Development and Characterization of an Electrically Rechargeable Zinc-Air Battery Stack
Abstract
:1. Introduction
2. Experimental Section
2.1. Design and Assembly of the Three-Cell Zinc Air Battery Stack

2.2. Performance Test of the Three-Cell Zinc Air Battery Stack
3. Results and Discussion
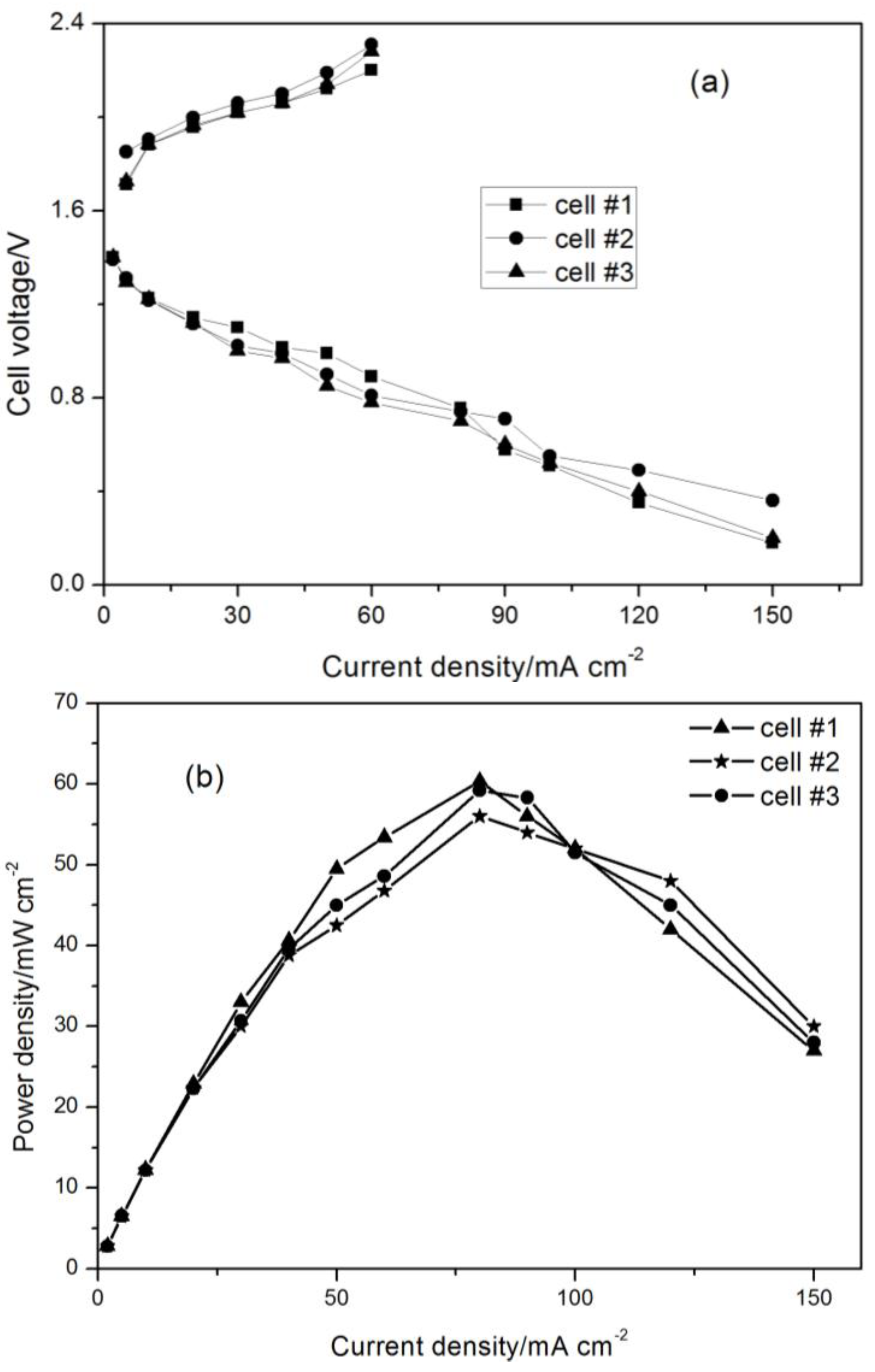
3.1. Polarization Performance for Each Cell in the Three-Cell Battery Stack
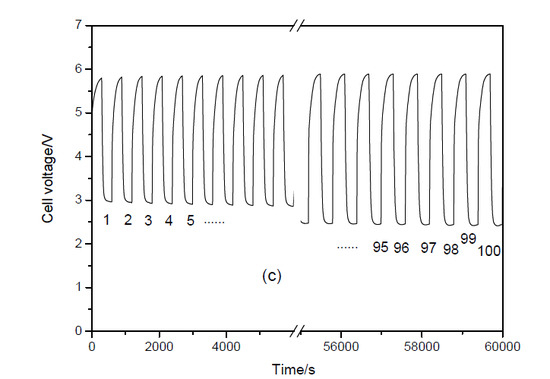
3.2. Cycling Performance of the Three-Cell Zinc-Air Battery Stack
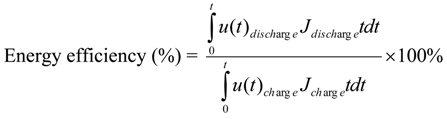

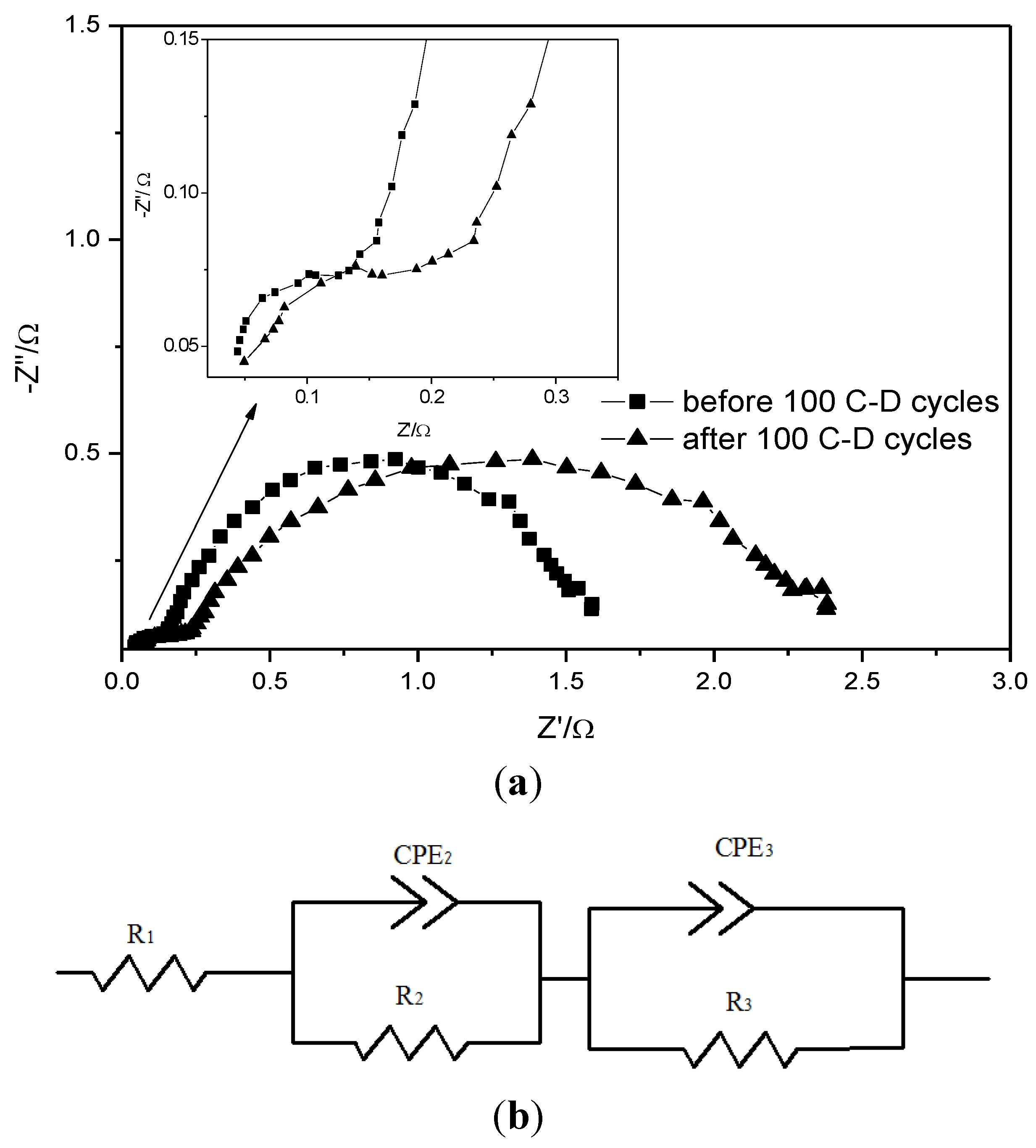
4. Conclusions
Glossary
| ORR | oxygen reduction reaction |
| OER | oxygen evolution reaction |
| C-D | charge-discharge |
| EVs | electric vehicles |
| EIS | electrochemical impedance spectroscopy |
| GDL | gas diffusion layer |
| CCL | catalytic coated layer |
| CNTs | carbon nanotubes |
| R1 | the ohmic resistance of the battery |
| R2 | the charge transfer resistance of negative reaction |
| CPE2 | the constant phase element associated with the double electric layer of negative reaction |
| R3 | the charge transfer resistance of positive reaction |
| CPE3 | the constant phase element associated with the double electric layer of positive reaction |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, Y.; Dai, H. Recent advances in zinc-air batteries. Chem. Soc. Rev. 2014, 43, 5257–5275. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Kim, Y.J.; Eom, S.W.; Choi, N.S.; Kim, K.W.; Cho, S.B. Improvement in self-discharge of Zn anode by applying surface modification for Zn-air batteries with high energy density. J. Power Sources 2013, 227, 177–184. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, A.; Higgins, D.; Li, H.; Wang, H.; Chen, Z. Highly active and durable core-corona structured bifunctional catalyst for rechargeable metal-air battery application. Nano Lett. 2012, 12, 1946–1952. [Google Scholar] [CrossRef] [PubMed]
- Takeguchi, T.; Yamanaka, T.; Takahashi, H.; Watanabe, H.; Kuroki, T.; Nakanishi, H.; Orikasa, Y.; Uchimoto, Y.; Takano, H.; Ohguri, N.; et al. Layered perovskite oxide: A reversible air electrode for oxygen evolution/reduction in rechargeable metal-air batteries. J. Am. Chem. Soc. 2013, 135, 11125–11130. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, S.T.; Cao, R.; Choi, N.S.; Liu, M.; Lee, K.T.; Cho, J. Metal-air batteries with high energy density: Li-air versus Zn-air. Adv. Energy Mater. 2011, 1, 34–50. [Google Scholar] [CrossRef]
- Pei, P.; Ma, Z.; Wang, K.; Wang, X.; Song, M.; Xu, H. High performance zinc air fuel cell stack. J. Power Sources 2014, 249, 13–20. [Google Scholar] [CrossRef]
- Cheng, F.; Chen, J. Metal-air batteries: From oxygen reduction electrochemistry to cathode catalysts. Chem. Soc. Rev. 2012, 41, 2172–2192. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Liu, X.; Zong, Y.; Hor, T.S.A.; Yu, A.; Liu, Z. Co3O4 nanoparticle-modified MnO2 nanotube bifunctional oxygen cathode catalysts for rechargeable zinc-air batteries. Nanoscale 2013, 5, 4657–4661. [Google Scholar] [CrossRef]
- Li, Y.; Gong, M.; Liang, Y.; Feng, J.; Kim, J.E.; Wang, H.; Hong, G.; Zhang, B.; Dai, H. Advanced zinc-air batteries based on high-performance hybrid electrocatalysts. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, A.; Ahmed, R.; Wang, H.; Li, H.; Chen, Z. Manganese dioxide nanotube and nitrogen-doped carbon nanotube based composite bifunctional catalyst for rechargeable zinc-air battery. Electrochim. Acta 2012, 69, 295–300. [Google Scholar] [CrossRef]
- Ma, H.; Wang, B. A bifunctional electrocatalyst α-MnO2-LaNiO3/carbon nanotubes composite for rechargeable zinc-air batteries. RSC Adv. 2014. [Google Scholar] [CrossRef]
- Lee, D.U.; Scott, J.; Park, H.W.; Abureden, S.; Choi, J.Y.; Chen, Z. Morphologically controlled Co3O4 nanodisks as practical bi-functional catalyst for rechargeable zinc-air battery applications. Electrochem. Commun. 2014, 43, 109–112. [Google Scholar] [CrossRef]
- Noack, J.; Cremers, C.; Bayer, D.; Tuebke, J.; Pinkwart, K. Development and characterization of a 280 cm2 vanadium/oxygen fuel cell. J. Power Sources 2014, 253, 397–403. [Google Scholar] [CrossRef]
- Toussaint, G.; Stevens, P.; Akrour, L.; Rouget, R.; Fourgeot, F. Development of a rechargeable zinc-air battery. ECS Trans. 2010, 28, 25–34. [Google Scholar]
- Drillet, J.F.; Adam, M.; Barg, S.; Herter, A.; Koch, D.; Schmidt, V.M.; Wilhelm, M. Development of a novel zinc/air fuel cell with a Zn foam anode, a PVA/KOH membrane and a MnO2/SiOC-based air cathode. ECS Trans. 2010, 28, 13–24. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Wang, B.; Fan, Y.; Hong, W. Development and Characterization of an Electrically Rechargeable Zinc-Air Battery Stack. Energies 2014, 7, 6549-6557. https://doi.org/10.3390/en7106549
Ma H, Wang B, Fan Y, Hong W. Development and Characterization of an Electrically Rechargeable Zinc-Air Battery Stack. Energies. 2014; 7(10):6549-6557. https://doi.org/10.3390/en7106549
Chicago/Turabian StyleMa, Hongyun, Baoguo Wang, Yongsheng Fan, and Weichen Hong. 2014. "Development and Characterization of an Electrically Rechargeable Zinc-Air Battery Stack" Energies 7, no. 10: 6549-6557. https://doi.org/10.3390/en7106549
APA StyleMa, H., Wang, B., Fan, Y., & Hong, W. (2014). Development and Characterization of an Electrically Rechargeable Zinc-Air Battery Stack. Energies, 7(10), 6549-6557. https://doi.org/10.3390/en7106549