Experimental Research on Heterogeneous N2O Decomposition with Ash and Biomass Gasification Gas
Abstract
:1. Introduction
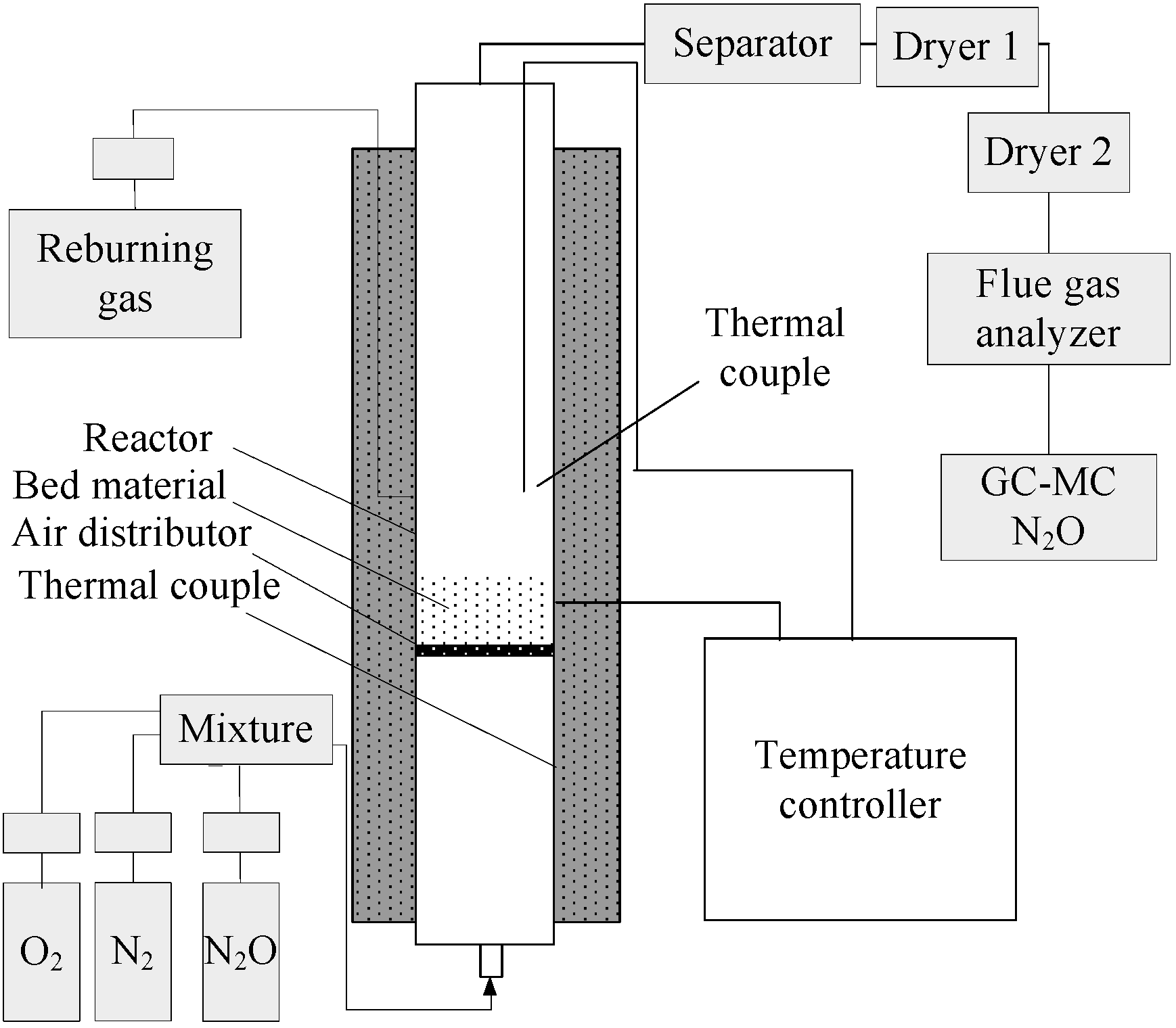
2. Experimental Devices and Method
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | TiO2 | CaSO4 | P2O5 | K2O | Na2O | |
|---|---|---|---|---|---|---|---|---|---|---|
| Circulating ash | 33.22 | 13.91 | 6.66 | 11.59 | 1.59 | 1.97 | 31.88 | 0.18 | 1.01 | 0.30 |
| : | mole of N2O at the reactor outlet, mol; |
| : | mole of N2O at the reactor inlet, mol; |
| : | mole of NO at the reactor outlet, mol; |
| : | mole of NO2 at the reactor outlet, mol; |
| : | flow of N2O inlet, mL/min; |
| : | time of collecting gas in sample bag, min; |
| : | volume of collecting gas in sample bag, mL; |
| : | concentration of NO in exhaust gas, ppm; |
| : | concentration of NO in exhaust gas, ppm. |
- : volume of biomass derived gas, mL;
- : volume of flue gas, mL.
3. Results and Discussion
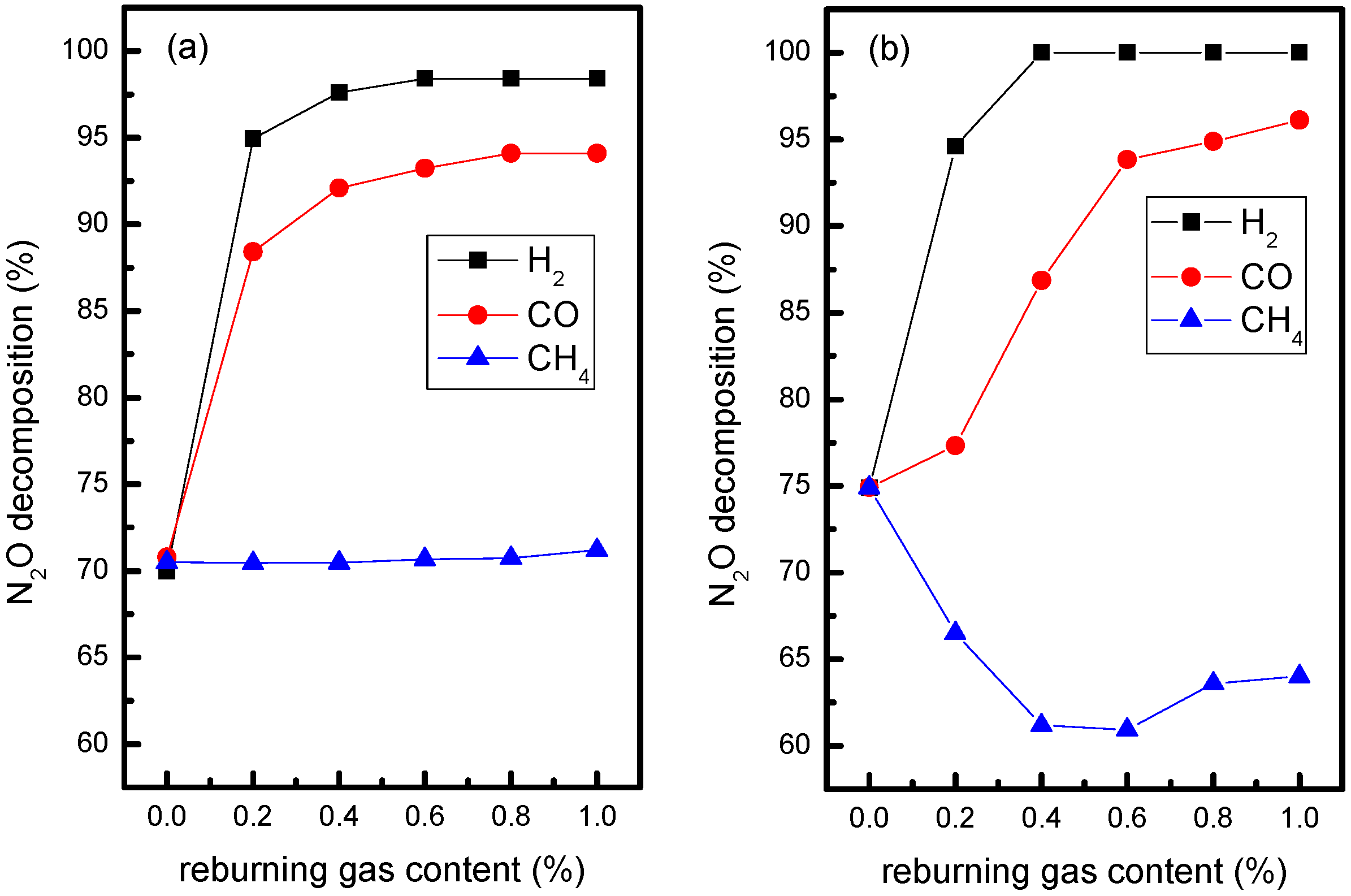
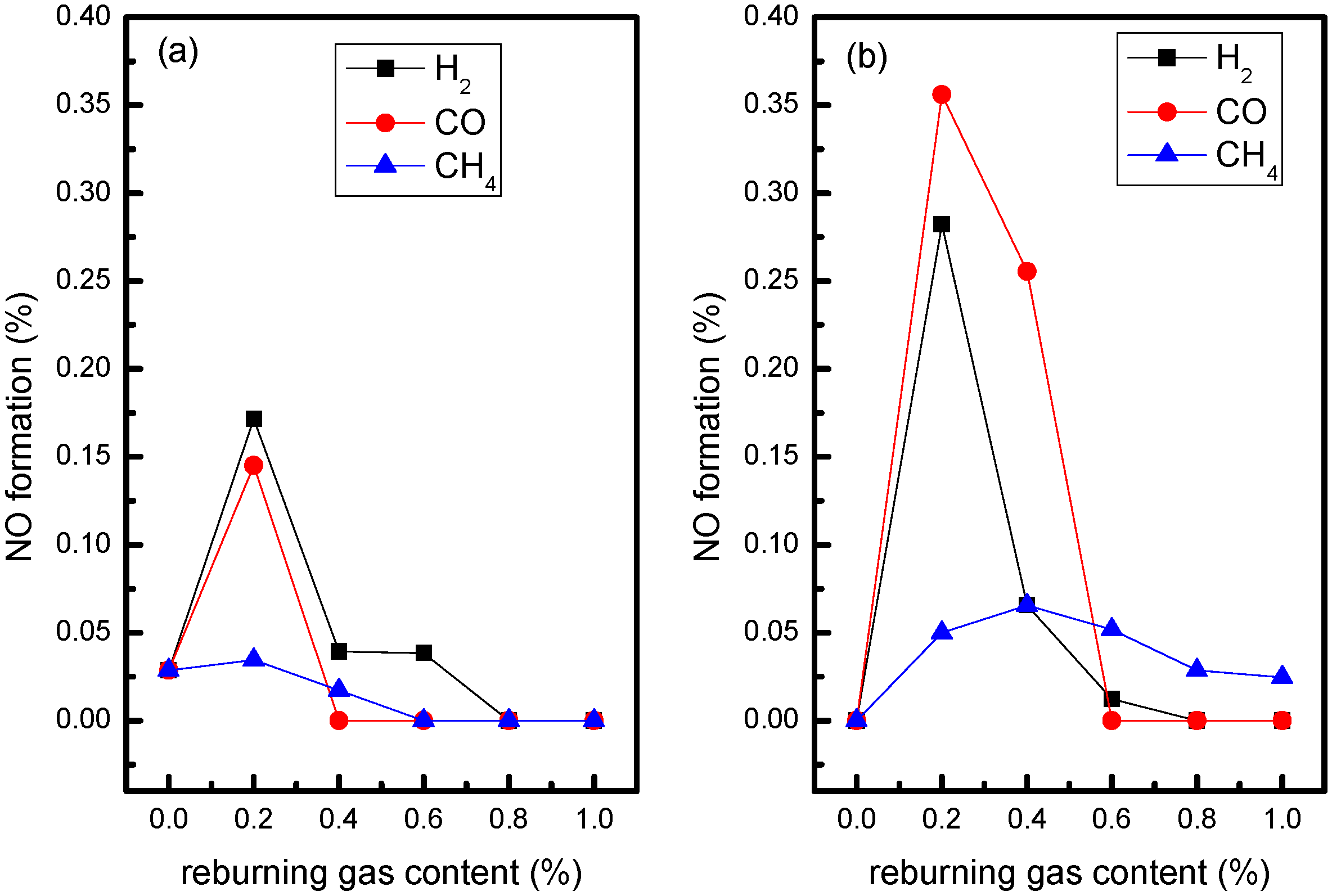
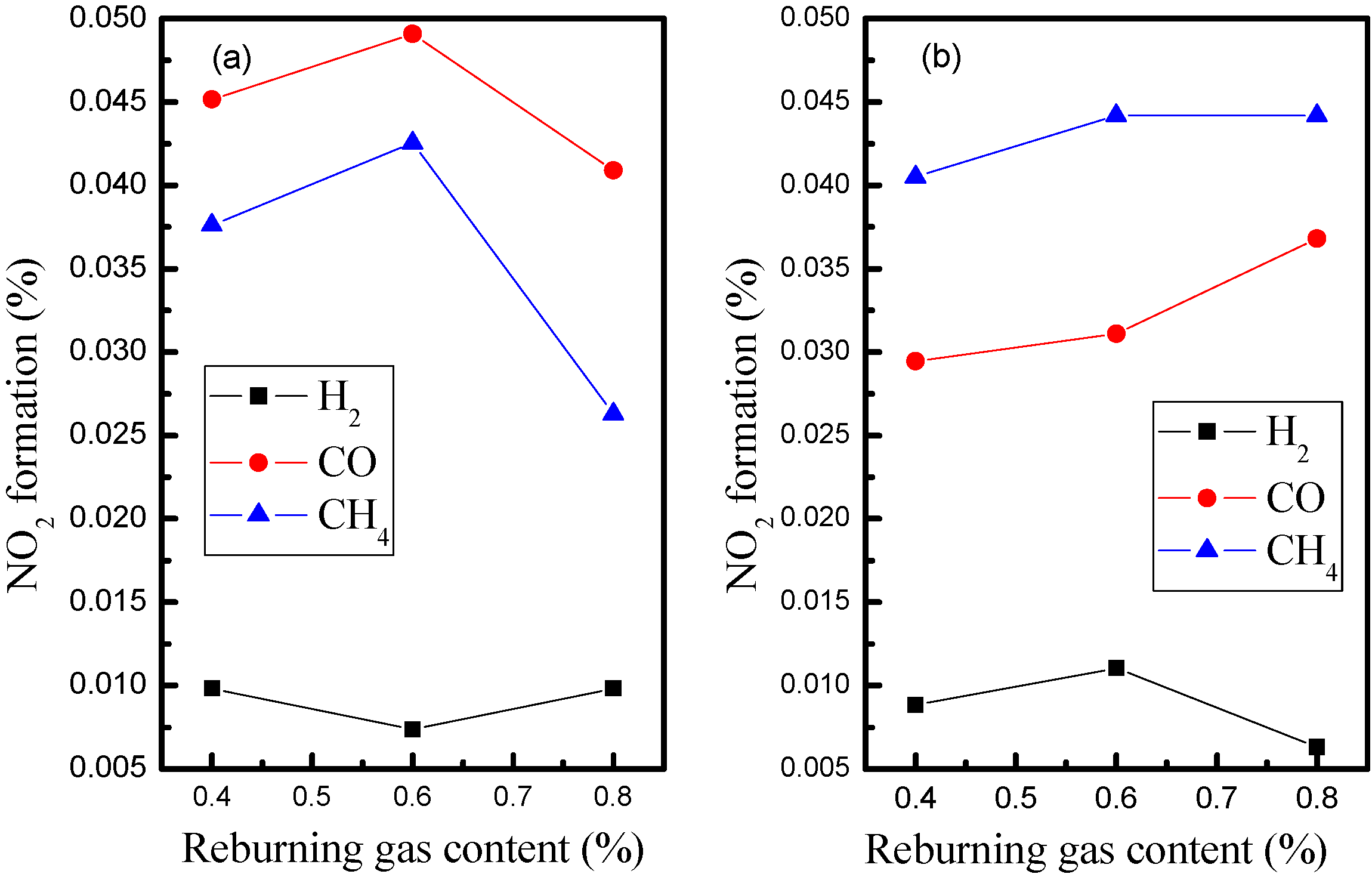
3.1. Influence of Biomass Gas Components without Oxygen in Flue Gas

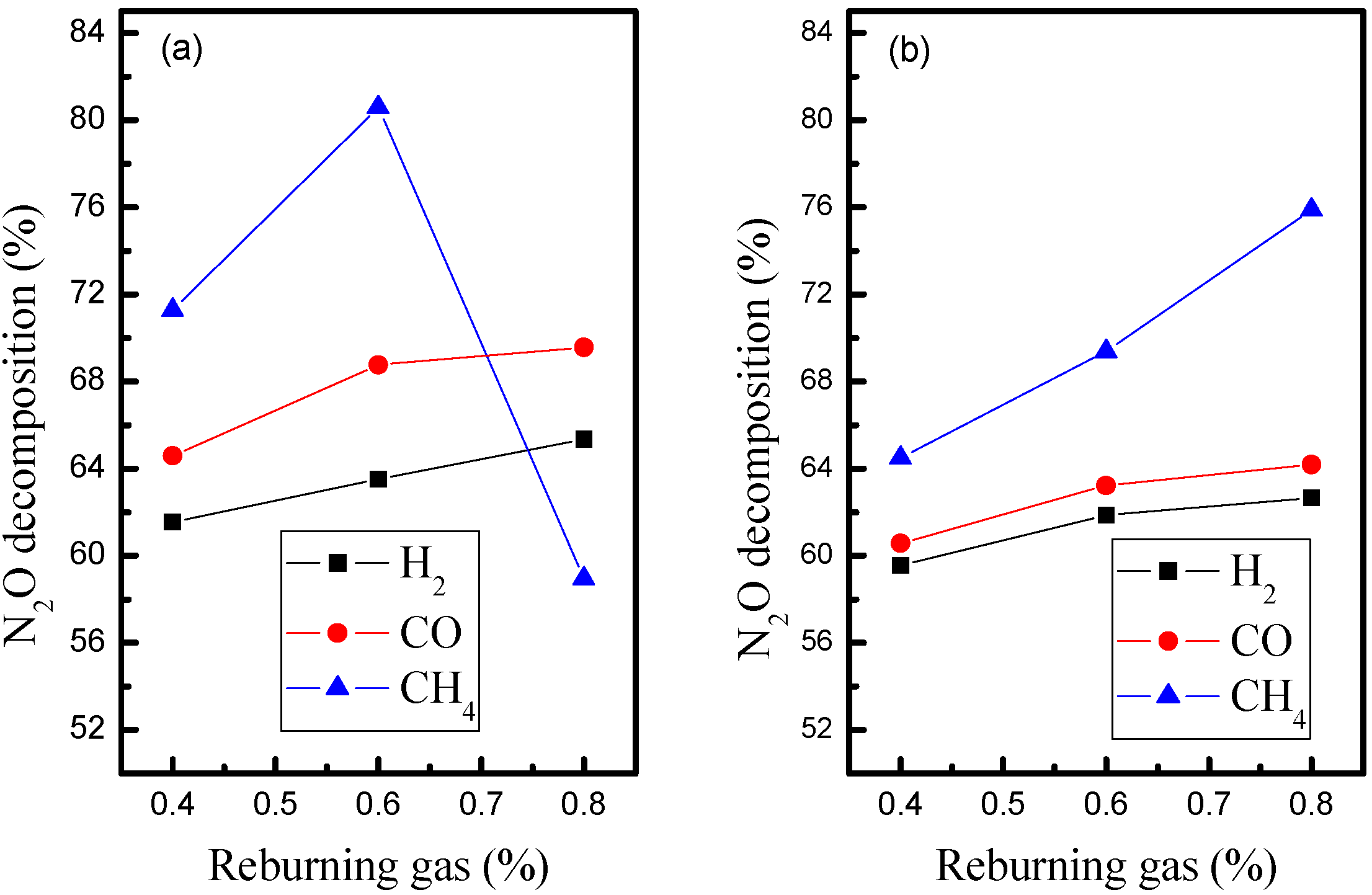
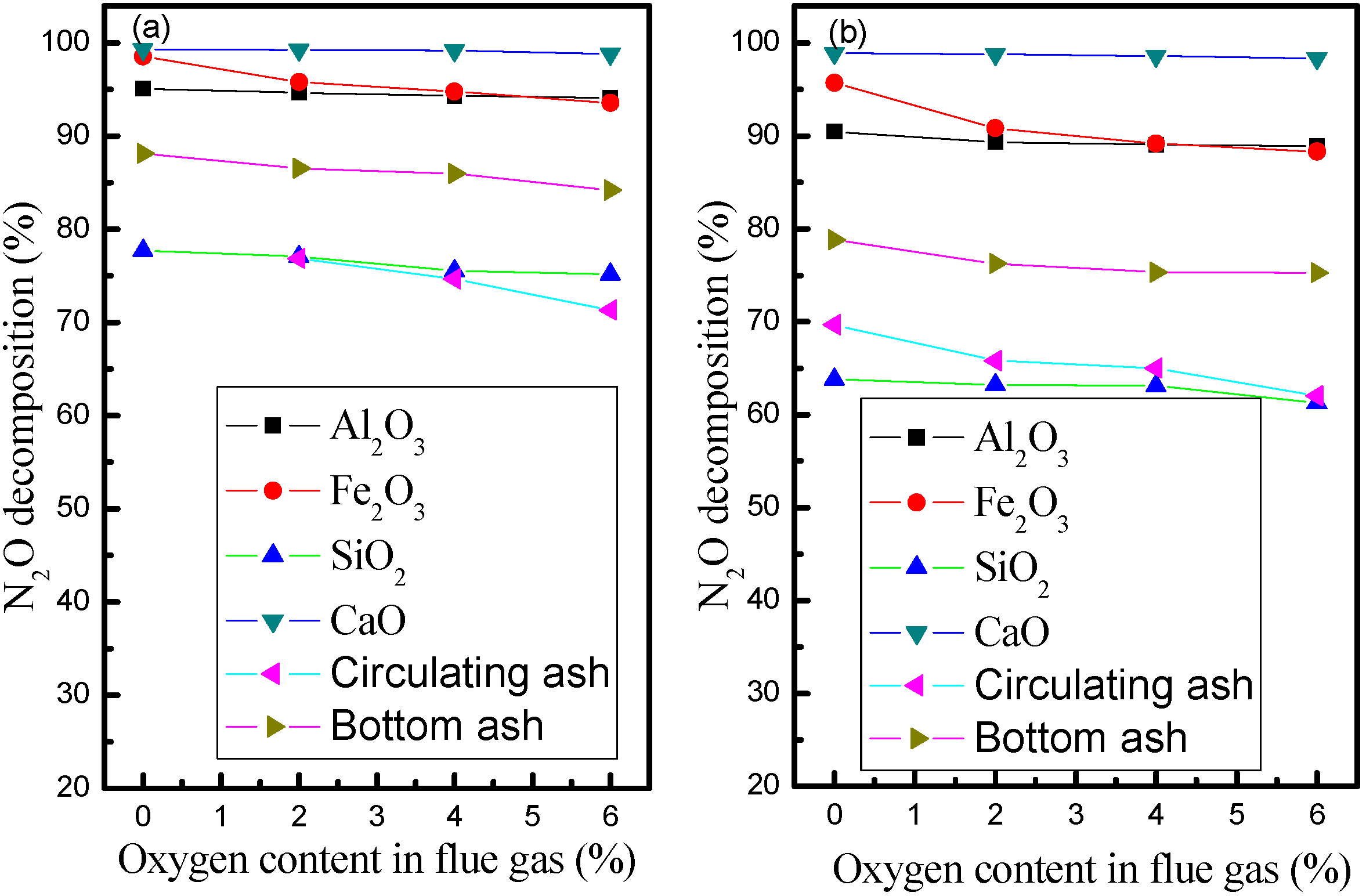
3.2. Influence of Biomass Gas Components with Oxygen Content in Flue Gas

3.3. Influence of Ash and Metal Oxides on Heterogenous N2O Decomposition
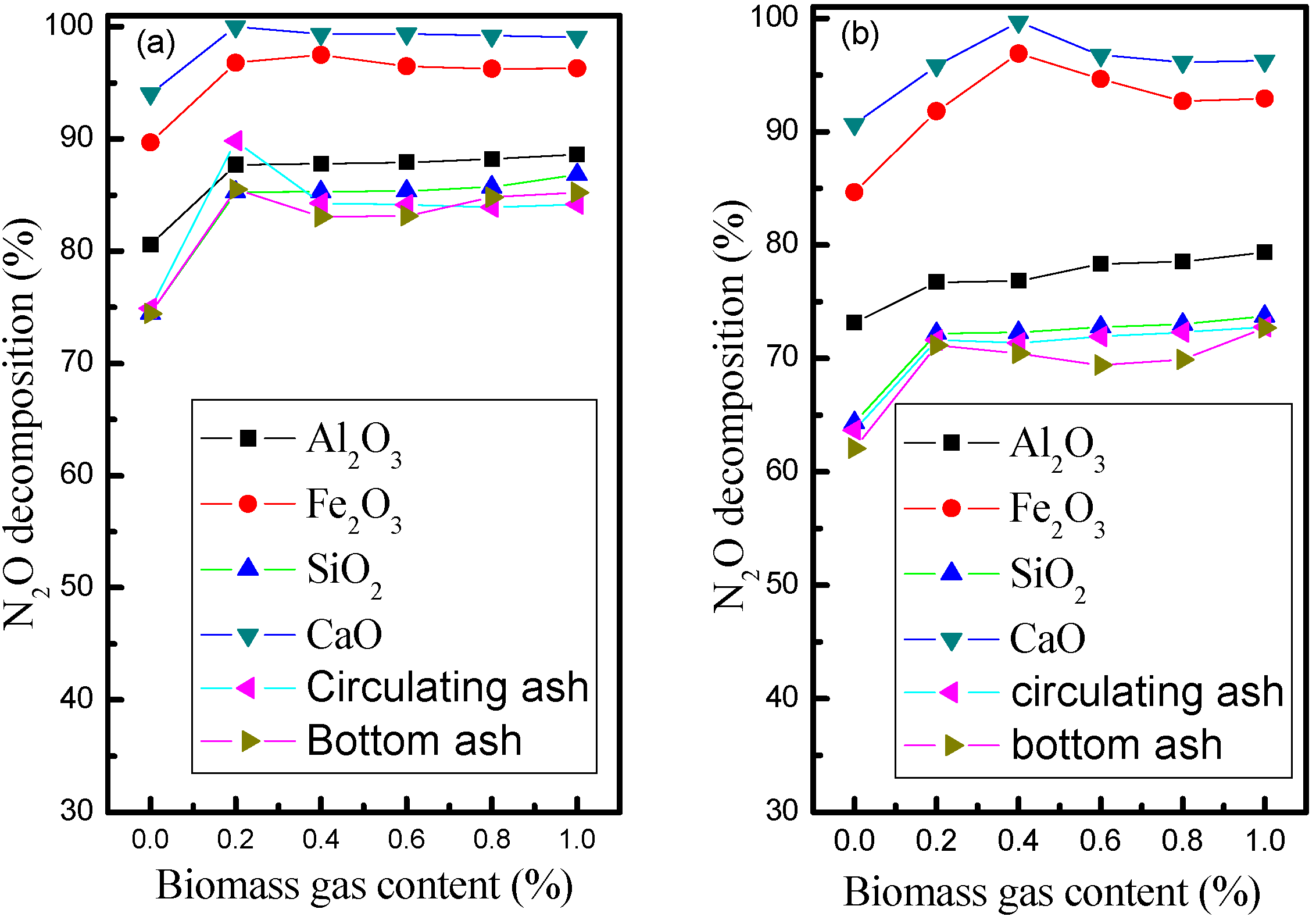

4. Conclusions
- (1)
- At the reaction temperature of 900 °C, the promoting effect order of CO, H2 and CH4 as biomass gas components on N2O decomposition is: H2 > CO > CH4.
- (2)
- Under the reductive conditions, the positive effect of H2 is greater than that of CH4 and CO on the conversion of heterogenous N2O with the circulating ash as bed material, whereas under oxidative conditions with the same bed material, the impact of CH4is proven to be much stronger than that of H2 or CO.
- (3)
- With four different typical solid oxides and the circulating ash as bed material, the catalytic effect of CaO on the conversion of N2O is more effective than that of Fe2O3, Al2O3 and SiO2.
Acknowledgments
References
- Liu, H.; Gibbs, B.M. Reduction of N2O emissions from a coal-fired circulating fluidized bed combustor by afterburning. Fuel 1998, 77, 1579–1587. [Google Scholar] [CrossRef]
- Armesto, L.; Boerrigter, H.; Bahillo, A. N2O emissions from fluidized bed combustion: The effect of fuel characteristics and operating conditions. Fuel 2003, 82, 1845–1850. [Google Scholar] [CrossRef]
- Bonn, B.; Pelz, G.; Baumann, H. Formation and decomposition of N2O in fluidized bed boilers. Fuel 1995, 74, 165–171. [Google Scholar] [CrossRef]
- Shen, B.X.; Mi, T.; Liu, D.C.; Feng, B.; Yao, Q.; Winter, F. N2O emission under fluidized bed combustion condition. Fuel Process. Technol. 2003, 84, 13–21. [Google Scholar] [CrossRef]
- Santiago, M.; Hevia, M.A.G.; Pérez-Ramírez, J. Evaluation of catalysts for N2O abatement in fluidized-bed combustion. Appl. Catal. B 2009, 90, 83–88. [Google Scholar] [CrossRef]
- Feng, B.; Yuan, J.W.; Lin, Z.J.; Cai, X.J.; Wu, H.W.; Liu, D.C. Mechanisms of heterogenous destruction of N2O under the conditions of fluidized bed combustion. J. Eng. Thermophys. 1995, 16, 111–114. [Google Scholar]
- Liu, H.; Lu, J.D.; Feng, B.; Wang, J.H.; Liu, D.C.; Lin, Z.J. Influence of non-homogenous reactions on N2O formation and destruction during Combustion of coal char in a circulating fluidized bed. Proc. CSEE 1998, 18, 237–340. [Google Scholar]
- Feng, J.K.; Yue, G.X.; Lv, J.F. Circulating Fluidized Bed Combustion Boiler; China Electric Power Press: Beijing, China, 2003. [Google Scholar]
- Labhsetwar, N.; Dhakad, M.; Biniwale, R.; Mitsuhashi, T.; Haneda, H.; Reddy, P.S.S.; Bakardjieva, S.; Subrt, J.; Kumar, S.; Kumar, V.; et al. Metal exchanged zeolites for catalytic decomposition of N2O. Catal. Today 2009, 141, 205–210. [Google Scholar] [CrossRef]
- Debbagh, M.N.; Bueno-López, A.; Salinas Martínez de Lecea, C.; Pérez-Ramírez, J. Kinetics of the N2O + CO reaction over steam-activated FeZSM-5. Appl. Catal. A 2007, 327, 66–72. [Google Scholar] [CrossRef]
- Fellah, M.F.; Onal, I. N2O decomposition on Fe- and Co-ZSM-5: A density functional study. Catal. Today 2008, 137, 410–417. [Google Scholar] [CrossRef]
- Haber, J.; Machej, T.; Janas, J.; Nattich, M. Catalytic decomposition of N2O. Catal. Today 2004, 90, 15–19. [Google Scholar] [CrossRef]
- Gluhoi, A.C.; Dekkers, M.A.P.; Nieuwenhuys, B.E. Comparative studies of the N2O/H2, N2O/CO, H2/O2 and CO/O2 reactions on supported gold catalysts: Effect of the addition of various oxides. J. Catal. 2003, 219, 197–205. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Pérez-Ramírez, J. Mechanistic peculiarities of the N2O reduction by CH4 over Fe-silicalite. Catal. Today 2007, 119, 243–246. [Google Scholar] [CrossRef]
- Shen, Q.; Li, L.D.; He, C.; Tian, H.; Hao, Z.; Xu, Z.P. A comprehensive investigation of influences of NO and O2 on N2O SCR by CH4 over Fe-USY zeolite. Appl. Catal. B 2009, 91, 262–268. [Google Scholar] [CrossRef]
- Yang, H.Q.; Hu, C.W.; Qin, S. Theoretical study on the reaction mechanism of CH4 with CaO. Chem. Phys. 2006, 330, 343–348. [Google Scholar] [CrossRef]
- Barišić, V.; Klingstedt, F.; Naydenov, A.; Stefanovb, P.; Kilpinena, P.; Hupaa, M. Catalytic activity of bed materials from industrial CFB boilers for the decomposition of N2O. Catal. Today 2005, 100, 337–342. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, J.; Yang, Y.; Hu, X.; Dong, C.; Lu, Q.; Qin, W. Experimental Research on Heterogeneous N2O Decomposition with Ash and Biomass Gasification Gas. Energies 2011, 4, 2027-2037. https://doi.org/10.3390/en4112027
Zhang J, Yang Y, Hu X, Dong C, Lu Q, Qin W. Experimental Research on Heterogeneous N2O Decomposition with Ash and Biomass Gasification Gas. Energies. 2011; 4(11):2027-2037. https://doi.org/10.3390/en4112027
Chicago/Turabian StyleZhang, Junjiao, Yongping Yang, Xiaoying Hu, Changqing Dong, Qiang Lu, and Wu Qin. 2011. "Experimental Research on Heterogeneous N2O Decomposition with Ash and Biomass Gasification Gas" Energies 4, no. 11: 2027-2037. https://doi.org/10.3390/en4112027
APA StyleZhang, J., Yang, Y., Hu, X., Dong, C., Lu, Q., & Qin, W. (2011). Experimental Research on Heterogeneous N2O Decomposition with Ash and Biomass Gasification Gas. Energies, 4(11), 2027-2037. https://doi.org/10.3390/en4112027





