Synthesis of Alcohols and Alkanes from CO and H2 over MoS2/γ-Al2O3 Catalyst in a Packed Bed with Continuous Flow
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of Catalysts
2.2. Characterization of Catalysts
2.3. Hydrogenation of CO
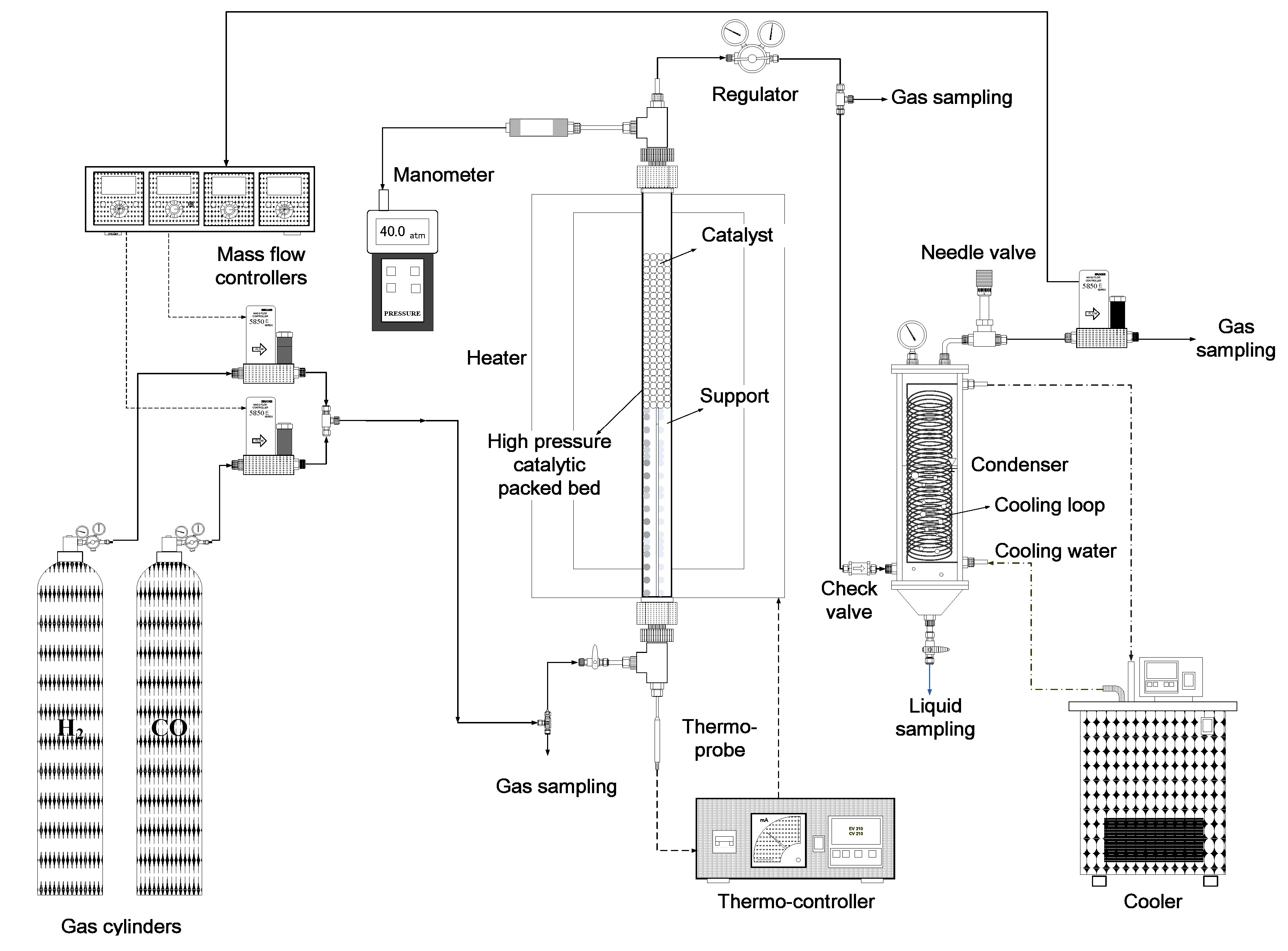
2.4. Analyses of Liquid and Gaseous Samples
3. Results and Discussion
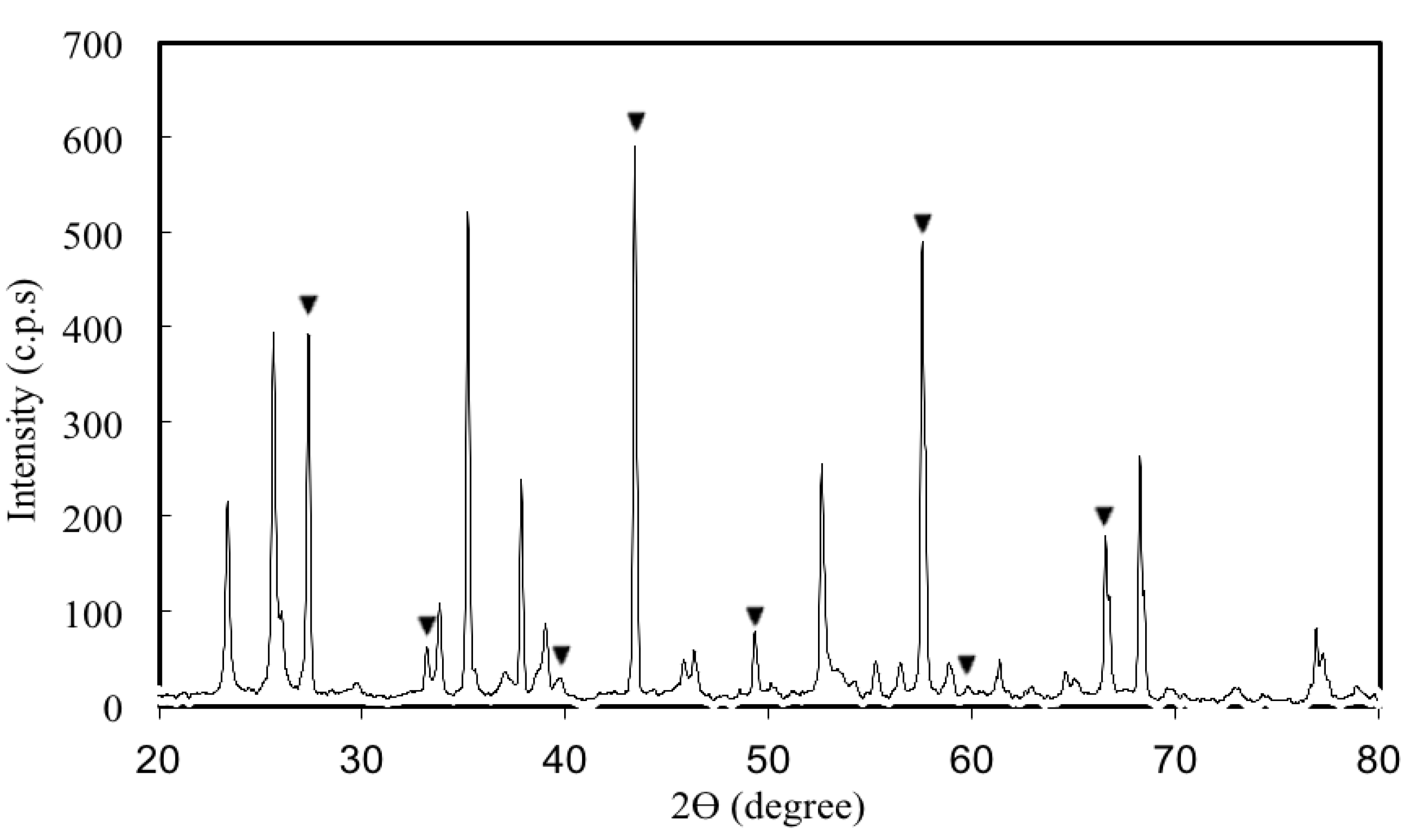
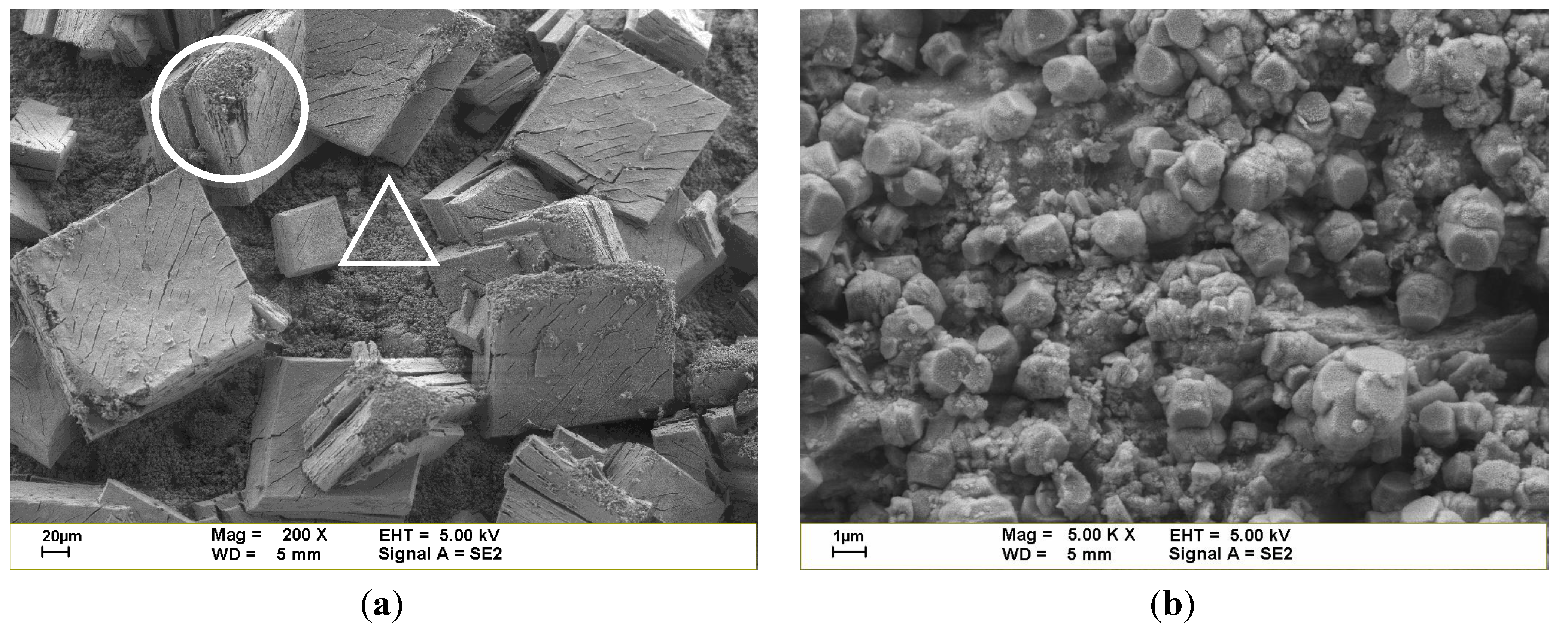
3.1. Properties and Characteristics of Catalysts
| Sample | SBET (m2 g−1) | Density (g cm−3) | Porosity | Pore size | |
|---|---|---|---|---|---|
| ρP | ρS | εP | (Å) | ||
| γ-Al2O3 | 280.46 | 1.27 | 2.89 | 0.528 | 55.5 |
| MoS2/γ-Al2O3 | 210.35 | 1.23 | 3.21 | 0.550 | 74.7 |
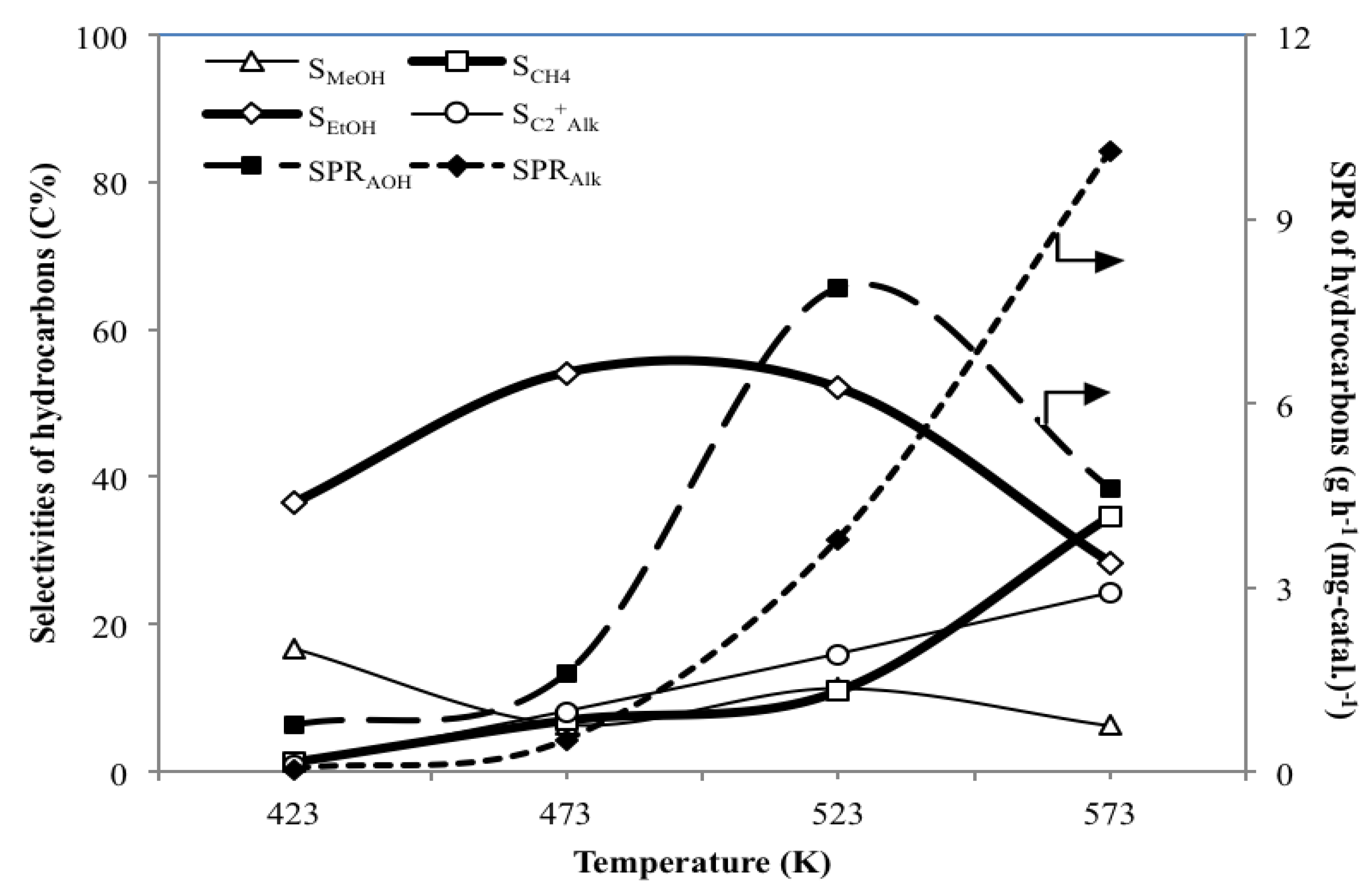
3.2. Effect of Temperature T on the Catalytic Performance of MoS2/γ-Al2O3
| Catalyst | T (K) | Conversion of CO (XCO) (C%) | SPRb (mg h−1 gcat−1) | Yieldc (C%) | Selectivity of hydrocarbon productd (C%) (SHC) | SPRHCsf | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SPRAlk | SPRAOH | YAlk | YAOH | SCH4 | SC2+Alk | SMeOH | SEtOH | SPrOH | SBuOH | SOtHCe | (mg h−1 gcat−1) | |||
| MoS2/γ-Al2O3 | 423 | 0.6 | 0.1 | 0.8 | 0.2 | 0.5 | 1.2 | 0.7 | 16.7 | 36.4 | 6.5 | 7.3 | 31.1 | 1.1 |
| 473 | 2.1 | 0.5 | 1.6 | 1.0 | 1.1 | 6.9 | 8.1 | 6.4 | 54.0 | 0.3 | 1.9 | 22.5 | 3.2 | |
| 523 | 8.1 | 3.8 | 7.9 | 2.0 | 5.0 | 10.9 | 15.8 | 11.3 | 52.0 | 2.3 | 0.3 | 7.5 | 13.2 | |
| 573 | 8.2 | 10.1 | 4.6 | 5.3 | 2.9 | 34.6 | 24.1 | 6.2 | 28.3 | 0.4 | 0.2 | 6.3 | 16.1 | |
| Rh-Mn/SiO2g | 563 | 3.6 | 46.1 | 205.8 | - | - | - | - | - | 56.8 | - | - | - | - |
| 573 | 4.8 | 53.4 | 260.7 | - | - | - | - | - | 67.0 | - | - | - | - | |
| 583 | 7.4 | 127.6 | 314 | - | - | - | - | - | 49.5 | - | - | - | - | |
| 593 | 9.5 | 189 | 427 | - | - | - | - | - | 44.9 | - | - | - | - | |
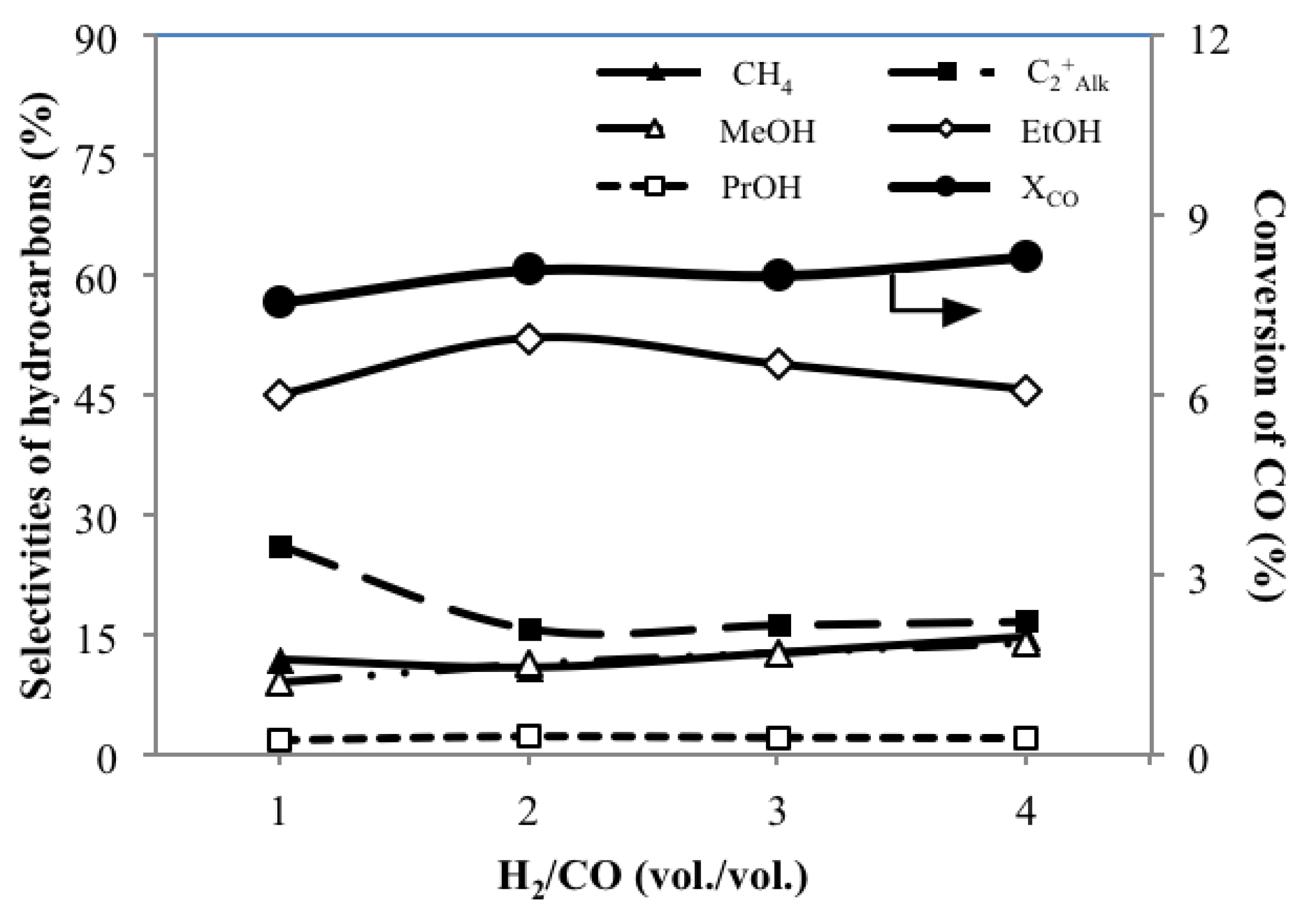
3.3. Effect of H2/CO Ratio MH/C on the Catalytic Performance
| Catalyst | H2/CO (vol./vol.) | XCO (C%) | SPRb (mg h−1 gcat−1) | Yieldc (C%) | SHCd (C%) | SPRHCsf | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SPRAlk | SPRAOH | YAlk | YAOH | SCH4 | SC2+Alk | SMeOH | SEtOH | SPrOH | SBuOH | SOtHCe | (mg h−1 gcat−1) | |||
| MoS2/γ-Al2O3 | 1 | 7.6 | 4.6 | 6.4 | 2.7 | 4.1 | 11.8 | 26.2 | 9.0 | 44.9 | 1.8 | 1.2 | 5.1 | 11.9 |
| 2 | 8.1 | 3.8 | 7.9 | 2.0 | 5.0 | 10.9 | 15.8 | 11.3 | 52.0 | 2.3 | 0.3 | 7.5 | 13.2 | |
| 3 | 8.0 | 4.1 | 7.8 | 2.2 | 4.8 | 12.7 | 16.3 | 12.7 | 48.7 | 2.1 | 0.5 | 7.0 | 13.3 | |
| 4 | 8.3 | 4.8 | 7.7 | 2.5 | 4.9 | 14.7 | 16.7 | 13.9 | 45.6 | 2.1 | 0.5 | 6.5 | 13.8 | |
| Rh-Mn-Li/TiO2g | 1 | 0.46 | - | - | 0.34 | 0.03 | 73.6 | - | 3.3 | 3.5 | - | - | 18.5 | - |
| 2 | 0.94 | - | - | 0.74 | 0.07 | 78.4 | - | 3.4 | 4.3 | - | - | 13.0 | - | |
| 3 | 1.57 | - | - | 1.27 | 0.13 | 80.8 | - | 3.4 | 4.8 | - | - | 10.3 | - | |
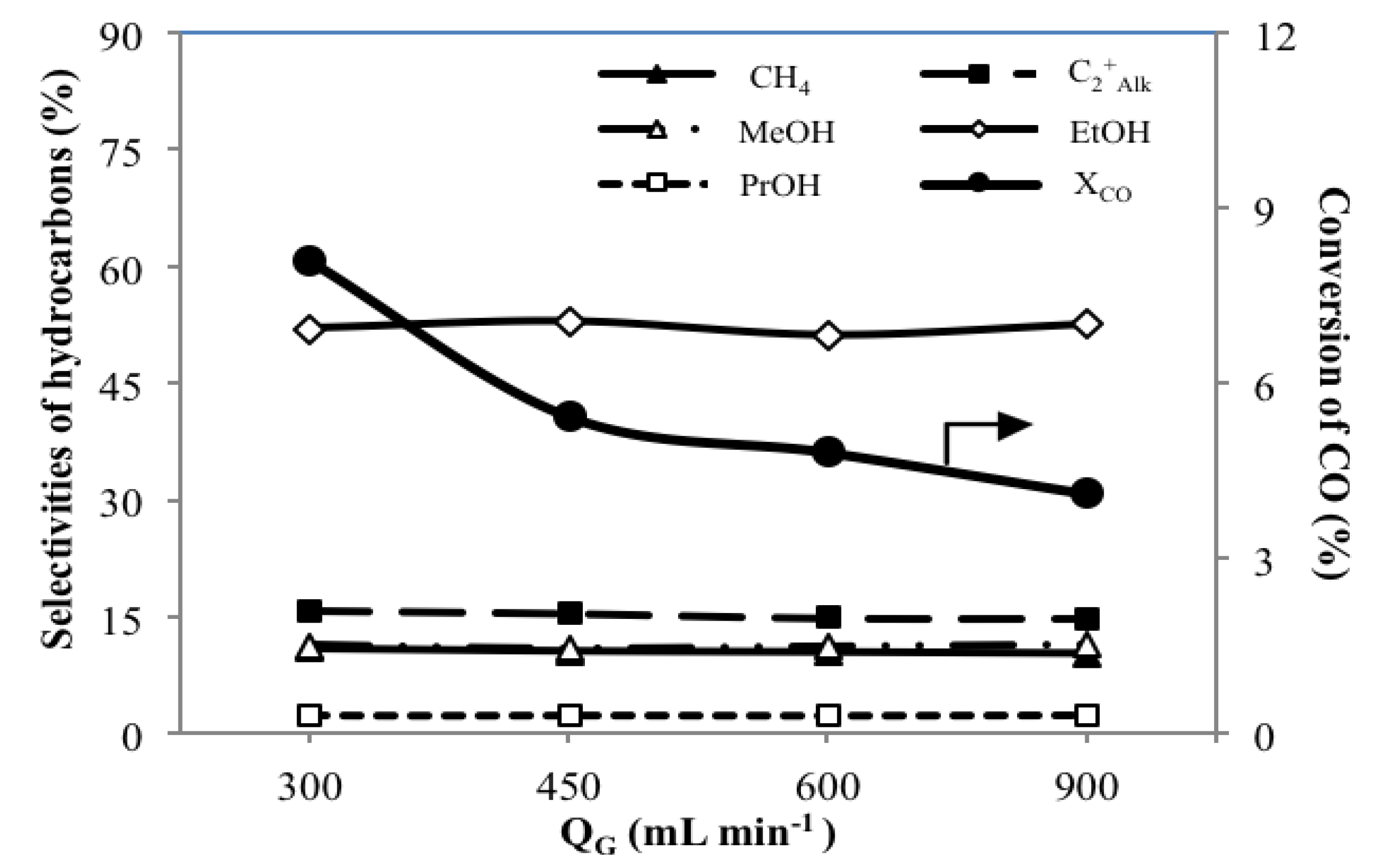
3.4. Effect of Total Gas Flow Rates QG on the Catalytic Performance
| Catalyst | QG (mL min−1) | GHSV (h−1) | WHSV (h−1) | XCO (C%) | SPRb (mg h−1 gcat−1) | Yieldc (C%) | SHCd(C%) | SPRHCsf (mg h−1 gcat−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SPRAlk | SPRAOH | YAlk | YAOH | SCH4 | SC2+Alk | SMeOH | SEtOH | SPrOH | SBuOH | SOtHCe | ||||||
| MoS2/γ-Al2O3 | 300 | 1,020 | 0.34 | 8.1 | 3.8 | 7.9 | 2.0 | 5.0 | 10.9 | 15.8 | 11.3 | 52.0 | 2.3 | 0.3 | 7.5 | 13.2 |
| 450 | 1,525 | 0.51 | 5.4 | 5.6 | 12.0 | 1.4 | 2.9 | 10.5 | 15.5 | 10.9 | 52.9 | 2.4 | 0.3 | 7.6 | 20.0 | |
| 600 | 2,035 | 0.69 | 4.8 | 6.4 | 14.3 | 1.2 | 2.6 | 10.4 | 14.9 | 11.2 | 51.1 | 2.3 | 0.3 | 7.8 | 23.6 | |
| 900 | 3,050 | 1.03 | 4.1 | 8.1 | 18.0 | 0.5 | 2.2 | 10.3 | 14.8 | 11.4 | 52.5 | 2.4 | 0.3 | 8.4 | 30.0 | |
| Rh-Mn/SiO2g | 1,700 | 38.7 | - | - | 15.6 | 21.9 | 40.2 | - | 2.8 | 53.9 | - | - | 3.1 | - | ||
| 3,750 | 24.6 | - | - | 9.45 | 14.8 | 38.4 | - | 3.9 | 56.1 | - | - | 1.6 | - | |||

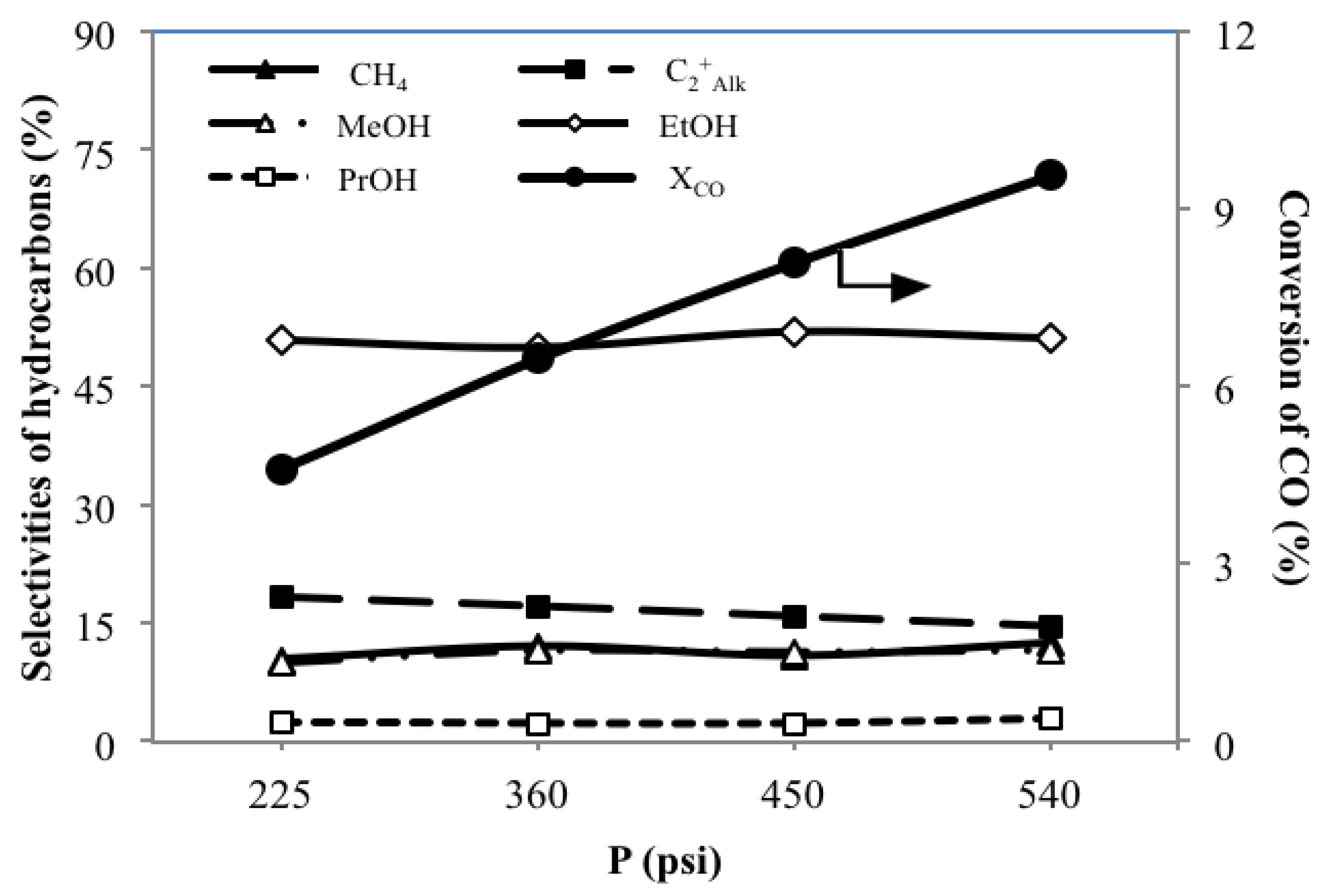
3.5. Effect of System Pressures PST on the Catalytic Performance
| Catalyst | PST (psi) | XCO (C%) | SPRb (mg h−1 gcat−1) | Yieldc (C%) | SHCd(C%) | SPRHCsf | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SPRAlk | SPRAOH | YAlk | YAOH | SCH4 | SC2+Alk | SMeOH | SEtOH | SPrOH | SBuOH | SOtHCe | (mg h−1 gcat−1) | |||
| MoS2/γ-Al2O3 | 225 | 4.6 | 2.2 | 4.2 | 1.2 | 2.7 | 10.3 | 18.3 | 9.9 | 50.9 | 2.5 | - | 8.1 | 7.4 |
| 360 | 6.5 | 3.3 | 6.2 | 1.8 | 3.9 | 12.1 | 17.1 | 11.5 | 50.0 | 2.3 | - | 7.1 | 10.7 | |
| 450 | 8.1 | 3.8 | 7.9 | 2.0 | 5.0 | 10.9 | 15.8 | 11.3 | 52.0 | 2.3 | 0.3 | 7.5 | 13.2 | |
| 540 | 9.6 | 4.7 | 9.3 | 2.4 | 6.1 | 12.5 | 14.6 | 11.5 | 51.2 | 2.9 | 0.3 | 7.1 | 15.8 | |
| Fe/TiO2g | 206 | 6.7 | - | - | - | - | 36.2 | 7.2 | 3.3 | 35.7 | - | - | 17.6 | - |
| 412 | 8.8 | - | - | - | - | 37.5 | 7.6 | 2.8 | 30.4 | - | - | 21.7 | - | |
4. Conclusions
- For the hydrogenation of CO using MoS2/γ-Al2O3 catalyst, T at 523 K is proper not only to yield SPRAOH and YAOH higher than SPRAlk and YAlk, respectively, but also to give a satisfactory high value of SEtOH of 52.0 C%.
- Compared to other settings of H2/CO, H2/CO at 2 gives highest SEtOH of 52.0 C%, which is in accordance with the stoichiometry of the formation of EtOH.
- A lower QG offers longer tR, resulting in higher reaction extents with higher XCO and YAOH along with a significantly high value of SEtOH of 52.0 C%.
- An increasing pressure enhances the XCO, YAlk and YAOH, while only slightly changing the SHCs of each HC.
- The beneficial use of MoS2/γ-Al2O3 in the hydrogenation of CO favors the production of ethanol with the proper operation conditions at T = 523 K, H2/CO = 2, lower flow rate and higher pressure.
Acknowledgements
References
- Spivey, J.J.; Egbebi, A. Heterogeneous catalytic synthesis of ethanol from biomass-derived syngas. Chem. Soc. Rev. 2007, 36, 1514–1528. [Google Scholar] [CrossRef] [PubMed]
- Subramani, V.; Gangwal, S.K. A review of recent literature to search for an efficient catalytic process for the conversion of syngas to ethanol. Energy Fuels 2008, 22, 814–839. [Google Scholar] [CrossRef]
- Mori, S. Development of utilization technologies of biomass energy. J. Environ. Eng. Manag. 2009, 19, 67–72. [Google Scholar]
- Pambudi, N.A.; Torii, S.; Saptoadi, H.; Sumbodo, W.; Syamsiyo, M.; Surono, U.B. Experimental study on combustion of biobriquettes jatropha curcas solid waste. J. Environ. Eng. Manag. 2010, 20, 133–136. [Google Scholar]
- Chuang, Y.S.; Chen, C.C.; Lay, C.H.; Sung, I.Y.; Wu, J.S.; Lee, S.C.; Sen, B.; Lin, C.Y. Optimization of incubation factors for fermentative hydrogen production from agricultural wastes. Sustain. Environ. Res. 2012, 22, 99–106. [Google Scholar]
- Syu, F.S.; Chiueh, P.T. Process simulation of rice straw torrefaction. Sustain. Environ. Res. 2012, 22, 177–183. [Google Scholar]
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; Mielenz, J.R.; Murphy, R.; Templer, R.; Tschaplinski, T. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Burch, R.; Hayes, M.J. The preparation and characterisation of Fe-promoted Al2O3-supported Rh catalysts for the selective production of ethanol from syngas. J. Catal. 1997, 165, 249–261. [Google Scholar] [CrossRef]
- Mazzocchia, C.; Gronchi, P.; Kaddouri, A.; Tempesti, E.; Zanderighi, L.; Kiennemann, A. Hydrogenation of CO over Rh/SiO2-CeO2 catalysts: Kinetic evidences. J. Mol. Catal. A Chem. 2001, 165, 219–230. [Google Scholar] [CrossRef]
- Ojeda, M.; Granados, M.L.; Rojas, S.; Terreros, P.; Garcia-Garcia, F.J.; Fierro, J.L.G. Manganese-promoted Rh/Al2O3 for C-2-oxygenates synthesis from syngas—Effect of manganese loading. Appl. Catal. A Gen. 2004, 261, 47–55. [Google Scholar] [CrossRef]
- Koizumi, N.; Murai, K.; Ozaki, T.; Yamada, M. Development of sulfur tolerant catalysts for the synthesis of high quality transportation fuels. Catal. Today 2004, 89, 465–478. [Google Scholar] [CrossRef]
- Li, D.B.; Yang, C.; Qi, H.J.; Zhang, H.R.; Li, W.H.; Sun, Y.H.; Zhong, B. Higher alcohol synthesis over a La promoted Ni/K2CO3/MoS2 catalyst. Catal. Commun. 2004, 5, 605–609. [Google Scholar] [CrossRef]
- Li, D.B.; Zhao, N.; Qi, H.J.; Li, W.H.; Sun, Y.H.; Zhong, B. Ultrasonic preparation of Ni modified K2CO3/MoS2 catalyst for higher alcohols synthesis. Catal. Commun. 2005, 6, 674–678. [Google Scholar] [CrossRef]
- Li, D.; Yang, C.; Zhao, N.; Qi, H.; Li, W.; Sun, Y.; Zhong, B. The performances of higher alcohol synthesis over nickel modified K2CO3/MoS2 catalyst. Fuel Process. Technol. 2007, 88, 125–127. [Google Scholar] [CrossRef]
- Woo, H.C.; Park, T.Y.; Kim, Y.G.; Nam, I.S.; Lee, J.S.; Chung, J.S. Alkali-promoted MoS2 catalysts for alcohol synthesis—The effect of alkali promotion and preparation condition on activity and selectivity. Stud. Surf. Sci. Catal. 1993, 75, 2749–2752. [Google Scholar]
- Sakashita, Y.; Araki, Y.; Shimada, H. Effects of surface orientation of alumina supports on the catalytic functionality of molybdenum sulfide catalysts. Appl. Catal. A Gen. 2001, 215, 101–110. [Google Scholar] [CrossRef]
- Qi, H.J.; Li, D.B.; Yang, C.; Ma, Y.G.; Li, W.H.; Sun, Y.H.; Zhong, B. Nickel and manganese co-modified K/MoS2 catalyst: High performance for higher alcohols synthesis from CO hydrogenation. Catal. Commun. 2003, 4, 339–342. [Google Scholar] [CrossRef]
- Huang, M.; Cho, K. Density functional theory study of CO hydrogenation on a MoS2 surface. J. Phys. Chem. C 2009, 113, 5238–5243. [Google Scholar] [CrossRef]
- Surisetty, V.R.; Dalai, A.K.; Kozinski, J. Effect of Rh promoter on MWCNT-supported alkali-modified MoS2 catalysts for higher alcohols synthesis from CO hydrogenation. Appl. Catal. A Gen. 2010, 381, 282–288. [Google Scholar] [CrossRef]
- Inoue, T.; Iizuka, T.; Tanabe, K. Hydrogenation of carbon dioxide and carbon monoxide over supported rhodium catalysts under 10 bar pressure. Appl. Catal. 1989, 46, 1–9. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Y.; Cao, C.; Elliott, D.C.; Stevens, D.J.; White, J.F. Conversion of biomass-derived syngas to alcohols and C2 oxygenates using supported Rh catalysts in a microchannel reactor. Catal. Today 2007, 120, 90–95. [Google Scholar] [CrossRef]
- Mazzocchia, C.; Tempesti, E.; Gronchi, P.; Giuffrè, L.; Zanderighi, L. Hydrogenation of CO over ZrO2-supported Rh catalysts: Role of experimental parameters in modifying the C2H5OHCH4 product ratio. J. Catal. 1988, 111, 345–352. [Google Scholar] [CrossRef]
- Egbebi, A.; Spivey, J.J. Effect of H2/CO ratio and temperature on methane selectivity in the synthesis of ethanol on Rh-based catalysts. Catal. Commun. 2008, 9, 2308–2311. [Google Scholar] [CrossRef]
- Berdinsky, A.S.; Chadderton, L.T.; Yoo, J.B.; Gutakovsky, A.K.; Fedorov, V.E.; Mazalov, L.N.; Fink, D. Structural changes of MoS2 nano-powder in dependence on the annealing temperature. Appl. Phys. A Mater. Sci. Process. 2005, 80, 61–67. [Google Scholar] [CrossRef]
- Ye, L.; Wu, C.; Guo, W.; Xie, Y. MoS2 hierarchical hollow cubic cages assembled by bilayers: One-step synthesis and their electrochemical hydrogen storage properties. Chem. Commun. 2006, 45, 4738–4740. [Google Scholar] [CrossRef]
- Luo, H.Y.; Lin, P.Z.; Xie, S.B.; Zhou, H.W.; Xu, C.H.; Huang, S.Y.; Lin, L.W.; Liang, D.B.; Yin, P.L.; Xin, Q. The role of Mn and Li promoters in supported rhodium catalysts in the formation of acetic acid and acetaldehyde. J. Mol. Catal. A Chem. 1997, 122, 115–123. [Google Scholar] [CrossRef]
- Haider, M.A.; Gogate, M.R.; Davis, R.J. Fe-promotion of supported Rh catalysts for direct conversion of syngas to ethanol. J. Catal. 2009, 261, 9–16. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chiang, S.-W.; Chang, C.-C.; Shie, J.-L.; Chang, C.-Y.; Ji, D.-R.; Tseng, J.-Y.; Chang, C.-F.; Chen, Y.-H. Synthesis of Alcohols and Alkanes from CO and H2 over MoS2/γ-Al2O3 Catalyst in a Packed Bed with Continuous Flow. Energies 2012, 5, 4147-4164. https://doi.org/10.3390/en5104147
Chiang S-W, Chang C-C, Shie J-L, Chang C-Y, Ji D-R, Tseng J-Y, Chang C-F, Chen Y-H. Synthesis of Alcohols and Alkanes from CO and H2 over MoS2/γ-Al2O3 Catalyst in a Packed Bed with Continuous Flow. Energies. 2012; 5(10):4147-4164. https://doi.org/10.3390/en5104147
Chicago/Turabian StyleChiang, Sheng-Wei, Chia-Chi Chang, Je-Lueng Shie, Ching-Yuan Chang, Dar-Ren Ji, Jyi-Yeong Tseng, Chiung-Fen Chang, and Yi-Hung Chen. 2012. "Synthesis of Alcohols and Alkanes from CO and H2 over MoS2/γ-Al2O3 Catalyst in a Packed Bed with Continuous Flow" Energies 5, no. 10: 4147-4164. https://doi.org/10.3390/en5104147
APA StyleChiang, S.-W., Chang, C.-C., Shie, J.-L., Chang, C.-Y., Ji, D.-R., Tseng, J.-Y., Chang, C.-F., & Chen, Y.-H. (2012). Synthesis of Alcohols and Alkanes from CO and H2 over MoS2/γ-Al2O3 Catalyst in a Packed Bed with Continuous Flow. Energies, 5(10), 4147-4164. https://doi.org/10.3390/en5104147





