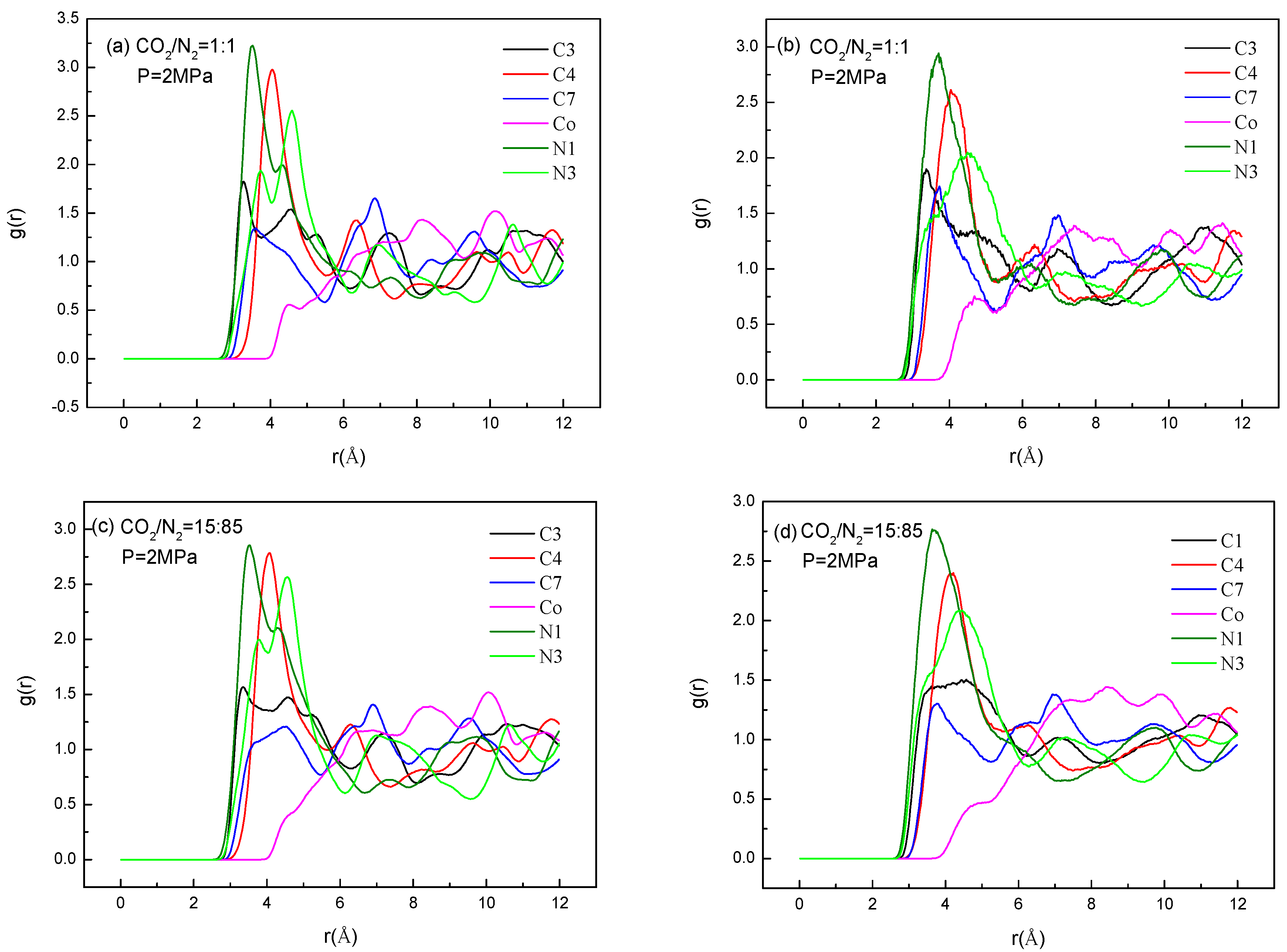

2.3. Effect of Electrostatic Interactions on Adsorption Selectivity in Bio-MOF-11
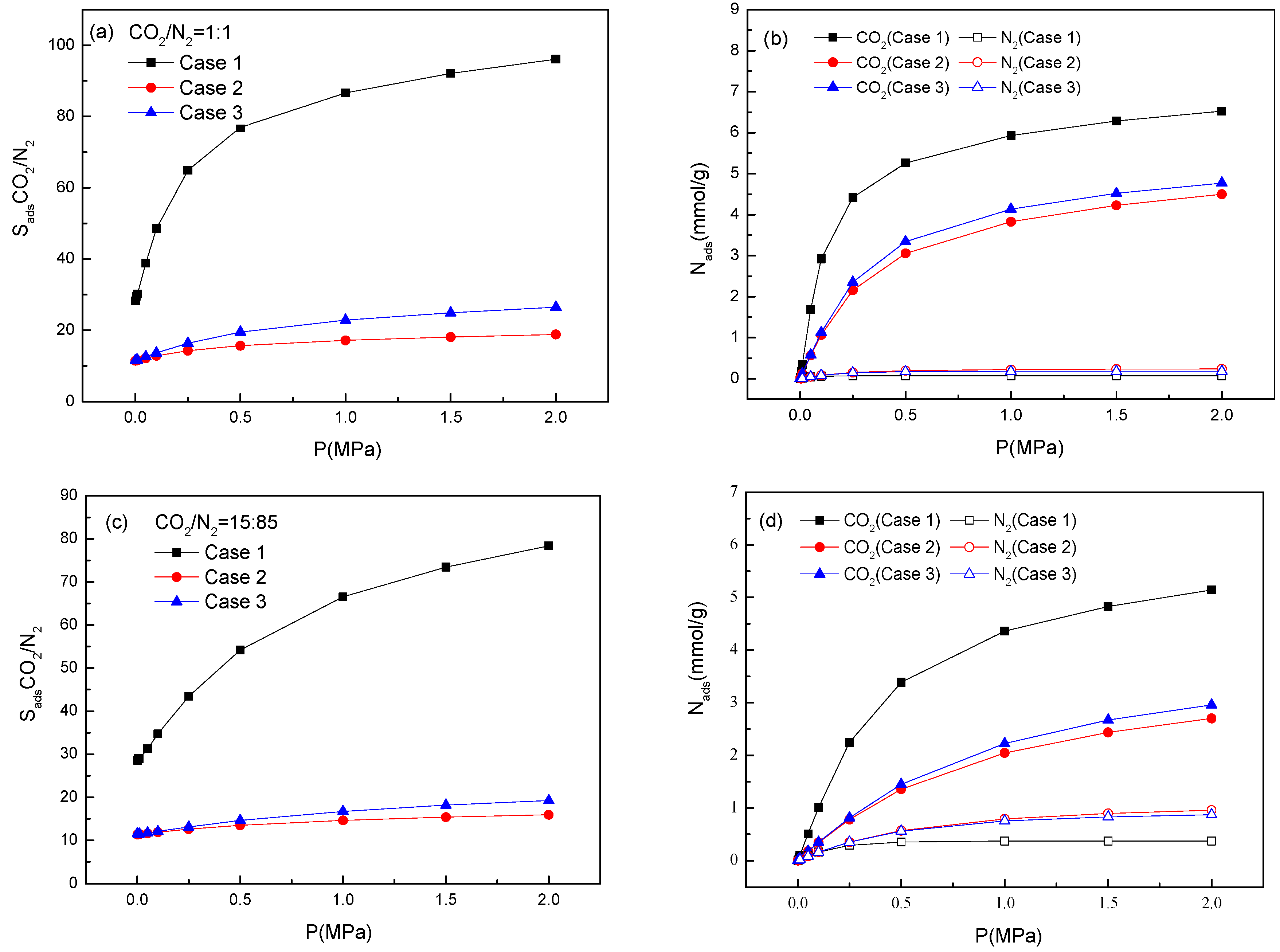
2.4. Effect of Temperature on Adsorption Selectivity in Bio-MOF-11

2.5. Gas Diffusion and Permeation Selectivity of Bio-MOF-11 Membranes
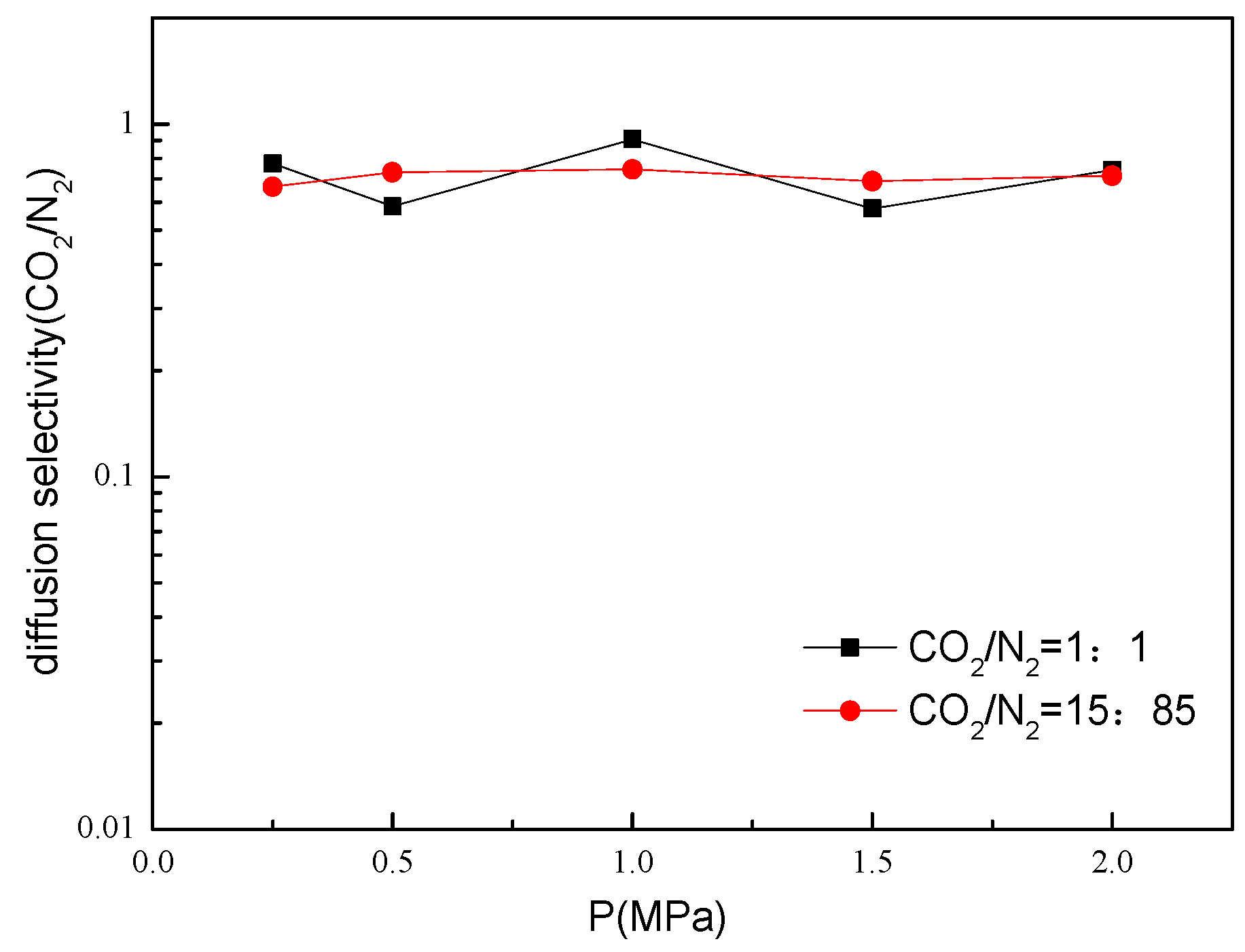
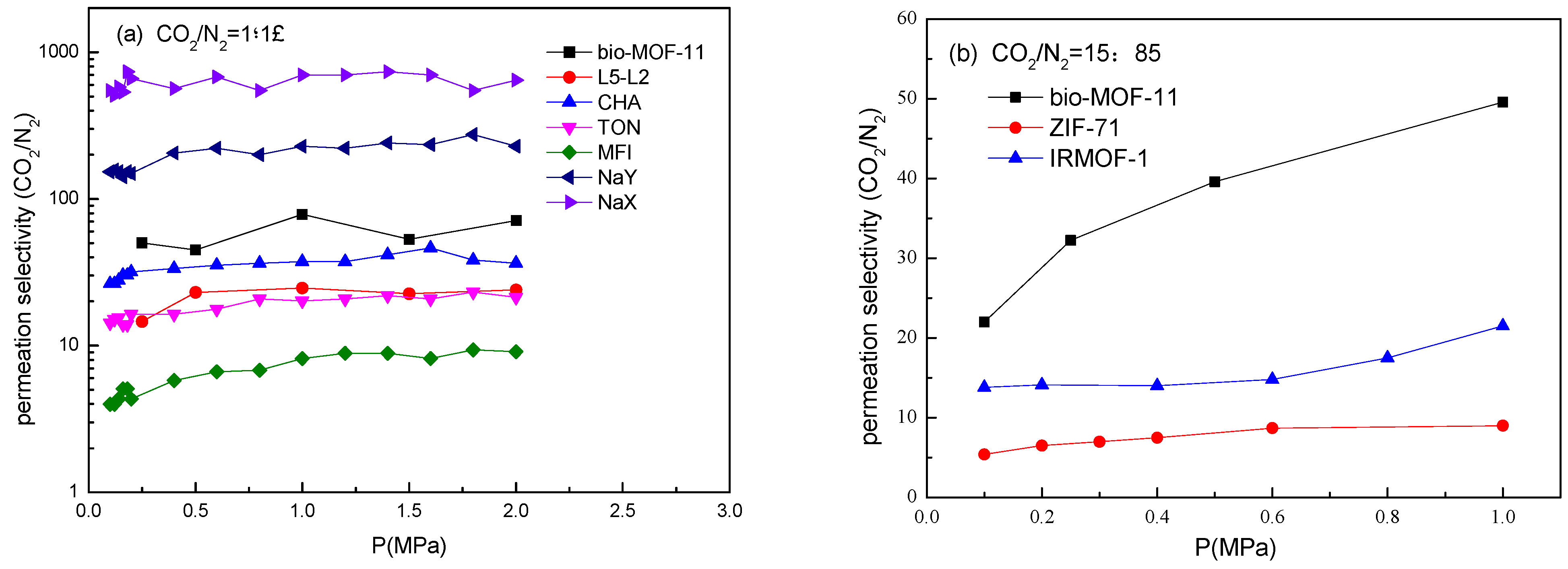
2.6. Gas Permeabilities and Selectivities of Bio-MOF-11 Membranes for CO2/N2 Mixtures.
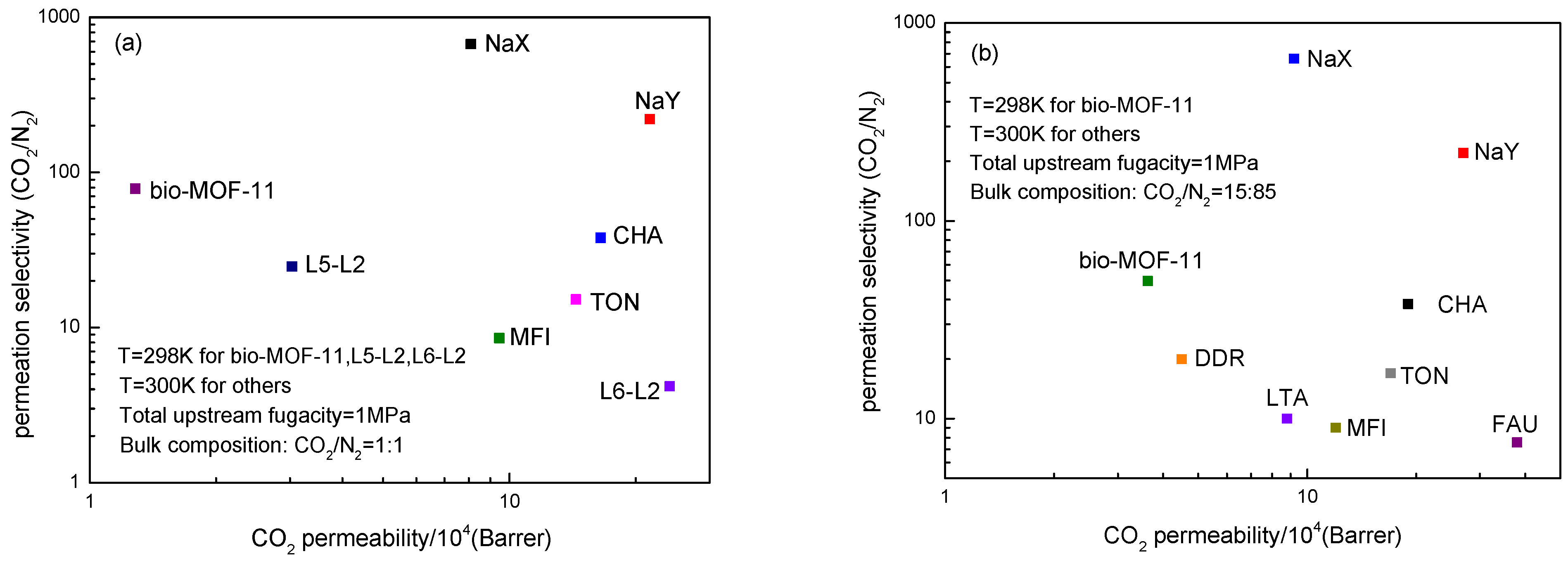
3. Simulation Section
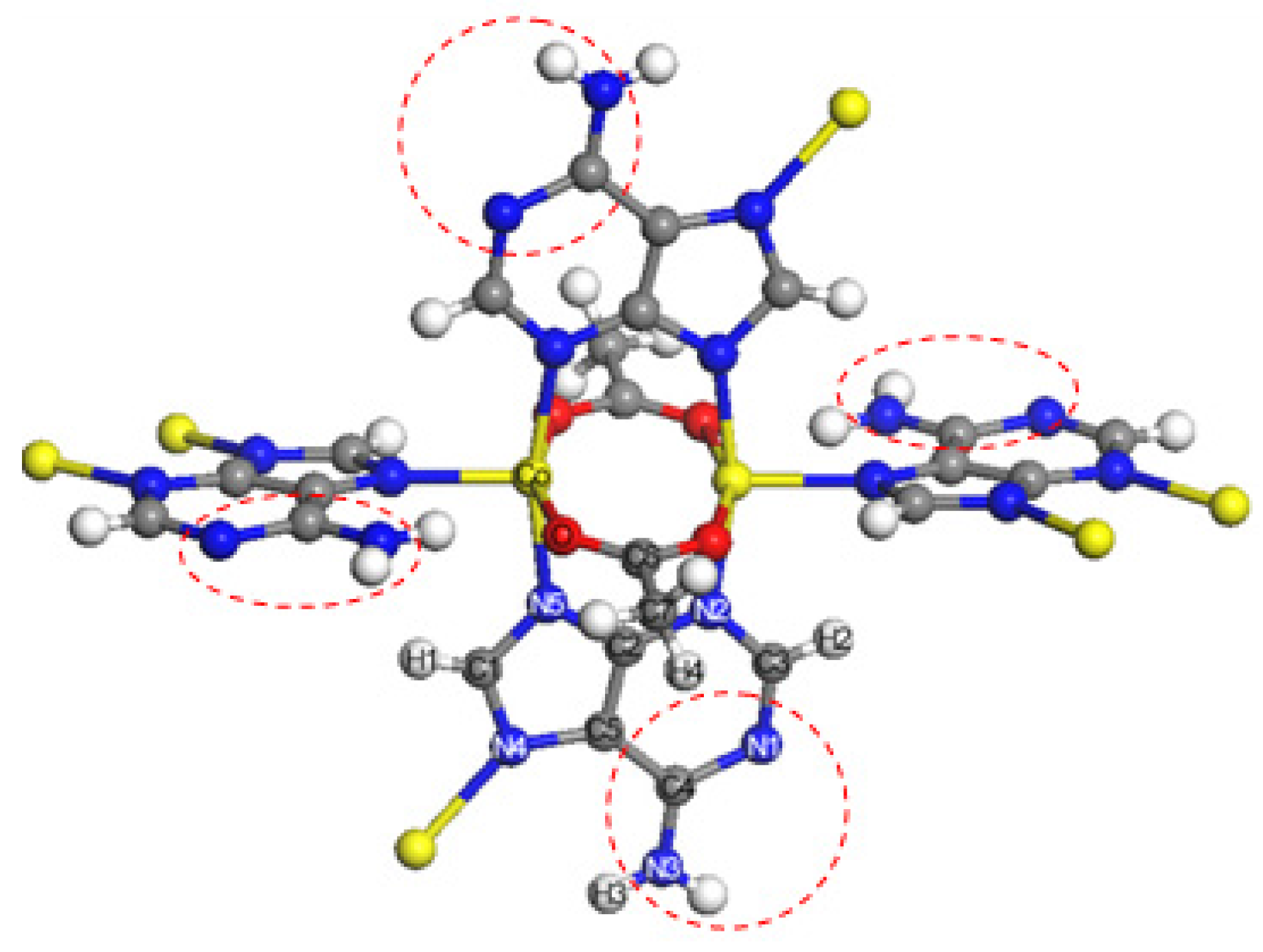
3.1. Bio-MOF-11 Structure
| Material | Topology a | Unit cell a (Å) | Cell Angle a (Deg) | ρcrys b (g/cm3) | Vfree b (cm3/g) | Porosity b |
|---|---|---|---|---|---|---|
| bio-MOF-11 | tetragonal | a = b = 15.43 c = 22.775 | α = β = γ = 90 | 1.234 | 0.318 | 0.393 |
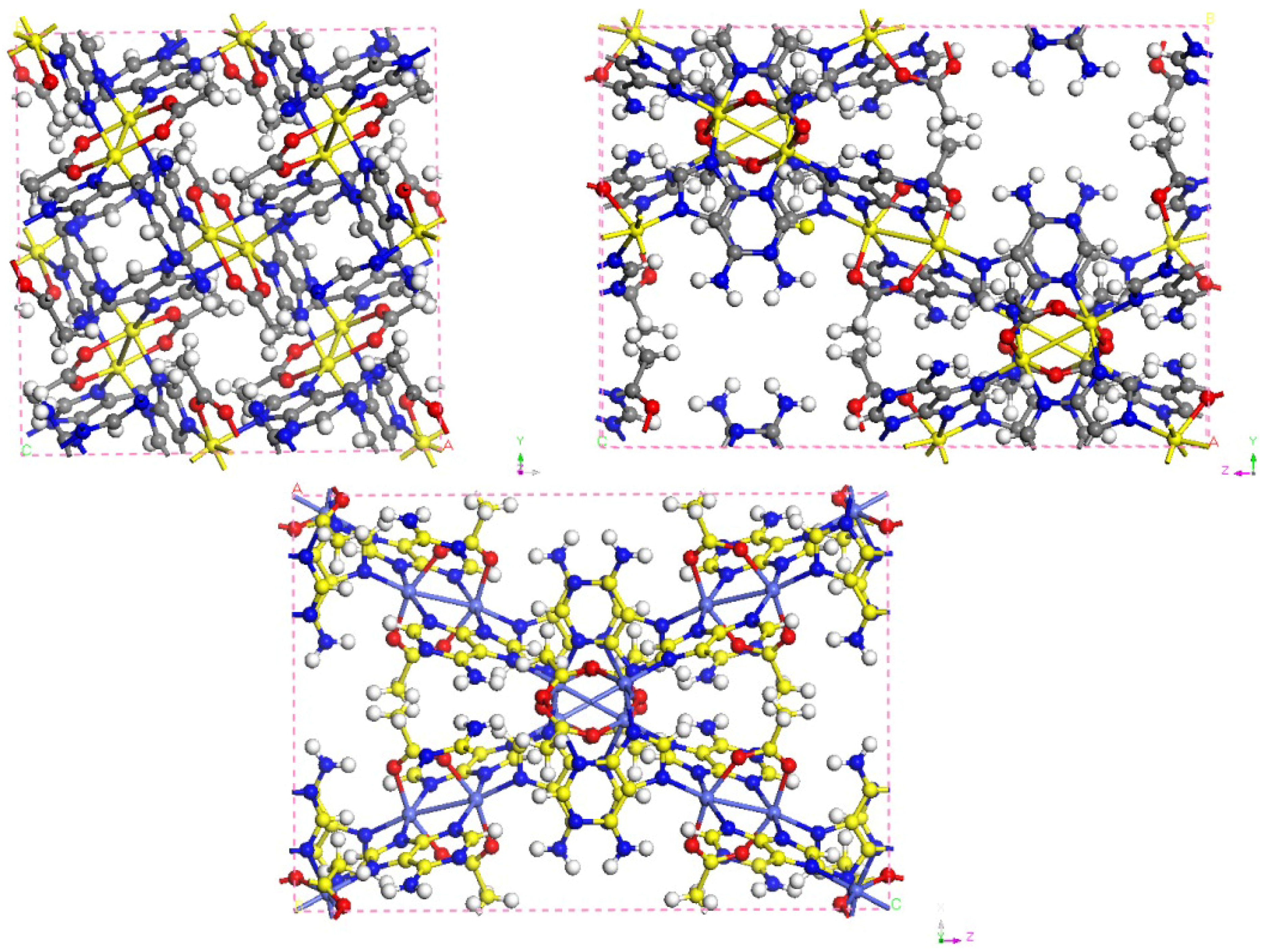
3.2. Force Field
| Atom | σ (Å) | ε/kB (K) |
|---|---|---|
| CO2_C | 2.80 | 27.0 |
| CO2_O | 3.05 | 79.0 |
| N2_N | 3.31 | 36.0 |
| MOF_Co | 2.56 | 7.05 |
| MOF_C | 3.43 | 52.84 |
| MOF_H | 2.57 | 22.14 |
| MOF_O | 3.12 | 30.19 |
| MOF_N | 3.26 | 34.72 |
| Atom | Co | O | C1 | C2 | C3 | C4 | C5 | C6 | C7 |
|---|---|---|---|---|---|---|---|---|---|
| Charge | 0.68 | −0.798 | 0.101 | 0.531 | 0.526 | 0.711 | 0.292 | 0.903 | −0.489 |
| Atom | N1 | N2 | N3 | N4 | N5 | H1 | H2 | H3 | H4 |
| Charge | −0.761 | −0.651 | −1.013 | −0.099 | −0.417 | 0.066 | 0.008 | 0.415 | 0.126 |
3.3. Simulation Details
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, Y.F.; Jiang, J.W. A bio-metal-organic framework for highly selective CO2 capture: A molecular simulation study. ChemSusChem 2010, 3, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Keskin, S.; Liu, J.C.; Rankin, R.B.; Johnson, J.K.; Sholl, D.S. Progress, opportunities, and challenges for applying atomically detailed modeling to molecular adsorption and transport in metal-organic framework materials. Ind. Eng. Chem. Res. 2009, 48, 2355–2371. [Google Scholar] [CrossRef]
- Mueller, U.; Schubert, M.; Teich, F.; Puetter, H.; Schierle-Arndt, K.; Pastre, J. Metal-organic frameworks-prospective industrial applications. J. Mater. Chem. 2006, 16, 626–636. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Yaghi, O.M. Strategies for hydrogen storage in metal-organic frameworks. Angew. Chem. Int. Ed. 2005, 44, 4670–4679. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Smit, B. Molecular simulation studies of separation of CO2/N2, CO2/CH4, and CH4/N2 by ZIFs. J. Phys. Chem. C 2010, 114, 8515–8522. [Google Scholar] [CrossRef]
- Yang, Q.Y.; Liu, D.H.; Zhong, C.L.; Li, J.R. Development of computational methodologies for metal-organic frameworks and their application in gas separations. Chem. Rev. 2013, 113, 8261–8323. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yang, Q.Y.; Zhong, C.L.; Liu, D.H.; Huang, H.L.; Zhang, W.J.; Maurin, G. Revealing the structure-property relationships of metal-organic frameworks for CO2 capture from flue gas. Langmuir 2012, 28, 12094–12099. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, C.C.; Liu, B.; Liu, D.H.; Yang, Q.Y.; Zhong, C.L. Large-scale computational screening of metal-organic frameworks for CH4/H2 separation. AIChE J. 2012, 58, 2078–2084. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, Q.; Zhang, L.; Liu, Y.C.; Kang, Y. Adsorption sites of hydrogen in zeolitic imidazolate frameworks. J. Phys. Chem. B 2009, 113, 11049–11053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Lively, R.P.; Zhang, C.; Koros, W.J.; Chance, R.R. Investigating the intrinsic ethanol/water separation capability of ZIF-8: An adsorption and diffusion study. J. Phys. Chem. C 2013, 117, 7214–7225. [Google Scholar] [CrossRef]
- Zhang, W.J.; Huang, H.L.; Zhong, C.L.; Liu, D.H. Cooperative effect of temperature and linker functionality on CO2 capture from industrial gas mixtures in metal-organic frameworks: A combined experimental and molecular simulation study. Phys. Chem. Chem. Phys. 2012, 14, 2317–2325. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.X.; Li, Z.; Lin, Y.S. Adsorption and diffusion of carbon dioxide on metal-organic framework (MOF-5). Ind. Eng. Chem. Res. 2009, 48, 10015–10020. [Google Scholar] [CrossRef]
- Zhao, Z.X.; Ma, X.L.; Kasik, A.; Li, Z.; Lin, Y.S. Gas separation properties of metal organic framework (MOF-5) membranes. Ind. Eng. Chem. Res. 2013, 52, 1102–1108. [Google Scholar] [CrossRef]
- Keskin, S.; Sholl, D.S. Efficient methods for screening of metal organic framework membranes for gas separations using atomically detailed models. Langmuir 2009, 25, 11786–11795. [Google Scholar] [CrossRef] [PubMed]
- Keskin, S.; Sholl, D.S. Assessment of a metal-organic framework membrane for gas separations using atomically detailed calculations: CO2, CH4, N2, H2 mixtures in MOF-5. Ind. Eng. Chem. Res. 2009, 48, 914–922. [Google Scholar] [CrossRef]
- Keskin, S. Atomistic simulations for adsorption, diffusion, and separation of gas mixtures in zeolite imidazolate frameworks. J. Phys. Chem. C 2011, 115, 800–807. [Google Scholar] [CrossRef]
- Huang, H.L.; Zhang, W.J.; Liu, D.H.; Liu, B.; Chen, G.J.; Zhong, C.L. Effect of temperature on gas adsorption and separation in ZIF-8: A combined experimental and molecular simulation study. Chem. Eng. Sci. 2011, 66, 6297–6305. [Google Scholar] [CrossRef]
- Yang, Q.Y.; Wiersum, A.D.; Llewellyn, P.L.; Guillerm, V.; Serred, C.; Maurin, G. Functionalizing porous zirconium terephthalate UiO-66(Zr) for natural gas upgrading: A computational exploration. Chem. Commun. 2011, 47, 9603–9605. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Deng, S.G. Structural stability of metal organic framework MOF-177. J. Phys. Chem. Lett. 2010, 1, 73–78. [Google Scholar] [CrossRef]
- Yu, J.M.; Balbuena, P.B. Water effects on postcombustion CO2 capture in Mg-MOF-74. J. Phys. Chem. C 2013, 117, 3383–3388. [Google Scholar] [CrossRef]
- Saha, D.; Bao, Z.B.; Jia, F.; Deng, S.G. Adsorption of CO2, CH4, N2O, and N2 on MOF-5, MOF-177, and zeolite 5A. Environ. Sci. Technol. 2010, 44, 1820–1826. [Google Scholar] [CrossRef] [PubMed]
- Wehring, M.; Gascon, J.; Dubbeldam, D.; Kapteijn, F.; Snurr, R.Q.; Stallmach, F. Self-diffusion studies in CuBTC by PFG NMR and MD simulations. J. Phys. Chem. C 2010, 114, 10527–10534. [Google Scholar] [CrossRef]
- Babarao, R.; Jiang, J.W. Unraveling the energetics and dynamics of ibuprofen in mesoporous metal-organic frameworks. J. Phys. Chem. C 2009, 113, 18287–18291. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, L.L.; Jiang, J.W. Adsorption of C1–C4 alcohols in zeolitic imidazolate framework-8: Effects of force fields, atomic charges, and framework flexibility. J. Phys. Chem. C 2013, 117, 25628–25635. [Google Scholar] [CrossRef]
- An, J.; Geib, S.J.; Rosi, N.L. Cation-triggered drug release from a porous zinc-adeninate metal-organic framework. J. Am. Chem. Soc. 2009, 131, 8376–8377. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Geib, S.J.; Rosi, N.L. High and selective CO2 uptake in a cobalt adeninate metal-organic framework exhibiting pyrimidine- and amino-decorated pores. J. Am. Chem. Soc. 2010, 132, 38–39. [Google Scholar] [CrossRef] [PubMed]
- Atci, E.; Erucar, I.; Keskin, S. Adsorption and transport of CH4, CO2, H2 mixtures in a bio-MOF material from molecular simulations. J. Phys. Chem. C 2011, 115, 6833–6840. [Google Scholar] [CrossRef]
- Liu, B.; Tang, L.X.; Lian, Y.H.; Li, Z.; Sun, C.Y.; Chen, G.J. Study on separation performance of metal-organic frameworks with interpenetration and mixed-ligand. Acta Chim. Sin. 2013, 71, 920–928. [Google Scholar] [CrossRef]
- Keskin, S. High CO2 selectivity of a microporous metal-imidazolate framework: A molecular simulation study. Ind. Eng. Chem. Res. 2011, 50, 8230–8236. [Google Scholar] [CrossRef]
- Ozturk, T.N.; Keskin, S. Predicting gas separation performances of porous coordination networks using atomistic simulations. Ind. Eng. Chem. Res. 2013, 52, 17627–17639. [Google Scholar] [CrossRef]
- Erucar, I.; Keskin, S. High CO2 selectivity of an amine-functionalized metal organic framework in adsorption-based and membrane-based gas separations. Ind. Eng. Chem. Res. 2013, 52, 3462–3472. [Google Scholar] [CrossRef]
- Keskin, S. Molecular simulation study of CH4/H2 mixture separations using metal organic framework membranes and composites. J. Phys. Chem. C 2010, 114, 13047–13054. [Google Scholar] [CrossRef]
- Krishna, R.; van Baten, J.M. In silico screening of zeolite membranes for CO2 capture. J. Membr. Sci. 2010, 360, 323–333. [Google Scholar] [CrossRef]
- Gupta, K.M.; Chen, Y.F.; Hu, Z.Q.; Jiang, J.W. Metal-organic framework supported ionic liquid membranes for CO2 capture: Anion effects. Phys. Chem. Chem. Phys. 2012, 14, 5785–5794. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.M.; Chen, Y.F.; Jiang, J.W. Ionic liquid membranes supported by hydrophobic and hydrophilic metal-organic frameworks for CO2 capture. J. Phys. Chem. C 2013, 117, 5792–5799. [Google Scholar] [CrossRef]
- Potoff, J.J.; Siepmann, J.I. Vapor-liquid equilibria of mixtures containing alkanes, carbon dioxide, and nitrogen. AIChE J. 2001, 47, 1676–1682. [Google Scholar] [CrossRef]
- Rappe, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A., III; Skiff, W.M. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar]
- Ewald, P.P. Die berechnung optischer und elektrostatischer gitterpotentiale. Ann. Phys. 1921, 369, 253–287. [Google Scholar] [CrossRef]
- Frenkel, D.; Smit, B. Understanding Molecular Simulation: From Algorithms to Applications, 2nd ed.; Academic Press: San Diego, CA, USA, 2002. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Xu, G.; Liu, B.; Lv, X.; Chen, G.; Sun, C.; Xiao, P.; Sun, Y. Molecular Simulation Studies of Flue Gas Purification by Bio-MOF. Energies 2015, 8, 11531-11545. https://doi.org/10.3390/en81011531
Li Z, Xu G, Liu B, Lv X, Chen G, Sun C, Xiao P, Sun Y. Molecular Simulation Studies of Flue Gas Purification by Bio-MOF. Energies. 2015; 8(10):11531-11545. https://doi.org/10.3390/en81011531
Chicago/Turabian StyleLi, Zhi, Gangqiang Xu, Bei Liu, Xin Lv, Guangjin Chen, Changyu Sun, Peng Xiao, and Yifei Sun. 2015. "Molecular Simulation Studies of Flue Gas Purification by Bio-MOF" Energies 8, no. 10: 11531-11545. https://doi.org/10.3390/en81011531
APA StyleLi, Z., Xu, G., Liu, B., Lv, X., Chen, G., Sun, C., Xiao, P., & Sun, Y. (2015). Molecular Simulation Studies of Flue Gas Purification by Bio-MOF. Energies, 8(10), 11531-11545. https://doi.org/10.3390/en81011531





