Biomass Steam Gasification with In-Situ CO2 Capture for Enriched Hydrogen Gas Production: A Reaction Kinetics Modelling Approach
Abstract
:Nomenclature
- C = Concentration (mol/m3)
- k = Arrhenius kinetic constant (s-1)
- r = Rate of reaction (mol/m3 s)
- Kw = Equilibrium constant (dimensionless)
- R = Volumetric rate of component (mol/m3 s)
- n = No of moles
- yi = Mole fraction of component i
- N = Total number of data points
Subscripts
- e = Experimental
- m = Modeling
1. Introduction
2. Model Formulation
2.1. Assumptions
- six reactions occur simultaneously in the gasifier including char gasification, Boudouard, methanation, methane reforming, water gas shift and carbonation [21].
2.2. Reaction Kinetics
| Reaction no, i | Name | Kinetics Scheme | Heat of Reaction, ∆H (kJ/mol) |
|---|---|---|---|
| 1 | Char Gasification | C + H2O→CO + H2 | +131.5 |
| 2 | Methanation | C + 2H2→CH4 | −74 |
| 3 | Boudouard | C + CO2→2CO | +172 |
| 4 | Methane Reforming | CH4 + H2O→CO + 3H2 | +206 |
| 5 | Water Gas Shift | CO + H2O↔CO2 + H2 | −41 |
| 6 | Carbonation | CO2 + CaO→CaCO3 | −178.3 |
| Reaction no, i | Kinetics Parameters, (mol/m3s) | References |
|---|---|---|
| 1 | 2.0×105 exp (−6,000/T) | [10] |
| 2 | 4.40 exp (−1.62×108/T) | [34] |
| 3 | 0.12 exp (−17,921/T) | [34] |
| 4 | 3x105 exp (−15,000/T) | [10] |
| 5 | 106 exp (−6,370/T) | [10] |
| Kw = 520 exp (−7,230/T) | ||
| 6 | 10.20 exp (−44.5/T) | [40] |
3. Results and Discussion
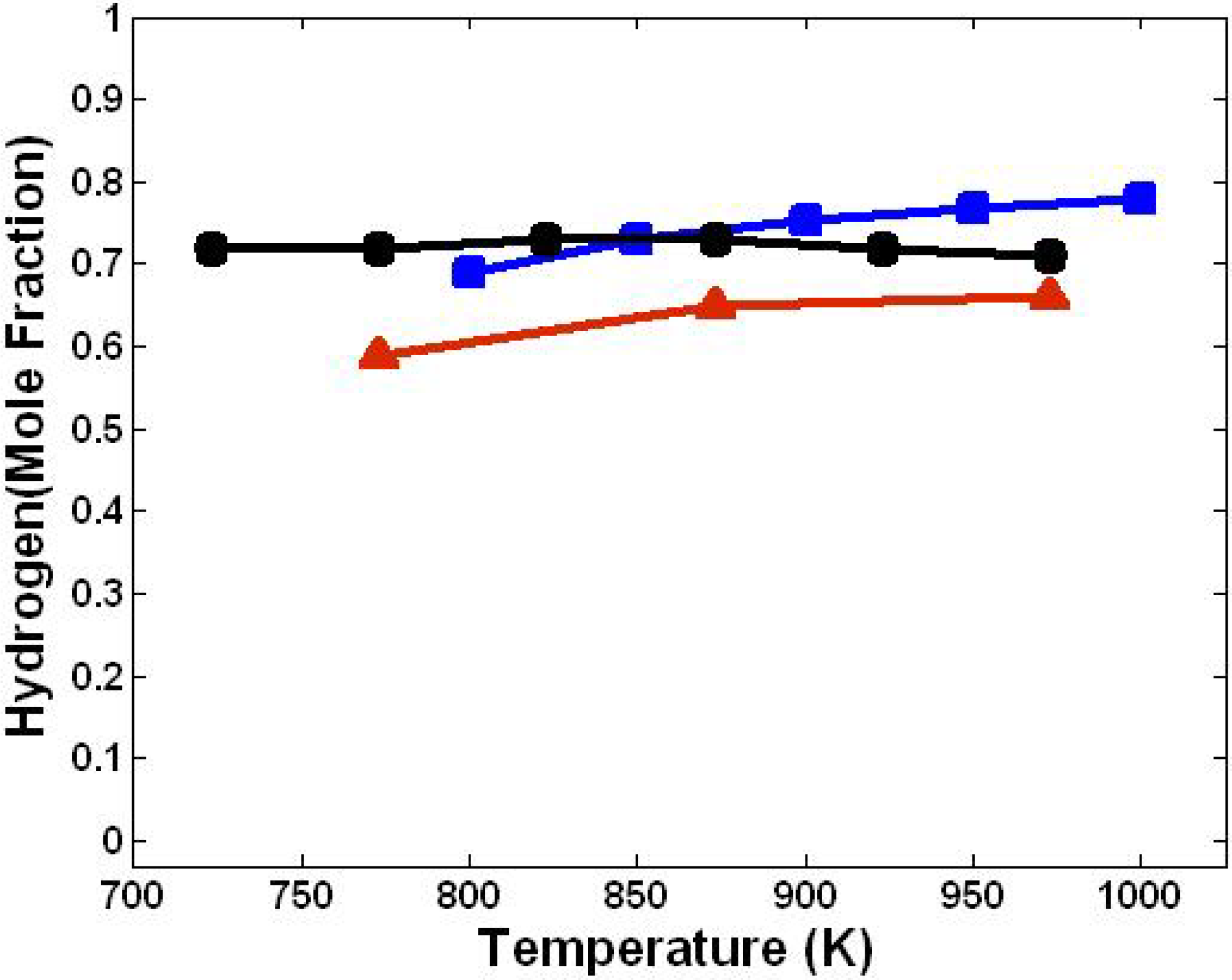
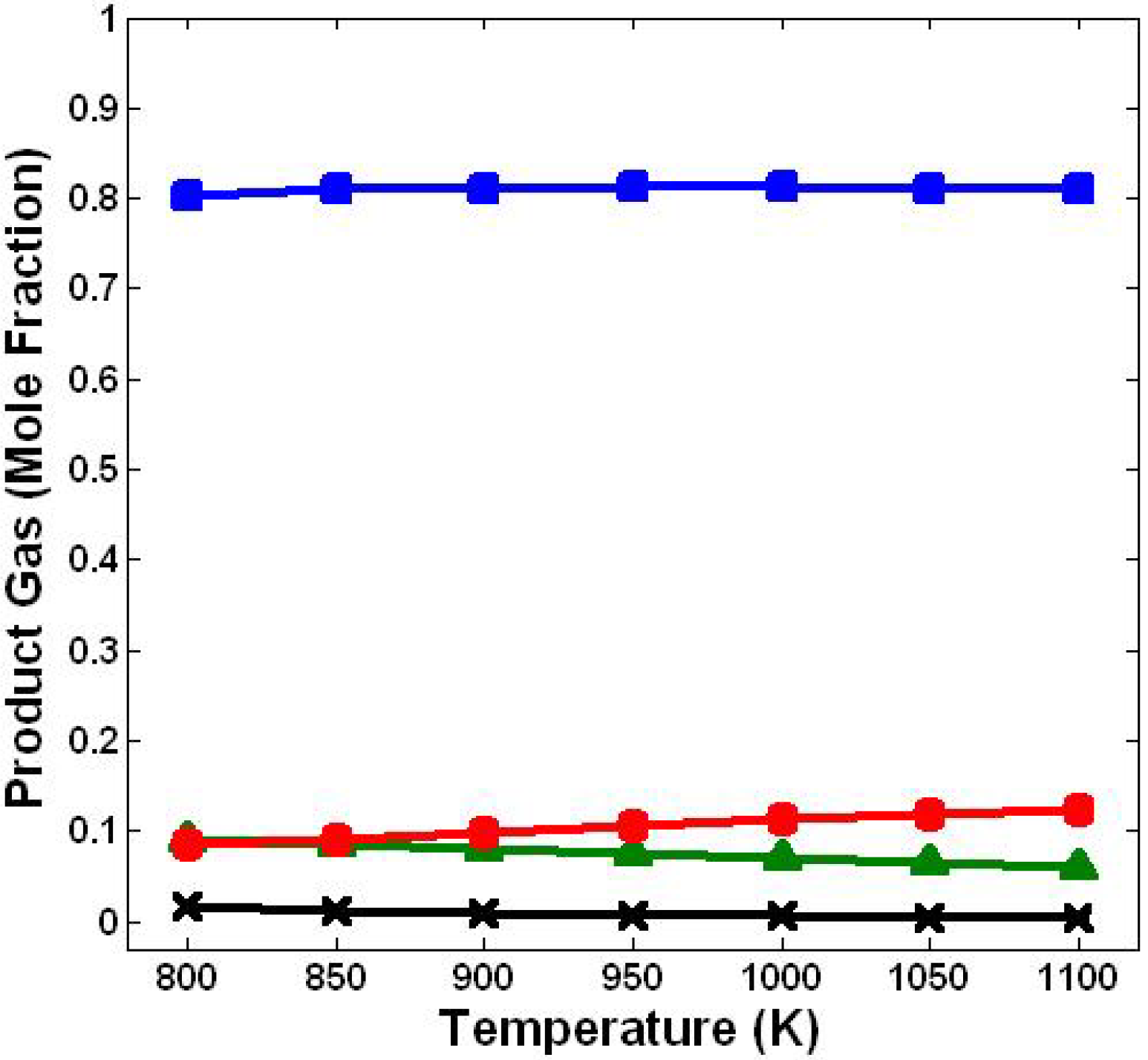
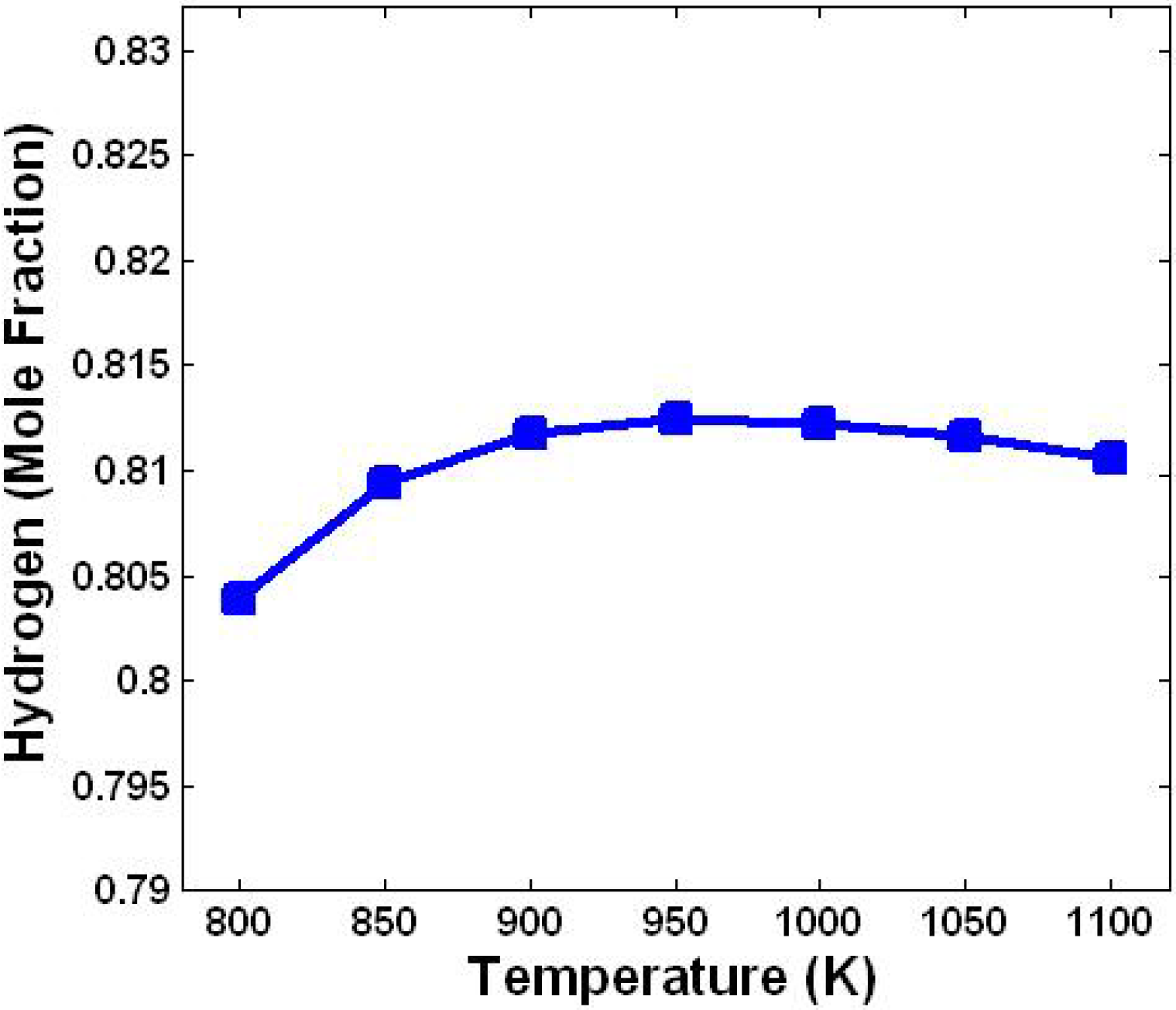
3.1. Effect of Temperature
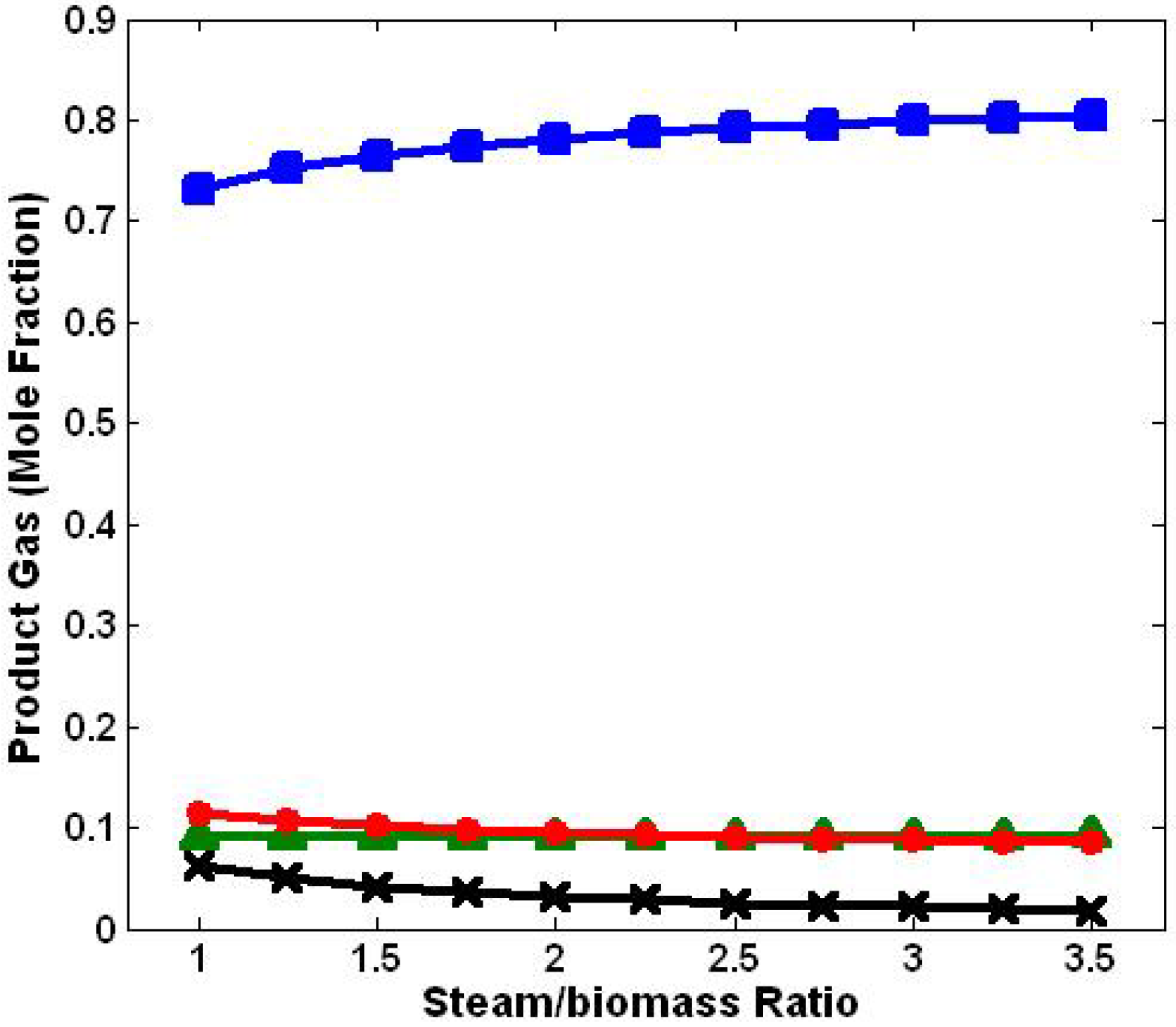
3.2. Effect of Steam/Biomass Ratio
3.3. Three Dimensional Results Based on the Effect of Temperature and Steam/Biomass Ratio
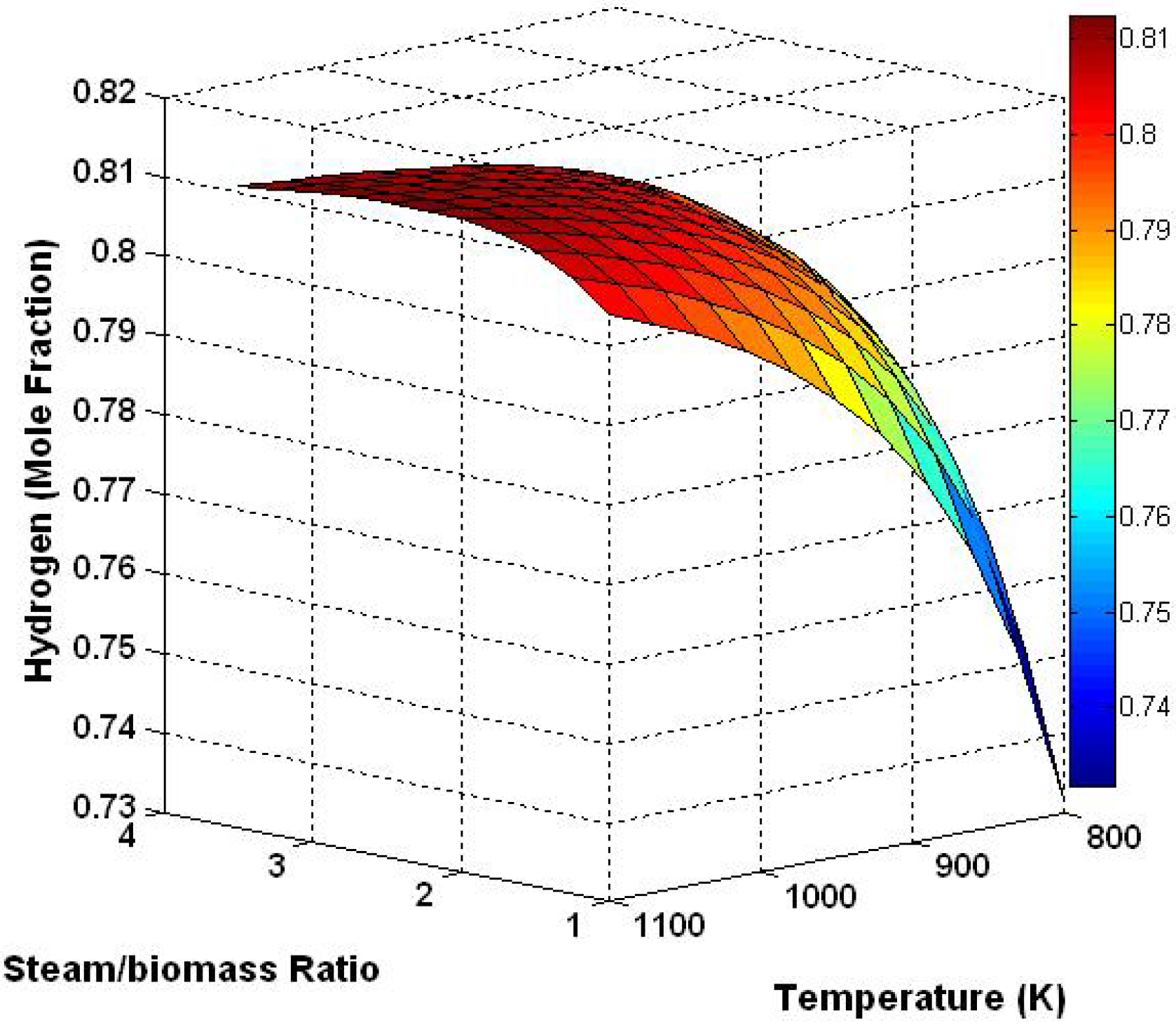

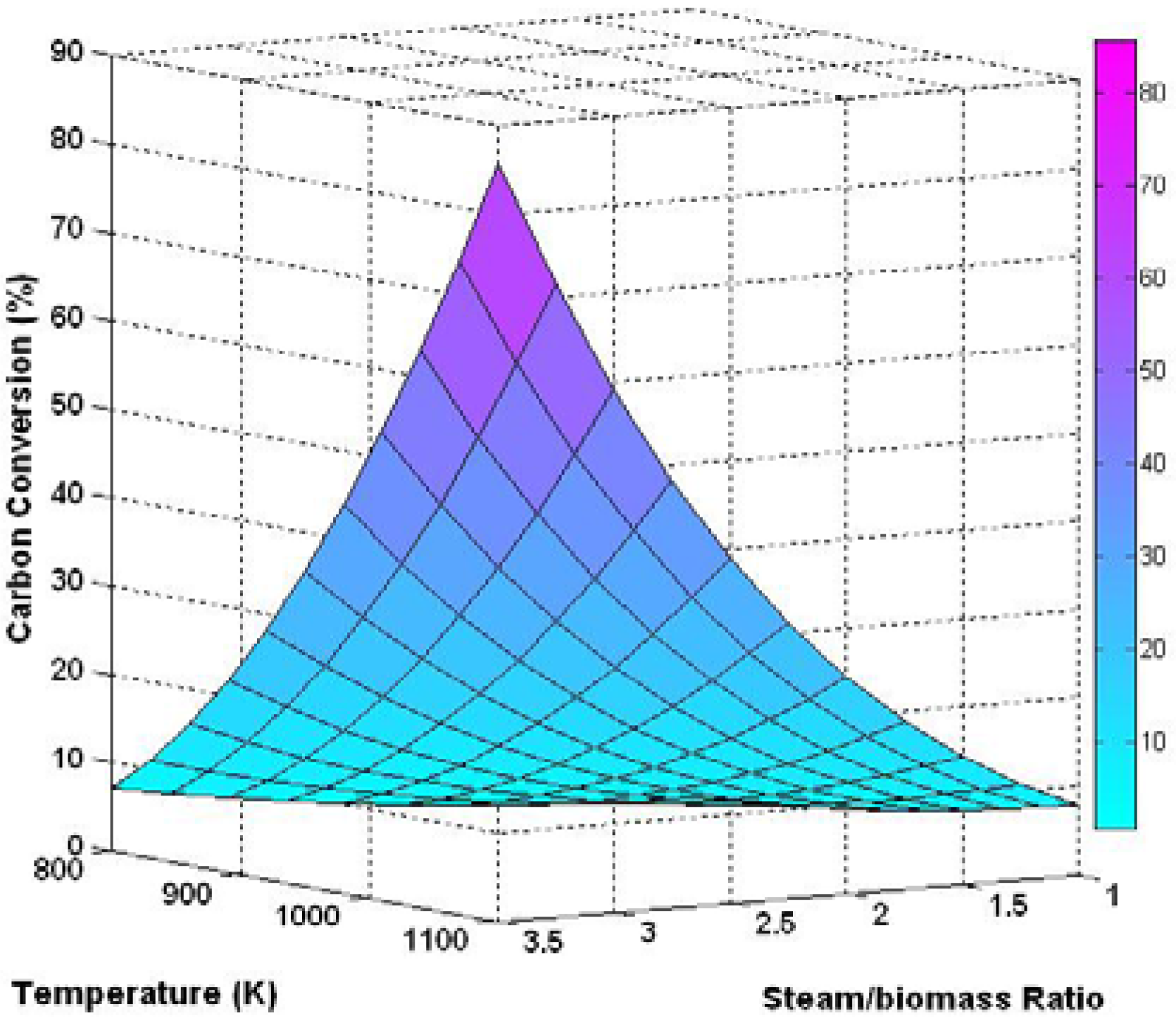
3.4. Effect of Sorbent/Biomass Ratio
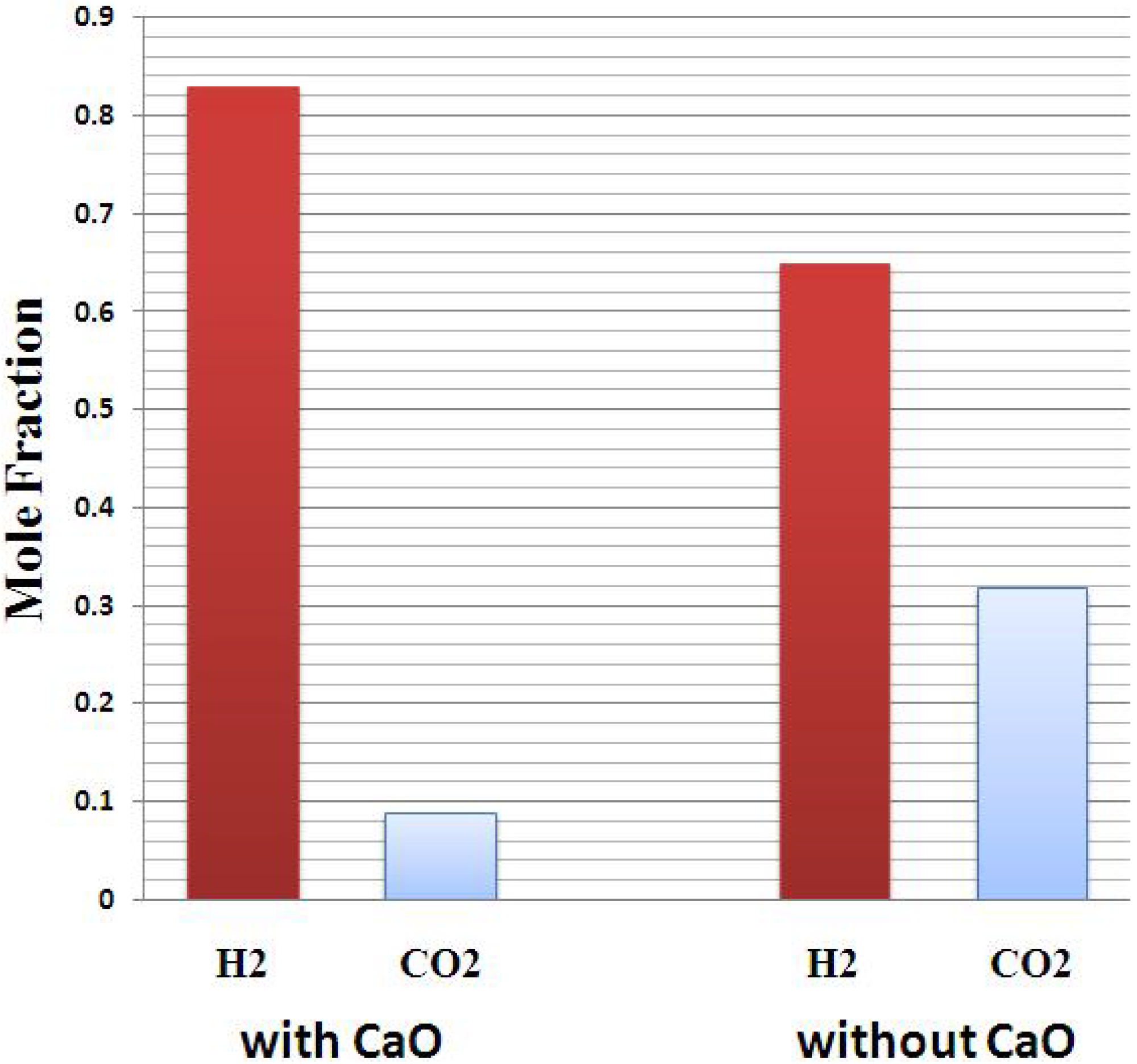
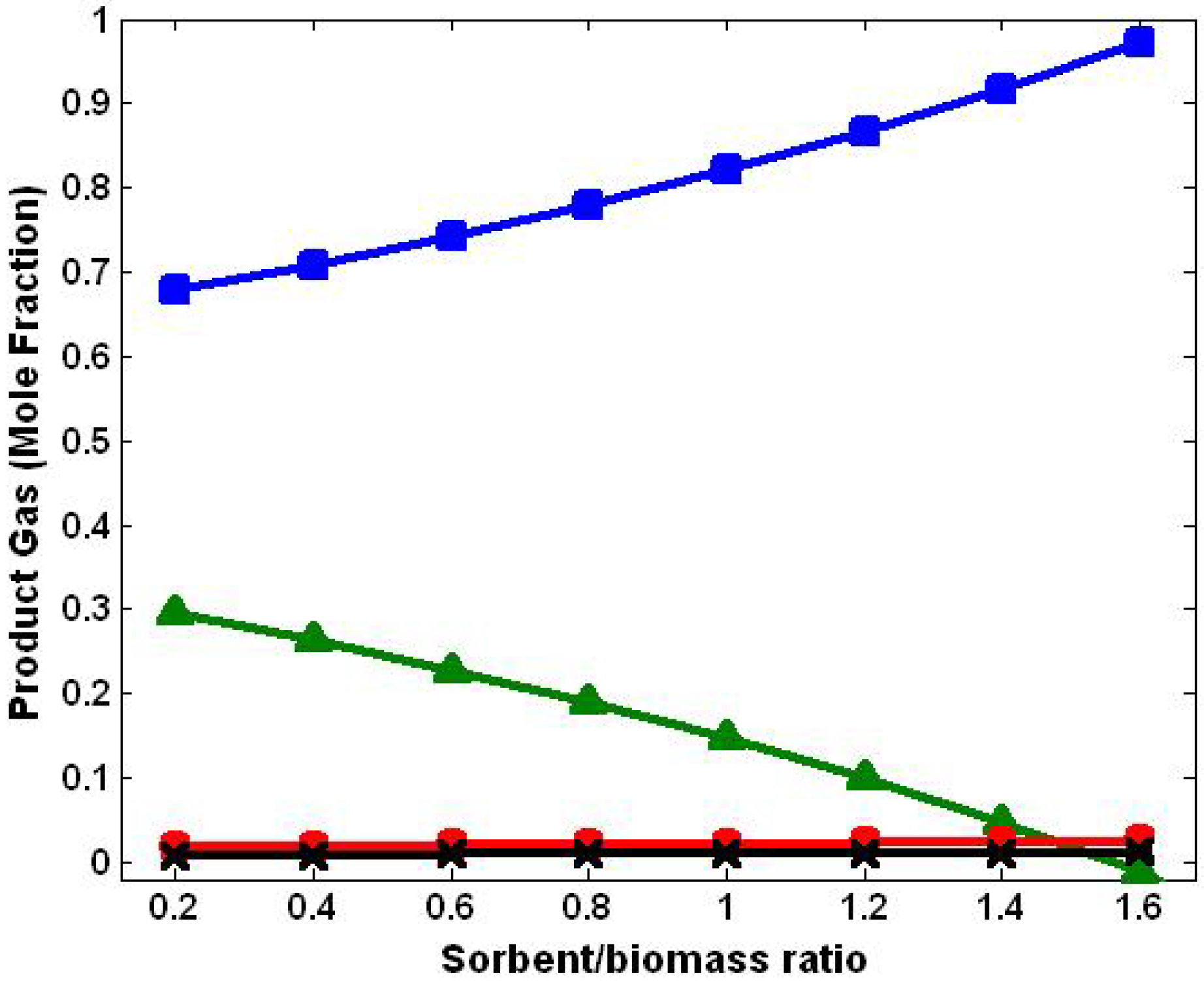
3.5. Comparison with Literature Data
| Parameters | This Model | Mahishi and Goswami [9] | Florin and Harris [18] |
|---|---|---|---|
| Approach | Kinetics Modelling | Experimental Work | Equilibrium Modelling |
| Gasification agent | Steam | Steam | Steam |
| Temperature range (K) | 800–1,000 | 773–973 | 723–973 |
| Pressure (atm) | 1 | 1 | 4.9 |
| Steam/biomass ratio | 2.0 | 1.0 | 2.0 |
| Sorbent/biomass ratio | 1.0 | 1.0 | 0.50 |
4. Conclusions
Acknowledgements
References
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Kelly-Yong, T.L.; Lee, K.T.; Mohamed, A.R.; Bhatia, S. Potential of hydrogen from oil palm biomass as a source of renewable energy worldwide. Energy Policy 2007, 35, 5692–5701. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 3): Gasification technologies. Bioresour. Technol. 2002, 83, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Wan Ab Karim Ghani, W.A.; Moghadam, R.A.; Salleh, M.A.M.; Alias, A.B. Air Gasification of Agricultural Waste in a Fluidized Bed Gasifier: Hydrogen Production Performance. Energies 2009, 2, 258–268. [Google Scholar]
- Khan, Z.; Yusup, S.; Ahmad, M.M.; Chok, V.S.; Uemura, Y.; Sabil, K.M. Review on hydrogen production technologies in Malaysia. Int. J. Eng. Technol. 2010, 10, 111–118. [Google Scholar]
- González, J.F.; Román, S.; Bragado, D.; Calderón, M. Investigation on the reactions influencing biomass air and air/steam gasification for hydrogen production. Fuel Process. Technol. 2008, 89, 764–772. [Google Scholar] [CrossRef]
- Florin, N.H.; Harris, A.T. Enhanced hydrogen production from biomass with in situ carbon dioxide capture using calcium oxide sorbents. Chem. Eng. Sci. 2008, 63, 287–316. [Google Scholar] [CrossRef]
- Kinoshita, C.M.; Turn, S.Q. Production of hydrogen from bio-oil using CaO as a CO2 sorbent. Int. J. Hydrogen Energy 2003, 28, 1065–1071. [Google Scholar]
- Mahishi, M.R.; Goswami, D. Y. An experimental study of hydrogen production by gasification of biomass in the presence of a CO2 sorbent. Int. J. Hydrogen Energy 2007, 32, 2803–2808. [Google Scholar] [CrossRef]
- Corella, J.; Sanz, A. Modeling circulating fluidized bed biomass gasifiers. A pseudo-rigorous model for stationary state. Fuel Process. Technol. 2005, 86, 1021–1053. [Google Scholar] [CrossRef]
- Melgar, A.; Pérez, J.F.; Laget, H.; Horillo, A. Thermochemical equilibrium modelling of a gasifying process. Energy Convers. Manag. 2007, 48, 59–67. [Google Scholar] [CrossRef]
- Mahishi, M.R.; Goswami, D.Y. Thermodynamic optimization of biomass gasifier for hydrogen production. Int. J. Hydrogen Energy 2007, 32, 3831–3840. [Google Scholar] [CrossRef]
- Sharma, A.K. Equilibrium modeling of global reduction reactions for a downdraft (biomass) gasifier. Energy Convers. Manag. 2008, 49, 832–842. [Google Scholar] [CrossRef]
- Nikoo, M.B.; Mahinpey, N. Simulation of biomass gasification in fluidized bed reactor using ASPEN PLUS. Biomass Bioenergy 2008, 32, 1245–1254. [Google Scholar] [CrossRef]
- Shen, L.; Gao, Y.; Xiao, J. Simulation of hydrogen production from biomass gasification in interconnected fluidized beds. Biomass Bioenergy 2008, 32, 120–127. [Google Scholar] [CrossRef]
- Pröll, T.; Hofbauer, H. H2 rich syngas by selective CO2 removal from biomass gasification in a dual fluidized bed system—Process modelling approach. Fuel Process. Technol. 2008, 89, 1207–1217. [Google Scholar] [CrossRef]
- Corella, J.; Toledo, J.-M.; Molina, G. Biomass gasification with pure steam in fluidised bed: 12 variables that affect the effectiveness of the biomass gasifier. Int. J. Oil Gas Coal Technol. 2008, 1, 194–207. [Google Scholar] [CrossRef]
- Florin, N.H.; Harris, A.T. Hydrogen production from biomass coupled with carbon dioxide capture: The implications of thermodynamic equilibrium. Int. J. Hydrogen Energy 2007, 32, 4119–4134. [Google Scholar] [CrossRef]
- Mahishi, M.R.; Sadrameli, M.S.; Vijayaraghavan, S.; Goswami, D.Y. A Novel Approach to Enhance the Hydrogen Yield of Biomass Gasification Using CO2 Sorbent. J. Eng. Gas Turbines Power 2008, 130, 011501. [Google Scholar] [CrossRef]
- Lv, P.; Yuan, Z.; Wu, C.; Ma, L.; Chen, Y.; Tsubaki, N. Bio-syngas production from biomass catalytic gasification. Energy Convers. Manag. 2007, 48, 1132–1139. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, J.; Shen, L. Simulation of Methanol Production from Biomass Gasification in Interconnected Fluidized Beds. Ind. Eng. Chem. Res. 2009, 48, 5351–5359. [Google Scholar] [CrossRef]
- Lü, P.; Kong, X.; Wu, C.; Yuan, Z.; Ma, L.; Chang, J. Modeling and simulation of biomass air-steam gasification in a fluidized bed. Frontiers Chem. Eng. Chin. 2008, 2, 209–213. [Google Scholar] [CrossRef]
- Gøbel, B.; Henriksen, U.; Jensen, T.K.; Qvale, B.; Houbak, N. The development of a computer model for a fixed bed gasifier and its use for optimization and control. Bioresour. Technol. 2007, 98, 2043–2052. [Google Scholar] [CrossRef] [PubMed]
- Gøbel, B. Dynamic Modelling of Gasification in a Fixed Char Bed. (Dynamisk modellering af forgasning i fixed koksbed). PhD Dissertation (ET-PhD 99-04), Technical University of Denmark, Lyngby, Denmark, 2000. [Google Scholar]
- Paviet, F.; Chazarene, F.; Tazerout, M. Thermo chemical equilibrium modelling of a biomass gasifying process using ASPEN PLUS. Int. J. Chem. React. Eng. 2009, 7, A 40. [Google Scholar]
- Gautam, G.; Adhikari, S.; Bhavnani, S. Estimation of Biomass Synthesis Gas Composition using Equilibrium Modeling. Energy Fuels 2010, 24, 2692–2698. [Google Scholar] [CrossRef]
- Schuster, G.; Löffler, G.; Weigl, K.; Hofbauer, H. Biomass steam gasification—An extensive parametric modeling study. Bioresour. Technol. 2001, 77, 71–79. [Google Scholar] [CrossRef]
- Kumar, A.; Jones, D.; Hanna, M. Thermochemical Biomass Gasification: A Review of the Current Status of the Technology. Energies 2009, 2, 556–581. [Google Scholar] [CrossRef]
- Ji, P.; Feng, W.; Chen, B. Comprehensive Simulation of an Intensified Process for H2 Production from Steam Gasification of Biomass. Ind. Eng. Chem. Res. 2009, 48, 3909–3920. [Google Scholar] [CrossRef]
- Raman, P.; Walawender, W.P.; Fan, L.T.; Chang, C.C. Mathematical model for the fluid-bed gasification of biomass materials. Application to feedlot manure. Ind. Eng. Chem. Process Des. Dev. 1981, 20, 686–692. [Google Scholar] [CrossRef]
- Gao, N.; Li, A. Modeling and simulation of combined pyrolysis and reduction zone for a downdraft biomass gasifier. Energy Convers. Manag. 2008, 49, 3483–3490. [Google Scholar] [CrossRef]
- Liu, H.; Gibbs, B.M. Modeling NH3 and HCN emissions from biomass circulating fluidized bed gasifiers. Fuel 2003, 82, 1591–1604. [Google Scholar] [CrossRef]
- Chejne, F.; Hernandez, J.P. Modelling and simulation of coal gasification process in fluidised bed. Fuel 2002, 81, 1687–1702. [Google Scholar] [CrossRef]
- Choi, Y.C.; Li, X.Y.; Park, T.J.; Kim, J.H.; Lee, J.G. Numerical study on the coal gasification characteristics in an entrained flow coal gasifier. Fuel 2001, 80, 2193–2201. [Google Scholar] [CrossRef]
- Mann, M.D.; Knutson, R.Z.; Erjavec, J.; Jacobsen, J.P. Modeling reaction kinetics of steam gasification for a transport gasifier. Fuel 2004, 83, 1643–1650. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, J.M.; Kim, S.D. Modeling of coal gasification in an internally circulating fluidized bed reactor with draught tube. Fuel 2000, 79, 69–77. [Google Scholar] [CrossRef]
- Macak, J.; Malecha, J. Mathematical Model for the Gasification of Coal under Pressure. Ind. Eng. Chem. Process Des. Dev. 1978, 17, 92–98. [Google Scholar] [CrossRef]
- Basu, P.; Kaushal, P. Modeling of pyrolysis and gasification of biomass in fluidized beds: A review. Chem. Prod. Process Model. 2009, 4, A 21. [Google Scholar]
- Nemtsov, D.A.; Zabaniotou, A. Mathematical modelling and simulation approaches of agricultural residues air gasification in a bubbling fluidized bed reactor. Chem. Eng. J. 2008, 143, 10–31. [Google Scholar] [CrossRef]
- Ar, I.; Dogu, G. Calcination kinetics of high purity limestones. Chem. Eng. J. 2001, 83, 131–137. [Google Scholar] [CrossRef]
- Yan, H.; Heidenreich, C.; Zhang, D. Mathematical modelling of a bubbling fluidised bed coal gasifier and the significance of net flow. Fuel 1998, 77, 1067–1079. [Google Scholar] [CrossRef]
- Li, X.T.; Grace, J.R.; Lim, C.J.; Watkinson, A.P.; Chen, H.P.; Kim, J.R. Biomass gasification in a circulating fluidized bed. Biomass Bioenergy 2004, 26, 171–193. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Inayat, A.; Ahmad, M.M.; Yusup, S.; Mutalib, M.I.A. Biomass Steam Gasification with In-Situ CO2 Capture for Enriched Hydrogen Gas Production: A Reaction Kinetics Modelling Approach. Energies 2010, 3, 1472-1484. https://doi.org/10.3390/en3081472
Inayat A, Ahmad MM, Yusup S, Mutalib MIA. Biomass Steam Gasification with In-Situ CO2 Capture for Enriched Hydrogen Gas Production: A Reaction Kinetics Modelling Approach. Energies. 2010; 3(8):1472-1484. https://doi.org/10.3390/en3081472
Chicago/Turabian StyleInayat, Abrar, Murni M. Ahmad, Suzana Yusup, and Mohamed Ibrahim Abdul Mutalib. 2010. "Biomass Steam Gasification with In-Situ CO2 Capture for Enriched Hydrogen Gas Production: A Reaction Kinetics Modelling Approach" Energies 3, no. 8: 1472-1484. https://doi.org/10.3390/en3081472
APA StyleInayat, A., Ahmad, M. M., Yusup, S., & Mutalib, M. I. A. (2010). Biomass Steam Gasification with In-Situ CO2 Capture for Enriched Hydrogen Gas Production: A Reaction Kinetics Modelling Approach. Energies, 3(8), 1472-1484. https://doi.org/10.3390/en3081472