Determination of Methane and Carbon Dioxide Formation Rate Constants for Semi-Continuously Fed Anaerobic Digesters
Abstract
:1. Introduction
2. Results and Discussion
2.1. Reactor Performance

2.2. Rate Constants
2.2.1. Determination of Kinetic Rate Constants
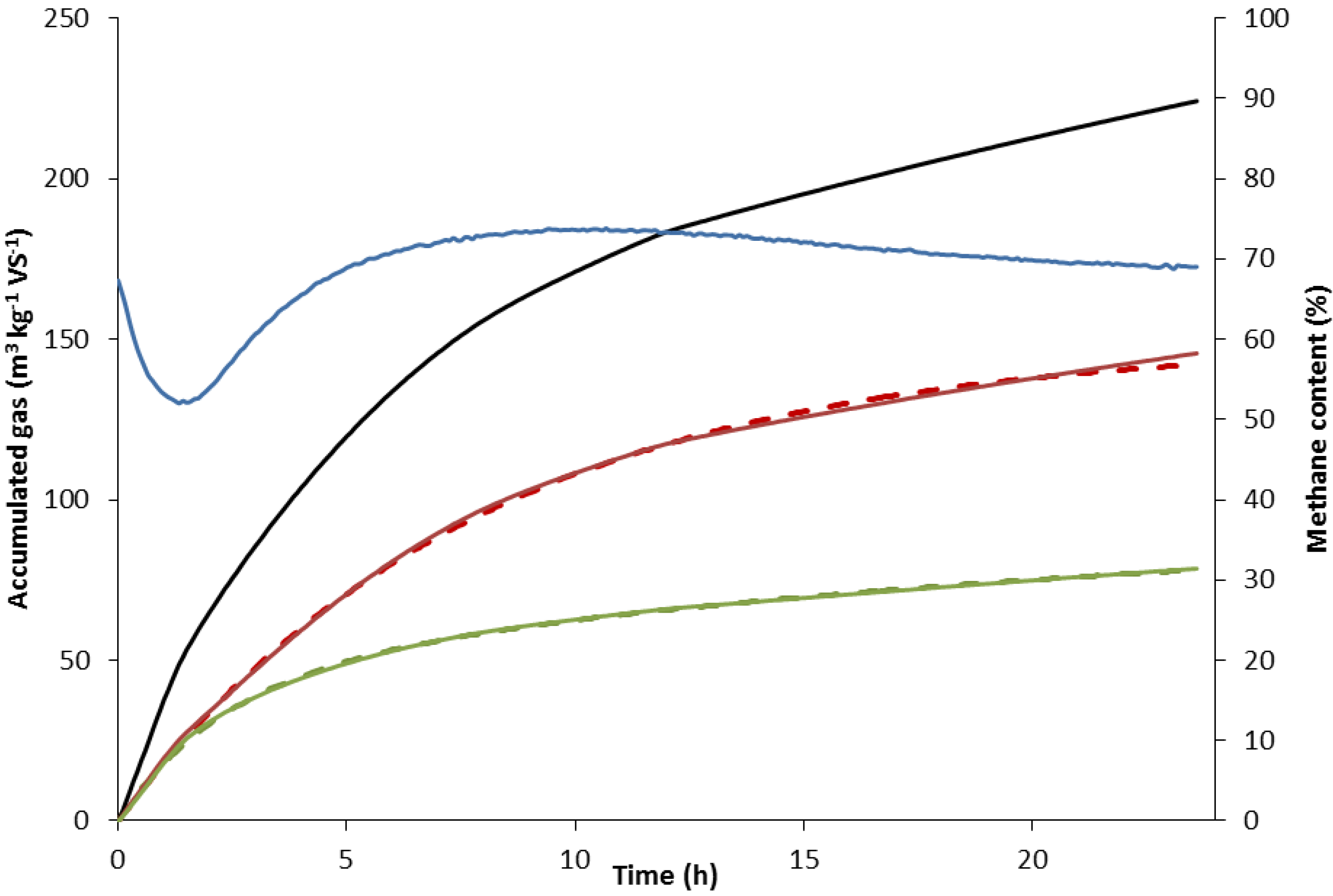
2.2.2. Rate Constant of Methane Formation (km)
2.2.3. Rate Constants of Carbon Dioxide Formation (kc1 and kc2)
3. Experimental Section
3.1. Reactor Set-Up
| Period (days) | OLR Slaughterhouse Waste (kg·VS·m−3·d−1) | OLR OFMSW (kg·VS·m−3·d−1) | OLR Glycerin (kg·VS·m−3·d−1) | HRT (Days) |
|---|---|---|---|---|
| −25 to −15 | 0.8 | 2.8 | 0 | 21 |
| −14 to 25 | 0.8 | 3.9 | 0 | 18 |
| 26 to 39 | 0.8 | 3.9 | 0.5–1.6 | 18 |
| 40 to 168 | 0.8 | 3.9 | 1.8 | 18 |
3.2. Experimental Monitoring and Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zinder, S.H. Microbiology of anaerobic conversion of organic wastes to methane: Recent developments. Am. Soc. Microbiol. News 1984, 50, 294–298. [Google Scholar]
- Angelidaki, I.; Karakashev, D.; Batstone, D.J.; Plugge, C.M.; Stams, A.J.M. Biomethanation and its potential. In Methods in Enzymology: Methods in Methane Metabolism; Rosenzweig, A.C., Ragsdale, S.W., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2011; Volume 494, pp. 327–351. [Google Scholar]
- Schnürer, A.; Houwen, F.P.; Svensson, B.H. Mesophilic syntrophic acetate oxidation during methane formation by a triculture at high ammonium concentration. Arch Microbiol. 1994, 162, 70–74. [Google Scholar] [CrossRef]
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lee, Y.W.; Jahng, D. Anaerobic co-digestion of food waste and piggery wastewater: Focusing on the role of trace elements. Bioresour. Technol. 2011, 102, 5048–5059. [Google Scholar] [CrossRef] [PubMed]
- Florencio, L.; Field, J.A.; Lettinga, G. Importance of cobalt for individual trophic groups in an anaerobic methanol-degrading consortium. Appl. Environ. Microbiol. 1994, 60, 227–234. [Google Scholar] [PubMed]
- Karlsson, A.; Einarsson, P.; Schnürer, A.; Sundberg, C.; Ejlertsson, J.; Svensson, B.H. Impact of trace element addition on degradation efficiency of volatile fatty acids, oleic acid and phenyl acetate and on microbial populations in a biogas digester. J. Biosci. Bioeng. 2012, 114, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Kida, K.; Shigematsu, T.; Kijima, J.; Numaguchi, M.; Mochinaga, Y.; Abe, N.; Morimura, S. Influence of Ni2+ and Co2+ on methanogenic activity and the amounts of coenzymes involved in methanogenesis. J. Biosci. Bioeng. 2001, 91, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Bayr, S.; Pakarinen, O.; Korppoo, A.; Liuksia, S.; Vaisanen, A.; Kaparaju, P.; Rintala, J. Effect of additives on process stability of mesophilic anaerobic monodigestion of pig slaughterhouse waste. Bioresour. Technol. 2012, 120, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, A.; Nordberg, A.; Jarlsvik, T.; Mathisen, B.; Svensson, B.H. Improvement of a grass-clover silage-fed biogas process by the addition of cobalt. Biomass Bioenergy 1997, 12, 453–460. [Google Scholar] [CrossRef]
- Gustavsson, J.; Svensson, B.H.; Karlsson, A. The feasibility of trace element supplementation for stable operation of wheat stillage-fed biogas tank reactors. Water Sci. Technol. 2011, 64, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Ahring, B.K.; Sandberg, M.; Angelidaki, I. Volatile fatty-acids as indicators of process imbalance in anaerobic digesters. Appl. Microbiol. Biotechnol. 1995, 43, 559–565. [Google Scholar] [CrossRef]
- Worm, P.; Müller, N.; Plugge, C.M.; Stams, A.J.M.; Schink, B. Syntrophy in methanogenic degradation. In (Endo)Symbiotic Methanogenic Archaea; Hackstein, J.H.P., Ed.; Springer: Berlin, Germany, 2010; Volume 19, pp. 149–173. [Google Scholar]
- Deublein, D.; Steinhauser, A. Biogas from Waste and Renewable Resources; Wiley-VCH: Mörlenbach, Germany, 2008. [Google Scholar]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; van Lier, J.B. Defining the biomethane potential (bmp) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Astals, S.; Nolla-Ardèvol, V.; Mata-Alvarez, J. Anaerobic co-digestion of pig manure and crude glycerol at mesophilic conditions: Biogas and digestate. Bioresour. Technol. 2012, 110, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Astals, S.; Venegas, C.; Peces, M.; Jofre, J.; Lucena, F.; Mata-Alvarez, J. Balancing hygienization and anaerobic digestion of raw sewage sludge. Water Res. 2012, 46, 6218–6227. [Google Scholar] [CrossRef] [PubMed]
- Ejlertsson, J. Method, a Device, and an Additive for Digesting Organic Matter. U.S. Patent 7947484, 16 July 2006. [Google Scholar]
- Pobeheim, H.; Munk, B.; Lindorfer, H.; Guebitz, G.M. Impact of nickel and cobalt on biogas production and process stability during semi-continuous anaerobic fermentation of a model substrate for maize silage. Water Res. 2011, 45, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Qiang, H.; Lang, D.L.; Li, Y.Y. High-solid mesophilic methane fermentation of food waste with an emphasis on iron, cobalt, and nickel requirements. Bioresour. Technol. 2012, 103, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.M.; Karlsson, A.; Svensson, B.H.; Bertilsson, S. Impact of trace element addition on biogas production from food industrial waste-linking process to microbial communities. FEMS Microbiol. Ecol. 2010, 74, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Vignais, P.M.; Billoud, B. Occurrence, classification, and biological function of hydrogenases: An overview. Chem. Rev. 2007, 107, 4206–4272. [Google Scholar] [CrossRef] [PubMed]
- Ferry, J.G. Enzymology of one-carbon metabolism in methanogenic pathways. FEMS Microbiol. Rev. 1999, 23, 13–38. [Google Scholar] [CrossRef] [PubMed]
- Buhr, H.O.; Andrews, J.F. The thermophilic anaerobic digestion process. Water Res. 1977, 11, 129–143. [Google Scholar] [CrossRef]
- De Vrieze, J.; Hennebel, T.; Boon, N.; Verstraete, W. Methanosarcina: The rediscovered methanogen for heavy duty biomethanation. Bioresour. Technol. 2012, 112, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vavilin, V.A.; Fernandez, B.; Palatsi, J.; Flotats, X. Hydrolysis kinetics in anaerobic degradation of particulate organic material: An overview. Waste Manag. 2008, 28, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, R.; Liu, G.; Chen, C.; He, Y.; Liu, X. Comparison of methane production potential, biodegradability, and kinetics of different organic substrates. Bioresour. Technol. 2013, 149, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Elbeshbishy, E.; Nakhla, G. Batch anaerobic co-digestion of proteins and carbohydrates. Bioresour. Technol. 2012, 116, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Batstone, D.J.; Tait, S.; Starrenburg, D. Estimation of hydrolysis parameters in full-scale anaerobic digesters. Biotechnol. Bioeng. 2009, 102, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Nordell, E.; Moestedt, J.; Karlsson, M. Biogas Producing Laboratory Reactor. SE Patent 1150954-4, 14 October 2011. [Google Scholar]
- Jonsson, S.; Borén, H. Analysis of mono- and diesters of o-phthalic acid by solid-phase extractions with polystyrene-divinylbenzene-based polymers. J. Chromatogr. A 2002, 963, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Vahlberg, C.; Nordell, E.; Wiberg, L.; Schnürer, A. Method for Correction of VFA Loss in Determination of Dry Matter in Biomass; SGC Rapport 2013:273; Gas Technology Centre: Malmö, Sweden, 2013. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moestedt, J.; Malmborg, J.; Nordell, E. Determination of Methane and Carbon Dioxide Formation Rate Constants for Semi-Continuously Fed Anaerobic Digesters. Energies 2015, 8, 645-655. https://doi.org/10.3390/en8010645
Moestedt J, Malmborg J, Nordell E. Determination of Methane and Carbon Dioxide Formation Rate Constants for Semi-Continuously Fed Anaerobic Digesters. Energies. 2015; 8(1):645-655. https://doi.org/10.3390/en8010645
Chicago/Turabian StyleMoestedt, Jan, Jonas Malmborg, and Erik Nordell. 2015. "Determination of Methane and Carbon Dioxide Formation Rate Constants for Semi-Continuously Fed Anaerobic Digesters" Energies 8, no. 1: 645-655. https://doi.org/10.3390/en8010645
APA StyleMoestedt, J., Malmborg, J., & Nordell, E. (2015). Determination of Methane and Carbon Dioxide Formation Rate Constants for Semi-Continuously Fed Anaerobic Digesters. Energies, 8(1), 645-655. https://doi.org/10.3390/en8010645





