Abstract
Offshore and coastal wind power is one of the fastest growing industries in many areas, especially those with shallow coastal regions due to the preferable generation conditions available in the regions. As with any expanding industry, there are concerns regarding the potential environmental effects which may be caused by the installation of the offshore wind turbines and their associated infrastructure, including substations and subsea cables. These include the potential impacts on the biological, physical and human environments. This review discusses in detail the potential impacts arising from offshore wind farm construction, and how these may be quantified and addressed through the use of conceptual models. It concludes that while not environmentally benign, the environmental impacts are minor and can be mitigated through good siting practices. In addition, it suggests that there are opportunities for environmental benefits through habitat creation and conservation protection areas.
1. Introduction
Environmentalists and environmental managers increasingly debate the installation of offshore wind generating capacity because of its current position as the most rapidly expanding sector of the renewable energy industry. These installations are increasing, for example in the UK, with the largest of the recent Round 3 zones, announced in January 2010, being over 8,600 km2 (see The Crown Estate website for further details (www.thecrownestate.co.uk). The potential for energy yield is greater at sea than an equivalent wind farm on land and, with technological advances, the capacity to install turbines and their associated infrastructure further and further offshore is becoming achievable, allowing access to even greater and more constant winds and energy yields. The move offshore also minimises public disturbance, overcoming objections to onshore wind farms due to the noise and visual impacts the structures.
The UK, for example, will rapidly increase the number of planned and operational wind farms in the near future. Using the latest turbine technology, the installation of 2,000 onshore and 1,500 offshore turbines should enable the UK government to achieve its 2010 energy targets [1]. Turbine design continues to develop, with larger and/or more efficient designs, e.g., 10 MW turbines are planned, such that generative capacity predictions and turbine numbers will increase in future.
Given these developments, there is the need to rigorously identify the environmental consequences of this activity. All human activities are required to have an environmental appraisal as they are assumed to have an environmental impact unless the developer can conclusively demonstrate otherwise. Hence a critical element in the development phase of an offshore wind farm is the Environmental Impact Assessment (EIA). At its best, an EIA should be simple—it is ‘what is the effect of this activity, at this place, at this time, carried out in this way, and how do we mitigate or compensate for any effect identified’. It is not a ‘helicopter survey’ (i.e. an academic exercise in which every aspect is studied irrespective of the probability of an effect by the development being detected. It should be a relatively straightforward process—to distinguish for any impact between substantial, moderate and slight according to the magnitude of impact itself, to assess the value and sensitivity of receiving landscape, and the sensitivity of the ‘potential receptors’. It should not be an unwieldy and excessively time consuming assessment of every component of the receiving area in which the output is measured by the size of the Environmental Statement (ES) (the resulting report of the EIA process). The EIA process should be a rigorous separation of the various phases of the project: pre-construction, during construction/pre operation, post-construction/during operation, post-operation/during decommissioning. There is the risk, in the EIA process, of making it too detailed, resulting in the presentation of too-detailed information, making the decision-making process not simpler, but more complicated than necessary.
This review aims to analyse the potential environmental impacts and benefits of an offshore wind farm, put them into context, and show the potential dangers of making the EIA more detailed than required. As with any activity of the scale of an offshore wind farm, there is the potential for impacts to occur within the local and surrounding environment. For ease, these may be divided into the four main sections of development. Firstly, exploration may involve installing a meteorological mast; undertaking geophysical surveys, initial surveys of the ecology of the area and other studies of the baseline environment and physical situation of the location, to determine suitability for development. During construction, depending on the foundations used for the development, monopile foundations may be driven or drilled into the seabed, or gravity bases placed on the surface of the seabed. The installation of sub-sea cables, and the presence of additional vessels in the area may also have an impact. In contrast, the operational phase of the wind farm is perhaps the phase with the least potential for impact, as once the structures are in place, maintenance for the projects is generally minimal where possible. Decommissioning of the project may have many of the impacts of construction, although possibly slightly less if sub-sea structures are left in situ once decommissioned.
Within all phases, the main potential threat to the surrounding environment is the disruption of natural processes, with the further potential to impact ecological functioning in the area. Therefore, we need to consider what components are affected and by what magnitude, and thus the links to ecosystem structure. Examples of interference with such processes are impeding migration routes and currents and thus sediment dispersal mechanisms. With the installation of new hard structures into the environment however, there is the potential for the foundations to act as artificial reefs, thereby creating habitat in place of that which is unavoidably lost through their installation. These new pockets of habitat can then act as stepping stones for colonisation, allowing the spread of both existing and new species across the area.
As with all projects, especially those of such a scale, monitoring elements of the project over its lifetime is a key way of identifying whether those impacts which were originally predicted did occur and, if so, did they occur to the extent originally anticipated. This not only increases the body of knowledge as to how structures such as offshore wind turbines interact with their environment, but can also feed into future EIAs, enhancing their value for future projects.
As with any marine activity, it is necessary to separate the effects into those which are unlikely, possible, probable and certain; given that we have a long history of putting structures into the environment, then there is a long case-history on which to base our assessments. There has to be a robust means of defining and quantifying an impact but, within a highly dynamic and variable system, of defining and detecting a defendable ‘signal to noise ratio’ and at the same time keeping the methods and effort of detection of effect in proportion. Hence the assessment has to include the air, water, sediment and their interfaces and in turn the effects on marine landscapes.
Offshore wind power has been described as a fledgling industry, and as such there was an initial suggestion in the UK that it should be treated gently while at the same time treated with rigour. Even early on, its consequences were described by NGOs as being ‘environmentally benign’. However, although we have a long history of understanding marine and estuarine activities [2,3], while there is an increasing number of EIA and ES for new schemes, as yet (if it ever occurs) there is little post-construction auditing, and we need to learn more from past experience. Thus there is the need to determine whether all elements of the EIA were needed, was there any non cost-effective (or even wasteful) monitoring and were the predictions of impact correct. It is of note that there is still a lot of qualitative prediction but also the need for post-operation monitoring to increase our confidence in assessments.
As a starting point and a means of both producing a logical sequence and communicating the potential for change to all audiences, all assessments of potential impacts of marine activities require a conceptual model [4]. While it is easy to create such models (referred to here as ‘horrendograms’) it is more difficult to determine the significance of the effects against the perceptions of change. Hence there is the need to differentiate the important features of change from the less important or even unlikely ones.
2. Conceptual Models for Offshore Wind Farm Environmental Problems
The assessment of the potential impacts of marine renewables, including offshore wind farms, requires a framework giving the significance of impacts relative to an undeveloped coastal or offshore site. This must include all relevant information on the potential impacts, including the severity (major, moderate), including no interaction, persistence (instantaneous through to two years), spatial extent (nearfield, far-field), areas of no knowledge or experience. Table 1 summarises the terminology that has been developed and applied to impact assessment. The significance of impacts is judged from informed sources (e.g., historic, contemporary studies, data, reports etc.) and from expert judgement, to range from major through no interaction to a positive impact on the environment.

Table 1.
Terminology for classifying and defining geo-environmental impacts.
| Impact | Adverse/beneficial | Definition |
|---|---|---|
| Major | adverse | The impact gives rise to serious concern; potentially it should be considered as unacceptable. |
| Moderate | adverse | The impact gives rise to some concern but it is likely tolerable (given its extent and duration). |
| Minor | adverse | The impact is undesirable but of limited concern. |
| Negligible | --- | The impact is not of concern. |
| No interaction | --- | No impact is found or it is undistinguishable from natural variation. |
| Positive | beneficial | The impact provides some gain to the environment. |
An independent view of the impacts of various offshore activities, for example for use within an EIA, requires knowledge of the importance of the footprints of the various activities. For example, within a wind farm, the footprint of a gravity base is greater than that of a monopile foundation, and as the size of blades increase, the distance between turbines can be up to 1 km, potentially reducing the impact on the seabed.
Within each phase (exploration, etc.) there is the need to separate the effects into those which are unlikely, possible, probable and certain. This should use the extensive case-history of similar activities (structures in the marine environment) as there is limited post-construction knowledge to illustrate the effects of offshore wind power generation on components in the air, water, sediment and their interfaces and in turn the effects on marine landscapes. To present the evaluation, each of these qualitative descriptors is assigned a colour (Table 2).
The persistence (duration) of each impact can then be scored relating to the timescale and significance of the impact. This can range from the short term, measured in days, to the entire duration of the project (from the site selection through to decommissioning). An indication of whether the impact is limited to the nearfield or extends to the farfield is also required where Nearfield: within 10 times the diameter for a single structure; the entire spatial extent of an array, plus 10 times the diameter of the outer array structures, for an array; Far-field:—to a distance of one tidal excursion from a single structure, or to a distance of one tidal excursion from the centroid of an array.

Table 2.
Summary of assessment criteria used to establish the significance of geo-environmental impacts.
| Qualitative Descriptor | Numerical Temporal Descriptor | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Short term (days) | Medium term (weeks) | Long-term (months) | Duration of development | |
| Major | ||||
| Moderate | ||||
| Minor | ||||
| Negligible | ||||
| No interaction | ||||
| Positive | ||||
| Nearfield impact only | Bold text | |||
| Far-field impact | Normal text | |||
| Unknown | ||||
| Research gap | ||||
The nearfield and far-field information in Table 2 is conveyed using bold and normal text fonts, respectively. For issues where the impacts on coastal processes are entirely unknown grey shading is used. Where the assessment concludes that impacts are not known or are only poorly known/understood (but nonetheless are considered worthy of attention in terms of future research), a bold outline box is used. As there is a limited amount of data and information available from operational projects, there are several research gaps which still need addressing (presented at the end of this review).
Impact assessment has largely been conducted at a generic level, and yet it is apparent there are site-specific differences even within the same sector due to a range of factors (e.g., proximity to the shore, wave climate), and certainly between sectors (for example other renewable technologies, oil and gas or port installations). Hence we need to consider and integrate these differences into the information presented to highlight and separate differing impacts. For example, different impacts are expected to arise from wave energy conversion devices which are floating and anchored (e.g., OPD’s Pelamis system (www.pelamiswave.com) in comparison to where these are mounted on the seabed (e.g., the Oyster device of Aquamarinepower). A further, quantitative comparison tool or matrix which allows a better comparison between sectors and activities in the offshore environment has been developed [5,6], and aims to capture many of the offshore activities currently being undertaken, and a wide range of potential impacts on the marine environment, both positive and negative, which might be caused by any of these activities.
This comparison matrix, shown in part in Table 3, aims quantify the severity of these potential impacts in terms of positive/negative impact on the environment, and the certainty of such an impact occurring as a result of the specific offshore activity being assessed. This allows each activity to be ‘scored’ in terms of a positive or negative overall impact on the marine environment (positive and negative numerical values representing positive and negative impacts respectively). For the ease of visual interpretation, each of the numerical scores in the section shown is illustrated with a colour, as described below.

Table 3.
Section of the offshore activity comparison matrix (showing previous scoring system, without positive and negative values, and physical pressures only—biological and chemical pressures are included in the full version).
| Coastal & Maritime Activities/Events | Sub-activities/events | Environmental Pressures | |||||||||||||||||
| Physical | |||||||||||||||||||
| Loss of Area (seabed) | Loss of Area (water column) | Loss of Area (airspace) | Substratum loss | Smothering | Changes in suspended sediment | Desiccation | Changes in emergence regime | Changes in water flow rate | Changes in currents | Changes in temperature | Changes in turbidity | Changes in wave exposure | Noise disturbance | Visual presence | Abrasion / physical disturbance | Displacement | Water abstraction | ||
| Aquaculture | Fin-fish | 2 | 2 | 0 | 0 | 2 | 2 | 0 | 0 | 2 | 2 | 0 | 2 | 0 | 1 | 1 | 2 | 0 | 0 |
| Macro-algae | 2 | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 2 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | |
| Predator control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | |
| Shellfisheries | 2 | 2 | 0 | 0 | 2 | 2 | 0 | 0 | 2 | 1 | 0 | 2 | 0 | 2 | 2 | 2 | 0 | 1 | |
| Climate Change | Current change | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sea level change | 2 | 2 | 0 | 0 | 0 | 0 | 2 | 2 | 2 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | |
| Temperature change | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Weather pattern change | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Coastal defence | Barrage | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 1 |
| Beach replenishment | 2 | 1 | 0 | 1 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | |
| Groynes | 1 | 1 | 0 | 1 | 1 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 0 | 1 | 0 | |
| Sea walls/breakwaters | 2 | 1 | 0 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 0 | 1 | 0 | |
As each individual activity is scored independently, it can provide an unbiased comparison of the activities, which can be used within Environmental Impact Assessments and other strategic decisions regarding planning in the offshore environment. The scores are allocated as follows:
- 0—Occurrence of impact/benefit is unlikely (white square)
- 1—Occurrence of impact/benefit is possible (yellow square)
- 2—Occurrence of impact/benefit is certain (red square)
This scoring system could be developed further, separating the likelihood of an impact taking place still further.
This approach has now been taken further to use the scores allocated to each activity in multivariate cluster analysis, followed by group average linkage to create the dendrogram (Figure 1). In this analysis, activities are grouped according to their similarity of the scoring of the suggested impacts and the resulting dendrogram shows that anthropogenic marine and coastal activities can be grouped into those relating to waste production, biological extraction (e.g., fisheries) and physical structures; offshore wind farms are included in the latter.
This review concentrates on the effects of the offshore and coastal wind power generation on the dominant and often high profile elements of the marine system—the seabed communities, offshore and coastal birds, bats, demersal and pelagic fishes and marine mammals. However, it is axiomatic that in order to understand and predict any changes to the biota requires an understanding of the physical and chemical characteristic of the marine space [3]. Hence in addition to the main ecological elements, the discussion below also includes the hydrographic and sedimentological aspects.
3. Environmental Impacts and Benefits of Offshore Wind Energy
3.1. Seabed Sediments, Hydrography and Benthic Invertebrates
The installation of offshore wind turbines means that there is unavoidable disturbance to the seabed in the vicinity of the turbine. For the majority of operational offshore wind farms to date, the turbines have been installed on monopile foundations, driven into the seabed sediment. On occasion, the nature of the seabed, for example the presence of hard chalk or other bedrock, demands that the monopiles be part-drilled (where the pile is driven as deep as possible, then the required depth achieved by drilling) to reach the required depth for turbine stability. Drilling into the seabed can result in the release of drill cuttings, fine-grained material which has the potential to remain in suspension before settling out. The introduction of fine particles into the existing sediment environment has the potential to alter the overall sediment structure in the surrounding area, thereby potentially affecting the biological community present [3].
Due to the potential environmental impact, the deposition of drill cuttings in many countries (e.g., the UK), is closely controlled by national legislation which demands monitoring be undertaken to ensure any drill cuttings are correctly deposited and the risk to the environment is kept to a minimum. Installing any structure will have an impact on the flow of currents in the immediate surrounding area. This alteration to flow patterns can result in scour around the base of the turbine tower, and has the potential to cause further changes to the the seabed.
The potential for impacts on the benthic community within an offshore wind farm is one of the major concerns which should be considered within any EIA, as there is often little mitigation possible to minimise disturbance to the communities directly impacted by the development. The alteration of the sediment structure and flow patterns around the foundations and towers can directly impact the adjacent habitats thereby potentially impacting the local communities. The increased turbulence may produce a coarser substratum which then becomes inhabited by coarse-sediment organisms whereas any pockets of fine sediments created by the local conditions would attract mud-tolerant organisms [3].
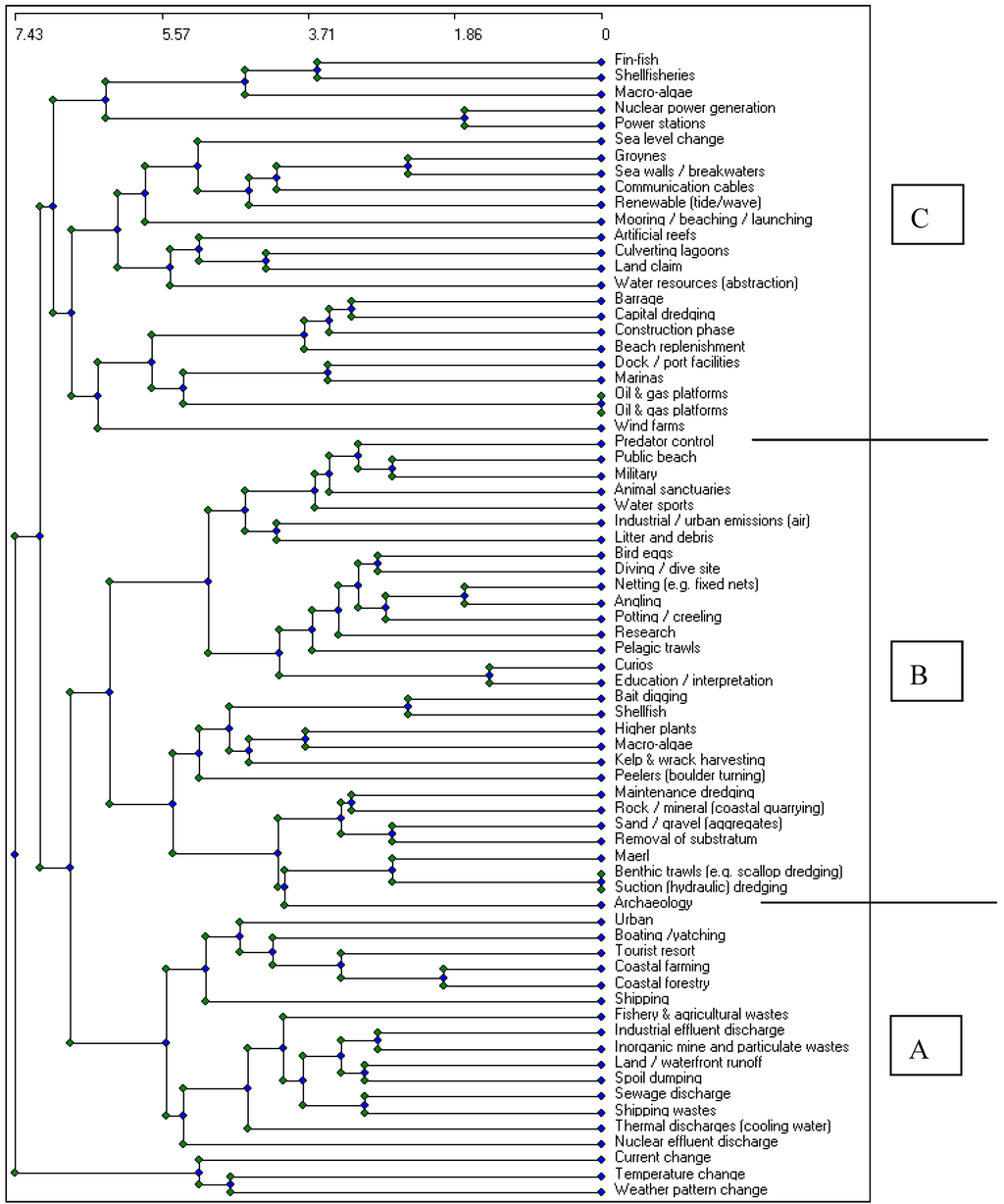
Figure 1.
Example of cluster analysis of comparison matrix, analysing waste activities, biological extraction and physical structures (A. waste discharges and waste activities; B. biological and physical extraction and activities; C. physical structures and physical interference).
This potential for change has the capacity to lead to further impacts up the food chain, and therefore the entire community may be altered. However, within offshore wind farms, to reduce the risk of wake effects impacting the efficiency of the generators, the turbines may be spaced up to seven times the diameter of the rotor blades apart. As technology improves and turbines and blades increase in size, the spacing between the individual turbines also increases so although the area of seabed impacted by each individual turbine may increase slightly due to the increased diameter of the pile, the size of the impacted area compared to the overall wind farm is greatly reduced.
Studies of the benthic changes before, during and after construction (Figure 2) show that while there are changes to the primary (species richness and abundance) and derived variables (e.g., diversity) adjacent to the wind farm and along cable routes, these changes are within a highly variable natural situation. The level of change therefore is related to the physical nature of the area and given that offshore wind farms are often built on sandbanks and gravel areas, by definition high energy mobile sites which are usually subject to substratum modification and turnover, then further anthropogenic change superimposed on natural change may be less detectable.
In addition to environmental changes through the installation of the offshore turbine structures and associated infrastructure, there is also the concern for direct habitat loss through site occupation by the monopile and foundations. With foundations of up to 6 m diameter, there is a minimum of 12.5 m2 lost per turbine (based on a 4 m diameter), plus a potential additional 452 m2 lost should scour protection be installed around the foundation (assuming an area 10 m out from the base of the turbine is protected [6]. However, it should again be noted that this area is relatively minor in relation to the total area within the wind farm site boundary. This equates to a small percentage of area potentially lost through turbine installation. At the Barrow Offshore Wind Farm, NW England, there are 30 turbines installed within an area of 10 km2, each occupying 452 m2, amounts to a total of 13,560 m2, assuming scour protection is deployed, less than 0.15% of the total area enclosed within the wind farm site boundary.
While other impacts on the benthos are possible, as yet there are no data. For example the potential for direct effects on stationary or slow-moving animals, which are unable to move therefore would be killed if directly beneath the jack-up vessel or pile. Similarly, there is the potential risk of smothering from deposition or accidental release of drill cuttings. With the deposition of drill cuttings from offshore oil and gas rigs, notable areas are affected even reaching many hectares [3] and these can cause benthic community changes where opportunistic species outcompete the less-tolerant species. However, the drill cuttings disposed from the construction of offshore turbines are likely to be of a much smaller volume.
Despite the loss of area, in terms of the physical area of disturbance, it has been shown that the installation of a turbine, with the right form of scour protection where appropriate, can increase the surface area available for organisms to colonise [6]. The area of habitat created depends on whether scour protection is installed, and if so, which type of material is used. Wilson and Elliott [6] showed that with synthetic fronds, which mimic seagrass, there is a potential for a direct area loss of 12.5 m2; however when boulder and gravel protection are used, the habitat gain is 577 m2 and 650 m2 respectively, assuming a 4 m diameter turbine, and 10 m of scour protection, as above.
Although there is the concern that wind turbines simply act as aggregation devices, attracting organisms from adjacent areas (see below), there is a large body of evidence which suggests that the towers and foundations act as artificial reefs, increasing productivity in the immediate vicinity, which has the potential to spread into the surrounding area [7].
Oil rigs, which are similar to offshore wind farms in terms of their subsurface structure act as successful fish and mobile invertebrate aggregation devices, with much anecdotal evidence of greater fishing in the areas surrounding platforms. Within the UK, evidence from operational offshore wind farms has found that the developments can have little effect on populations. Surveys undertaken within the Barrow Offshore Wind Farm, off the north-west coast of England, eighteen months after construction, found that similar catch rates of lobster were found both inside and outside the wind farm boundary [8]. Numbers of legally-sized crabs caught were also found to be similar. However, the abundance of undersized crabs caught was much greater inside the wind farm boundary than outside, suggesting that the wind farm area was acting as a haven for juvenile crabs [8].
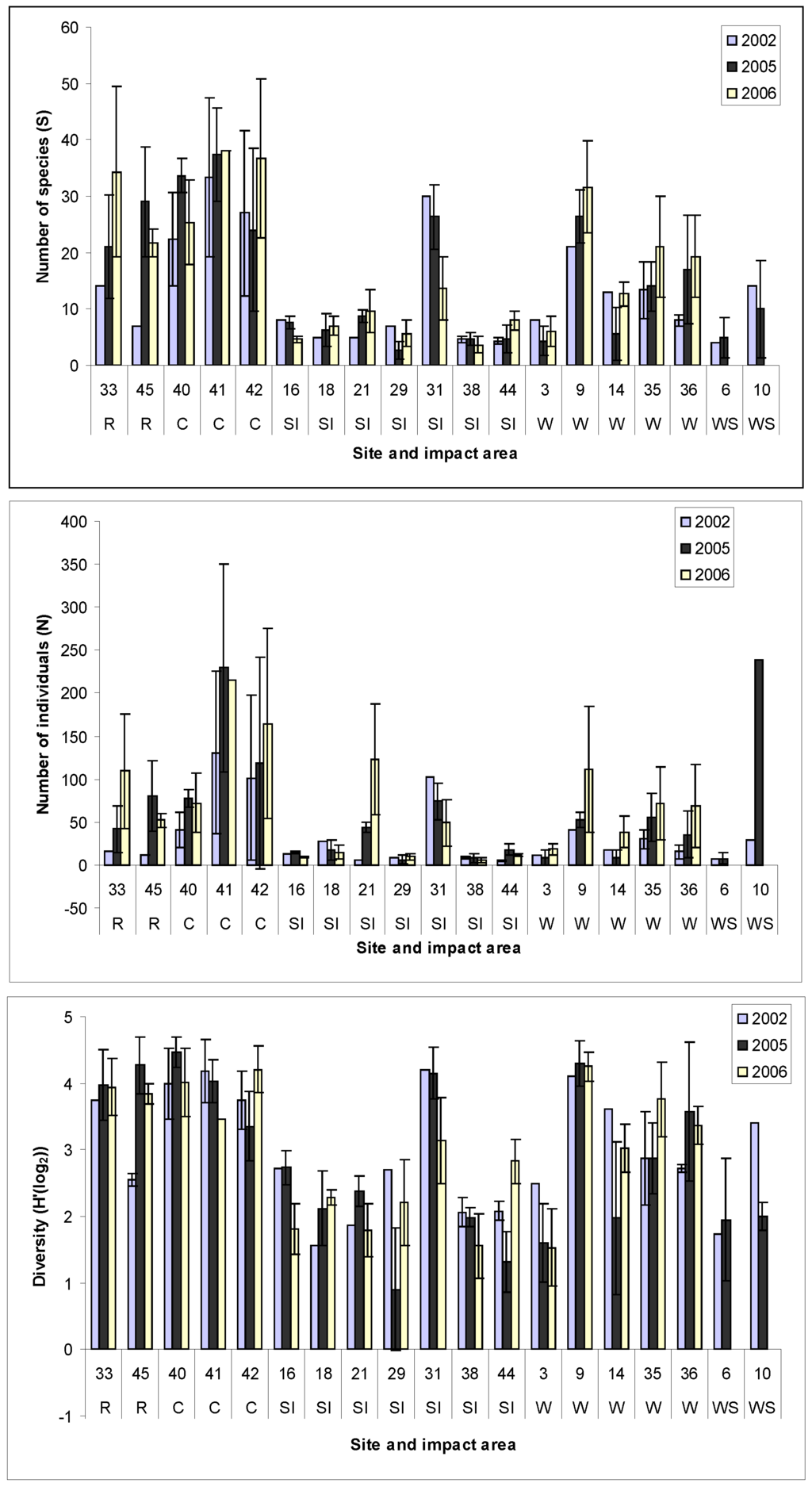
Figure 2.
a–c Macrobenthic faunal changes (S, A and H’) for a UK wind farm showing within and outside the areas of impact between 2002 (pre-construction) and 2006 (post construction). R—Reference Site; C—Cable route; SI—Secondary impact (outside the development area but within the tidal excursion); W–Wind farm development site; WS—Scour assessment (within the vicinity of turbines) [Unpublished data].
It is of note that the creation of complex habitats, which may mimic rocky substratum with crevices, that adjoins onto sandy substrata is the preferred habitat for commercially-caught crab and lobster. Hence the creation of this mixture of habitat by wind turbine monopiles and scour protection will be beneficial to those populations. Building on such information, wind farms may have the capacity to increase benthic populations for commercial reasons, particularly if this is built-in to the project at the earliest planning and design phases. As the currents around the base of wind turbines require scour protection to ensure the stability of the turbine, the scour protection can act as habitat creation [6], thereby increasing the environmental benefits. As certain species prefer specific habitats, for example near rocky outcrops, animals such as crab and lobster are likely to be found in higher numbers than on a flat sandy-bed habitat. One of the most common forms of scour protection is to deploy large boulders around the base of the tower. This mimics a rocky outcrop environment and therefore may increase lobster, crab and reef fish within the wind farm boundary. Given that the perceived impact on commercial fisheries is often one of the greatest objections against offshore wind farm developments, this has the potential for a win-win situation, as both the benthic population and commercial fishermen may benefit from the installation of the turbines.
3.2. Demersal and Pelagic Fishes
Alterations in the physical environment due to noise, electromagnetic fields, water clarity, nature of the benthic substrata and hydrodynamic field, are of concern with regards to interactions between offshore wind farms and fish communities [9,10,21] or community-controlling coastal processes [11]. Water quality issues such as pollution have a comparatively reduced footprint and duration, although effects may occur during the construction and decommissioning phases or as consequences of operational accidents.
These effects on the physical and biological aspects of habitats receiving wind turbines and operational pressures are considered here as having negative environmental impacts ranging from moderate to minor, although the direction and magnitude of the response is still unclear (Table 4). Offshore wind power structures can also act as both artificial reefs and fish aggregation devices (FAD), which have been used to restore damaged ecosystems. Moreover, and directly relevant to fish and other commercially-important groups, is the creation of de facto fishing exclusion zones which act as marine protected areas, which have proven successful in managing and protecting biodiversity and fisheries [13,14,15,16]. Recent proposals for wind farms off the Dutch coast have been examined in the light of their beneficial role in preventing beam-trawling, itself regarded as a damaging activity [3] on fish populations which are far from their historical abundance, diversity and overall community integrity [17]. Therefore increased benefits to biodiversity and fisheries may result when wind farms are placed in nursery grounds and when combined with other measures of reducing fishing mortality. Benefits to commercially-fished stocks may arise from several factors such as reduced juvenile by-catch mortality, increased spawning biomass, over-spill of large size fish to fishing grounds, etc.

Table 4.
Potential impacts of offshore wind farms: marine fishes (Modified from [12]). The evaluation of the severity of impacts is presented between brackets, however, the impacts will differ significantly between the construction, operational and decommissioning stages [10].
| Potential impact | Likely to occur around offshore wind farms? | Likely to cause significant impact? |
|---|---|---|
| Noise | Yes, during the consenting and construction phase (notably seismic surveys and piling) and later during the operational phase. | Species dependent with hearing specialist being the most vulnerable (Moderate to Minor) |
| Electro-magnetic field | Yes, export cable route and their connecting cables. | Depends on the species, and their level of vulnerability/sensitivity. Potentially more severe effects on elasmobranchs (Moderate to Minor) |
| Habitat loss/degradation | The seabed habitat will be lost (monopile) or replaced (cable route) through installation. | Potentially no, as the loss is usually small compared to the total available habitat. Creation of new hard habitat (foundations and scour protection) may be beneficial to fish. (Minor) |
| Increased turbidity | Yes, during the initial construction phase, e.g., as cables are installed. Impact should be reduced after construction. | Increased turbidity may impact on fish with benthic eggs through egg smothering, Reduction in the ability to feed for visual predators or indirect effects by affecting primary production. May also release sediment-bound chemical pollutants. (Minor, Undetectable?) |
| Alteration of community composition | Yes, due to changes in habitats and conditions leading to altered interactions between species (predator-prey, competition,) | Yes for those species being removed, or with increased vulnerability but no for those entering the area. Overall there may be a benefit to the surrounding environment (reef effect) (Unknown) |
3.2.1. Underwater Structures
Much interest has been placed on turbine underwater structures functioning as artificial reefs and FAD [18,21]. For example, a study in the Adriatic Sea proposed a beneficial link between offshore wind farms and bluefin tuna [15]. Castro et al. (2002) reviewed the effect of floating structures on fish aggregating behaviour suggesting that wind farms may act as fish aggregation devices, resulting in catch rates in the immediate area between ten and a hundred times greater than in the surrounding open ocean [84]. The cause may be a combination of the shelter provided by the structures, and the reduced fishing effort which is often found around offshore wind farms (although in most cases not specifically prohibited unless on safety grounds).
Despite this there is the need for more evidence for underwater structures being generally beneficial for fish. The FAD may simply be a change in distribution with no direct effects on averaged long-term gains of fish biomass and yield and even potential detrimental effects such as increased vulnerability to predators or exposure to fishing devices. Indirect evidence for the likely ecological effects can be obtained from available literature on artificial reefs and other underwater structures. This experimental evidence suggests that the aggregating effect is rapid, with noticeable effects occurring within a very short time from the erection of underwater structures [19,20] which suggests a redistribution of fish available in the area. However, the presence of such directed behaviours may indicate an overall beneficial effect for populations such as increased food availability, shelter, presence of mates or suitable spawning surface as conceptually proposed in the literature [84].
Wind turbine field studies suggest a strong species-specific response with increased abundance over longer periods and changes of assemblage composition (fish and macrocrustaceans) [21,20]. However, there is the need for the study of long-term interactions between fish and offshore turbine structures. Furthermore, the role of underwater structures as FAD or artificial reefs may be modified by other alterations of the physical environment as result of other perturbations such as underwater noise, electromagnetic fields and disturbance of the seabed.
3.2.2. Sound Pollution Effects on Fishes
Natural sounds in the ocean are produced by abiotic (breaking waves, currents and drifting materials on the seabed, rain, etc.) or biotic sources (vocalization, snapping sounds, bubble ejection, echolocation chirps, etc.). Given the physiology of sound perception in fish and the physics of underwater sound waves (i.e. fast speed and long propagation distance), natural sound and pressure waves create a sound spectrum which could be distorted if interfered by sound pollution from the creation and operation of wind turbines. At an intense level, this could have significant effects, occasionally causing physical injury or death as demonstrated in fish affected by underwater explosions or pile driving (most likely during the scoping and construction phases) [22,85]. More importantly, there may be a range of sublethal effects leading to ecological implications given that fish use sounds to gather information about predators, prey, competitors, and mates, for the location of migration routes or feeding grounds [22]. Mueller-Blenkle et al. [86] recently found responses to distant piling noise in cod Gadus morhua and sole Solea solea in controlled field experiments. Therefore noise pollution, when interfering with or masking natural signals, could have consequences at the ecosystem level such as community composition changes due to avoidance behaviours, migration failure due to navigation impairment, mortality due to increased exposure to predators, growth reduction and reproductive impairment due to reduced access to prey or spawning partners.
3.2.3. Electromagnetic Fields
Fish are sensitive to electromagnetic (EM) fields [3,10]. Experimentally demonstrable EM clues used by fish include the Earth’s magnetic field, marine currents and weak electric fields caused by electrophysiological activity of muscles and nerves of benthic organisms. These clues have long been considered to play roles in spatial orientation and prey detection [24,25,26] especially in elasmobranchs but also widespread in many teleosts [25]. In a typical offshore wind farm, EM fields arise from the buried electrical cables interconnecting wind turbines with the consumer grid. The intensity of the induced EM field around these cables depends on many design factors such as type and magnitude of current, conductor core geometry, insulation type, nature of the seabed, depth of the cable if buried, etc. [25]. There is the potential for induced EM fields at the seabed surface to exceed the sensitivity threshold of electro-sensitive species [24,25] although the strength of EM fields quickly diminishes with distance from the cable (theoretical decay rate = 1/distance3) [23]. Therefore directed behaviours, both attraction and avoidance responses, are restricted to a narrow corridor along cable routes. Potentially the width of the impacted area, i.e. where EM fields are at or above the lower sensitivity limit measured in the laboratory, could for the more sensitive species extend hundreds of metres from a cable [23]. Possible ecological effects of EM field may include poor hunting performance in these areas or failure to complete migrations if fish, especially demersal and benthic species travelling along the coast, have to migrate over these cables. However, little direct behavioural evidence is available and there are inconclusive results on affected fish behaviours of electro-sensitive species due to EM fields [23].
3.2.4. Habitat Alteration
Hard structures used to support the turbines or to protect their foundations represent artificial hard bottom habitats and opportunities for fish or their larvae to settle. This is accompanied by a loss of original bottom habitat due to direct physical destruction (i.e. turbines foundations, cable trenching and armour deposition) or indirectly by modifications in the hydrodynamic field resulting in increased scouring of soft sediments. The effects could extend into the water column affecting pelagic fish and larvae especially during the construction and decommissioning of wind farms as sediment plumes may increase turbidity or lead to the re-mobilization of sediment-bound contaminants. Therefore, while the construction of offshore wind farms increases the complexity of the habitats, this may lead to changes in the nature of the local fish assemblage. From the conservation view, this may cause the fish community to return to their original (pre-anthropogenically-altered) condition and is consequently perceived as beneficial. However, it may also cause further deviation from the reference community natural to the area and may be considered undesirable [18].
In coastal temperate waters, where wind power is more likely to develop, initial fouling assemblages on underwater wind power structures differ from those inhabiting adjacent natural hard substrata and thus influence surrounding natural assemblages [27,28]. The nature and complexity of man-made structures influence assemblages of benthic crustaceans more than fish [19,20]. Enhancement of the structures with features such as holes and artificial seagrass beds have been proposed to increase complexity and microhabitat choice, measures that are considered to increase the ecological value of artificial hard substrata and have positive effects on the complexity of the resulting fish assemblage [10,18].
Once the changes to the physical environment have changed the local fish community it is possible that alteration could extend further from what is purely expected as a result of biological interactions, with these effects occurring at different scales. Predation pressure, competition for resources, input of invasive species, settling of larvae, diseases, species dominance and many other effects are much harder to identify with precision as many possible feedback loops between fishes and fish and other biological components of the ecosystem may operate at any given time and scale. The potential effects on the ecology of adjacent coastal waters are then difficult to predict.
3.2.5. Fish Sampling Methods
Survey methods need adapting to the particular behaviour of each species. Ideally, the sampling method should indicate abundance by being quantitative. It is also important to obtain information on the fish prey and predator fields for ecosystem-based assessments. Traditional fishing gear (trawls, pots, creels, gillnets, plankton nets, etc.) preferentially target a single habitat (e.g., pelagic or demersal) and often produce a sample that contains a complex mixture of fish and shellfish. These data give the primary community variables such as species richness, abundance and biomass to indicate the community structure. However, this will only be true when the fishing method is un-selective and operates effectively and reproducibly. On many occasions, sampling biases dictate that alternative gear or two or more complementary gear types must be used. Using different gear types (i.e. multigear assessments) requires more complex analysis and often the different outputs cannot be combined but it undoubtedly produces a more valid overall assessment [87].
Newer methods for fisheries assessment use acoustic and visual or video-based survey techniques. Acoustic methods use sound waves to echolocate fish targets which are necessarily grouped in coarse taxonomic or functional groups such as ‘pelagic clupeids’. For practical and technical reasons acoustic methods are best suited to provide overall fish biomass of pelagic species but less or not effective on benthic fishes. Visual scuba censuses are increasingly used in studies focussing on the habitat enhancement role of wind farm structures. The advantages of this are the precise spatial resolution provided and the non-destructive nature of the sampling. It has, however, fundamental disadvantages in areas where visibility is poor or in deep waters and where species identification or diver access may be difficult. Remote techniques such as towed video or baited video stations may expand the operational range of visual scuba censuses but are not currently used. Stereoscopic video systems could be used to describe the size structure of the fish being observed but, as with any other survey gear may be subject to bias as its efficiency may be limited on small cryptic fish species. Importantly, remote video or scuba surveys non-intrusive survey methods are often well-received as they promote welfare of fish and, in the case of video observations, provide a permanent visual record that can be assessed in multiple ways.
Finally, given the limitations of field surveys, specific manipulative methods and experimental designs are needed to test hypotheses, including different levels of complexity in underwater structures and cascade effects on fish. These experiments will require adding test structures or exclusion trials where the access of fish or other biological components to the structures is controlled [20,27,28]. Similarly the effects of noise or EM fields on fish behaviour will need better knowledge of the sensitivity of key fish species and responses to actual stimuli in the field. Active acoustic tracking has been used with success to follow fish responses in control exposure experiments in the field [23].
3.3. Coastal and Inland Birds
Coastal and inland birds may be using the marine areas as passage migrant routes, and so infrastructural and environmental considerations need to be met e.g., wind farms cannot be constructed in all locations, and some locations are better than others. The ‘quality’ of the wind supply in an area results in siting the turbines in open, exposed areas such as coastal margins and offshore areas (Drewitt and Langston 2006 [1]). Such areas are often also important and sensitive habitats for a range of plants and animals, for instance for coastal and inland birds for feeding, roosting and migrating. Potential impacts and hazards to coastal and inland birds have been summarized [29] as:
- Behavioural: birds avoiding the areas around turbines as a response to a visual or audio cue; the turbines act as a barrier to movement;
- Physical habitat: birds respond to destruction, modification or creation of habitat associated with wind turbines construction and operation;
- Direct population change: birds collide with turbine structures.
Within each of these, the indirect impacts of wind turbine construction and operation for birds include disrupting foraging behaviour, breeding activities and migratory patterns whereas direct impacts include increased mortality, alterations in the availability of food, roost and nesting resources, and in increased predation risk.
3.3.1. Collision Risk from Coastal and Offshore Wind-farms and Turbines
The large numbers of diurnal and nocturnal migrants including coastal and land birds, which move through offshore areas, vary with migration intensity, time, altitude and species depending on external factors such as season and weather [30]. Hϋppop et al., [30] found that in the German Bight, half of these migratory flights were at heights coinciding with turbine rotor-blade heights. Given the current move from 30–40 m to >70 m blades then the area of influence will increase. For migrant birds, turbine structures on land appear only to cause a significant problem when they are situated on exposed sites with high migration densities i.e. passes, straits and peninsulas [30]. Gull species are frequent victims as they are abundant and widespread and so the likelihood of encounters with turbines are greater. Waterfowl such as cormorants and geese species are also at risk although when measured collision rates for these are very low. Raptors also have low collision rates but these increase where very large numbers occur in areas with high densities of turbines [31]. For example, the white-tailed eagle Haliaeetus albicilla is an important and charismatic coastal raptor and has been of particular concern with respect to collision mortalities. It is listed as threatened and there have been documented incidents of collisions from 2001. In Germany the number of white-tailed eagles found during occasional searches is increasing annually [32] and these incidents are most likely to occur in areas where concentrations are high and there is a large prey base as with other raptors. Passerine migrants also collide with turbines at a very low rate given the large numbers passing through wind farms [31]. It is therefore considered that of key concern for collision risk are those species which pass through a wind farm (or wind farms) on a regular basis (e.g., on a daily basis between foraging and breeding sites, and in particular in poor light or weather conditions), are long-lived (usually with a correspondingly low annual reproductive output), and are large (usually with a correspondingly low manoeuvrability) (see below).
In general, collision risk depends on several different factors related to bird species, numbers and behaviour, weather conditions and topography and the nature of the wind farm/turbine itself including the use of lighting. The risk is greater on or close to areas that are frequently used for feeding, roosting or migratory pathways and local flight paths. This is particularly important for wading birds and gulls when turbines are located in a coastal or estuarine site, as these birds often move daily between inland roosting and coastal feeding grounds. This diurnal movement is often linked to tidal cycles.
Large birds such as swans and geese tend to be less manoeuvrable and may be at a greater risk of colliding with a turbine structure. However some studies have shown geese to be adept at avoiding collision, for example Fernley et al., [33] studying goose collisions at operating wind farms, recorded an avoidance rate of up to 100% at some sites – this was based on the main species at the study sites: Canada, Snow and Brent geese (respectively Branta canadensis, Anser caerulescens and Branta bernicla).
Flight pattern is an important factor with regards to collision risk. Raptors often drift on wind currents, and if those currents flow through a wind farm, then a collision may occur. Raptors also practice contour flying close to the ground and a sudden updraft may force the bird into the rotor swept area [34]. The risk to birds from collision may further increase with age and decreasing physiological condition, this being particularly important for birds on long migrations such as many wader species and passerines.
Reduced visibility due to poor weather conditions and darkness may increase the potential collision risk. This can be difficult to measure with present monitoring techniques, although with new technologies being developed, such as thermal imaging, additional data may become available. Strong winds may also affect collision potential, reducing the ability of some species to avoid structures and the rotor swept area and/or forcing flights into the rotor swept area of a turbine due to a reduction in the available flight height. However, the impact of these potential collision risks may be reduced as the birds may not fly under such conditions. Dirsken et al., [35] for example found that pochard Aythya ferina and tufted duck Aythya fuligula regularly flew through a wind farm in the night under moonlit conditions but avoided the area in dark and foggy conditions.
Artificial illumination of turbine structures offshore can affect collision risk. In general, and under normal conditions, migrating birds are able to avoid structures although Hϋppop et al., [30] demonstrated that under poor visibility, birds, and in particular terrestrial birds, were attracted to offshore illumination and thus drawn into wind farm sites (which are continually illuminated), thus increasing the risk of collision incidents.
Habituation can be important with regard to avian mortalities by wind turbines [36] as birds become habituated to the presence of wind turbines after the first few years of operation [37]. In some areas, particularly where other anthropogenic structures exist, such as chimneys and other large structures, inland and some coastal birds may be habituated to avoiding large structures, recognising them as a hazard to avoid, and thus reducing mortality rates. In other areas birds may learn with time. For example, avoidance responses have been observed by large gull species near to the wind turbines at Europoort, Rotterdam (pers. obs.), with some individuals displaying a modified roost flight-line through the wind turbines at a substantial distance (estimated at over 1 km), whilst others were observed under the same conditions and at the same time of day to fly to within a few 100 m of an indivudal turbine and then undertake a rapid direction and altitude change in order to avoide the rotor sweep. Whether this variation avoidance was due to individual preferences or habituation of some individuals is unclear, but demonstrates that the habituation concept, whilst undoubtedly reducing collision risk, can also be difficult to quantify accurately. Despite this, habituation will reduce collision impacts over-time, although this depends on the species and their fidelity to an area. In addition, recent changes in turbine technology have led to a decrease in death rates of all flying animals [36] as turbines have larger blades that rotate more slowly and, with the advancement of tracking technology, wind farms are located more appropriately and care is taken to avoid diurnal and seasonal migratory flyways.
3.3.2. Displacement/Disturbance/Avoidance
Coastal and inland birds may be displaced from a habitat due to the construction or operation of a wind farm/turbine. Displacement distances, i.e. the distance around a wind farm where bird activity is reduced or absent, vary considerably. Displacement distances of up to 800 m have been demonstrated by Pederson and Poulsen [38] for the coastal species lapwing Vanellus vanellus, golden plover Pluvialis apricaria and gull species near to a single turbine located on coastal grassland. Larsen and Madsen [39], found that field use by wintering pink-footed geese Anser brachyrynchus in Danish farmland was affected by the presence of wind turbines, with avoidance distances varying between wind farms with a linear turbine layout (avoidance distance of c.100 m) and a clustered layout (c.200 m). The study also highlighted the fact that habitat loss was reduced where turbines were located close to other avoidance zones that were associated with existing physical elements in the landscape such as roads or other large structures.
In the UK at the coastal wind farm at Blyth, Northumberland, Still et al., [40] found no significant disturbance effects despite the area supporting internationally important numbers of wintering purple sandpiper Calidris maritima which roost on the seawall at the wind farm site. Similar situations have been highlighted elsewhere for example golden plover roosting within the Havergigg coastal grassland wind farm site in Cumbria, NW England (SGS Environment 1994, in Percival [31]).
Whilst avoidance is necessary to prevent collisions by migratory shore and land birds, this will in itself have an energetic cost to individuals, particularly if they have to make large diversions to avoid wind farm sites on migratory flyways. In some circumstances, such required ‘diversions’ could become so great that the wind farm in effect becomes a barrier. The extra energy required to make such diversionary flights will reduce the condition of the bird and effects on breeding and survival rates may affect overall population size. In extreme cases, the barrier effect may render a preferred functional site unavailable, e.g., a barrier between a breeding and feeding area may, if alternative feeding areas (even potentially sub-optimal) cannot be found, lead to a loss of that breeding site function.
Noise emitted from turbines and wind farms may potentially cause disturbance to coastal and land birds. These noises are typically masked by background natural sounds but it has been suggested that in some cases these noises may interfere with wildlife and birds, for example by masking alarm calls preventing birds from alerting others to dangers [34].
3.3.3. Habitat Changes/Loss
Habitat loss can affect the overall population size of an inland or coastal bird species by reducing food availability or forcing birds to travel further afield to obtain food, thus expending additional energy. These factors lead to changes in fecundity and survival of individuals, hence affecting population size and viability. The loss of a roost site effects birds in a similar way, as safe roosting is important for energy conservation and birds will travel large distances to find a suitable roost. For coastal and land birds, habitat loss would be particularly important where a wind farm was located in an estuary or coastal position as these areas provide rich feeding grounds and roosting areas for waders and waterfowl. The total direct loss of habitat from a wind turbine installation and its infrastructure, (cable lines etc.), is dependent on the overall size of the development but is likely to be small. About 2–5% of actual habitat loss over the development area will arise from the turbines themselves. However interactions with the surrounding landscape, geomorphology and hydrology may increase this loss [1]. Human land use in estuarine locations has historically been high, and this continues. Similarly, the provision of roost sites close to estuarine feeding sites for many species of wildfowl is extremely important, with the viability of such roosts being dependent on several factors including proximity to feeding grounds (terrestrial and estuarine), land use (including agricultural crop type), the availability of sight and flight lines and ambient disturbance levels. These key location determinants mean that only a limited number of inland roost sites are tenable for most species, and these are therefore used by large numbers on a regular basis. Species such as golden plover and lapwing, which feed and roost in very large densities on both estuarine and adjacent terrestrial habitats, and undertake regular (daily or tidal) movements between both habitats, require viable flightlines between sites. Many of these movements occur at an altitude within most operational rotor sweeps, and the design of most wind farms in such areas usually are linearly along the estuary bank, thus readily providing a potential barrier for such estuary-to-field and return movements by wader species. Consideration and knowledge of such areas and the habitat needs for key species is therefore necessary during the EIA, particularly when considering the effects of a wind farm development in the context of other adjacent developments (i.e. cumulative effects).
Cumulative effects may occur if wind farm sites are poorly planned in conjunction with other neighbouring sites, for example where more than one wind farm site exists along a flyway corridor of a given population. Primarily, this may become a concern where rare species, in particular those restricted geographically, or long-lived species with low productivity experience a cumulative mortality across sites although this can also be an important aspect for many populations. For instance a ‘chain’ of small, linear wind farms along the banks of an estuary could have a significant detrimental effect on a population which regularly moves between the estuary and hinterland, as well as wider implications for migratory movements into and out of the estuary, and the status of the estuary in the wider international flyway context.
3.3.4. Detection of Impacts
During the EIA and consenting process for the development of wind farms, additional assessment work is necessary in the more sensitive areas (e.g., in Europe development sites within or adjacent to sites of conservation value designated under the EU Wild Birds and Habitats Directives). To assess the impacts of the construction and operation of a wind farm on the avifauna of an area, a baseline study is needed to provide seasonal data on species, their abundance, distribution and movements, including altitude and direction of flights, in relation to the likely height of rotor operation, as well as the wind farm layout. Tidal cycles, light and weather conditions should be noted as these may affect the presence and behaviour of coastal and inland birds in an area, and their susceptibility of interactions with turbines. Several methods are available to identify diurnal usage by birds in and around a wind farm, with a series of prescribed survey methodologies available, primarily using expert observation from differing survey platforms. As this review cannot give all methods and their applicability for all wind farm options, other sources should be consulted, e.g., the UK Collaborative Offshore Wind Research into the Environment (COWRIE) and The Royal Netherlands Institute for Sea Research (NIOZ). The following gives a short description of the some of the survey requirements and/or options available.
Infrared and radar technologies can detect nocturnally active species such as the Golden plover (Pluvialis apricaria) (and bats), although such technologies are currently being developed and refined. In predicting the effects of a wind farm on such bird and bat populations, there is a small but increasing body of research available from exsiting wind farm developments, with predictive models of impact having been developed, based on these data. Such models are of potential use in predicting collision rates of avian species, following the construction of a wind farm as they enable factors such as turbine design and operational parameters such as wind farm layout to be addressed, in addition to site-specific data on the seasonality, main flightlines and abundance levels of key species and generic data on the size and avoidance behaviour of these species (or surrogates) when interacting with turbines. Such models broadly indicate likely impact rates but rely upon both good baseline datasets and accurate avoidance rate data for turbines. Baseline data collection techniques are generally prescribed and robust, although there may be site-specific aspects that require additional data to provide a full picture of aviafaunal usage. However, avoidance rate data, particularly at sea and at night are not well defined, and further research on these aspects is needed. As such, the output from current collision risk models should be used with caution, with their greatest strengths being in providing a standardised approach to the assessment of a generic collision risk. This can allow for intra- and inter-site mortality rates to be compared based on a range of wind farm layout designs at an early stage of the development, and in the wider cumulative impact characterisation stage. Wider Population Viability Assessments (PVAs) may be required for certain species, depending on their local distribution and the functional value of the area. Upon project consent, pre and post construction monitoring should follow a standard Before-After/Control-Impact (BACI) approach [3] with prescribed, standardised and repeatable methods of data collection and analysis allowing for a comparison of impacts. Onshore, scavenging trials can be used to provide information on the rates of scavenger predation at wind farm sites, and the results of such studies then used to adjust collision mortality rates determined from standard carcass retrieval studies.
3.4. Sea Birds
The highly mobile marine birds (considered here to include seabirds, seaducks and divers) undertake not only long distance migratory flights but also daily foraging trips, and have flight responses to advancing weather systems, compensation flights for tidal displacements and ad hoc responses to sudden localised feeding opportunities. As the result, marine birds are not only at risk to interaction with marine structures (such as wind turbines) during migration but at all times as they spend most of their lifecycle at sea only returning to land to breed. As with inland and coastal birds, the two main issues considered for marine birds in relation to offshore wind farms have centred around the potential problem of direct mortality resulting from collisions and concerns surrounding energy budget depletion and barrier effects for birds that are avoiding the wind farm areas [1,41,42].
Direct mortality and/or lethal injury to birds can occur not only from collisions with the stationary superstructure and the rotating rotor blades but also as the result of the turbulent airflow associated with the blades around the sweep area. Collision mortality rate data attributed to offshore wind farms are currently very limited for marine birds, largely because of the difficulties associated with the detection of collisions and recovery of carcasses at sea [1], particularly during periods of darkness and poor weather. To date, measurement of collision rates at sea for species and/or sites has proven to be very difficult, with the most effective method being infra-red thermal imagery technology to gather data from sampled sections of the turbine sweep area, recording being triggered by warm-bodied objects entering the field of view [29]. Further research into such techniques is ongoing as there is the need for operational monitoring of collision impacts and associated risk as more wind farm developments become operational and renewable energy targets require a substantial increase in generation capacity.
Radar studies undertaken at several offshore wind farms document a substantial avian avoidance response, with the birds modifying their flight trajectories to avoid the turbines and therefore reducing the risk of collision [43] (see Figure 3). Species such as common eider Somateria mollissima modified their flight trajectories at an average distance of 3km from the Nysted offshore wind farm (Denmark) during daylight (less by night) compared to pre-construction flight patterns [43,44]. At the Tunø Knob offshore wind park in the Kattergat (Denmark), Larsen and Guillemette [45] found that common eiders mostly avoided flying close to and within the wind park and that this avoidance was caused not by the action of the rotors but by the presence of the turbine structure themselves. The avoidance behaviour implied that risk of collision at the Tunø Knob and Nysted offshore wind farm was negligible, although it could be potentially higher during poor visibility [43,45].
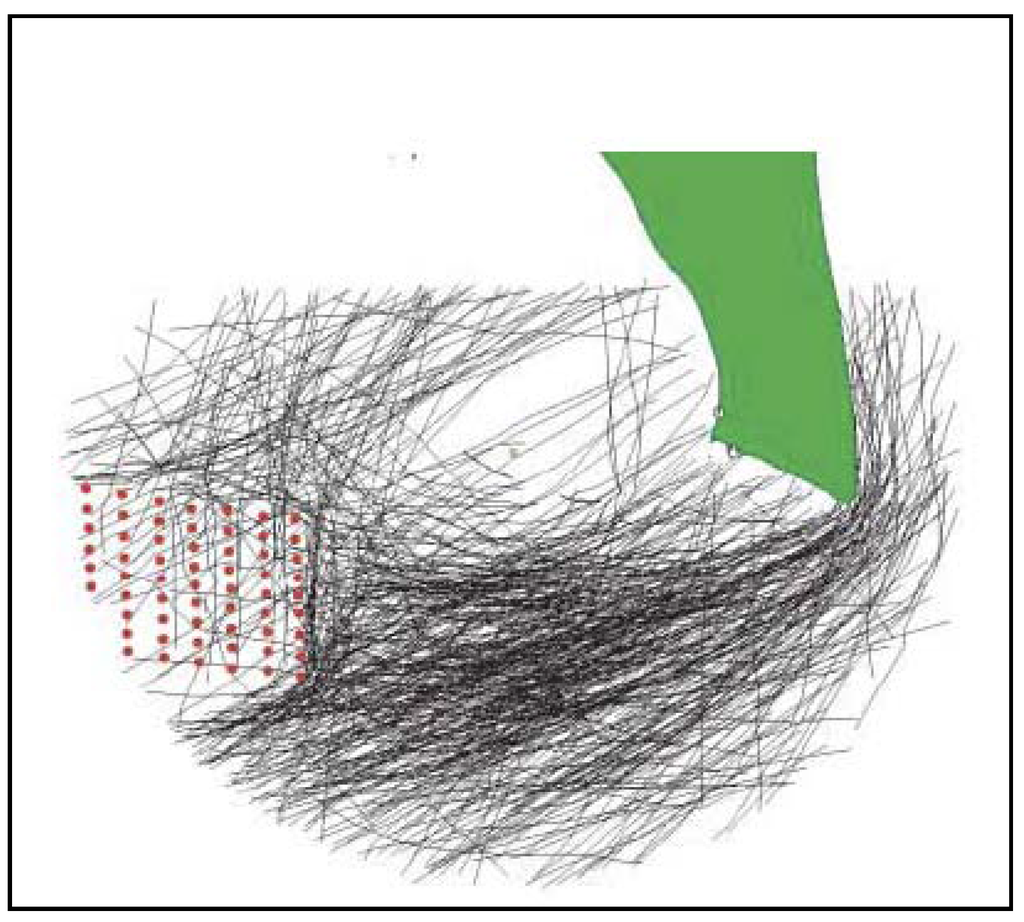
Figure 3.
Radar registration from the Nysted offshore wind farm applied on a GIS-platform. Red dots indicate individual wind turbines, green area the land, green dot the sitting of the radar and black lines migrating waterbird flocks determined visually at the Nysted offshore wind farm. Adapted from Kahlert et al. [44].
As there are limited data available on the collision mortality of seabirds with offshore wind farm structures [46], caution should be taken in drawing general conclusions. The further development of radar and thermal imaging hardware and associated software is therefore required to gather data on actual collision rates for a range of species. However, whilst it is important to quantify the actual collision rates of seabirds at offshore wind farms, it is also necessary to model the effect of such mortality rates on populations at a range of scales. The significance of individual mortalities of species is linked to population dynamics for each species, and can have a wide variance in overall ecological importance. This is because the populations of species with high adult survival rates and correspondingly low breeding rates, such as many seabird species and raptors, may be more susceptible to relatively small sale additive mortality impacts (and in particular the mortality of adult breeding birds) than, for instance, passerine species. Longer lived species are generally less able to rapidly replace any population losses than species with a relatively high annual mortality and correspondingly short lifespan. Therefore, the loss of a long-lived species such as the northern gannet (Morus bassanus), has a potentially high impact on the population status, and possibly conservation importance, than for most passerines with a lifespan of perhaps 4 or 5 times less. However, there are widely varying relative abundances between such species with extremely large migratory flocks of some passerines undertaking long distance offshore migrations, passing through wind farm areas. Such relative abundance levels will influence relative collision rates between species although, to some extent, such effects may be dampened by relative avoidance potential, where larger birds are perhaps less likely to be able to avoid individual turbine collisions than smaller more agile species. All of these factors, as well as others such as a range of operational parameters associated with turbine design and operation, need to be considered in addressing the severity of any avian collision risk. Such criteria have been used to produce Collision Risk Models, such as that of Band et al. [47], which is routinely used as a basic predictive tool within the EIA process. However, such tools are only as good as the data on which they are based and, as already noted, collision mortality rates for individual species of seabirds with wind farm structures is poorly documented. Whilst there is agreement that collision mortalities are low and acceptable for most offshore wind farms, if appropriately sited, this is based on limited direct data, and considerable reliance on surrogate datasets. As such, more comprehensive data are required thus allowing refining the predictive modelling routines and a greater confidence in prediction during EIA.
More general avoidance behaviour occurs when birds are scared off by wind turbines so that they are either unable to use an area around the turbine(s), or they modify their behaviour within an area. Such behaviour reduces collision risk but means that offshore wind farms can both produce areas of effective ‘habitat loss’ for some seabird species, and/or represent a barrier to movement either to local feeding and roosting flights, or to longer migratory flights [1,46,48]. Whether or not marine birds will be affected at an offshore wind farm site will be dependent on several factors, including the species of marine birds using the area (this related to individual species vulnerability to disturbance), the functional use of the surrounding waters and airspace, and wind farm design. For instance, seaducks and divers are more prone than seabirds to disturbance by the visual stimulus of rotating turbines or displacment by the boat /helicopter traffic associated with maintenance [49,50]. Moreover, behavioural responses vary between species, depending on such factors as stage of life cycle (wintering, moulting and breeding), flock size and degree of habituation [1]. Such avoidance behaviour may have several consequences, one of which is an increase in the energy demands of an individual bird, because of increased distances flown in order to avoid a wind farm. The impact of extended or modified flight lengths due to the presence of a wind farm site may be small in energetic terms for migratory birds [48] but could have greater consequences for wintering birds commuting daily between feeding and roosting areas e.g., common eider and common scoter Melanitta nigra [46,48]. A study at the Nysted offshore wind farm reported an added distance of c.500 m to migratory common eider as the consequence of the wind farm’s presence in their migratory flight path. Hence, the cost of avoidance is trivial but the construction of further wind farms along the migration route could have cumulative effects on the population. Similarly, and as with inland and coastal birds, potentially more significant effects could also occur if a wind farm were inappropriately sited between breeding and feeding sites, particularly for colonial species with limited breeding site availability (e.g., many seabird species).
Avoidance may also result in the general displacement of birds from a preferred feeding distribution if the birds avoid the entire wind farm area and a strip around the turbines. Petersen et al. [51] found that the numbers of common scoters and red-throated divers Gavia stellata greatly declined after the construction of the Horns Rev offshore wind farm. A smaller decline in long tailed duck Clangula hyemalis was also observed at the Nysted offshore wind farm [51].
Given the recent introduction of offshore wind farms, there are few comprehensive studies of the pre- and post-construction effects. The most comprehensive study is that by Desholm [29] which presents an extensive and very comprehensive assessment of the Nysted wind farm area, Denmark. This illustrates the habituation of the birds to the development (Figure 4 and Figure 5).
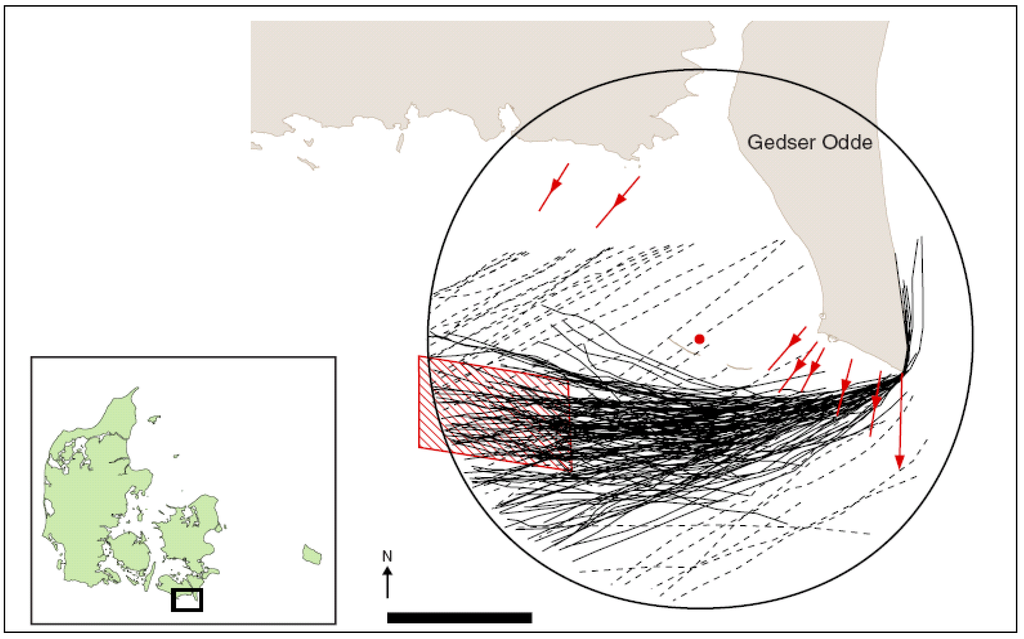
Figure 4.
The Gedser Odde peninsula, Denmark, as the proposed site of the Nysted offshore wind farm showing the preconstruction migration pattern of geese (broken lines) and common eider (solid lines) recorded by radar; red lines denote land migration patterns, the red hatched area is the proposed wind farm site and the red dot is the radar tower [29].
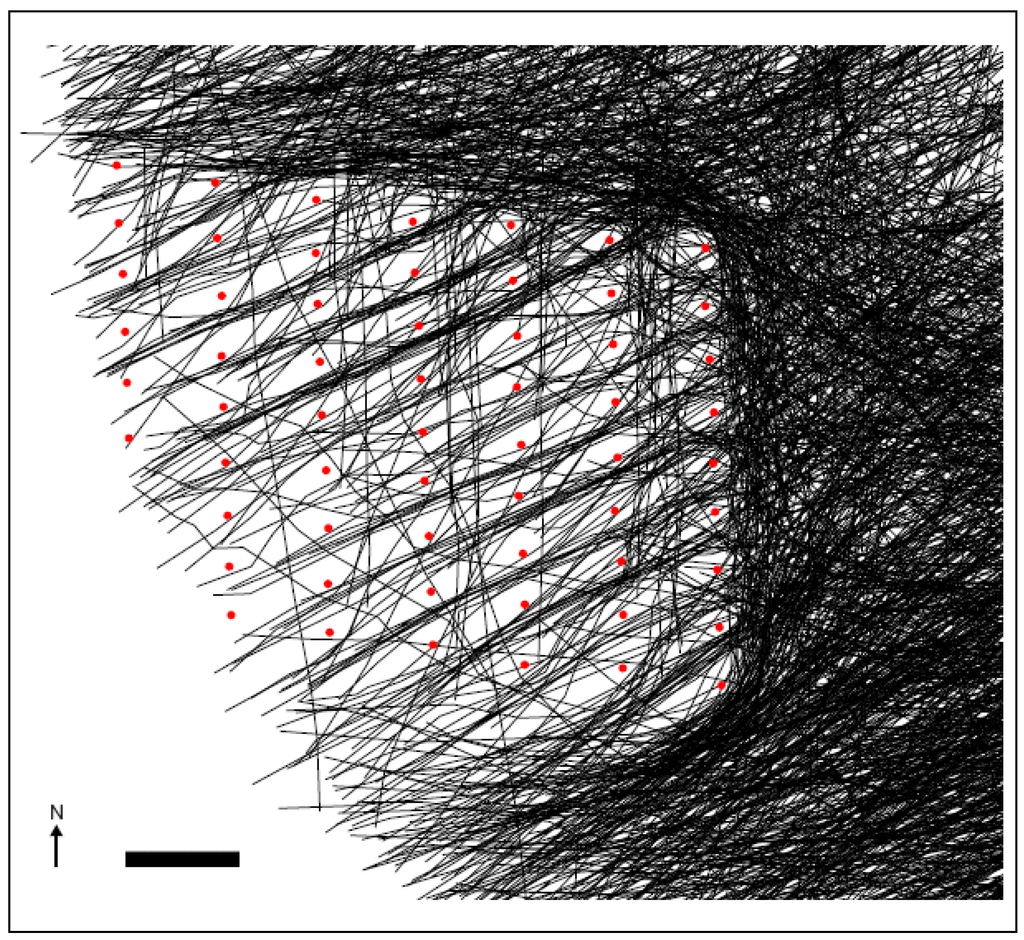
Figure 5.
The south-west flight paths of autumn-migrating waterbirds at the Nysted wind farm, Denmark. Red dots indicate individual turbines and black lines migrating waterbird flocks [29].
Such effects underscore the need for a rigorous baseline seabird survey and data collation component to any EIA with the need to address both the development area and adjacent communities and ecological function. As indicated above, the EIA also requires cumulative impacts to be considered on the seabird community especially where other likely plans and projects might affect the seabird community. This is especially the case within designated conservation sites such as European Marine Sites which require an Appropriate Assessment (Article 6(3) of the EU Habitats and Species Directive). This requires not only good biological and effects data for the specific development area, but for a substantially wider area that may be affected through the cumulative or in-combination approach. For instance, for some species of migratory seabird, it may be necessary to address the likely impact of a series of wind farm developments along a linear coastal feature, in terms both of avoidance and barrier effects (and associated energetic implications) and the more direct collision mortality effects. As discussed above, such criteria are extremely difficult to predict accurately, given current data gaps, and any assessments of impacts are potentially additionally hampered by inertia in data release from other wind farm developments within the assessment area, who not unreasonably have their own commercial concerns to consider. In the UK, the creation of a central repository for wind farm biological data and a requirement for developers to lodge data with the repository has gone some way to addressing this issue although there remain issues, particularly along the north-west European coastline, where trans-national boundaries can severely hamper such collaborative initiatives.
The above text has largely addressed the likely negative impacts that wind farms may have on the seabird ecology of an area. Undoubtedly there are several potential significant areas of impact possible from such structures. However, experience has shown that with suitable background data and an increasing body of effects information, mitigation measures can be employed to reduce such impacts. By far the most effective pathway for impact reduction is through the correct siting of wind farms, away from key migratory and other important functional areas. However, there are additional measures that can be undertaken, including layout design. It is also important to emphasise that positive effects to seabird populations are also possible from wind farm developments, for instance where wind farms may provide nursery or refuge areas for fish, increasing the prey availability in an area, or where turbine bases and scour protection provide a greater diversity of substratum and associated fish assemblage, again increasing the prey potential for some species.

Table 5.
Potential impacts of offshore wind farms: birds (modified from Wilson [12]).
| Potential impact | Likely to occur around offshore wind farms? | Likely to cause significant impact? |
|---|---|---|
| Mortality through collision | Depends on conditions, species and location of wind farm | No—figures indicate a very low risk. (Very minor) |
| Mortality through disruption of feeding grounds | Depends on the species present and location of wind farm | No—if careful planning means development away from important areas. (Undetectable?) |
| Disruption of migration routes | Depends on location of wind farm and distance from shore | No—if wind farm is not too close to shore, and major known routes are avoided in planning. (Minor) |
3.5. Mammals (Bats)
In addition to the effects of wind farms on bird populations, and given that bats also make migrations over open sea and coastal areas, then it is necessary briefly to mention any potential impacts. However, given that there are few studies on this aspect then any conclusions are tentative. Despite this, some of the comparisons made between birds and bats in relation to the impacts wind turbines and wind farms have upon them may be relevant and give an indication of the potential effects. It is emphasised, however, that there are fundamental differences not only in the biology of these animals but in the way in which they respond behaviourally at such installations. Patterns of mortality reflect these differences. Bats will actively investigate wind turbines, a behaviour which has not been demonstrated in birds, and migrating bats appear to be attracted to the structures. It is also of note that whereas bats are prone to depressurisation injuries (barotraumas), birds appear to be less susceptible [52]. Despite this, it is still largely unclear as to why bats are killed by wind turbines. Table 6 gives possible reasons for these mortalities. In the UK, the placement of turbines may be an issue for bats, not only because of the risk of direct collision if turbines are placed on migration or commuting routes, but also because of displacement from foraging habitat [53].

Table 6.
Possible reasons for bat fatalities associated with wind turbines (adapted from Kunz et al, [54]).
| Hypotheses for bat fatalities appropriate to coastal locations | |
|---|---|
| Roost attraction | Bats perceive wind turbines as potential roosts |
| Food Attraction | Insects that bats feed on are present around the wind farm sites as a result of either the altered landscapes or are attracted by the heat produced by the turbines |
| Acoustic Attraction | Bats are attracted to audible and or ultrasonic sound produced by turbines |
| Echolocation Failure | Bats may not acoustically detect turbines or rotor movement |
| Electromagnetic Field Disorientation | Complex electromagnetic fields produced by turbines confuse and disorientate bats. |
| Barotraumas | The sudden decrease in air pressure near to moving turbine blades cause tissue damage to air-containing structures and the bats die of internal injuries. |
3.6. Mammals (Marine Mammals)
As with the other ecological components described above, there is the need to consider the biological features of cetaceans and other sea mammals which make them susceptible to adverse effects of offshore wind turbines [55]. Notably, cetaceans (whales, dolphins and porpoises) have highly sensitive auditory systems, most of them are highly vocal and many also exhibit a greatly developed echolocation capacity. These attributes are essential for communication (possibly over hundreds of kilometres) and to recognize their surrounding environment, allowing them to navigate, avoid obstacles and predators, forage for food and find other individuals [56,57]. Baleen and toothed whales have distinct auditory capacities: baleen whales are considered as low frequency sound producers (from below 10 Hz to 25 kHz) and their low frequency moans, calls and songs have been suggested for use for long-distance communication [58,59]; toothed whales produce high frequency sounds (from a few kHz to 150 kHz) and are assumed to be true echolocators [58].
Pinnipeds (seals and sealions) also vocalize, both in and out of water, and use calls to determine territory and dominance out of water [60]. True seals (Phocidae) appear to hear higher frequencies underwater than eared seals (Otariidae), and vice-versa in relation to airborne sounds [61]. True seals are known to have underwater audiograms ranging from 1 kHz to 50 kHz (with sensitivities of 60–82 dB–all dB values hereinafter are re. 1 µPa @ 1 m.), while out of water, seal hearing sensitivity ranges from 2 kHz to 20 kHz (similar to humans). However, they are probably able to hear lower frequency sounds (100 Hz at 96 dB) either in and out of water [62].
Given their great auditory capabilities, marine mammals are highly sensitive to noise in the ocean, particularly that coming from human activities [62]. Maximum sound pressure levels to avoid auditory damage from single pulses have been estimated to be of 180 dB for cetaceans and 190 dB for pinnipeds [63]. A cumulative Energy Flux Density value (EFD, which takes into account both cumulative duration and the level of exposure) has also been estimated at 195 dB re. 1 µPa2s as leading to a auditory Temporary Threshold Shift (TTS) and at 215 dB re. 1 µPa2s as producing Permanent Threshold Shift (PTS) to marine mammals [64].

Table 7.
Potential impacts of offshore wind farms: marine mammals (modified from Wilson [12]).
| Potential impact | Likely to occur around offshore wind farms? | Level of significance |
|---|---|---|
| Mortality through collision with increased boat traffic | Unlikely during operation, as collisions with boats are rare. May be an issue during construction due to damage to hearing/orientation | Low, as animals will generally be able to avoid a collision. (Undetectable/minor?) |
| Leaving area due to disturbance | Depends on species, but a certain level of avoidance is expected | Relative low, as long as the wind farm was not on a key migration route or in a major feeding ground (Detectable?) |
| Noise damage | Yes, during construction, with lower levels during operation. | Potentially high during piling operations, but much reduced once installed, thereby reducing the risks. (Minor) |
| Disruption of normal behaviour | Yes, in the initial construction phase, but less likely once marine mammals have become accustomed to the operational levels of noise and activity | Relatively low once operations have been ongoing for a period of time. (Detectable?) |
Several behavioural changes have been reported in marine mammals adjacent to offshore wind farms, such as temporary displacement from the area and changes in echolocation rates [65,66]. Behavioural reactions to noise can occur up to 20 km from the sound source [9], although individuals of the same species may react differently in different locations [67]. In particular, baleen whales are estimated to show behavioural avoidance of noises around 130–170 dB and suffer physical damage at 220 dB [68]. Conversely, very few data are available on the physiological effects of anthropogenic noise and very little is known on what frequencies and sound intensities may be responsible of such damage. Nevertheless, documented effects on pinnipeds, for example, are usually related to high intensity sounds [62] and hearing loss may occur at 1.8 km in harbour porpoises and 400 m in seals during pile-driving [9].
Offshore wind farms may have numerous impacts on marine mammals, during exploration, construction, operation and decommissioning activities (Figure 6), but the direct effects of noise and vibration seem to be those posing a bigger threat [69]. Usually higher frequency sounds are generated during construction and decommissioning, while low frequency sounds occur during operation [66]. High levels of noise may induce temporary or permanent displacement of marine mammals from the affected areas (largely harmful in preferred habitats), particularly during construction and decommissioning operations [10,60,65,70]. Disruption of feeding and social behaviours [60] may also occur and these can generate stress and, ultimately, lead to death [70]. Noise and visual presence may be particularly damaging in mating and breeding areas (e.g., seal haul-outs), thereby reducing breeding success and growth and/or recovery of cetacean populations, which may be particularly harmful for threatened species [60].
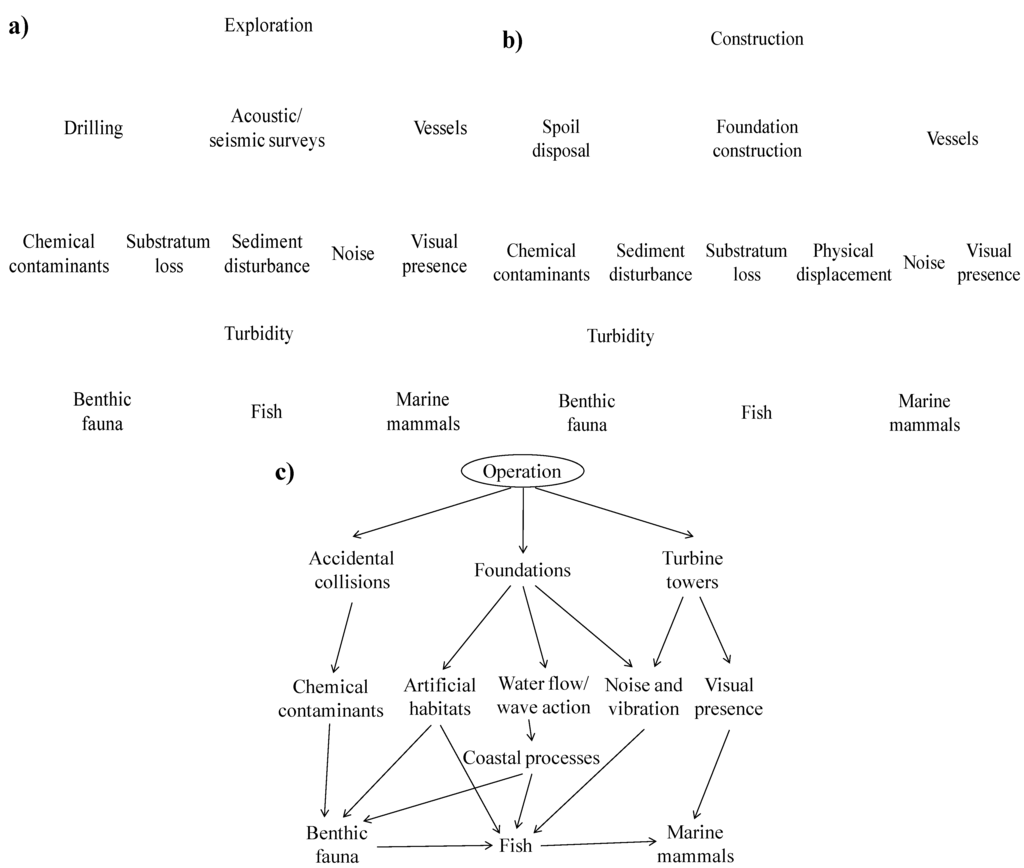
Figure 6.
Environmental impacts of offshore wind farms during (a) pre-installation exploration, (b) construction (similar effects are likely to occur during decommissioning) and (c) operation (adapted from Gill [10]; Elliott [4]).
During exploration, seismic surveys are a major noise source, and avoidance behaviour has been reported in baleen whales at distances of up to 370 km from the source during such operations [62]. Based on maximum energy emitted from airguns (20–160 Hz) and peak frequencies at which baleen whales emit sound, species which will most likely be affected by seismic surveys are fin whales Balaenoptera physalus, minke whales Balaenoptera acutorostrata and humpback whales Megaptera novaeangliae [55,68]. Additionally, deep diving odontocetes may be particularly vulnerable to seismic exploration, once sounds may concentrate within some water layers (e.g., the SOFAR channel) and be transmitted over greater distances than usual [68]. Avoidance reactions by seals have also been observed although these were only short-term responses [62].
Throughout the construction phase, several behavioural changes by marine mammals have been reported, such as temporary displacement from the area and changes in echolocation rates [65]. For example, harbour porpoise avoidance behaviour was reported in the Horns Rev and Nysted offshore wind farms sites (Denmark), as well as in the reference site, which was 10 km away [67]. This reaction persisted up to the first two years of operation in Nysted, where abundance was then still lower than expected. Nevertheless, it is not certain what specifically triggered this behavior; whether it was the noise, visual presence, vessels or changes in prey availability. Harbour porpoise avoidance has been particularly related to pile-driving activities, and hearing impairment is a possible consequence at close range [66]. Furthermore, seals have been reported (in the Näsrevet offshore wind farm, Sweden) to avoid vessels passing close to haul-out sites [62], and masking may occur in harbour seals up to 80 km distance [9,60].
While in operation, offshore wind farms have been registered to produce noise peaks at 120 dB at 16 Hz, therefore exceeding ambient noise levels only at very low frequencies (<1 kHz). As baleen whales are low frequency sound producers, they are expected to be the most affected species by noise produced during offshore wind farm operation, particularly open ocean species. Such noise may interfere with communication and navigation, thus affecting baleen whale migration success [66,68]. Conversely, for bottlenose dolphins, noise at 6 kHz has to exceed ambient noise levels (~80 dB) by 22 dB while for harbour seals at 1100 Mz it should be 16 dB above ambient levels. Both of these levels are unexpected to be produced in offshore wind farms at such high frequencies [62]. Despite this, experimental results suggest that harbour porpoises and harbour seals can detect low-frequency sounds such as those produced during wind-farm operation [71], showing that research is still needed on this subject. Additionally, airborne noise of offshore wind farms operation is expected to be heard by pinnipeds, but should only be 10–20 dB above their lowest audible threshold at the base of the turbine, thus not causing great disturbance. In addition, seals commonly show habituation to noise and vibration, once no threat is perceived, thus avoidance responses are only short-termed [62].
Despite all of these potential effects of noise, it has been suggested that, for example, harbour porpoises and seals may be more tolerant to disturbance in areas of greater importance for feeding or reproduction, thus more easily abandoning areas of lower interest, without major consequences [67]. Therefore, it is of note that impacts of noise from offshore wind farms on marine mammals will depend on the low-frequency hearing abilities of the different species, on sound-propagation conditions and on ambient noise levels, either due to natural or anthropogenic sound (e.g., from shipping) [72].
Given the above effects, it is recognised that there should be controls on exploration and construction activities in known or anticipated breeding areas (particularly during breeding season) and along cetacean migration routes. The use of Acoustic Deterrent Devices (ADDs) has been suggested as a possible mitigation measure during construction operations, possibly providing an effective and less expensive method of minimising adverse effects [73]. Despite this, research is still needed on the efficacy of this and on the appropriate types of signals and measure of animals' responses to their action. Research is also required on noise emissions of offshore wind farms' related activities and the continuing of extremely important monitoring programmes in areas of implementation. For marine mammals, particular focus should be given on the breeding and/or migration success of each species or population in relation to offshore activities including wind farms as well as on cumulative impacts with other human activities.
4. Final Discussion
The present review has summarised the environmental impacts due to offshore and coastal wind energy generation. The earlier assessment and conceptual models (‘horrendograms’) produced by Elliott [4] (Figure 6) showed the potential impacts across all features of the exploration, construction, operation and decommissioning. In the intervening years, we now have a better empirical database of the impacts in areas where wind farms have been built. In general, the impacts due to wind farms are little different from any marine structure, in that the main, impacts are due to interferences with processes because of a physical structure and its construction. Even at a preliminary analysis, the effects are similar to other marine constructions in which scale, duration and extent are paramount. However, existing studies show the need for putting the effects in context and to quantify more fully the spatial extent—temporal duration of those effects. It illustrates the problems of scale which appear even more critical in the case of an activity such as offshore wind power—at the monopile, between monopiles, within the farm, and between farms levels. Furthermore, it is necessary to separate the significance of effect in various terms where we can discuss statistical significance, given the appropriate amount of data, the environmental-ecological significance and the social significance—i.e. if society thinks that these devices create a problem then that may be of sufficient concern even in the scientific evidence does not support that concern. The benthic data shown here, for example, illustrate the fact that inherent variability in an ecological component will make detection of the signal of change difficult. Each of the types of significance has a different degree of robustness and ease of detection.
The aim of the impact prevention and mitigation process is the protection of critical, fundamental marine processes [74,75,76,77]. These processes then need to be related to the monitoring requirements: for surveillance and condition monitoring, compliance monitoring and investigative/diagnostic monitoring [3]. As with other marine stressors, it is easy to require developers to undertake monitoring out of proportion to the perceived or actual effects but there needs to be a defendable and proportionate monitoring strategy. It is acknowledged that the OWP industry has suffered from an adverse media response/public perception which has been transferred from land-based schemes and we argue that while we need to have continued debate on the value of offshore wind in the context of a national energy policy, the concerns have to be kept in proportion.
We emphasise here that there is the need to separate real from perceived impacts of offshore wind farms and separate valuable monitoring of the important features from ‘helicopter surveys’ (i.e. where all aspects are studied irrespective of the chance that impacts will be detected). For example, suggestions of the need to investigate plankton changes due to OWP have been rebutted being both unnecessary and futile. This emphasises that there should be science which concentrates on the ‘need to know’ from the ‘nice to know’—i.e. the science needed to answer precise questions. But also there is the need to separate adverse impacts from benefits—for example the possibility for habitat creation (see Wilson [12]) for dual benefits (getting clean energy and increased ecological functioning) and also perhaps for a third benefit—of creating de facto no-trawl zones which can be linked to fisheries enhancement. Hence there is the need to consider not only cumulative impacts but also cumulative benefits within complex marine areas and spatial planning [78]. This shows that we should learn from mitigation measures and available experience, but also the potential for true and adventurous marine management.
Furthermore, and as discussed above, the wider EIA process urgently needs a more comprehensive body of data on the effects of wind farm operations on biological communities. To some, before and after construction effects can be assessed using surrogate information from other anthropogenic structures, but the longer-term effects of operational stages of offshore wind farms are still poorly understood. Detecting changes in community structure and function between pre-construction, operation and post-operation phases of a wind farm requires both a long time-frame sampling programme and a large sampling coverage in order to account for the natural variability in communities. Currently, for example, aerial and ship-based seabird surveys are generally undertaken using a Before After Control Impact study (BACI) in order to compare bird distribution and density within the wind farm site [79,80], both survey techniques using a distance sampling method [81]. However there may be constraints to such techniques during the operational phase of the development, and as such, remote sensing techniques are being developed to provide additional data gathering routines. Such programmes have both time and cost implications, but once a sufficient body of data have been gathered, it is likely that their scope and frequency can be reduced (e.g., for future developments).
The potential impacts may be summarized. In addition to noise effects, and as with any marine activities, sediment disturbance occurring during exploration and construction/decommissioning operations leads to high turbidity and may disperse and attenuate echolocation signals, thus disorientating the animals [60]. Moreover, alteration/loss in bottom habitats and benthic fauna will probably affect food web dynamics, species competition and predator-prey relationships, thus reducing breeding success of fish and, consequently, of marine mammals [4,10,51,60]. One of the greatest concerns in the construction offshore wind farms is the potential for piling noise from the installation of piled foundations to impact upon marine mammals, as discussed above. However, marine mammals are not the only species in the offshore environment susceptible to underwater noise. The presence of swim bladders in fin-fish makes them vulnerable to the pressure of the piling activities, which can be fatal.
There is the need to focus on robust means of defining and quantifying an impact, but within a highly dynamic and variable system; defining and detecting a defendable ‘signal to noise ratio’ and at the same time keep the methods and effort of detection of effect in proportion. For the constructional phases, experience from operational wind farms in the UK and elsewhere shows that the destruction of habitats will amount to a very small area or percentage of the total construction area of the development, and that damaged vegetation and fauna have the capacity to recover within few years of the disturbance. Hence recommendations for monitoring programmes must include endangered or protected species and ecosystem key organisms. The survey design must also take into account potential fouling organisms which may rapidly colonise the turbine towers and foundations.
In terms of the quantitative assessment of the potential impacts of an offshore wind farm, the analysis started by Hemingway et al. [82] has now been expanded to take each activity within the coastal and marine environment, identify its effects, allocate a ’score’ to these effects and perform a cluster analysis (Table 3, Figure 1). This shows that offshore wind power has similarities with other activities which involve putting a structure into the marine environment. Hence it is the presense of the structure, rather than the operation, which may be of greater significance to the ecosystem; however it must be noted that the marine system is able to adjust to new structures in the sea, and these may even have the potential to act as a benefit to their receiving environment.
As more wind farms come into operation, it is essential to obtain a greater knowledge base of what actually happens around the bases of the turbines, and how they are colonised by benthic communities. Work has shown how scour protection and towers may create hard substrata and thus act as artificial reefs, thereby increasing production on these ‘reefs’ and creating organic material as enrichment for the local marine environment. However, this potential benefit needs to be studied in greater detail, allowing it to be taken into consideration when undertaking impact assessments on the benthic community.
Nevertheless, underwater structures may produce artificial reefs, and offshore wind farm areas could be marked as "no-take" zones, thus producing potential marine conservation and biodiversity benefits [60]. As shown from the surveys undertaken, offshore wind farms may also have the capacity to act as havens for juvenile fish and shellfish, which will have the greater benefit of potentially increasing production which could spread into the surrounding area. Although fishing within a wind farm boundary is not prohibited, and it has been shown on many occasion that successful fishing can be undertaken among the turbines, many fishermen still believe they cannot fish within the wind farm. Indirectly then, the wind farm can act as a reduced-take zone, with lower numbers of fishermen using the area. Despite this, other types of fishing and even aquaculture could be practiced within the wind farm area.
It is clear that there are substantial gaps in our understanding of how offshore wind farms will impact on the local ecology. There have been some advances but we are still in a position where we cannot predict all impacts or benefits [10,21]. Given the general perception that offshore wind power and marine renewable in general may bring interesting possibilities for the restoration of coastal fish assemblages to past levels of diversity and abundance it is necessary to produce the necessary scientific evidence to support management plans and legislation (e.g., the European Marine Strategy Framework Directive). Part of the efforts should be directed to create better assessment tools such as fish indices of biotic integrity to assess change (i.e. recovery or degradation) using fish communities as ecological endpoints. For example, many methods are currently being developed in connection with the European Water Framework Directive and some have been adapted recently for use in coastal waters [83]. In the coming years, these integrated approaches will be used for offshore wind power developments.
4.1. Gaps in Knowledge and Suggestions for Further Study
As with all relatively ‘young’ industries, there are still gaps in the knowledge of offshore wind farm development, with areas of limitations including:
- detection of statistically robust changes to the ecological components and the detection of the signals from the noise, the inherent variability;
- identification of sub-lethal and behavioural effects of noise and EM fields and consequences for fish populations through effects on feeding and interference with migration routes at open sea scales;
- identification of effects on receiving ecosystems carrying capacity and its modification on, around and between introduced artificial habitats;
- the effects of fish aggregations around the structures on abundance and diversity of associated and surrounding biota;
- the effects of structural complexity of wind turbine foundations and the cascade effect on fish assemblages;
- analysis of biotic interactions and cascade effects on community structure;
- assessment of fish changes with non-invasive techniques (i.e. acoustic, video tracking);
- expected reference condition of fish assemblage integrity and identification of disturbance-sensitive species;
- landscape integration with other marine renewable installations and marine protected areas to maximize environmental benefits of creating no-take zones;
- interference to seabird and coastal bird migration patterns, feeding areas, breeding areas, roosting areas;
- collision risk for birds and bats – actual and predicted, value of models;
- types of seabirds and bats at risk, examples of mortalities or causes of deviation to migration patterns;
- perceived risk versus evidence-based conclusions for all aspects;
- analyses of scale for the different components—within and between monopiles and wind farms;
- disruption to large sea areas and the potential effects with footprints getting bigger.
5. Concluding Remarks
The science described here has to be used in managing this new industry. Within this we have to use the DPSIR approach as a philosophy to determine/quantify the effects on the natural and human systems and then look for sustainable responses [2,4]—within this we look at Drivers (such as the need for energy) leading to Pressures (such as the placement of structures) leading to State changes on the natural environment. In turn these lead to Impacts on the human system, e.g., society is prevented from using certain areas of seabed, and so we need Responses which may be social, economical, legal or technological instruments to control the adverse consequences and generate benefits of the activity. The aim then of management of such activities to allow the sustainable use of the marine space and so this requires a multidisciplinary approach, what has been called the 7-tenets as an indication of sustainable solutions to potential environmental problems—that our solutions should be environmentally/ecologically sustainable, economically viable, technologically feasible, socially desirable/tolerable, administratively achievable, legally permissible and politically expedient [3,77]. The aim has to be to maintain and protect the ecological goods and services, for the well-being of the natural system, while at the same time delivering economic goods and services for the well-being of society.
Despite the increasing number of EIA and Environmental Statements produced for new wind farm developments, as yet there is insufficient post-construction environmental auditing. Once that information is available, we can determine if all elements of the EIA were needed (i.e. was there any non-cost effective (or even wasteful) monitoring) and were the predictions of impact correct (or even in the right magnitude). As indicated here, there is still a lot of qualitative prediction but also the need for post-operation monitoring to increase our confidence in assessments. The weight of evidence presented here shows that while coastal and offshore wind farms are not totally environmentally benign, their environmental impacts may be relatively minor compared to many other marine activities. Indeed, as shown here, there are even opportunities for other environmental benefits besides the creation of green, renewable energy. However, there is still the need to put the effects in context and to more fully quantify their spatial extent—temporal duration, to address the problems of scale which are even more critical in this case—at the monopile, between monopiles, within the farm, and between farms, and to determine the significance of effects.
Therefore it is concluded that in order to gain acceptance of an industry we need to search for joint wins—for habitat loss and gain, for component loss and gain and for the use of those components e.g., in fisheries. As shown here, there is the need to consider the cumulative impacts of all marine activities and to look at pressures all together. Finally, we need a rigorous post-EIA auditing to check the accuracy of predictions and a more rigorous decision-tree approach and logical planning framework approach to determining the nature and amount of monitoring required.
Acknowledgements
This review presents the views of the authors and not necessarily those of any organizations with whom they have worked. We gratefully acknowledge the discussions of many colleagues and also the detailed comments provided by anonymous referees who have helped to improve the paper.
References
- Drewitt, A.L.; Langston, R.H.W. Assessing the impacts of wind farms on birds. IBIS 2006, 148, 29–42. [Google Scholar] [CrossRef]
- McLusky, D.S.; Elliott, M. The Estuarine Ecosystem: Ecology, Threats and Management; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Gray, J.S.; Elliott, M. Ecology of Marine Sediments: Science to Management; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Elliott, M. The role of the DPSIR approach and conceptual models in marine environmental management: An example for offshore wind power. Mar. Pollut. Bull. 2002, 44, iii–vii. [Google Scholar] [CrossRef]
- Aubry, A.; Elliott, M. The use of environmental integrative indicators to assess seabed disturbance in estuaries and coasts: Application to the Humber estuary, UK. Mar. Pollut. Bull. 2006, 53, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.C.; Elliott, M. The habitat-creation potential of offshore wind farms. Wind Energy 2009, 12, 203–212. [Google Scholar] [CrossRef]
- Linley, E.A.S.; Wilding, T.A.; Black, K.D.; Hawkins, A.J.S.; Mangi, S. Review of the Reef Effects of Offshore Wind Farm Structures and Potential for Enhancement and Mitigation; Technical Report to the Department for Business; Enterprise and Regulatory Reform (BERR) by PML Applications Ltd in association with Scottish Association of Marine Sciences (SAMS): London, UK, 2007. [Google Scholar]
- Centrica Energy. Race Bank Offshore Wind Farm—Environmental Statement. 2009. Available online: http://www.centricaenergy.com/files/pdf/RaceBank_NonTechnical_Summary.pdf (Accessed on 7 July 2010).
- Thomsen, F.; Lüdemann, K.; Kafemann, R.; Piper, W. Effects of Offshore Wind Farm Noise on Marine Mammals and Fish; Technical Report for COWRIE Ltd.: Hamburg, Germany, July 2006; p. 62. [Google Scholar]
- Gill, A.B. Offshore renewable energy: Ecological implications of generating electricity in the coastal zone. J. Appl. Ecol. 2005, 42, 605–615. [Google Scholar] [CrossRef]
- Broström, G. On the influence of large wind farms on the upper ocean circulation. J. Mar. Syst. 2008, 74, 585–591. [Google Scholar] [CrossRef]
- Wilson, J.C. Offshore wind farms: Their impacts and potential habitat gains as artificial reefs, in particular for fish. M.Sc. Dissertation, University of Hull & The Institute of Estuarine and Coastal Studies, Semptember 2007. Available on line: http://www.hull.ac.uk/iecs/pdfs/wilsonmsc2007.pdf (accessed on 14 July 2010). [Google Scholar]
- Willis, T.J.; Millar, R.B.; Babcock, R.C. Protection of exploited fish in temperate regions: High density and biomass of snapper Pagrus auratus (Sparidae) in northern New Zealand marine reserves. J. Appl. Ecol. 2003, 40, 214–227. [Google Scholar] [CrossRef]
- Pomeroy, R.S.; Watson, L.M.; Parks, J.E.; Cid, G.A. How is your MPA doing? A methodology for evaluating the management effectiveness of marine protected areas. Ocean Coastal Manage. 2005, 48, 485–502. [Google Scholar] [CrossRef]
- Fayrama, A.H.; de Risi, A. The potential compatibility of offshore wind power and fisheries: An example using bluefin tuna in the Adriatic Sea. Ocean Coastal Manage. 2007, 50, 597–605. [Google Scholar] [CrossRef]
- Horwood, J.W.; Nichols, J.M.; Milligan, S. Evaluation of closed areas for fish stock conservation. J. Appl. Ecol. 1998, 35, 893–903. [Google Scholar] [CrossRef]
- Heike, K.; Lotze, H.K.; Lenihan, B.J.; Bourque, R.H.; Bradbury, R.G.; Cooke, M.C.; Kay, S.M.; Kidwell, M.X.; Kirby, C.H.; Peterson, J.B.C. Depletion, Degradation, and Recovery Potential of Estuaries and Coastal Seas. Science 2006, 312, 1806–1809. [Google Scholar]
- Inger, R.; Attrill, M.J.; Bearhop, S.; Broderick, A.C.; Grecian, W.J.; Hodgson, D.J.; Mills, C.; Sheehan, E.; Votier, S.C.; Witt, M.J.; Godley, B.J. Marine renewable energy: Potential benefits to biodiversity? An urgent call for research. J. Appl. Ecol. 2009, 4, 1145–1153. [Google Scholar] [CrossRef]
- Wilhelmsson, D.; Saleh, A.; Yahya, S.; Őhman, M.C. Effects of high-relief structures on cold temperate fish assemblages: A field experiment. Mar. Biol. Res. 2006, 2, 136–147. [Google Scholar] [CrossRef]
- Langhamer, O.; Wilhelmsson, D. Colonisation of fish and crabs of wave energy foundations and the effects of manufactured holes—A field experiment. Mar. Environ. Res. 2009, 68, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmsson, D.; Malm, T.; Őhman, M.C. The influence of offshore windpower on demersal fish. ICES J. Mar. Sci. 2006, 63, 775–784. [Google Scholar] [CrossRef]
- Popper, A.N.; Hastings, M.C. The effects of anthropogenic sources of sound on fishes. J. Fish Biol. 2009, 75, 455–489. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.B.; Huang, Y.; Gloyne-Philips, I.; Metcalfe, J.; Quayle, V.; Spencer, J.; Wearmouth, V. EMF-Sensitive Fish Response to EM Emissions from Sub-Sea Electricity Cables of the Type Used by the Offshore Renewable Energy Industry; Technical Report for COWRIE 2.0 Electromagnetic Fields (EMF) Phase 2 (Project Reference COWRIE-EMF-1-06): London, UK, March 2009. [Google Scholar]
- Kalmijn, A.J. Electric and magnetic field detection in elasmobranch fishes. Science 1982, 218, 916–918. [Google Scholar] [CrossRef] [PubMed]
- Őhman, M.C.; Sigray, P.; Westerberg, H. Offshore windmills and the effects of electromagnetic fields on fish. Ambio 2007, 36, 631–634. [Google Scholar] [CrossRef]
- Filer, J.L.; Booker, C.G.; Sims, D.W. Effects of environment on electric field detection by small spotted catshark Scyliorhinus canicula (L.). J. Fish Biol. 2008, 72, 1450–1462. [Google Scholar] [CrossRef]
- Wilhelmsson, D.; Malm, T. Fouling assemblages on offshore wind power plants and adjacent substrata. Estuarine Coastal Shelf Sci. 2008, 79, 459–466. [Google Scholar] [CrossRef]
- Andersson, M.H.; Berggren, M.; Wilhelmsson, D.; Öhman, M.C. Epibenthic colonization of concrete and steel pilings in a cold-temperate embayment: A field experiment. Helgoland Mar. Res. 2009, 63, 249–260. [Google Scholar] [CrossRef]
- Desholm, M.; Fox, A.D.; Beasley, P.; Kahlert, J. Remote techniques for counting and estimating the number of bird-wind turbine collisions at sea: A review. In Wind, Fire and Water: Renewable Energy and Birds. Ibis 2006, 148 (Suppl. 1), 76–89. [Google Scholar] [CrossRef]
- Huppop, O.; Dierschke, J.; Exo, K.M.; Fredrich, E.; Hill, R. Bird migration studies and potential collision risk with offshore wind turbines. Ibis 2006, 148, 90–109. [Google Scholar] [CrossRef]
- Percival, S.M. Birds and wind turbines in Britain. Br. Wildl. 2000, 12, 8–15. [Google Scholar]
- Everaert, J.; Stienen, E.W.M. Impact of wind turbines on birds in Zeebrugge (Belgium). Significant effect on breeding tern colony due to collisions. Biodivers. Conserv. 2007, 16, 3345–3359. [Google Scholar] [CrossRef]
- Fernley, J.; Lowther, S.; Whitfield, P. A Review of Goose Collisions at Operating Wind Farms and Estimation of the Goose Avoidance Rate; Technical Report by Natural Research Ltd, West Coast Energy and Hyder Consulting: London, UK, 2006. [Google Scholar]
- Kikuchi, R. Adverse impacts of wind power generation on collision behaviour of birds and anti-predator behaviour of squirrels. J. Nat. Conserv. 2000, 16, 44–55. [Google Scholar] [CrossRef]
- Dirksen, S.; Van der Winden, J.; Spanns, A.L. Nocturnal collision risks of birds with wind turbines in tidal and semi offshore areas. In Proceedings of the International Workshop on Wind Energy and Landscape, Genoa, Italy, 26th June–27th June 1997.
- Sovacool, B.K. Contextualizing avian mortality: A preliminary appraisal of bird and bat fatalities from wind, fossil fuel, and nuclear electricity. Energy Policy 2009, 37, 2241–2248. [Google Scholar] [CrossRef]
- Smallwood, K.S.; Thelander, C.G. Bird mortality at the Altamont Pass Wind Resource Area: March 1998–September 2001. National Renewable Energy Laboratory. Available online: http://www.nrel.gov/docs/fy05osti/36973.pdf (accessed on 21 Jan 2010).
- Drewitt, A.L.; Langston, R.H.W. Assessing the impacts of wind farms on birds. Ibis 2006, 148, 29–42. [Google Scholar]
- Larsen, J.K.; Madsen, J. Effects of wind turbines and other physical elements on field utilization by pink footed geese (Anser brachyrynchus): A landscape perspective. Landscape Ecol. 2000, 15, 755–764. [Google Scholar] [CrossRef]
- Still, D.; Little, B.; Lawrence, S. The Effect of Wind Turbines on the Bird Population at Blyth Harbour; Report to Energy Technology Support Unit (ETSU) on Behalf of the Department of Trade and Industry: Didcot, UK, 1996; p. 34.
- Percival, S.M. Assessment of the Effects of Offshore Wind Farms on Birds; Technical Report for Department of Trade and Industry (DTI) Sustainable Energy Programmes; East Tennessee State University (ETSU): Didcot, UK, 2001. [Google Scholar]
- Langston, R.H.W.; Pullan, J.D. Windfarms and birds: an analysis of windfarms on birds, and guidance on environmental assessment criteria and site selection issues; Technical Report to the International to the Council of Europe (Bern Convention); Royal Society for Protection of Birds (RSPB)/Birdlife: Strasbourg, France, 2003. [Google Scholar]
- Desholm, M.; Kahlert, J. Avian collision risk at an offshore wind farm. Biol. Lett. 2005, 1, 296–298. [Google Scholar] [CrossRef] [PubMed]
- Kahlert, J.; Petersen, I.K.; Fox, A.D.; Desholm, M.; Clausager, I. Investigations of Birds during Construction and Operation of Nysted Offshore Wind Farm at Rødsand; Annual Status Report 2003; National Environmental Research Institute: Kalø, Denmark, 2004; p. 82. [Google Scholar]
- Larsen, J.K.; Guillemette, M. Effects of wind turbines on flight behaviour of wintering common eider: Implications for habitat use and collision risk. J. Appl. Ecol. 2007, 44, 516–522. [Google Scholar] [CrossRef]
- Fox, A.D.; Desholm, M.; Kahlert, J.; Christensen, T.K.; Krag Petersen, I.B. Information needs to support environmental impact assessment of the effects of European marine offshore wind farms on birds. Ibis 2006, 148 (Suppl. 1), 129–144. [Google Scholar] [CrossRef]
- Band, W.; Madders, M.; Whitfield, D.P. Developing field and analytical methods to assess avian collision risk at wind farms. In Birds and Wind Farms: Risk Assessment and Mitigation; de Lucas, M., Janss, G.F.E., Ferrer, M., Eds.; Quercus Editions: Madrid, Spain, 2007; pp. 259–275. [Google Scholar]
- Masden, E.A.; Haydon, D.T.; Fox, A.D.; Furness, R.W.; Bullman, R.; Desholm, M. Barriers to movement: Impacts of wind farms on migrating birds. ICES J. Mar. Sci. 2009, 66, 746–753. [Google Scholar] [CrossRef]
- Garthe, S.; Hüppop, O. Scaling possible adverse effects of marine wind farms on seabirds: Developing and applying a vulnerability index. J. Appl. Ecol. 2004, 41, 724–734. [Google Scholar] [CrossRef]
- Exo, K.-M.; Hüppop, O.; Garthe, S. Birds and offshore wind farms: A hot topic in marine ecology. Wader Study Group Bull 2003, 100, 50–53. [Google Scholar]
- Petersen, I.K.; Christensen, T.K.; Kahlert, J.; Desholm, M.; Fox, A.D. Final Results of Bird Studies at the Offshore Wind Farms at Nysted and Horns Rev, Denmark; National Environmental Research Institute Report; National Environmental Research Institute: Rønde, Denmark, 2006. [Google Scholar]
- Willis, C.K.R.; Barclay, R.M.R.; Boyles, J.G.; Brigham, R.M.; Brack, V., Jr.; Waldien, D.L.; Reichard, J. Bats are not birds and other problems with Sovacool’s (2009) analysis of animal fatalities due to electricity generation. Energy Policy. in press. [CrossRef]
- Bat Conservation Trust. Available online: http://www.bats.org.uk/ (accessed on 21 January 2010).
- Kunz, T.H.; Arnett, E.B.; Erickson, W.P.; Hoar, A.R.; Johnson, G.D.; Larkin, R.P.; Strickand, M.D.; Thresher, R.W.; Tuttle, M.D. Ecological impacts of wind development on bats: Questions, research needs, and hypotheses. Front Ecol. Environ. 2007, 5, 315–324. [Google Scholar] [CrossRef]
- Mendão, V.; Elliott, M.; Assis, C.A.; dos Santos, M.E. Towards the definition of cetacean guilds: Human pressures and habitat needs of whales and dolphins in the North-East Atlantic. Mar. Ecol. Prog. Ser. submitted.
- Gordon, J.; Tyack, P.L. Sound and cetaceans. In Marine Mammals: Biology and Conservation; Evans, P.G.H., Raga, J.A., Eds.; Klüwer Academic/Plenum Publishers: New York, NY, USA, 2001; pp. 139–196. [Google Scholar]
- Richardson, W.J.; Greene, C.R., Jr.; Malme, C.I.; Thomson, D.H. Marine Mammals and Noise; Academic Press: San Diego, CA, USA, 1995; p. 576. [Google Scholar]
- Waller, G.; Burchett, M.; Dando, M. Sealife: A Complete Guide to the Marine Environment; Pica Press: Mountfield, Sussex, UK, 1996; p. 504. [Google Scholar]
- Wartzok, D.; Ketten, D.R. Marine mammals sensory systems. In Biology of Marine Mammals; Reynolds, J.E., Rommel, S.A., Eds.; Smithsonian Institution Press: Washington, DC, USA, 1999; pp. 117–175. [Google Scholar]
- Hiscock, K.; Tyler-Walters, H.; Jones, H. High Level Environmental Screening Study for Offshore Wind Farm Developments—Marine Habitats and Species Project; Technical Report for The Department of Trade and Industry New & Renewable Energy Programme; The Marine Biological Association: Plymouth, UK, 2002; p. 156. [Google Scholar]
- Schusterman, R.J. Behavioral capabilities of seals and sea lions: A review of their hearing, visual, learning and diving skills. Psychological Rec. 1981, 31, 125–143. [Google Scholar]
- Vella, G.; Rushforth, I.; Mason, E.; Hough, A.; England, R.; Styles, P.; Holt, T.; Thorne, P. Assessment of the Effcts of Noise and Vibration from Offdhore Wind Farms on Marine Wildlife; ETSU W/13/00566/REP; University of Liverpool, Centre for Marine and Coastal Studies Environmental Research and Consultancy: Liverpool, UK, 2001; p. 107. [Google Scholar]
- NOAA Small Takes of Marine Mammals Incidental to Specified Activities; Seismic Hazards Investigation in Southern California. Available online: http://www.epa.gov/fedrgstr/EPA-SPECIES/1999/June/Day-11/e14902.htm (accessed on 19 July 2010).
- NOAA Small Takes of Marine Mammals Incidental to Specified Activities; Rim of the Pacific (RIMPAC) Antisubmarine Warfare (ASW) Exercise Training Events Within the Hawaiian Islands Operating Area (OpArea). Available online: http://www.epa.gov/fedrgstr/EPAIMPACT/2006/April/Day-24/i3831.htm (accessed on 19 July 2010).
- Henriksen, O.D.; Teilmann, J.; Carstensen, J. Effects of the Nysted Offshore Wind Farm Construction on Harbor Porpoises—The 2002 Annual Status Report for the Acoustic T-POD Monitoring Programme; National Environmental Research Institute, Ministry of the Environment: Aarhus, Denmark, 2003; p. 45.
- Parsons, E.C.M.; Clark, J.; Ross, A.; Simmonds, M.P. The Conservation of British Cetaceans: A Review of the Threats and Protection Afforded to Whales, Dolphins and Porpoises in UK Waters; Whale and Dolphin Conservation Society: Chippenham, Wiltshire, UK, 2007; p. 123. [Google Scholar]
- Teilmann, J.; Tougaard, J.; Carstensen, J. Effects from offshore wind farms on harbour porpoises in Denmark. In Proceedings of The European Cetacean Society’s 21st Annual Conference, ASCOBANS/ECS Workshop: Offshore Wind Farms and Marine Mammals: Impacts and Methodologies for Assessing Impacts, San Sebastian, Spain, 22 April–25 April, 2007; Evans, P.G.H., Ed.; European Cetacean Society: Aberdeen, UK, 2008; p. 68. [Google Scholar]
- Evans, P.G.H. Biology of cetaceans of the North-east Atlantic (in relation to Seismic Energy). In Proceedings of the Seismic and Marine Mammals Workshop, London, UK, 23 June-25 June 1998; Tasker, M.L., Weir, C., Eds.; Sea Mammal Research Unit: St Andrews, UK, 2002; pp. 1–35. [Google Scholar]
- Nedwell, J.R.; Parvin, S.J.; Edwards, B.; Workman, R.; Brooker, A.G.; Kynoch, J.E. Measurement and Interpretation of Underwater Noise During Construction and Operation of Offshore Windfarms in UK Waters; Subacoustech: London, UK, 2007; p. 80. [Google Scholar]
- Kannen, A. The need for integrated assessment of large-scale offshore wind farm development. In Managing European Coasts: Past, Present, and Future; Vermaat, J., Bouwer, L., Turner, K., Salomons, W., Eds.; Springer: New York, NY, USA, 2004; pp. 365–378. [Google Scholar]
- Koschinski, S.; Culik, B.M.; Henriksen, O.D.; Tregenza, N.; Ellis, G.; Jansen, C.; Kathe, G. Behavioural reactions of free-ranging porpoises and seals to the noise of a simulated 2 MW windpower generator. Mar. Ecol. Prog. Ser. 2003, 265, 263–273. [Google Scholar] [CrossRef]
- Madsen, P.T.; Wahlberg, M.; Tougaard, J.; Lucke, K.; Tyack, P. Wind turbine underwater noise and marine mammals: Implications of current knowledge and data needs. Mar. Ecol. Prog. Ser. 2006, 309, 279–295. [Google Scholar] [CrossRef]
- Gordon, J.; Thompson, D.; Gillespie, D.; Lonergan, M.; Calderan, S.; Jaffey, B.; Todd, V. Assessment of the Potential for Acoustic Deterrents to Mitigate the Impact on Marine Mammals of Underwater Noise Arising from the Construction of Offshore Windfarms; COWRIE Ltd: London, UK, 2007; p. 71. [Google Scholar]
- Elliott, M.; Burdon, D.; Hemingway, K.L. Marine Ecosystem Structure, Functioning, Health and Management and Potential Approaches to Marine Ecosystem Recovery: A Synthesis of Current Understanding; Unpublished Report: YBB092-F-2006 for the Countryside Council of Wales by the Institute of Estuarine and Coastal Studies; University of Hull: Hull, UK; p. 102.
- Elliott, M.; Burdon, D.; Hemingway, K.L.; Apitz, S. Estuarine, Coastal and Marine Ecosystem Restoration: Confusing management and science—A revision of concepts. Estuarine Coastal Shelf Sci. 2007, 74, 349–366. [Google Scholar] [CrossRef]
- Borja, A.; Elliott, M. What does ‘Good Ecological Potential’ mean, within the European Water Framework Directive? Mar. Pollut. Bull. 2007, 54, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Mee, L.D.; Jefferson, R.L.; Laffoley, Dd’A.; Elliott, M. How good is good? Human values and Europe’s proposed Marine Strategy Directive. Mar. Pollut. Bull. 2008, 56, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Boyes, S.J.; Elliott, M.; Thomson, S.M.; Atkins, S.; Gilliland, P. A proposed multiple-use zoning scheme for the Irish Sea. An Interpretation of current legislation through the use of GIS-based zoning approaches and effectiveness for the protection of nature conservation interests. Mar. Policy 2007, 31, 287–298. [Google Scholar]
- Camphuysen, C.J.; Fox, T.J.; Leopold, M.F.; Petersen, I.K. Towards Standardised Seabirds at Sea Census Techniques in Connection with Environmental Impact Assessments for Offshore Wind Farms in the UK; Technical Report for Royal Netherlands Institute for Sea Research; COWRIE Ltd.: London, UK, 2003. [Google Scholar]
- Maclean, I.M.D.; Wright, L.J.; Showler, D.A.; Rehfish, M.M. A Review of Assessment Methodologies for Offshore Windfarms; British Trust for Ornithology Report Commissioned by COWRIE: London, UK, May 2009. [Google Scholar]
- Buckland, S.T.; Anderson, D.R.; Burnham, K.P.; Laake, J.L.; Borchers, D.L.; Thomas, L. Introduction to Distance Sampling: Estimating Abundance of Biological Populations, 2nd ed.; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Hemingway, K.L.; Cutts, N.D.; Boyes, S.; Allen, J.; Elliott, M.; Travers, S. Marine Species Protection: A Review of Risk and Considerations for Improvement; Defra Living Land and Seas Science Division: London, UK, 2006. Available online: http://www.defra.gov.uk/wildlifecountryside/resprog/findings/marine-spec/cr0354-species-protection.pdf (accessed on 9 July 2010).
- Henriques, S.; Pais, M.P.; Costa, M.J.; Cabral, H. Efficacy of adapted estuarine fish-based multimetric indices as tools for evaluating ecological status of the marine environment. Mar. Pollut. Bull. 2008, 56, 1696–1713. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.J.; Santiago, J.A.; Santana-Ortega, A.T. A general theory on fish aggregation to floating objects: An alternative to the meeting point hypothesis. Rev. Fish Biol. Fish. 2002, 11, 255–277. [Google Scholar] [CrossRef]
- Popper, A.N.; Fewtrell, J.; Smith, M.E.; McCauley, R.D. Anthropogenic sound: Effects on the behavior and physiology of fishes. Mar. Technol. Soc. J. 2004, 37, 35–40. [Google Scholar] [CrossRef]
- Mueller-Blenkle, C.; McGregor, P.K.; Gill, A.B.; Andersson, M.H.; Metcalfe, J.; Bendall, V.; Sigray, P.; Wood, D.T.; Thomsen, F. Effects of Pile-Driving Noise on the Behaviour of Marine Fish; COWRIE: London, UK, 2010. [Google Scholar]
- Andersson, M.H.; Gullstrom, M.; Asplund, M.E.; Ohman, M.C. Importance of Using Multiple Sampling Methodologies for Estimating of Fish Community Composition in Offshore Wind Power Construction Areas of the Baltic Sea. Ambio 2007, 36, 634–636. [Google Scholar] [PubMed]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).