Abstract
The aim of this study was to induce lipid accumulation in Chlorella cells by creating stressful growth conditions. Chlorella vulgaris CCALA 896 was grown under various batch growth modes in basal and modified BG-11 and Kolkwitz culture broths, using a continuous light regimen of 150 µE/m2/s, at 30 °C. In order to perform the experiments, two indoor photobioreactor shapes were used: a cylindrical glass photobioreactor (CGPBR) with a working volume of 350 mL, and a flat glass photobioreactor (FGPBR) with a working volume of 550 mL. Stress-eliciting conditions, such as nitrogen and phosphorous starvation, were imposed in order to induce lipid accumulation. The results demonstrated that more than 56% of the lipids can be accumulated in Chlorella biomass grown under two-phase batch growth conditions. The highest biomass productivity of 0.30 g/L/d was obtained at the highest nominal dilution rate (0.167 day−1) during a semi-continuous regimen, using a modified Kolkwitz medium. During the pH-stress cycles, the amount of lipids did not increase significantly and a flocculation of Chlorella cells was noted.
1. Introduction
The production of biodiesel has recently received worldwide attention, because of its capability to be carbon neutral [1] and due to the fact that it can be produced intensively on relatively small areas of marginal land [2]. It has been demonstrated that vegetable oils and fats used as alternative engine fuels are extremely viscous, with viscosities ranging from 10 to 17 times greater than those of petroleum diesel fuel. On the other hand, the energy density of biodiesel is comparable to that of petroleum diesel [3,4,5]. The main advantages of biodiesel can be recognized in its transportability, its ready availability, its renewability, its higher combustion efficiency, and its low sulphur and aromatic content [6]. Large commercial producers of biodiesel often make use of vegetable oils [2]. Common oilseeds for biodiesel production include soybean, rapeseed/canola, palm, corn, sunflower, cottonseed, peanut and coconut oils, as well as algae oils [7]. In general, the oil contents are similar in seed plants and microalgae, although there are significant variations in the biomass productivity and in the resulting production of oil. Algal biofuels have a clear potential for contributing to environmental, social and economic sustainability [8]. Microalgae have a higher photosynthetic efficiency, biomass productivity, and growth rate than do oilseed crops [9,10,11,12,13]. High lipid productivity of fast growing algae is a major requirement for the commercial production of biodiesel. However, under optimal growth conditions, large amounts of algal biomass are produced, but with relatively low lipid content, whereas species with high lipid content are typically slow growing. Major advances in this area can be made through the induction of lipid biosynthesis by means of environmental stresses [14]. Normally, abiotic factors such as light (quality, quantity), temperature, nutrient concentration, O2, CO2, pH, and salinity are necessary in order to maintain optimal alga growth conditions. However, some of these factors can themselves become an environmental stress which can induce a lipid accumulation in microalgae. Of the many factors affecting the growth and biochemical composition of microalgae, nitrogen concentration and light intensity are the most effective ones. Nitrogen deprivation has become one of the most common strategies for simulating high lipid accumulation in algal cells. Lipid content could easily be doubled when a culture is subjected to nitrogen deficiency [15,16,17] and, in the meanwhile, a degradation of certain proteins occurs [18]. However, carbohydrate storage occurs when growth is arrested, due to phosphorus starvation. As in the modelling reported by Jiang et al. [19] regarding polyhydroxyalkanoate storage kinetics, it is assumed that cells accumulate carbohydrates and lipids at the highest rates when there are none inside the cell: these gradually decrease their accumulation rate as the maximum storage capacity is approached [20].
Chlorella vulgaris is known as one of the fastest growing green microalgae. Pulz [21] reported that by using a tubular system on an industrial scale (700 m3) in a glasshouse area of 10,000 m2, an annual production of 130–150 tons of Chlorella dry biomass could be obtained. Furthermore, during the nutrient starvation phase, the lipid content in C. vulgaris could be increased significantly, i.e., between 50% and 70% [22,23,24]. Even if the biofuel production is not yet competitive, mainly due to the impact of the photobioreactor technology on the process cost, the relevant advances in photobioreactors for intensive microalga productions have been recently reported by Olivieri et al. [25]. Moreover, the renewed interest in developing photosynthesis-based reactor technology is demonstrated by the number of novel applications proposed in the last 3 years, i.e., life support systems for space missions, artificial photosynthetic photovoltaic panels, and optofluidic-based micro-photobioreactors [25].
This paper focuses on an autotrophic cultivation of Chlorella vulgaris, in low nitrogen-content media, using indoor photobioreactors. Nutritional factors, which controlled the Chlorella growth and the chemical composition of cells (i.e., proteins, carbohydrates, lipids), were studied. The moderate feeding of both N and P and/or their starvation in the photobioreactor were investigated in order to induce a high accumulation of lipids into Chorella cells.
2. Materials and Methods
2.1. Organism and Culture Conditions
Chlorella vulgaris (CCALA 896) was obtained from the Culture Collection of Algae, Institute of Botany (Trebon, Czech Republic). The strain was maintained and cultivated in modified basal, Kolkwitz and BG-11 media (for the compositions see Table 1). The initial culture concentration for all the experimental sets was 40 mg/L of dry weight biomass; the culture temperature was 30 ± 0.2 °C, and was maintained by a heat exchanger-Julabo water bath. The cultures were mixed by means of an air flow mixture (98% air and 2% of CO2) that made it possible to maintain the pH value within a range of 7.0 to 7.8. Further specific variations in the pH values are reported in the figure captions or within the text of the paper. All experiments were carried out using OSRAM Biolux lamps (36 W/72) under a continuous light intensity of 150 µE/m2/s. The light intensity that impinged on the photobioreactors was measured at their external wall. The experiments were performed by irradiating the photobioreactors from one side, with one exception (described in the text, Section 3.5), during which the photobioreactor was illuminated on both sides. The light intensity was measured with the use of a Quantum/Radiometer/Photometer (model LI-185B, Li-COR, Lincoln, NE, USA).
2.2. Photobioreactor Shapes
Two photobioreactor shapes were used in order to perform the experiments: (i) a cylindrical glass photobioreactor (CGPBR) with an internal diameter (i.d.) of 4.6 cm and a working volume of 350 mL, and (ii) a flat glass photobioreactor (FGPBR) with a 10.0 cm × 4.0 cm-wide cross section and a working volume of 550 mL. The CGPBR was utilised in order to perform the experiments under both batch growth and semi-continuous conditions. The FGPBR was employed in order to carry out the experiments during the two batch-growth phases. Both photobioreactor types (CGPBR and FGPBR) were provided with an internal glass tube (Ø = 10 mm) equipped with an air stone sparger. Compressed air + CO2 flowed inside the aforesaid tube, and this made it possible both to mix the Chlorella culture and to control the culture pH. The FGPBR was equipped with three probes for the continuous control of culture parameters such as temperature, pH and dissolved oxygen concentration. The probes were connected to a control unit (Chemitec srl, Florence, Italy).

Table 1.
Chemical composition of the culture broths tested.
| Macroelements | Culture broth compositions | ||
|---|---|---|---|
| Modified Kolkwitz medium (g/L) | Modified BG-11 medium (g/L) | Modified basal medium (g/L) | |
| NaNO3 | 0.5 | ||
| KNO3 | 0.59 | 0.59 | |
| K2HPO4 | 0.14 | 0.04 | 0.038 |
| MgSO4 · 7H2O | 0.09 | 0.075 | 0.02 |
| CaCl2 · 2H2O | - | 0.036 | - |
| Na2CO3 | - | 0.2 | - |
| Citric acid | - | 0.006 | - |
| FeEDTA | 1 (mL) | 1 (mL) | - |
| Microelements | (mg/L) | (mg/L) | (mg/L) |
| H3BO3 | 2.86 | 2.86 | 0.05 |
| MnCl2·4H2O | 1.81 | 1.81 | 0.10 |
| ZnSO4·7H2O | 0.22 | 0.22 | 0.01 |
| Co(NO3)2 · 6H2O | 0.05 | 0.05 | 0.01 |
| CuSO4 · 5H2O | 0.08 | 0.08 | 2.50 × 10−6 |
| Na2MoO4 · 2H2O | 0.39 | 0.39 | 0.01 |
| FeSO4 · 7H2O | - | - | 3.50 |
| EDTA | - | - | 4.00 |
2.3. Culture Operations
Three modified culture broths (basal medium, BG11, Kolkwitz) were tested under the batch growth conditions in order to select the best one in terms of growth. Culture samples were periodically withdrawn from the photobioreactors in order to check the Chlorella growth and to perform analyses. During the semi-continuous regimen (repetitive batch growth), 50% of the culture volume was withdrawn from the reactor and replaced with an equal volume of fresh medium. Three different repetitive batch regimens of growth were tested: the volume was withdrawn, and was then replaced every 3, 4 and 5 days. Chlorella biomass was collected, as reported by Carlozzi [26], when a semi-steady state condition was reached.
2.3.1. Nitrogen and Phosphorus Starvation Conditions
In order to investigate the lipid accumulation under N starvation conditions, Chlorella was grown in three modified culture broths in which the nitrogen (N) content was set at 82 mg/L. This value corresponded to the salt concentrations of 0.50 g/L of NaNO3 in the modified BG-11 medium and of 0.59 g/L of KNO3 in both the modified Kolkwitz and basal culture broths, as shown in Table 1. No changes were made in the phosphorus (P) content in the said culture broths.
2.4. Analytical Methods
The dry-weight biomass concentration was determined by using the method reported by Carlozzi and Pinzani [27]. The chlorophyll-a (Chl-a) content was determined spectrophotometrically according to the method reported by Strickland and Parson [28].
Cell number counts were performed on samples of microalgal culture that had been suitably diluted using a Thoma cell counting chamber. Nitrate and phosphate concentrations were determined by using a C99 Multiparameter Bench Photometer (Hanna, Lucca, Italy) and reagents. The protein content was determined according to Lowry’s method [29]. Bovine serum albumin (BSA) was used as a standard. The carbohydrate content of the biomass was determined with the use of the phenol-sulphuric acid method [30]. Glucose solution was used as a standard. The lipid content was determined according to Bligh and Dyer [31], after carbonization of the material extracted, using a 2:1 methanol/chloroform solution [32]. Tripalmitin (Sigma-Aldrich, Milan, Italy) was used as a standard. The lipid productivity (Plipid) was determined by using the following Equation (1):
where Bp is the biomass productivity and Lc is the lipid content.
Plipid (g/L/d) = Bp (g/L/d) × Lc (%)
The microalgal biomass productivity (Pbiomass) was determined by using the following Equation (2):
where BC2 and BC1 are, respectively, the biomass concentrations (g/L) at times t2 and t1 (day). The specific growth rate attained during exponential growth was determined by using the following Equation (3):
where µe is the specific growth rate (h−1); and BC2 and BC1 are, respectively, the biomass concentrations (g/L) at times t2 and t1 (h) [33]. Analyses were performed in triplicate, and all values quoted in this study are means ± standard deviation (SD).
Pbiomas (g/L/d) = (BC2 – BC1)/(t2 – t1)
µe = (ln BC2 – ln BC1)/(t2 – t1)
2.5. Statistical Analyses
The analysis of biomass composition variance was performed for the cultures grown in the different culture broths. The effect of the culture broths on the lipid content in the biomass was analysed statistically. Significantly different mean values were established by means of a t-test and variance tests (P > 0.05). Furthermore, the Pearson correlation was used to estimate relationships (range from −1 to 1) between lipid accumulation and nutrient depletion. Statistical analyses were carried out using the Sigma Plot 12.5 package.
3. Results and Discussion
3.1. Cultivation of Chlorella vulgaris CCALA 896 Using Three Different Culture Broths
In order to grow Chlorella vulgaris CCALA 896 for producing biomasses rich in lipids, three different synthetic culture broths were tested. At first, in order to select the suitable medium, the experiments were performed under batch growth conditions using basal, Kolkwitz and BG-11 culture broths. The results, as reported in Figure 1, show the changes in the Chl-a and cell numbers. The trends of the Chl-a indicate that the basal medium had a lower growth capacity than the two others tested. Similar results were obtained as far as the number of cell trends was concerned (Figure 1b).
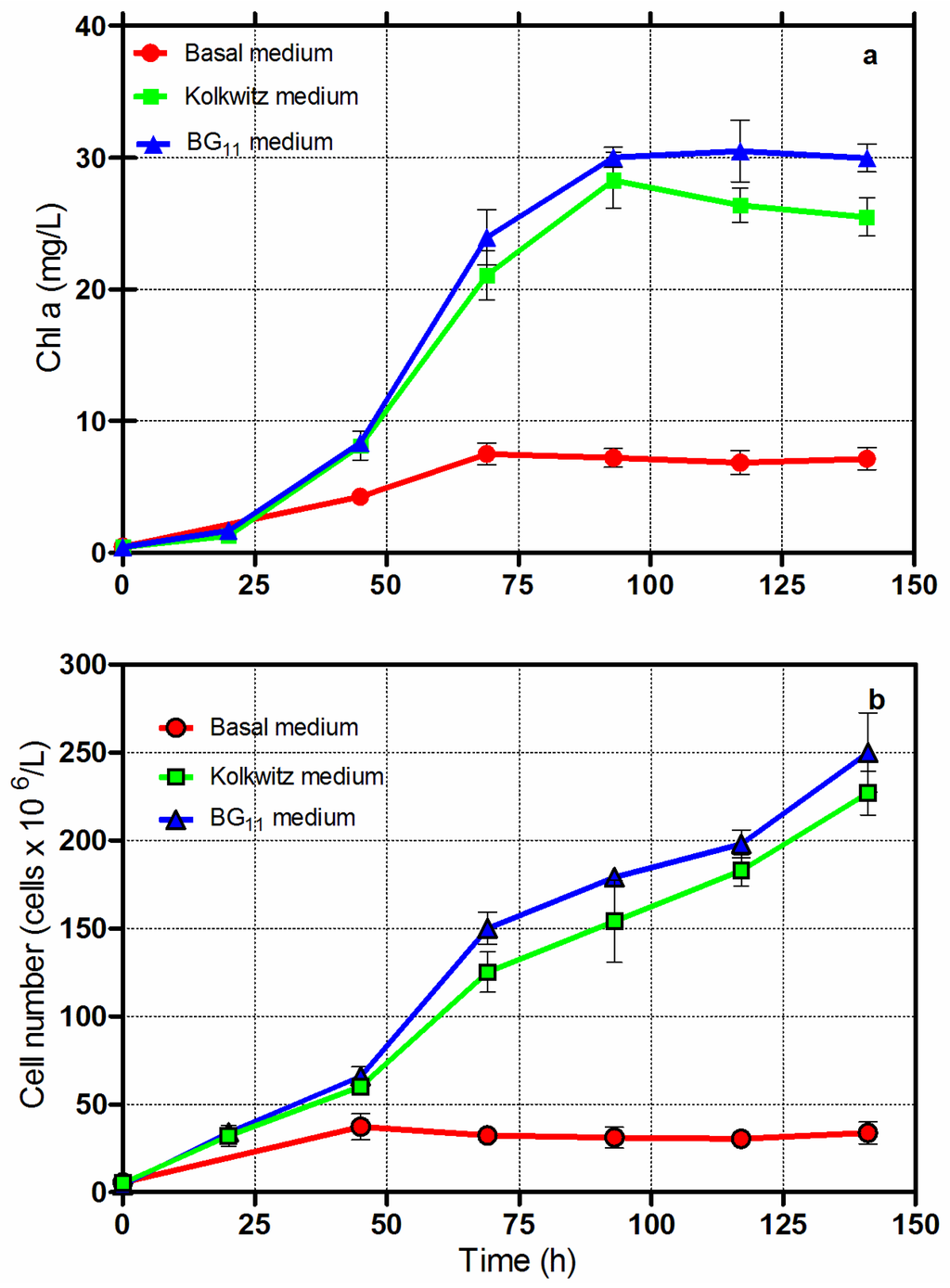
Figure 1.
Batch growth of Chlorella vulgaris CCALA 896 using three different synthetic culture broths: Basal; Kolkwitz and BG-11. All experiments were carried out at 30 °C, and with a light intensity of 150 µE/m2/s.
Figure 1.
Batch growth of Chlorella vulgaris CCALA 896 using three different synthetic culture broths: Basal; Kolkwitz and BG-11. All experiments were carried out at 30 °C, and with a light intensity of 150 µE/m2/s.
The Chlorella vulgaris CCALA 896 cultivation under batch conditions showed similarities in growth in the BG-11 and Kolkwitz culture broths. The final dry-biomass concentrations were 0.7 g/L in the culture grown in the basal medium, 2.04 g/L in the Kolkwitz, and 2.2 g/L in the BG-11. Table 2 illustrates the biomass composition of the Chlorella cells harvested at the end of the batch growth regimen using three different culture broths. No relevant changes in the biomass composition were found; the amount of lipids ranged from 25.3% to 27.9%.

Table 2.
Biomass composition of the Chlorella cells harvested at the endof the batch growth regimen using three different culture broths.
| Culture broths | Proteins (%) | Carbohydrates (%) | Lipids (%) |
|---|---|---|---|
| Basal | nd 1 | nd | 27.9 ± 0.2 |
| BG-11 | 39.2 ± 1.1 | 24.5 ± 0.3 | 25.3 ± 0.1 |
| Kolkwitz | 37.2 ± 0.8 | 27.7 ± 0.2 | 26.8 ± 0.2 |
Note: 1 Not determined.
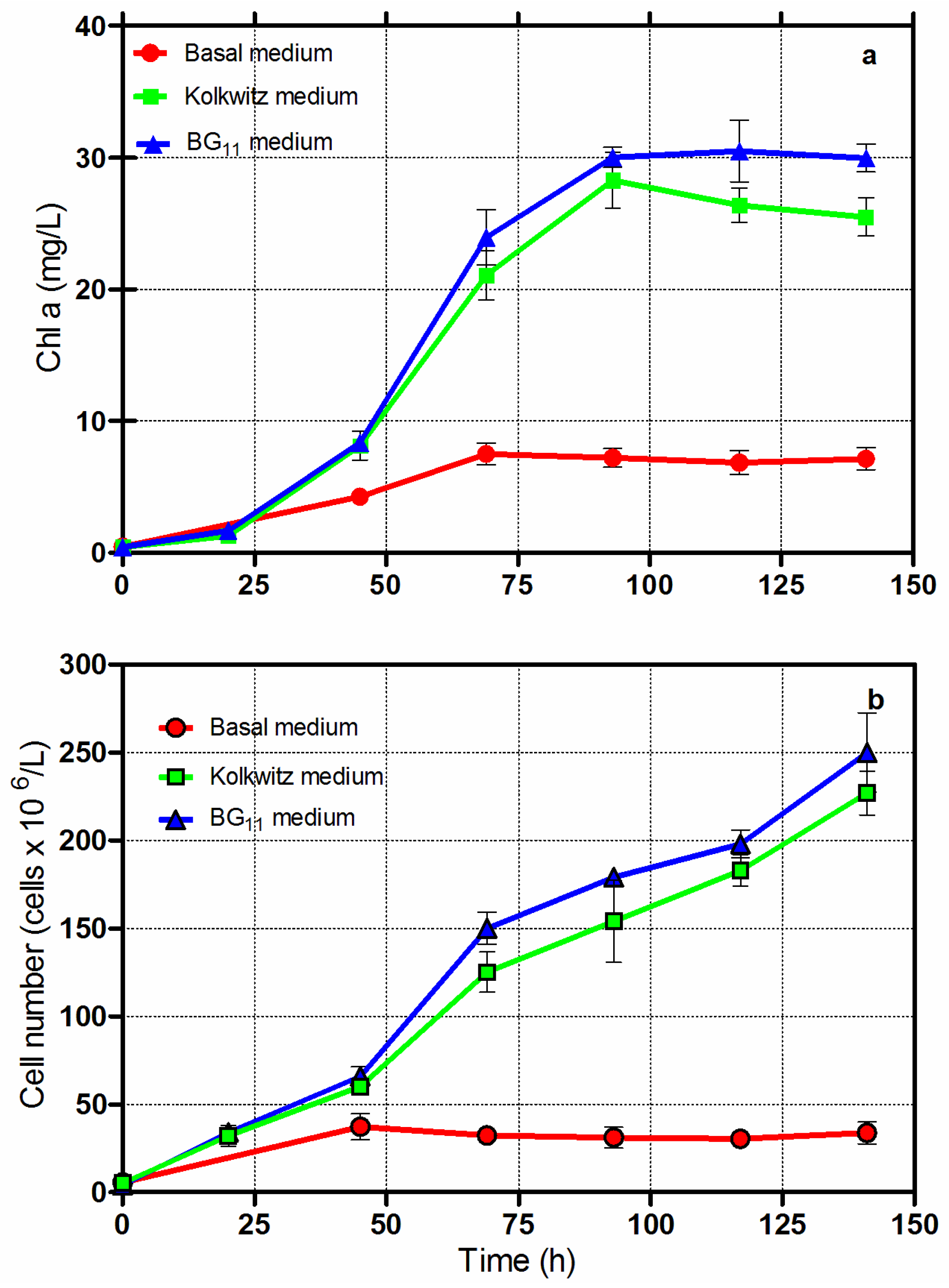
The basal medium was also demonstrated to be unfit for growing Chlorella vulgaris CCALA 896 under the repetitive batch growth mode. Every 72 h, an appropriate culture volume (175 mL) was withdrawn from the reactors and replaced with an equal volume of fresh culture broth. The results are shown in Figure 2. The dry-biomass concentrations attained at the end of the experimental sets (repetitive batch-growth regimen) were 0.66 g/L in the culture grown in the basal medium and 1.64 g/L in the Kolkwitz medium.
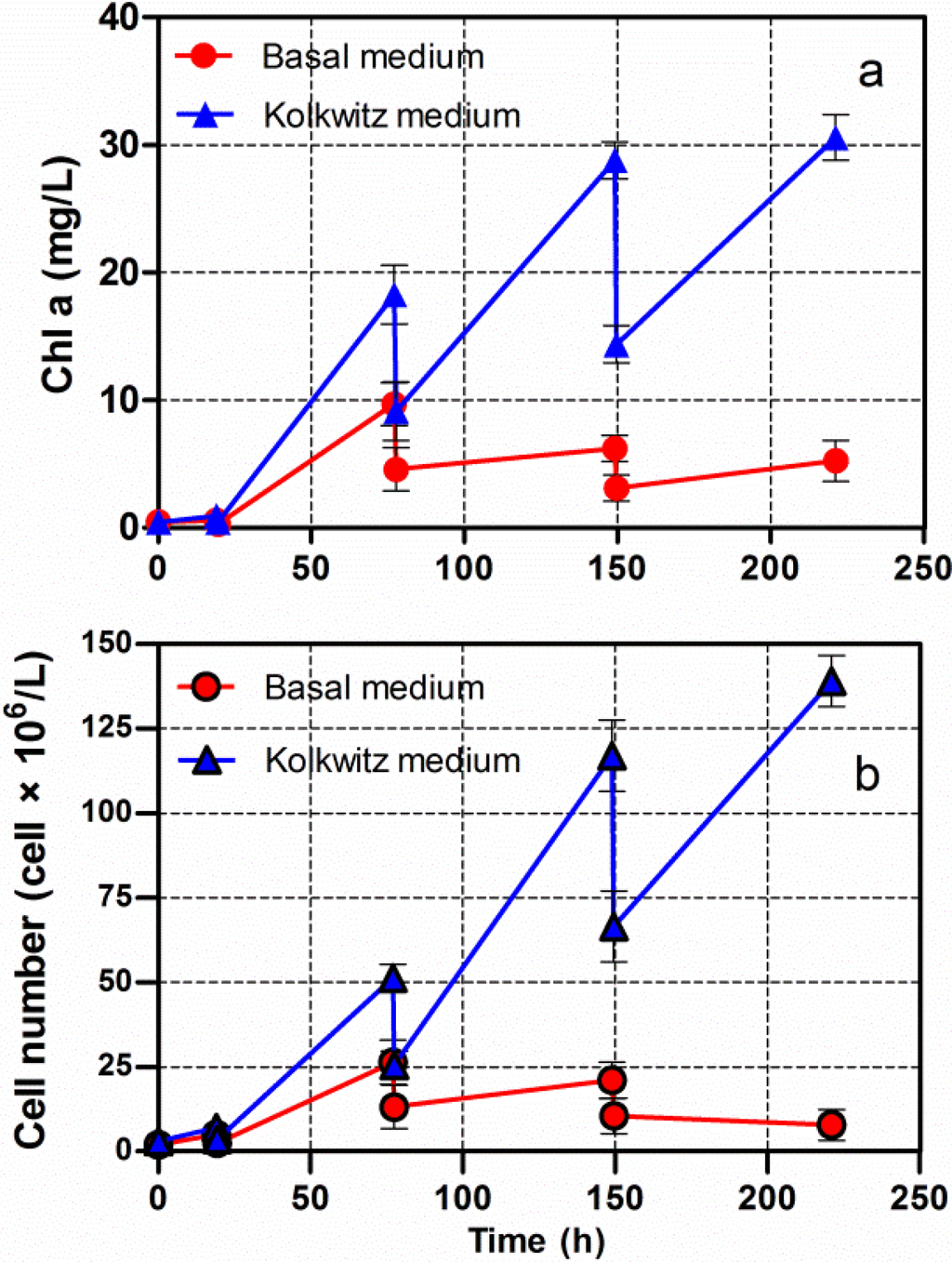
Figure 2.
(a) Changes in Chl-a and (b) cell numbers versus time in Chlorella vulgaris CCALA 896 grown under repetitive batch (i.e., semi-continuous) regimen conditions. Every 72 h, a culture volume of 175 mL was withdrawn from the CGPBR (working volume of 350 mL) and replaced with an equal volume of fresh medium. Experiments were performed by culturing Chlorella in two different culture broths: basal and Kolkwitz.
Figure 2.
(a) Changes in Chl-a and (b) cell numbers versus time in Chlorella vulgaris CCALA 896 grown under repetitive batch (i.e., semi-continuous) regimen conditions. Every 72 h, a culture volume of 175 mL was withdrawn from the CGPBR (working volume of 350 mL) and replaced with an equal volume of fresh medium. Experiments were performed by culturing Chlorella in two different culture broths: basal and Kolkwitz.
Because of the scanty growth that Chlorella obtained in the basal medium (six times lower than in the Kolkwitz medium), it was not investigated further for the production of Chlorella dry-biomass. On the contrary, the modified Kolkwitz and BG-11 culture broths demonstrated that they can be profitably used for the production of oil-rich biomass.
3.2. Cumulative Lipids in Chlorella vulgaris CCALA 896 Grown under Nutritional Starvation Conditions
Several nitrogen molecules (ammonia, nitrate, nitrite and urea) can be used as a nitrogen source for growing microalgae. The content of lipids in microalgae can be increased by means of several growth conditions, such as nitrogen deprivation [16,34,35]. The amount of nitrogen available to the alga appear to determine the amount of lipids accumulated in two ways: (i) by limiting the growth by means of nitrogen deficiency, which causes photosynthetic activity to be directed at the synthesis of reserve materials, including lipids; and (ii) by altering the ratio of lipid to carbohydrate formation as the nitrogen content of the alga changes [36].
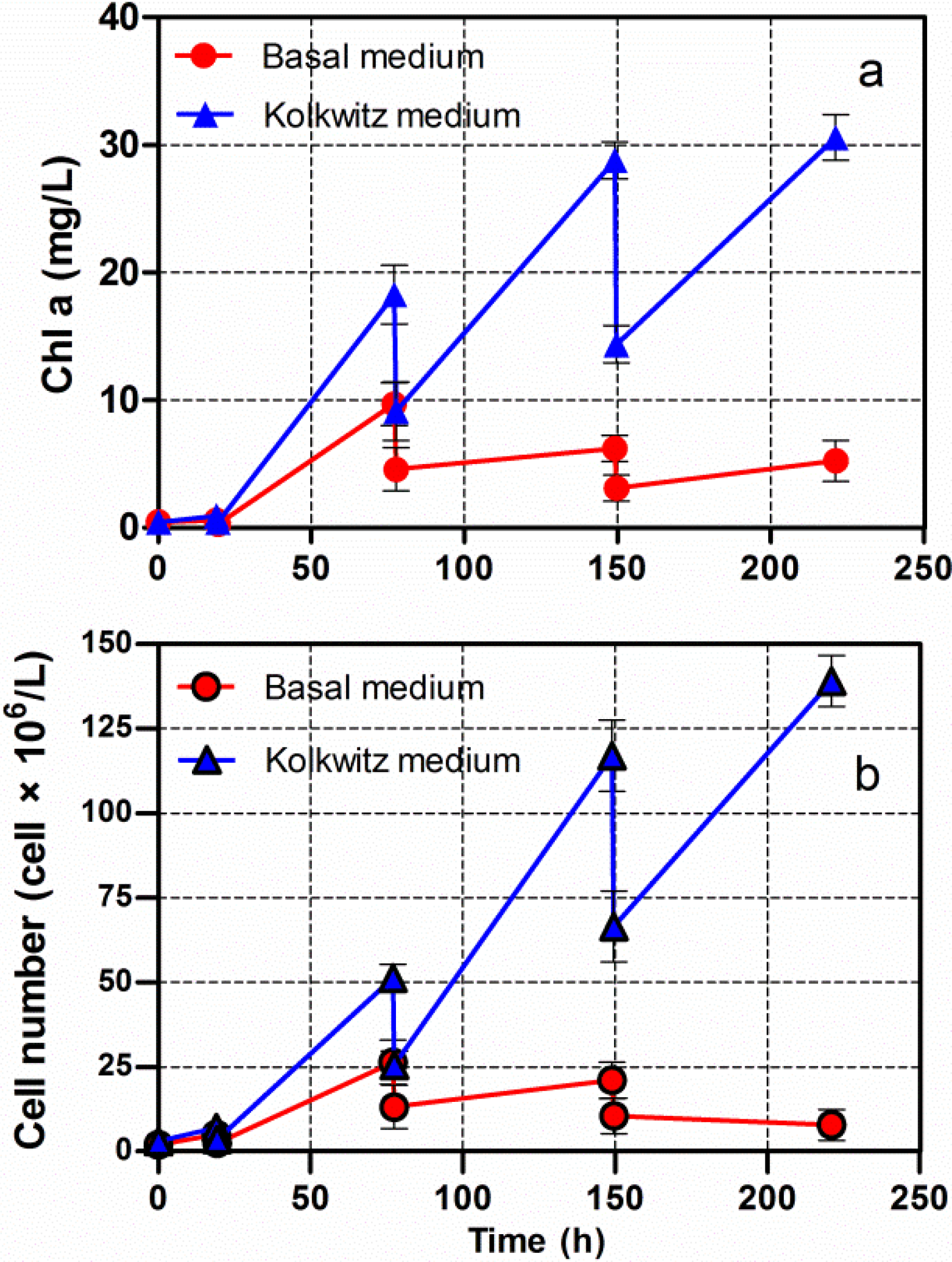
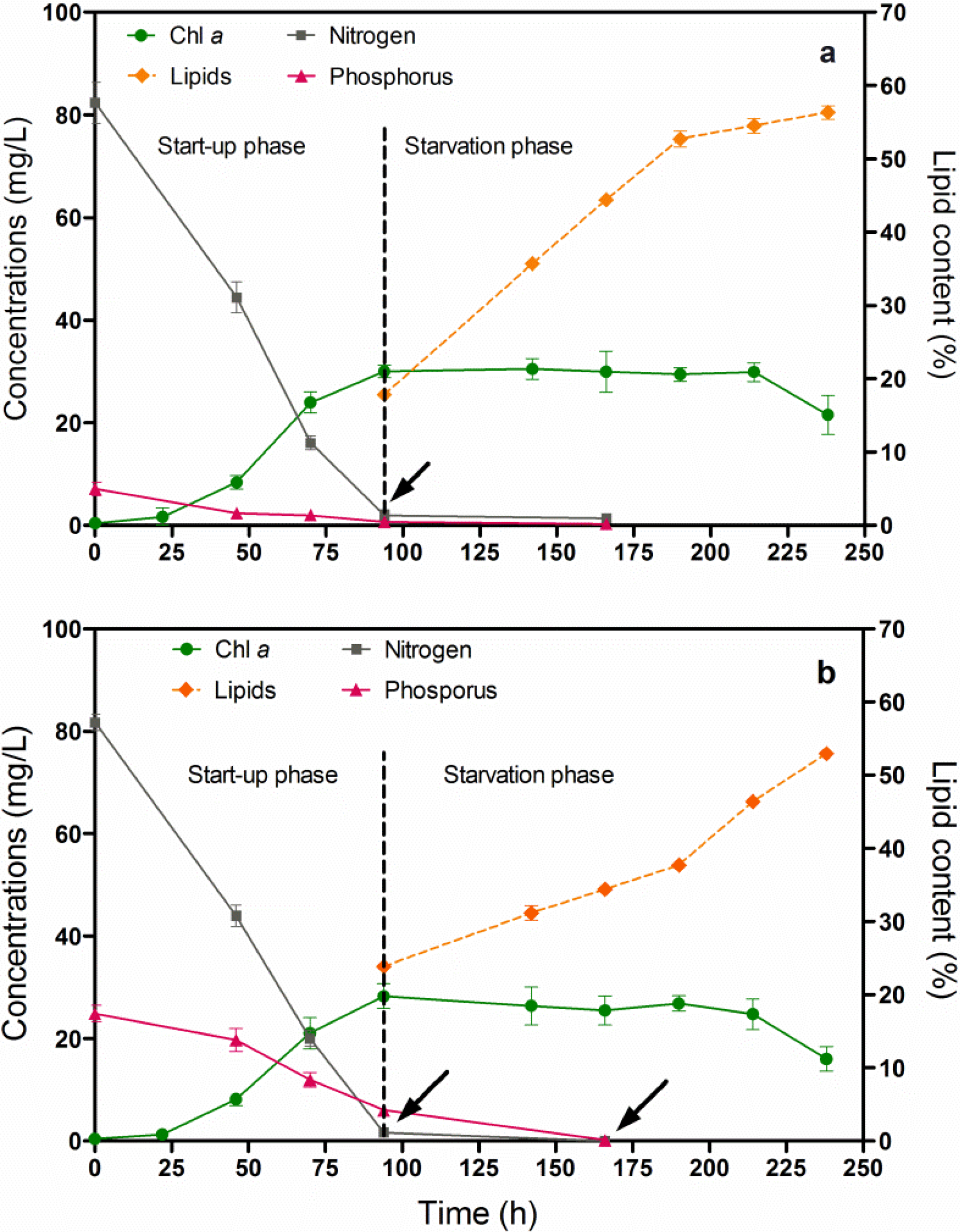
Chlorella vulgaris CCALA 896 was grown in two different culture broths (BG-11 and Kolkwitz) in order to produce lipid-rich biomasses. The investigation was carried out under two different batch growth phase conditions: (i) a start-up phase and (ii) a nutrient-starvation phase as shown in Figure 3. The first phase involved a nutrient-sufficient condition, while the second one involved nitrogen (N) and phosphorus (P) starvations. The latter was performed in order to induce lipid accumulation. The Figure 3 illustrates the main changes in growth and lipid content in Chlorella biomass produced under nutritional starvation conditions. A major quantity of nitrogen and phosphorus were consumed over 94 h of Chlorella growth (start-up phase). From this point on, indicated by the arrows, the lipid content in the Chlorella biomass was checked every 24 h.
By using a modified BG-11 during the N and P starvation phases, the Chl-a concentration, merged with a stable behaviour; on the contrary, the lipid content, increased in the Chlorella biomass from 17.9% to 56.3% (Figure 3a). By using the Kolkwitz medium, the Chl-a concentration began to be stable at about 25 mg/L, and the lipid content also increased from 23.9% to 53.0% in the dry-weight biomass (Figure 3b). The T-test was used for making a comparison of the final mean lipid contents. No significant differences in the lipid percentage were observed in either of the culture broths (t = 0.0753, P = 0.9436). It is, thus, possible to assert that the choice of the cultural media does not affect the final lipid content of Chlorella biomass. Moreover, there was a significantly negative linear relationship between lipid accumulation and nitrogen and phosphorus depletion in both the Chlorella cultures (Figure 3a,b), (r = −1.0).
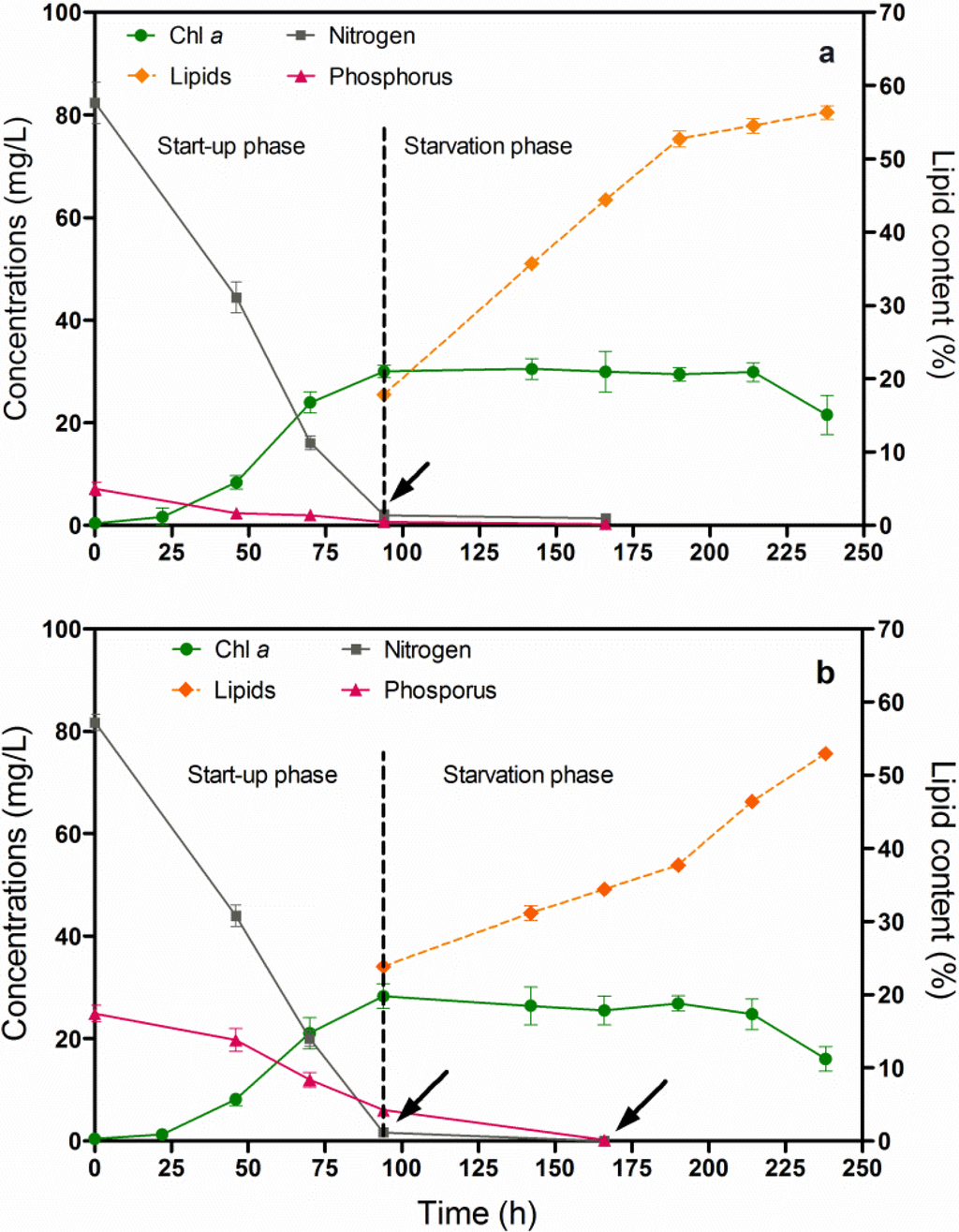
Figure 3.
Changes in the Chl-a concentration and lipid content versus time, of Chlorella vulgaris CCALA 896 grown under batch growth conditions using the modified BG-11 medium (a) and modified Kolkwitz medium (b). The nitrogen (N) and phosphorus (P) concentrations were checked as well. The lipid content in the Chlorella biomass was checked, starting from the N and P starvation conditions, which are indicated by the arrows.
Figure 3.
Changes in the Chl-a concentration and lipid content versus time, of Chlorella vulgaris CCALA 896 grown under batch growth conditions using the modified BG-11 medium (a) and modified Kolkwitz medium (b). The nitrogen (N) and phosphorus (P) concentrations were checked as well. The lipid content in the Chlorella biomass was checked, starting from the N and P starvation conditions, which are indicated by the arrows.
3.3. Changes in the Dry-Biomass Composition of Chlorella vulgaris CCALA 896 Cultured by Using Two Phases: A Cultivation Strategy (A Nutrient-Sufficient Phase Followed by a N- and P- Deprived Phase)
During the nutrient-sufficient (start-up) phase of the Chlorella growth, no relevant changes in the biomass composition were noted (Table 3). On the contrary, during the following N- and P-deprived phase, a cumulative lipid condition was experienced by the Chlorella cells. Some changes in the carbohydrate and protein contents were also observed in the Chlorella biomass. Table 3 illustrates a high lipid accumulation versus the carbohydrate and protein content in the C. vulgaris CCALA 896 grown in the modified BG-11 medium. Starting from the beginning of the N and P starvation (T95), the lipid content increased 3 times; moreover, the maximum lipid content in the dry-biomass (56.3%) was reached at the cultivation time of T238 h. Similar results were obtained by growing Chlorella in a modified Kolkwitz medium (the maximum lipid content in the dry-biomass was 53.0%). It was also observed that the lower protein and carbohydrate contents were reached in Chlorella grown in the BG-11 as compared with the Kolkwitz medium.

Table 3.
Changes of Chlorella vulgaris CCALA 896 biomass composition versus time, in both modified BG-11 and Kolkwitz culture broths, during a nutrient sufficient phase (T0–T94) followed by a N and P deprived phase (T95–T238).
| Time (h) | Biomass composition | |||||
|---|---|---|---|---|---|---|
| Lipids (%) | Proteins (%) | Carbohydrates (%) | ||||
| BG-11 | Kolkwitz | BG-11 | Kolkwitz | BG-11 | Kolkwitz | |
| T0 | 20.9 ± 0.07 | nd 1 | 45.1 ± 0.03 | nd | 27.6 ± 0.02 | nd |
| T94 | 17.9 ± 0.01 | 23.9 ± 0.01 | 42.6 ± 1.03 | 37.5 ± 0.17 | 28.8 ± 0.20 | 28.7 ± 0.15 |
| T142 | 35.7 ± 0.06 | 31.1 ± 0.06 | 30.3 ± 0.40 | nd | 30.7 ± 0.15 | nd |
| T166 | 44.4 ± 0.05 | 34.4 ± 0.03 | nd | 27.2 ± 0.01 | nd | 34.7 ± 0.18 |
| T190 | 52.7 ± 0.11 | 46.4 ± 0.03 | 25.9 ± 0.11 | nd | 18.6 ± 0.08 | nd |
| T214 | 54.5 ± 0.10 | 49.7 ± 0.02 | nd | nd | nd | 24.5 ± 0.01 |
| T238 | 56.3 ± 0.09 | 53.0 ± 0.02 | 24.1 ± 0.10 | 25.6 ± 0.12 | 15.2 ± 0.13 | nd |
Note: 1 Not determined.
A summary of the results obtained by investigating both modified culture broths (BG11 and Kolkwitz) during the two batch-growth phases (the start-up phase and the following starvation phase) are provided in Table 4. The highest results as regards the final lipid content (56.3%), lipid productivity (0.113 g/L/d), and specific growth rate (0.0892 h−1) were attained by cultivating C. vulgaris CCALA 896 in a modified BG-11 medium.

Table 4.
Specific growth rate, biomass productivity, final lipid content and lipid productivity of Chlorella vulgaris CCALA 896 grown under N and P starvation conditions. Experiments were performed using two different culture broths.
| Medium | Specific growth rate (h−1) | Biomass productivity (g (dw)/L/d) | Final lipid content (%) | Lipid productivity (g/L/d) |
|---|---|---|---|---|
| Modified BG-11 | 0.0892 ± 0.0061 | 0.200 ± 0.047 | 56.3 ± 0.9 | 0.113 ± 0.050 |
| Modified Kolkwitz | 0.0773 ± 0.0080 | 0.190 ± 0.028 | 53.0 ± 0.2 | 0.101 ± 0.030 |
3.4. Cultivation of Chlorella vulgaris CCALA 896 in A Modified Kolkwitz Medium under the Conditions of Three Different Repetitive Batch-Growth Regimens
Further investigations into lipid production by means of microalga cultivation were carried out by growing Chlorella vulgaris CCALA 896 in a modified Kolkwitz medium, which was performed under conditions of three different repetitive (i.e., also semi-continuous) batch growth regimens. Half of the photobioreactor working volume (175 mL) was removed and replaced with an equal volume of fresh medium, every three, four and five days. The results achieved under these three semi-continuous regimens are shown in Figure 4. Semi-steady-state conditions were attained when a constant up-and-down pattern in the Chl-a concentration was observed. At the highest nominal dilution rate (0.167 day−1), when the Chl-a concentration stabilized (semi-steady-state condition), the up-and-down Chl pattern ranged from 29.8 mg/L to 15.2 mg/L. At the middle nominal dilution rate (0.125 day−1), the Chl-a concentration ranged from 23.6 mg/L to 12.37 mg/L. At the lowest nominal dilution rate (0.100 day−1), the Chl-a concentration ranged from 20.1 mg/L to 10.1 mg/L.
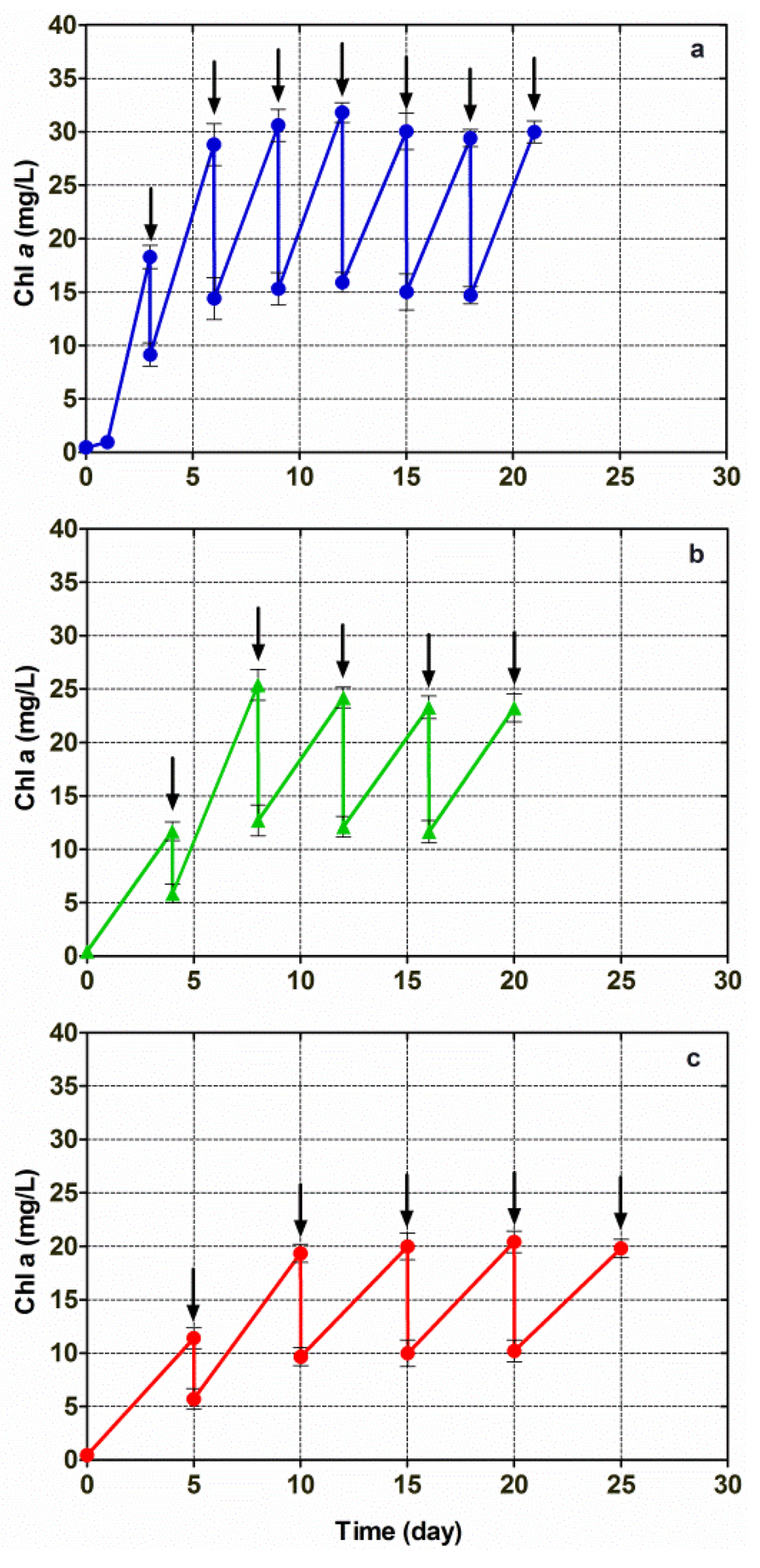
Figure 4.
Changes in the Chl-a concentration versus time, in Chlorella vulgaris CCALA 896 grown under three different repetitive batch-growth (i.e., semi-continuous) regimens and using a modified Kolkwitz medium. The Chlorella cultures were diluted by removing half of the reactor working volume: (a) every 3 days (nominal dilution rate = 0.167 day−1); (b) every 4 days (nominal dilution rate = 0.125 day−1) and (c) every 5 days (nominal dilution rate = 0.100 day−1). Arrows indicate when the dilutions were performed.
Figure 4.
Changes in the Chl-a concentration versus time, in Chlorella vulgaris CCALA 896 grown under three different repetitive batch-growth (i.e., semi-continuous) regimens and using a modified Kolkwitz medium. The Chlorella cultures were diluted by removing half of the reactor working volume: (a) every 3 days (nominal dilution rate = 0.167 day−1); (b) every 4 days (nominal dilution rate = 0.125 day−1) and (c) every 5 days (nominal dilution rate = 0.100 day−1). Arrows indicate when the dilutions were performed.
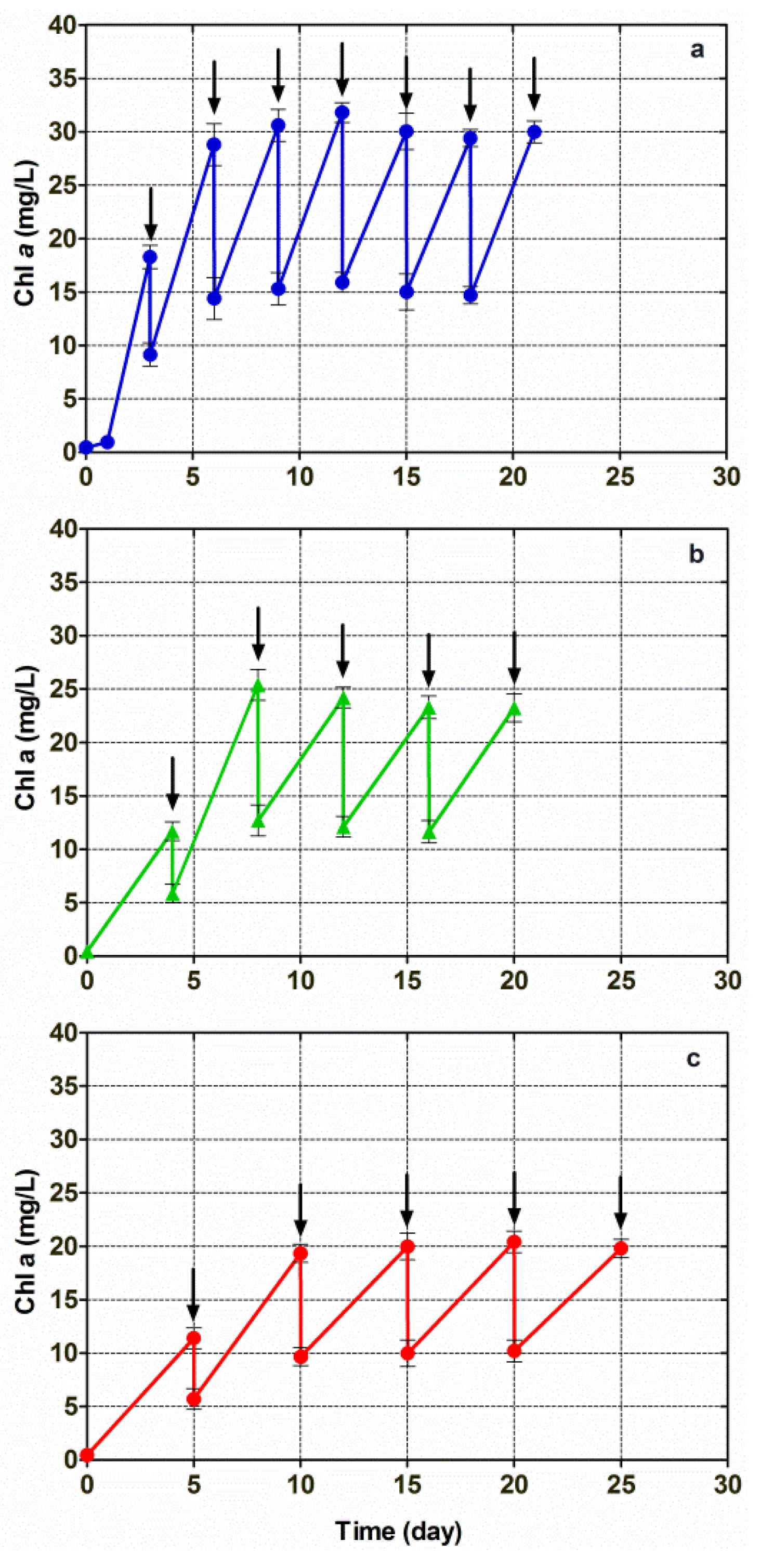
Figure 5 illustrates the lipid content in the dry-biomass of Chlorella harvested during the three semi-steady-state conditions. Volumetric productivities of dry-biomass and lipids have been reported as well. As shown in the figure, the productivity of lipids decreased when the nominal dilution rate was the lowest (0.100 day−1). A similar trend was observed for the Chlorella biomass productivity. On the contrary, the lipid content in dry-biomass increased, depending on the nominal dilution rate: the highest lipid content in the dry-biomass of Chlorella (34.2% ± 6.8%) was attained by diluting the Chlorella culture every 5 days, which corresponds to a nominal dilution rate of 0.100 day−1. Despite the fact that the highest lipid content in the Chlorella biomass was achieved at the lowest nominal dilution rate (0.100 day−1), the highest productivity of lipids (0.102 ± 0.004 g/L/d) was obtained at the highest nominal dilution rate (0.167 day−1).
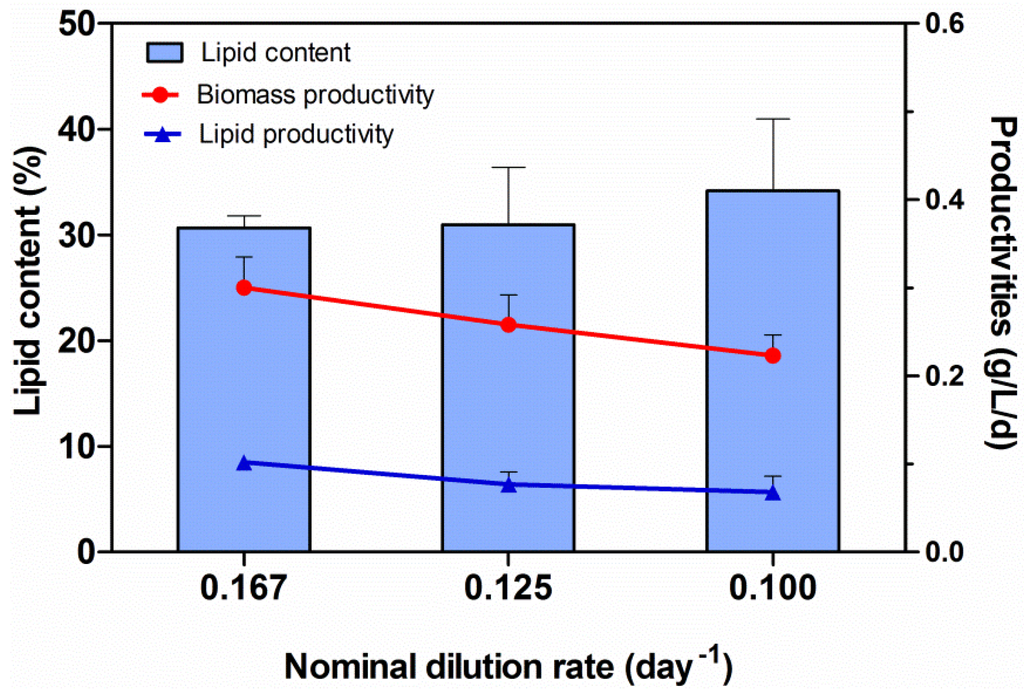
Figure 5.
Lipid content in the dry-biomass of Chlorella and lipid and biomass productivities. Experiments were performed using a CGPBR (working volume of 350 mL). Chlorella vulgaris CCALA 896 was grown by using a modified Kolkwitz medium under three different repetitive batch-growth regimens, as reported in the previous Figure 4. The data represent the mean ±SD.
Figure 5.
Lipid content in the dry-biomass of Chlorella and lipid and biomass productivities. Experiments were performed using a CGPBR (working volume of 350 mL). Chlorella vulgaris CCALA 896 was grown by using a modified Kolkwitz medium under three different repetitive batch-growth regimens, as reported in the previous Figure 4. The data represent the mean ±SD.
Over the years, several studies regarding microalgae cultivation have focused on oil-rich biomass productivity performed under batch-growth regimen [37,38,39]. A comparison of our results with some of the others reported in the literature and achieved using different Chlorella strains are shown in Table 5. In order to optimize microbial processes for producing raw biomasses or biomolecules, the culture operations are performed under a continuous regimen, in which a steady-state condition is a basic requirement before setting up the process. Nevertheless, in several commercial microbial processes, the procedures used are prevalently in a batch, fed-batch, or semi-continuous regimen (i.e., half of the reactor working volume is withdrawn every day from the reactor, to then be replaced with an equal volume of fresh medium). When these growth strategies are applied, it means that the growth of microalgae is performed in the absence of a true steady state, and this adds a certain complexity to the above-mentioned processes.
In the present investigation, the operation mode selected to enhance the biomass productivity was the semi-continuous regimen, which is also known as repetitive batch-growth, in which a semi-steady state condition can be reached [26]. This growth regimen was compared with the batch-growth one. By culturing Chlorella vulgaris CCALA 896 in batch mode: (i) during the start-up phase, the lipid content in the biomass was low, but the specific growth rate was high; (ii) during the starvation phase, under both N and P starvation, the lipid content was high, but the growth was scanty. A similar observation was also reported by Xin et al. [37] in culturing the freshwater microalga Scenedesmus sp. LX1. Moreover, in accordance with Guest et al. [20], N starvation consistently resulted (as expected) in an accumulation of lipids; on the contrary, P starvation alone did not result in any appreciable accumulation of lipids (data not shown). When both N and P starvation were investigated by culturing Chlorella under batch-mode conditions, the maximum percentage of lipids (56.3%) was found in the dry-biomass. Remarkable changes in the lipid content and its accumulation in Chlorella cells, together with the biomass productivity, were obtained when the photobioreactor was operated under the repetitive batch-growth regimen.

Table 5.
Comparison of our results with some other Chlorella strains found in the literature.
| Chlorella (strains) | PBRs 1 | Growth mode | Light intensity (µE/m2/s) | Media | T (°C) | Biomass productivity (g/L/d) | Lipids | References | |
|---|---|---|---|---|---|---|---|---|---|
| Content (%) | Productivity (g/L/d) | ||||||||
| C. vulgaris CCALA 896 | Cylindrical | B 2 | 150 | BG-11 | 30 | 0.200 | 56.3 | 0.113 | Our results |
| C. sp. | Rectangular | B | 600 | ASW 5 | 30 | - | 52.2 | 0.124 | [40] |
| C. sp. | Rectangular | SCR | 600 | ASW | 30 | - | 43.65 | 0.139 | [40] |
| C. vulgaris CCAP 211/11B | Helical | B | 130 | -- | 25 | 0.024 | 58.0 | - | [23] |
| C. vulgaris AG10032 | Column | B/FB 4 | 200 | BG-11 | 18/25 | 0.145 | 53.0 | 0.077 | [41] |
| C. vulgaris 2714 | Flasks | B | - | OCM 6 | 26 | - | 27.38 | - | [42] |
| C. vulgaris ESP-31 | ns | B | 60 | Basal | 25 | - | 22.0 | 0.056 | [24] |
| C. vulgaris CCAP 211 | Erlenmeyer flasks | B | 70 | BBM 7 | 30 | - | - | 0.008 | [43] |
| C. vulgaris | ns 8 | B | 800 lux | - | 22 | - | 62.9 | - | [22] |
| C. vulgaris KCTCAG10032 | ns | B | 150 | BG-11 | 25 | 0.105 | - | 0.007 | [44] |
Notes: 1 Photobioreactors; 2 Batch; 3 Semicontinuous regimen; 4 Fed-batch; 5 Artificial sea water; 6 Optimized culture medium; 7 Bold basal medium; 8 Not specified.
The accumulation of lipids in Chlorella cells is strongly related to the nitrogen concentration [40]. By culturing Chlorella under semi-continuous regimen conditions at a low initial urea concentration (0.025 g/L), the above-cited authors obtained a maximum lipid productivity of 0.139 g/L/day. In the present study, the highest lipid productivity (0.102 g/L/day) was attained under nitrate-replete growth conditions by using the repetitive batch-growth regimen. The difference in the lipid production obtained between the present study and that by Hsieh and Wu [40] can be attributed to the lower light intensity that we used (150 µE/m2/s) as compared to that of the others (600 µE/m2/s).
Since oil-rich Chlorella biomass also contains proteins and carbohydrates (Table 3), in accordance with Chisty [12], the residual biomass from biodiesel production processes can potentially be used as animal feed. Moreover, as suggested by the same author, some of the residual biomass could be used to produce methane by means of anaerobic digestion, for generating the electrical power necessary to run the microalgal biomass production facility. Recently, the microalgal-biodiesel residue of Chlorella biomass, after lipid extraction, has been investigated for methane production by Li et al. [45].
3.5. Cultivation of Chlorella vulgaris CCALA 896 in a Modified BG-11 Medium Using a Flat-Glass Photobioreactor (FGPBR)
In order to complete our investigation of lipid photoproduction by means of Chlorella vulgaris CCALA 896, a new experiment was performed using a FGPBR. The culture system used to carry out the investigation was connected to a control unit in order to keep a check on the oxygen and pH parameters. The light intensity that impinged on the reactor wall was the same as the one used in previous experiments (150 µE/m2/s); however, it was provided on both sides of the photobioreactor.
The modified BG-11 medium was used to feed the Chlorella cells; the culture temperature was 30 ± 0.5 °C. The investigation was performed in two sequential batch-growth phases: (i) a Start-up phase and (ii) a Starvation phase. The results are shown in Figure 6.
During the start-up phase, the Chl-a concentration increased up to 38.6 mg/L, while a depletion in both N and P was observed at the culture time of 110 h (Figure 6a). Moreover, during this Start-up phase, the pH value remained constant (7.2), due to the flowing of CO2 into the Chlorella culture. The said flow was regulated by means of a solenoid valve connected to the control unit. At the same time a significant increase in the oxygen concentration (10.4 mg/L) was observed (Figure 6b). During the subsequent N and P starvation phase, the Chl-a concentration began to decrease, while the lipid content in Chlorella cells increased from 22.7% to 34.6% at T182. It was clear that the response of the Chlorella strain under N and P starvation conditions drove the metabolism towards lipid synthesis, thus causing a cumulative amount of lipids in the Chlorella cells. Also in this case, a negative linear relationship between nitrogen and phosphorus depletion and lipid accumulation could be observed (Figure 6a) (r = −1.0). After that, in order to investigate the effect of pH on cumulative lipids, at the culture time of 142 h the flow of CO2 was turned off and its effect was monitored: the pH increased to 10.64; moreover, the oxygen concentration quickly decreased to 8.0 mg/L at the culture time of 156 h. Subsequently, a second pH-stress cycle was imposed on the Chlorella culture, and the changes in the pH and oxygen parameters were then monitored (Figure 6b). The amount of the lipid content during the pH-stress cycles did not increase significantly (Figure 6a); however, a flocculation of Chlorella cells was noted, a fact that was due to the high pH value. Subsequently, a sedimentation in the Chlorella cells was observed.
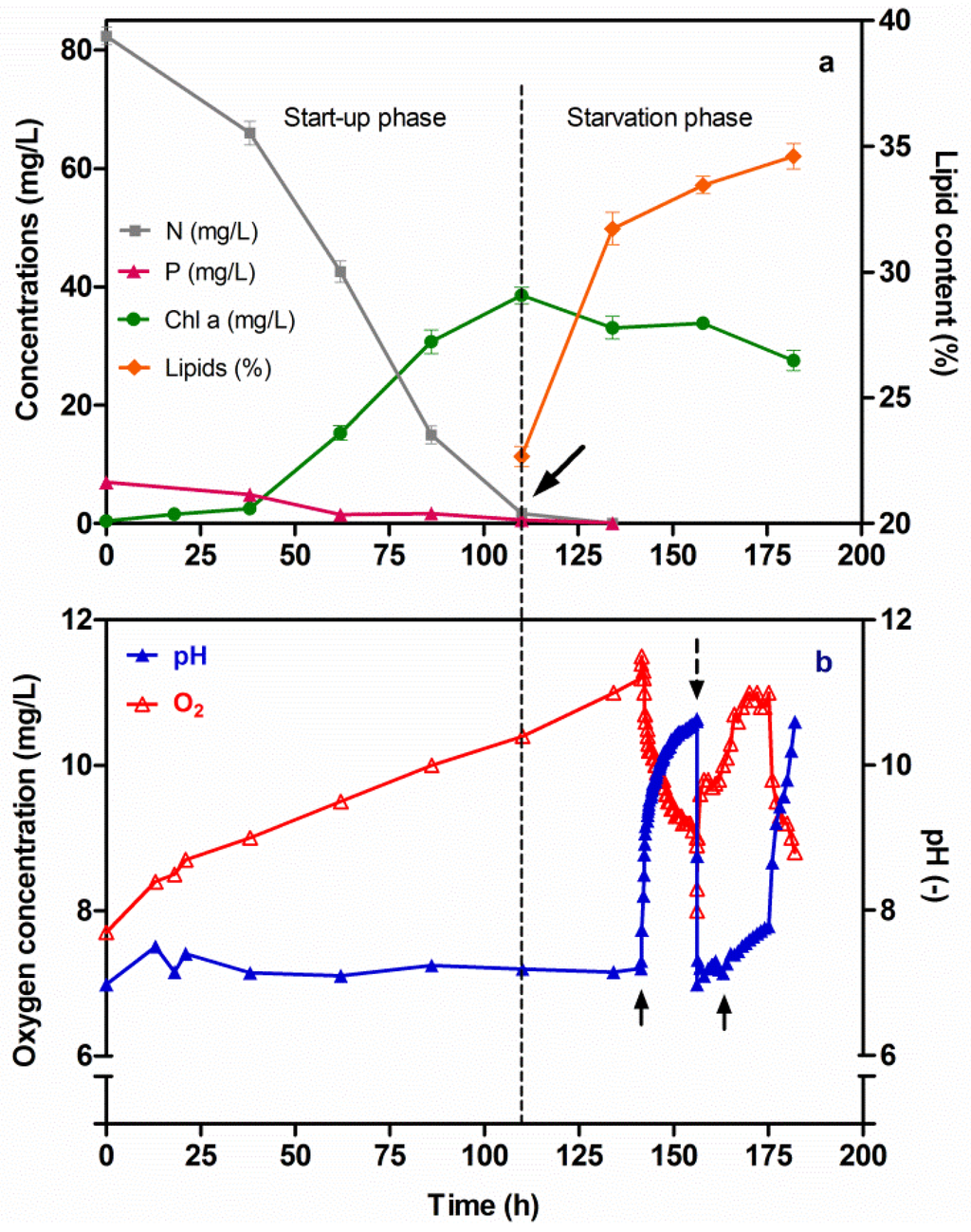
Figure 6.
Changes in the Chl-a, N and P concentrations and lipid content versus time, under two sequential phases (Start-up and Starvation) of Chlorella vulgaris CCALA 896 growth (a); Changes in the pH and O2 versus time (b); The arrow in Figure (a) indicates the beginning of the N and P starvation condition; the two arrows in Figure (b) indicate a halt into the inflow of CO2 in the culture; instead, the dotted row indicates the turn on to the inflow of CO2. A FGPBR with a working volume of 550 mL was used.
Figure 6.
Changes in the Chl-a, N and P concentrations and lipid content versus time, under two sequential phases (Start-up and Starvation) of Chlorella vulgaris CCALA 896 growth (a); Changes in the pH and O2 versus time (b); The arrow in Figure (a) indicates the beginning of the N and P starvation condition; the two arrows in Figure (b) indicate a halt into the inflow of CO2 in the culture; instead, the dotted row indicates the turn on to the inflow of CO2. A FGPBR with a working volume of 550 mL was used.
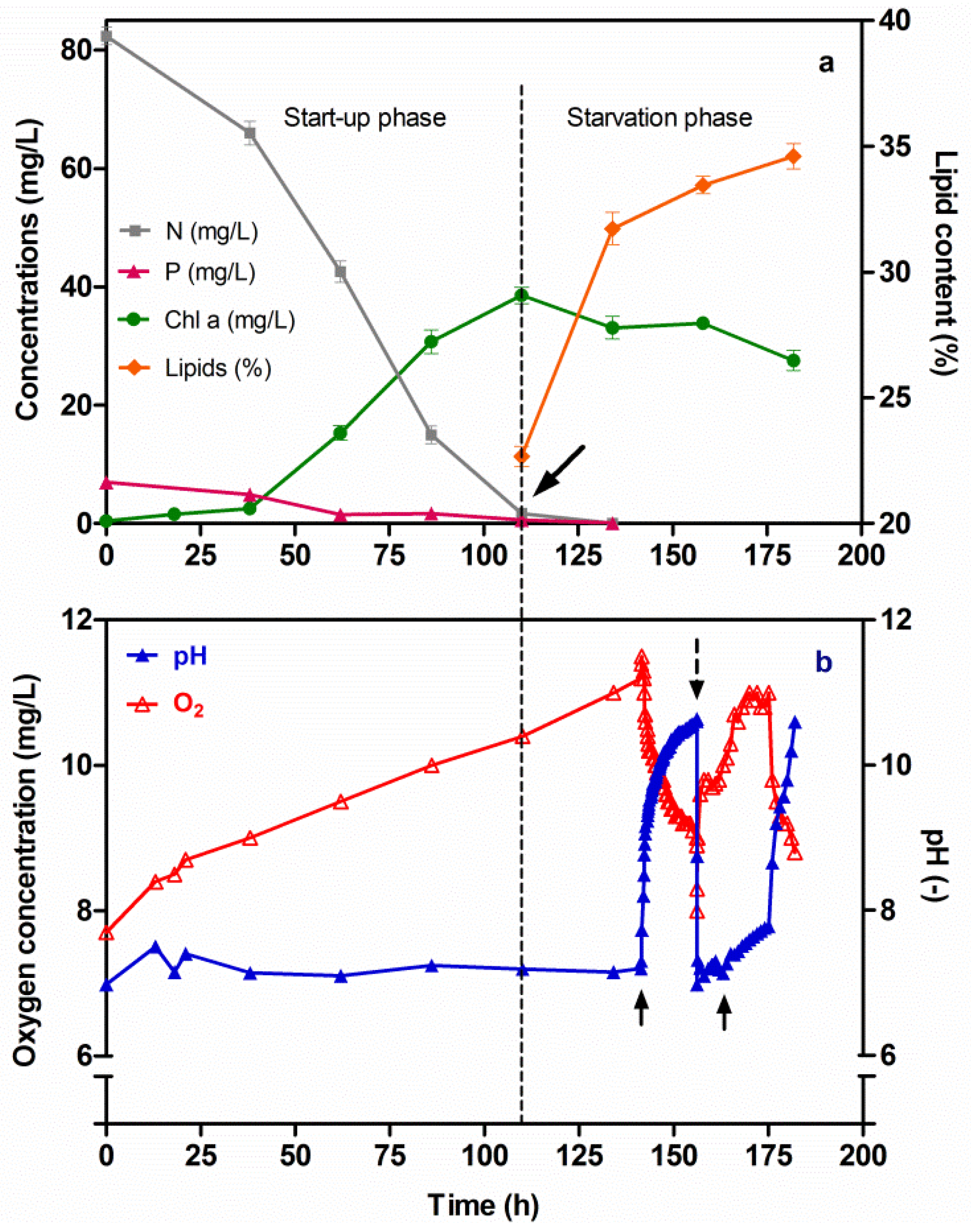
Figure 7 shows the changes in the Chlorella biomass composition during the N and P starvation phase and under stressed-pH conditions. During the first 24 h of starvation time (hours 110 to 134), the lipid content increased significantly from 22.7% to 31.7% while the protein content decreased slightly. Subsequently, we could see that the effect of the pH-stressed conditions (indicated by the arrow) did not cause significant changes in the biomass composition of Chlorella vulgaris CCALA 896 (Figure 7). The protein content remained stable; the lipid content increased, reaching the maximum value (34.6%), and the carbohydrate content increased slightly.
In 2013, an interesting investigation has been reported that concerns technologies for harvesting the biomass of microalgae by increasing the pH value in order to induce the flocculation–sedimentation of microalgae, such as Scenedesmus obliquus and Chlorella vulgaris [46]. The authors demonstrated that flocculation of microalgae can be induced by adding NaOH or Ca(OH)2 to the culture. Although the highest pH value (about 10.7) reached in the flat photobioreactor used in the present study was not high enough to attain a total flocculation-sedimentation in the Chlorella vulgaris CCALA 896, this technology could certainly be improved. In order to cut down on the high costs of microalgae harvesting, which is one of the most expensive steps in microalgae production, the flocculation-sedimentation of Chlorella cells caused by a pH higher than 10.8 with the addition of Ca(OH)2 [46] should be performed at the end of the Chlorella growth, when a pH value of 10.7 has already been attained. By doing this, the amount of Ca(OH)2 required for the flocculation-sedimentation of cells could be reduced significantly.
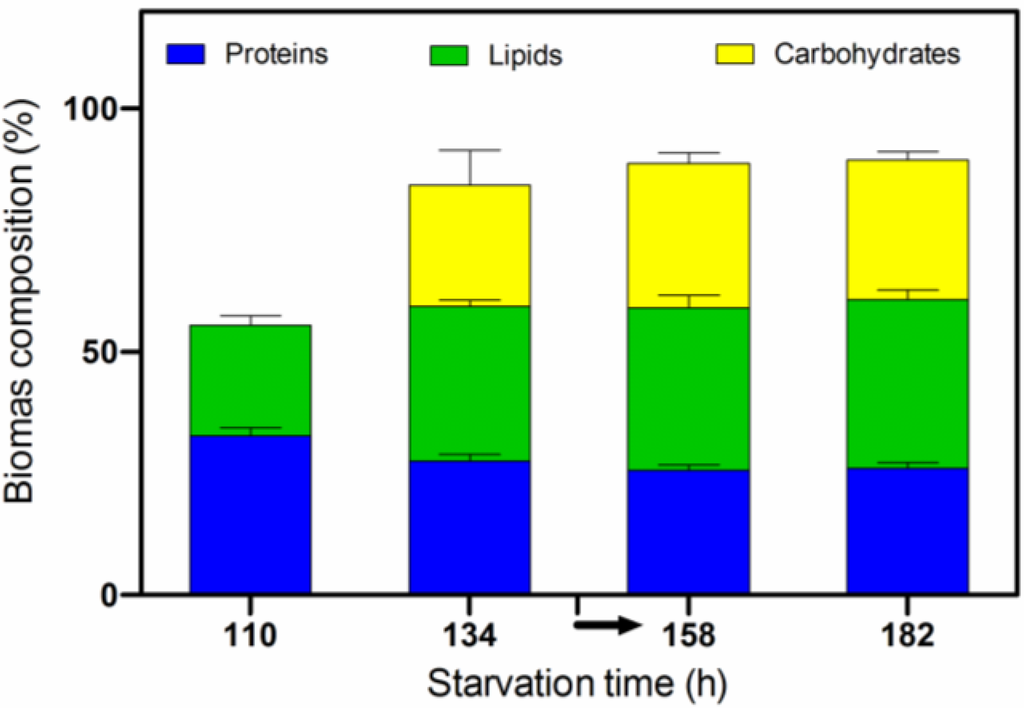
Figure 7.
Changes in biomass composition in Chlorella vulgaris CCALA 896 versus time, during the N and P starvation phase. The arrow indicates the beginning of the pH-stressed conditions. A FGPBR with a working volume of 550 mL was used. The data represent the mean ± SD.
Figure 7.
Changes in biomass composition in Chlorella vulgaris CCALA 896 versus time, during the N and P starvation phase. The arrow indicates the beginning of the pH-stressed conditions. A FGPBR with a working volume of 550 mL was used. The data represent the mean ± SD.
4. Conclusions
Lipid production, for biodiesel conversion can be successfully accomplished by culturing Chlorella vulgaris CCALA 896 under repetitive batch growth regimen conditions, with the replacement of fresh culture broths deprived of nitrogen. Judging from the small amount of data published so far, we should take into consideration the fact that the repetitive batch-growth mode needs to be further investigated. The high pH-stressed conditions imposed at the end of the growth cycle have been shown to be a suitable technique for reducing the high cost involved in harvesting Chlorella biomass.
Acknowledgments
This investigation was partially supported by the projects financed by the Ente Cassa di Risparmio di Firenze (n. 2011.0587) and the European Community’s Seventh Framework Programme [FP7/2007-2013]-Oli-PHA project n. 280604. The authors wish to thank the Department of Botany and Genetics, Faculty of Natural Sciences, Vilnius University, Lithuania for providing the necessary facilities and for supporting their young students.
Author Contributions
Sigita Vaičiulytė and Giulia Padovani carried out all the experiments reported in the present paper. They also analysed the samples and elaborated the data. Sigita Vaičiulytė wrote the first draft of this paper. Jolanta Kostkevičienė contributed to perform the draft and cooperated for elaborating some data. Pietro Carlozzi designed the experiments and was responsible for drafting writing and revising the whole investigation. All the authors were also involved in drafting and gave some esteemed comments on revising on the last paper revision.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Demirbas, A.; Demirbas, M.F. Green Energy and Technology, Algae Energy, Algae as a New Source of Biodiesel, 1st ed.; Springer: London, UK, 2010; pp. 139–157. [Google Scholar]
- Campbell, M.N. Biodiesel: Algae as a renewable source for liquid fuel. Guelph Eng. J. 2008, 1, 2–7. [Google Scholar]
- Demirbas, A. Fuel properties and calculation of higher heating values of vegetable oils. Fuel 1998, 77, 1117–1120. [Google Scholar] [CrossRef]
- Rakopoulos, C.D.; Antonopoulos, K.A.; Rakopoulos, D.C.; Hountalas, D.T.; Giakoumis, E.G. Comparative performance and emissions study of a direct injection diesel engine using blends of diesel fuel with vegetable oils or bio-diesels of various origins. Energy Convers. Manag. 2006, 47, 3272–3287. [Google Scholar]
- Xu, H.; Miao, X.; Wu, Q. High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J. Biotechnol. 2006, 126, 499–507. [Google Scholar] [CrossRef]
- Demirbas, A. Importance of biodiesel as transportation fuel. Energy Policy 2007, 35, 4661–4670. [Google Scholar] [CrossRef]
- Moser, B.R. Biodiesel production, properties, and feedstocks. In Biofuels: Global Impact on Renewable Energy, Production Agriculture, and Technological Advancements; Tomas, D., Lakshmanan, P., Songstad, D., Eds.; Springer New York: New York, NY, USA, 2011; pp. 285–347. [Google Scholar]
- Chisti, Y. Fuels from microalgae. Biofuels 2010, 1, 233–235. [Google Scholar] [CrossRef]
- Milne, T.A.; Evans, R.J.; Nagle, N. Catalytic conversion of microalgae and vegetable oils to premium gasoline, with shape-selective zeolites. Biomass 1990, 21, 219–232. [Google Scholar] [CrossRef]
- Dote, Y.; Sawayama, S.; Inoue, S.; Minowa, T.; Yokoyama, S. Recovery of liquid fuel from hydrocarbon-rich microalgae by thermochemical liquefaction. Fuel 1994, 73, 1855–1857. [Google Scholar] [CrossRef]
- Milnowa, T.; Yokoyama, S.; Kishimoto, M.; Okakura, T. Oil production from algae cells of Dunaliella tereiolata by direct thermochemical liquefaction. Fuel 1995, 74, 1735–1738. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Sharma, K.K.; Schuhmann, H.; Schenk, P.M. High lipid induction in microalgae for biodiesel production. Energies 2012, 5, 1532–1553. [Google Scholar] [CrossRef]
- Rodolfi, L.; Zittelli, G.C.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar]
- Illman, A.M.; Scragg, A.H.; Shales, S.W. Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzyme Microb. Technol. 2000, 27, 631–635. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Yeh, K.-L.; Aisyah, R.; Lee, D.-J.; Chang, J.-S. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: A critical review. Bioresour. Technol. 2011, 102, 71–81. [Google Scholar] [CrossRef]
- Hu, Q. Environmental effects on cell composition. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Richmond, A., Ed.; Blackwell Science Ltd.: Oxford, UK, 2004; Volume 1, pp. 83–93. [Google Scholar]
- Jiang, Y.; Hebly, M.; Kleerebezem, R.; Muyzer, G.; van Loosdrecht, M.C.M. Metabolic modeling of mixed substrate uptake for polyhydroxyalkanoate (PHA) production. Water Res. 2011, 45, 1309–1321. [Google Scholar]
- Guest, J.S.; van Loosdrecht, M.C.M.; Skerlos, S.J.; Love, N.G. Lumped pathway metabolic model of organic carbon accumulation and mobilization by the alga Chlamydomonas reinhardtii. Environ. Sci. Technol. 2013, 47, 3258–3267. [Google Scholar]
- Pulz, O. Photobioreactors: Production systems for phototrophic microorganisms. Appl. Microbiol. Biotechnol. 2001, 57, 287–293. [Google Scholar] [CrossRef]
- Piorreck, M.; Baasch, K.H.; Pohl, P. Biomass production, total protein, chlorophylls, lipids and fatty acids of freshwater green and blue-green algae under different nitrogen regimes. Phytochemistry 1984, 23, 207–216. [Google Scholar] [CrossRef]
- Scragg, A.H.; Illman, A.M.; Carden, A.; Shales, S.W. Growth of microalgae with increased calorific values in a tubular bioreactor. Biomass Bioenergy 2002, 23, 67–73. [Google Scholar] [CrossRef]
- Yeh, K.-L.; Chang, J.-S. Effects of cultivation conditions and media composition on cell growth and lipid productivity of indigenous microalga Chlorella vulgaris ESP-31. Bioresour. Technol. 2012, 105, 120–127. [Google Scholar] [CrossRef]
- Olivieri, G.; Salatino, P.; Marzocchella, A. Advances in photobioreactors for intensive microalgal production: Configurations, operating strategies and applications. J. Chem. Technol. Biotechnol. 2014, 89, 178–195. [Google Scholar] [CrossRef]
- Carlozzi, P. Hydrogen photoproduction by Rhodopseudomonas palustris 42OL cultured at high irradiance under a semicontinuous regime. J. Biomed. Biotechnol. 2012, 2012. [Google Scholar] [CrossRef]
- Carlozzi, P.; Pinzani, E. Growth characteristics of Arthrospira platensis cultured inside a new close-coil photobioreactor incorporating a mandrel to control culture temperature. Biotechnol. Bioeng. 2005, 90, 675–684. [Google Scholar] [CrossRef]
- Strickland, J.D.H.; Parsons, T.R. A Practical Hand Book of Seawater Analysis, 2nd ed.; Fisheries Research Board of Canada: Woods Hole, MA, USA, 1972; pp. 185–199. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method for total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Marsh, J.B.; Weinstein, D.B. Simple charring methods for determination of lipids. J. Lipid Res. 1966, 7, 574–576. [Google Scholar]
- Carlozzi, P. The effect of irradiance growing on hydrogen photoevolution and on the kinetic growth in Rhodopseudomonas palustris, strain 420L. Int. J. Hydrog. Energy 2009, 34, 7949–7958. [Google Scholar] [CrossRef]
- Takagi, M.; Watanabe, K.; Yamaberi, K.; Yoshida, T. Limited feeding of potassium nitrate for intracellular lipid and triglyceride accumulation of Nannochloris sp. UTEX LB1999. Appl. Microbiol. Biotechnol. 2000, 54, 112–117. [Google Scholar]
- Li, Y.; Horsman, M.; Wang, B.; Wu, N.; Lan, C.Q. Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl. Microbiol. Biotechnol. 2008, 81, 629–636. [Google Scholar] [CrossRef]
- Fogg, G.E.; Collyer, D.M. Accumulation of lipids by algae. In Algal Culture: From Laboratory to Pilot Plant; Burlew, J.S., Ed.; Carnegie Institution of Washington: Washington, DC, USA, 1953; Volume 1, pp. 177–181. [Google Scholar]
- Xin, L.; Hu, H.; Ke, G.; Sun, Y. Effects of different nitrogen and phosphorus concentrations on the growth, nutrient uptake, and lipid accumulation of freshwater microalga Scenedesmus sp. Bioresour. Technol. 2010, 101, 5494–5500. [Google Scholar] [CrossRef]
- Pittman, J.K.; Dean, A.P.; Osundeko, O. The potential of sustainable algal biofuel production using wastewater resources. Bioresour. Technol. 2011, 102, 17–25. [Google Scholar]
- Yeh, K.-L.; Chen, Y.-C.; Chang, J.-S. pH-stat photoheterotrophic cultivation of indigenous Chlorella vulgaris ESP-31 for biomass and lipid production using acetic acid as the carbon source. Biochem. Eng. J. 2012, 64, 1–7. [Google Scholar] [CrossRef]
- Hsieh, C.-H.; Wu, W.-T. Cultivation of microalgae for oil production with a cultivation strategy of urea limitation. Bioresour. Technol. 2009, 100, 3921–3926. [Google Scholar] [CrossRef]
- Mujtaba, G.; Choi, W.; Lee, C.-G.; Lee, K. Lipid production by Chlorella vulgaris after a shift from nutrient-rich to nitrogen starvation conditions. Bioresour. Technol. 2012, 123, 279–283. [Google Scholar] [CrossRef]
- Heredia-Arroyo, T.; Wei, W.; Ruan, R.; Hu, B. Mixotrophic cultivation of Chlorella vulgaris and its potential application for the oil accumulation from non-sugar materials. Biomass Bioenergy 2011, 35, 2245–2253. [Google Scholar]
- Converti, A.; Casazza, A.A.; Ortiz, E.Y.; Perego, P.; Borghi, M.D. Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem. Eng. Process. Process Intensif. 2009, 48, 1146–1151. [Google Scholar] [CrossRef]
- Yoo, C.; Jun, S.-Y.; Lee, J.-Y.; Ahn, C.-Y.; Oh, H.-M. Selection of microalgae for lipid production under high levels carbon dioxide. Bioresour. Technol. 2010, 101, S71–S74. [Google Scholar] [CrossRef]
- Li, Y.; Hua, D.; Zhang, J.; Gao, M.; Zhao, Y.; Xu, H.; Liang, X.; Jin, F.; Zhang, X. Influence of inoculum to substrate ratios (ISRs) on the performance of anaerobic digestion of algal residues. Ann. Microbiol. 2013. [Google Scholar] [CrossRef]
- Castrillo, M.; Lucas-Salas, L.M.; Rodriguez-Gil, C.; Martinez, D. High pH-induced flocculation-sedimentation and effect of supernatant reuse on growth rate and lipid productivity of Scenedesmus obliquus and Chlorella vulgaris. Bioresour. Technol. 2013, 128, 324–329. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
