Gasification of Biochar from Empty Fruit Bunch in a Fluidized Bed Reactor
Abstract
:1. Introduction
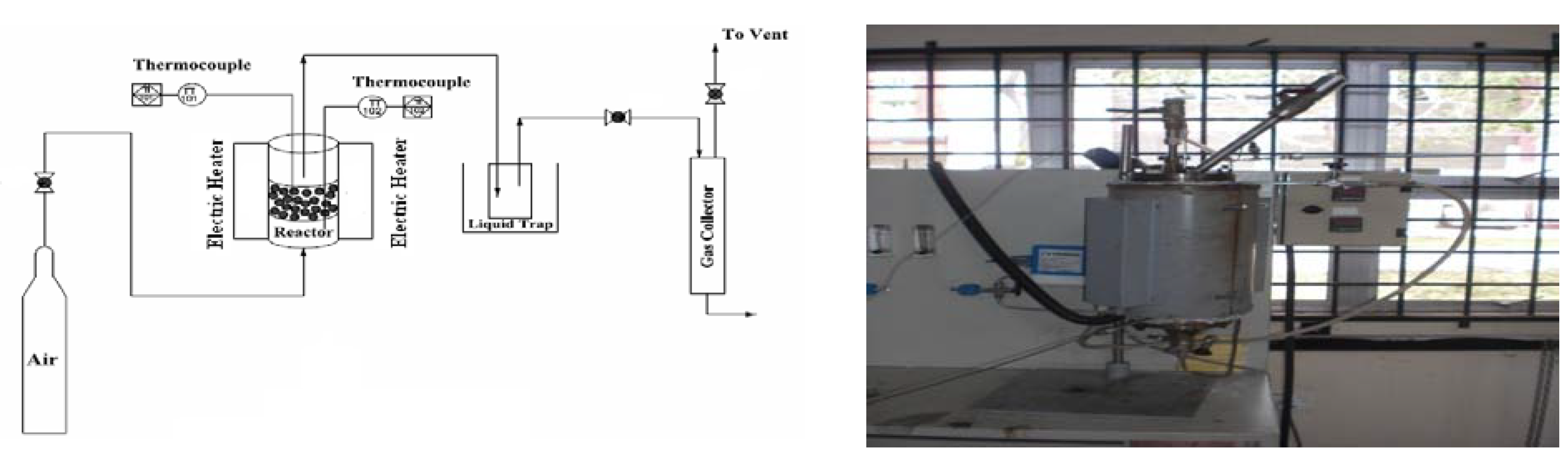
2. Methodology
2.1. Material and Methods
2.2. Gasification
| Size = 500 μm | Temp (°C) | Volume (L) | Ash (grams) | |||
|---|---|---|---|---|---|---|
| Original mass (grams) | H2 | C0 | C02 | CH4 | ||
| 50.00 | 500 | 1.992 | 0.620 | 0.997 | 0.144 | 12.00 |
| 58.00 | 600 | 4.272 | 0.650 | 0.914 | 0.603 | 11.77 |
| 50.00 | 700 | 5.40 | 0.615 | 0.873 | 0.900 | 9.55 |
| 50.00 | 750 | 6.14 | 0.564 | 0.830 | 1.604 | 8.45 |
| 51.11 | 800 | 9.52 | 0.984 | 0.711 | 1.794 | 8.45 |
| 50.00 | 850 | 10.07 | 1.000 | 0.698 | 1.680 | 7.95 |
3. Results and Discussion
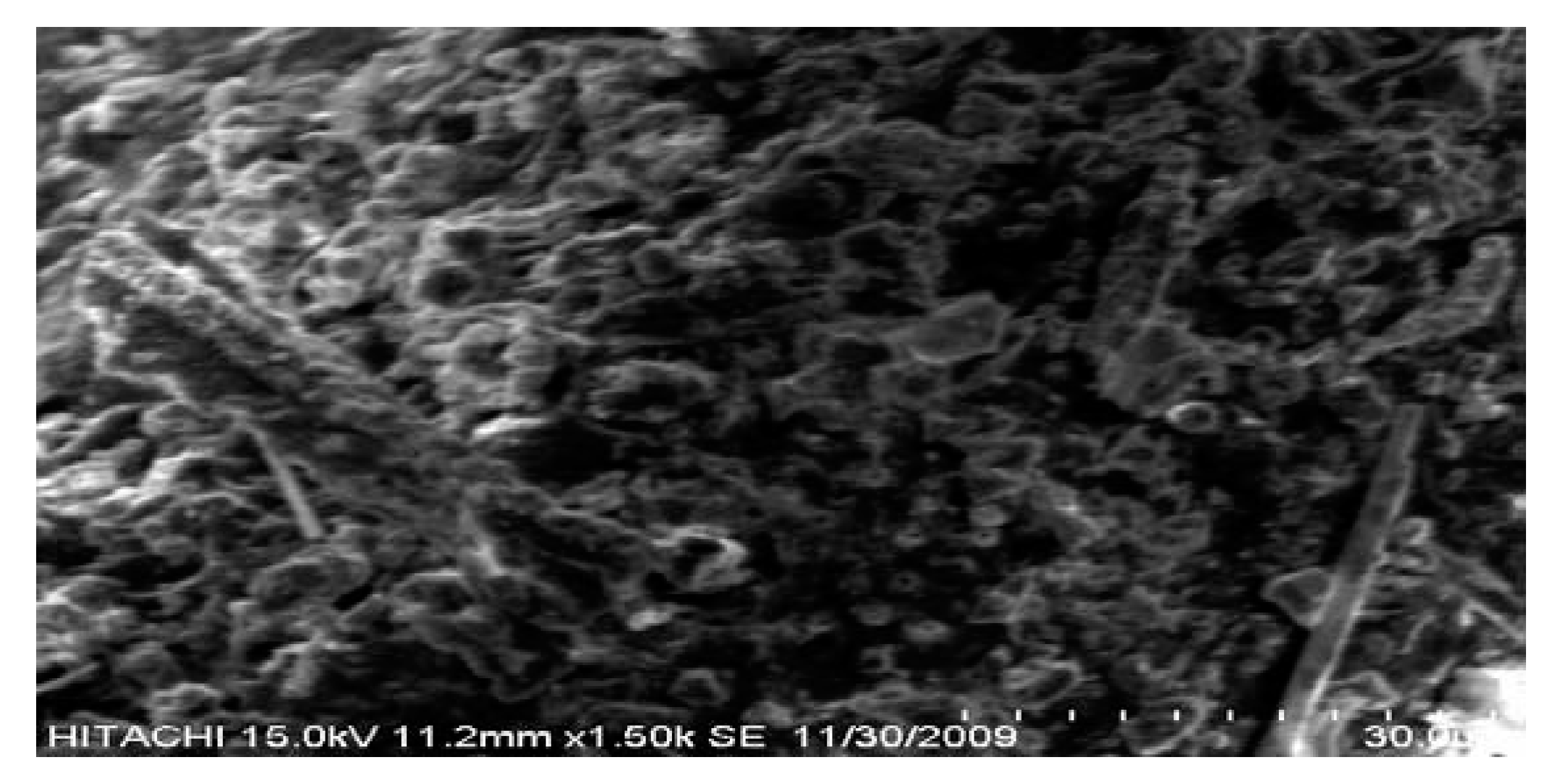
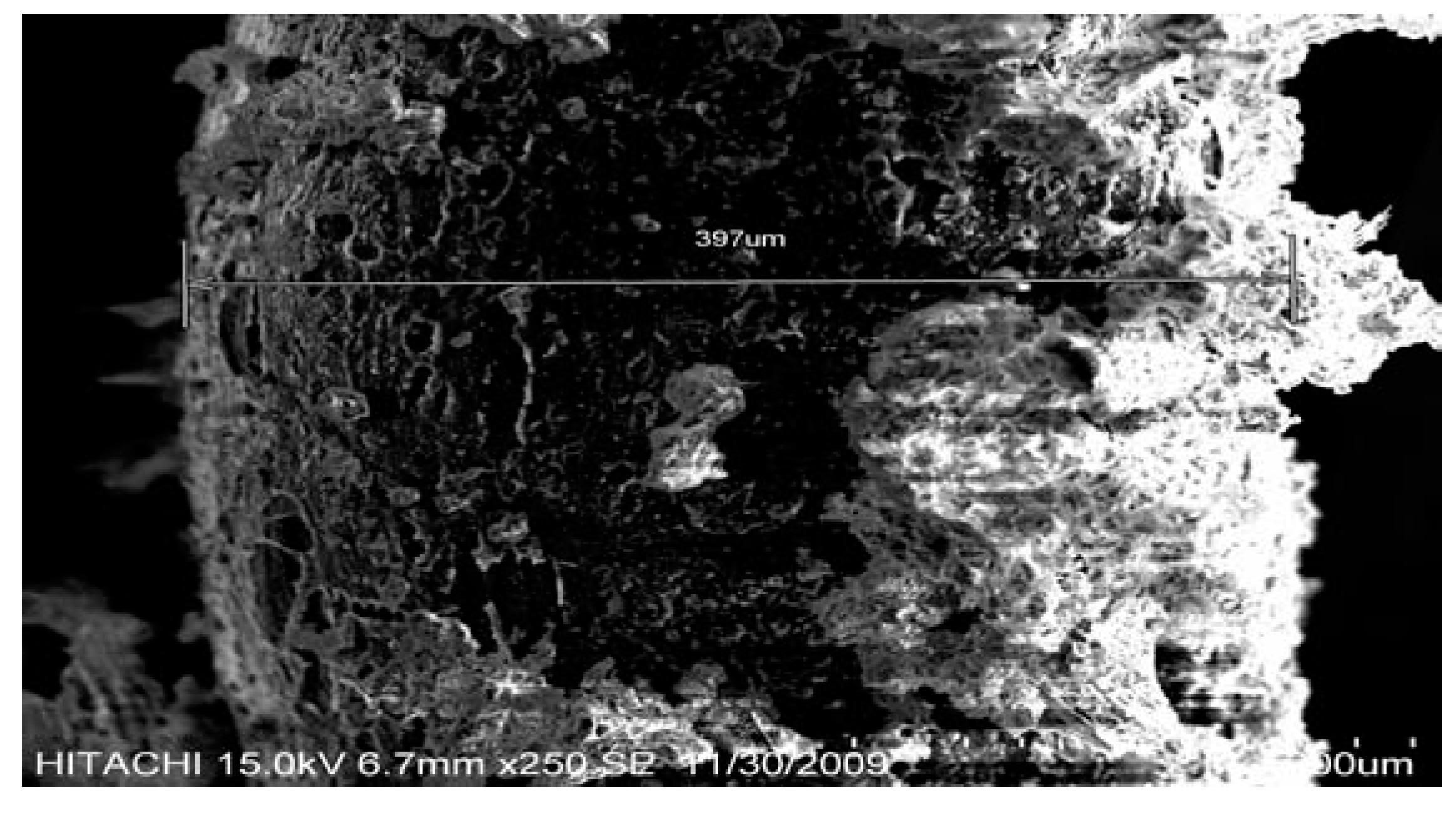
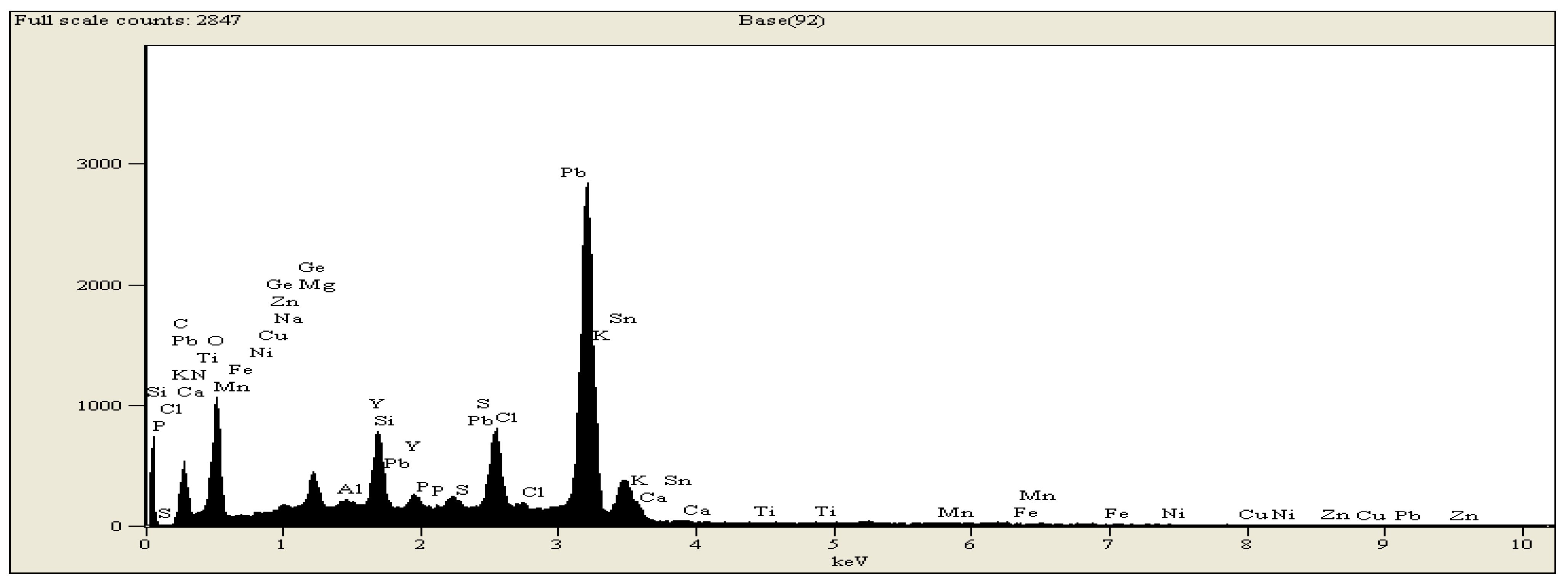
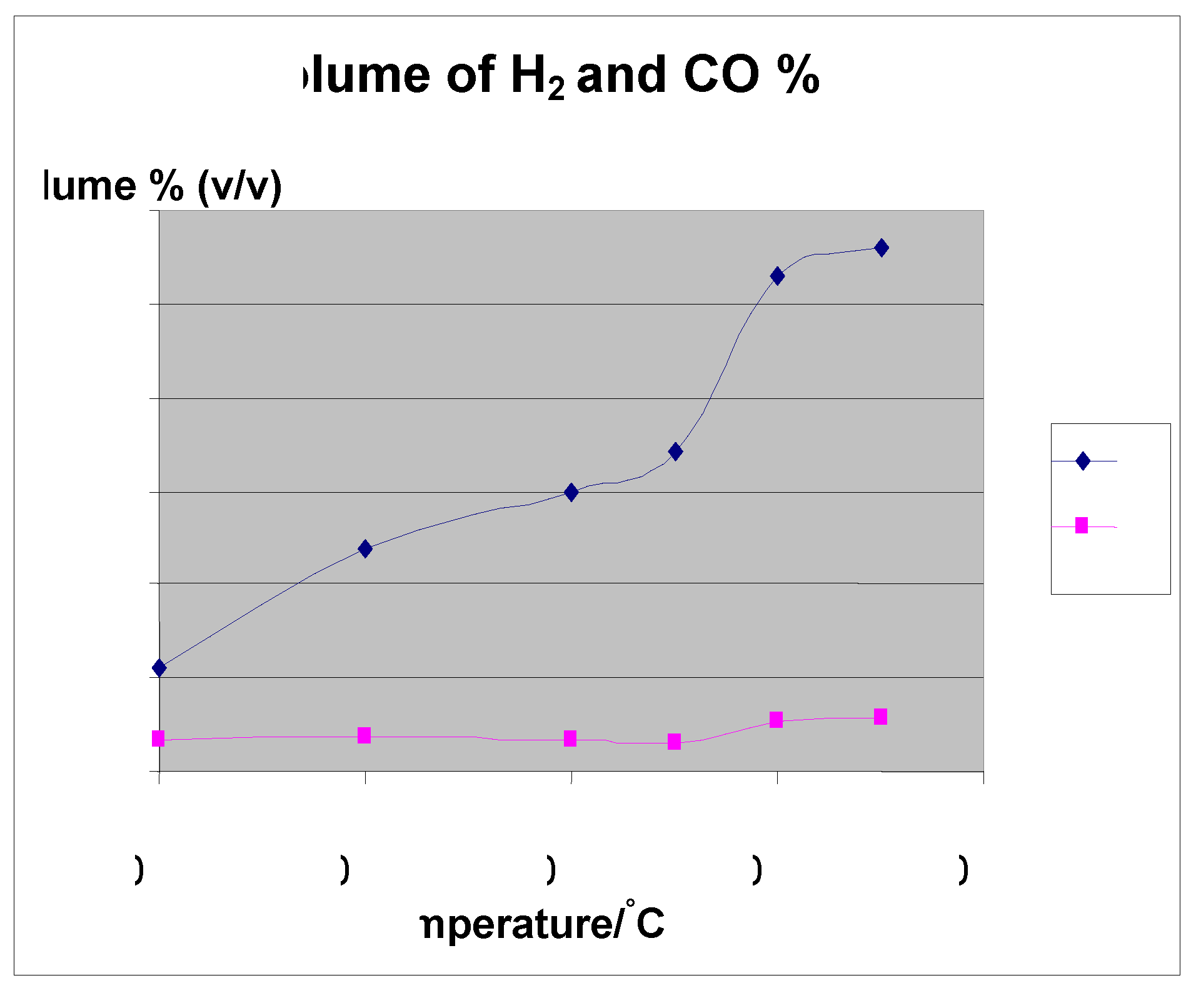
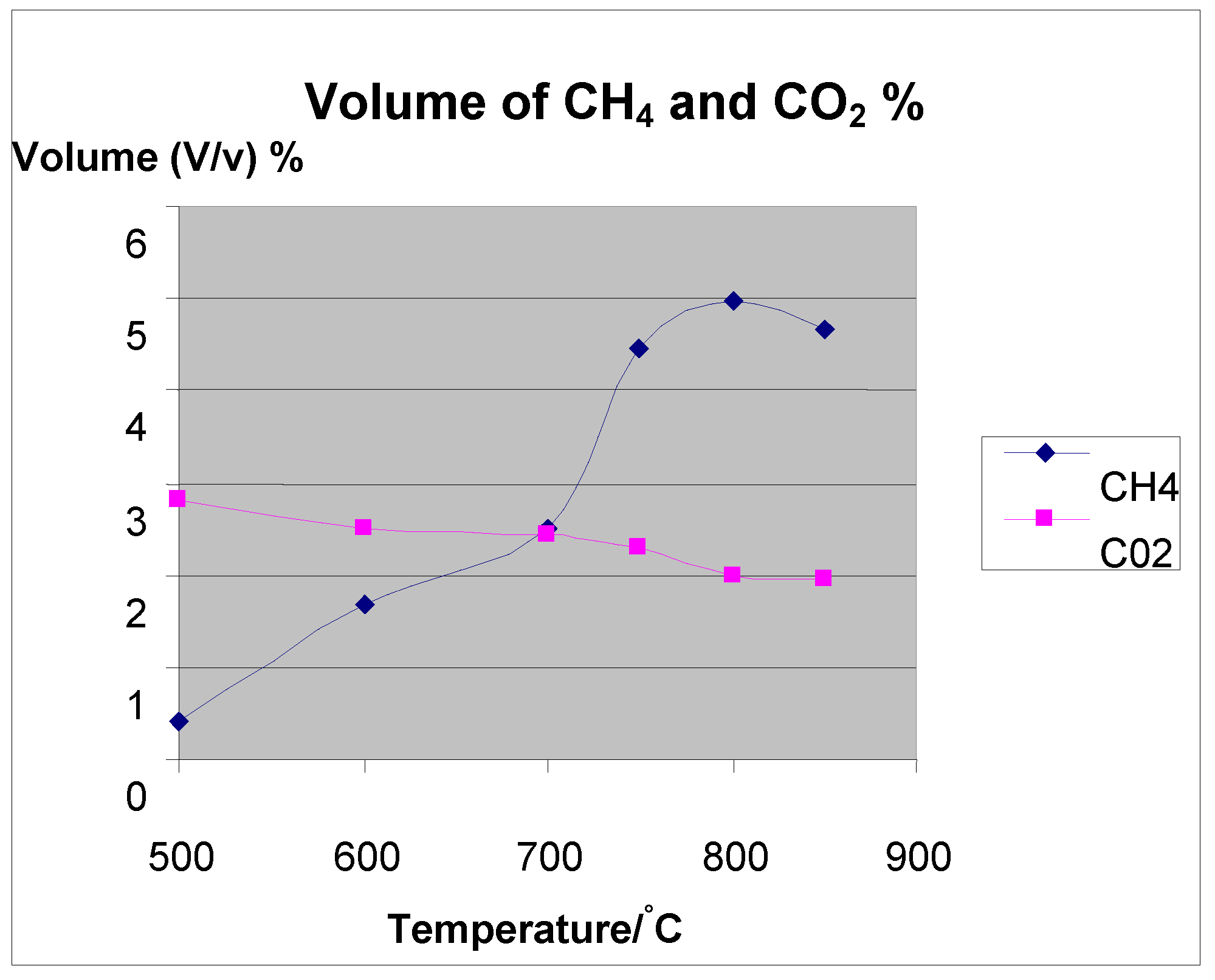
| Size = 500 μm, flowrate = 5 L/min | Temp (°C) | Volume in ml/gram | Conversion % | |||
|---|---|---|---|---|---|---|
| Original mass (grams) | H2/g | CO/g | CO2/g | CH4/g | ||
| 50.00 | 500 | 39.84 | 12.4 | 19.94 | 2.88 | 76.00 |
| 58.00 | 600 | 73.65 | 11.18 | 15.75 | 10.38 | 79.77 |
| 50.00 | 700 | 108 | 12.3 | 17.45 | 18 | 80.90 |
| 50.00 | 750 | 111.63 | 10.25 | 15.09 | 29.17 | 82.00 |
| 51.11 | 800 | 173.1 | 17.89 | 12.93 | 32.6 | 83.00 |
| 50.00 | 850 | 201.4 | 20 | 13.95 | 33.6 | 84.00 |
4. Effect of Temperature
5. Carbon Conversion Efficiency and HHV
6. Conclusions
Acknowledgments
References
- Malaysian Palm Oil Industry Performance 2008. Global Oils & Fats Business Magazine. 2009, Volume 6. Available online: http://www.mpoc.org.my/gofbm_download.aspx?id=ff6cb4c8-9863-49b2-ae12-ee5e2d5d1f0a (accessed on 30 May 2010).
- Choo, Y.M.; Ngan, M.; Chan, K.W.; Basiron, Y. An option for greenhouse gas mitigation in the energy sector. J. Oil Palm Res. 2005, 17, 47–52. [Google Scholar]
- CO2 Emissions from Fuel Combustion 1971-2000, 6th ed.; International Energy Agency, OECD: Paris, France, 2002; p. 105.
- World Growth Palm Oil Green Development Campaign. Palm Oil—The Sustainable Oil A Report by World Growth. Available online: http://www.worldgrowth.org/assets/files/Palm_Oil.pdf (accessed on 30 May 2010).
- Sahabat Empty Fruit Bunch Biomass Project. Available online: http://cdm.unfccc.int/UserManagement/FileStorage (accessed on 30 May 2010).
- Rao, T.R.; Ashish, B.; Bheemarasetti, J.V.R. Kinetics of rice husk char gasification. Energy Convers. Manag. 2001, 42, 2061–2069. [Google Scholar] [CrossRef]
- Ocampo, A.; Arenas, E.; Chejne, F.; Espinela, J.; Londonˆo, C.; Aguirrea, J.; Perez, J.D. An experimental study on gasification of Colombian coal in fluidized bed. Fuel 2003, 82, 161–164. [Google Scholar] [CrossRef]
- Bayarsaikhan, B.; Sonoyama, N.; Hosokai, S. Inhibition of steam gasification of char by volatiles in a fluidized bed under continuous feeding of a brown coal. Fuel 2006, 85, 340–349. [Google Scholar] [CrossRef]
- Chatterjee, P.K.; Datta, A.B.; Kundu, K.M. Fluidized bed gasification of coal. Can. J. Chem. Eng. 1995, 73, 204–210. [Google Scholar] [CrossRef]
- Huang, J.; Fang, Y.; Chen, H.; Wang, Y. Coal gasification characteristic in a pressurized fluidized bed. Energy Fuels 2003, 17, 1474–1479. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, J.M.; Kim, S.D. Coal gasification characteristics in an internally circulating fluidized bed with draught tube. Fuel 1997, 76, 1067–1073. [Google Scholar] [CrossRef]
- Kikuchi, K.; Suzuki, A.; Mochizuki, T.; Endo, S.; Imai, E. Ash agglomerating gasification of coal in a spouted bed reactor. Fuel 1985, 64, 368–372. [Google Scholar] [CrossRef]
- Gutierrez, L.A.; Watkinson, P.A. Fluidized-bed gasification of some Western Canadian coals. Fuel 1982, 61, 133–138. [Google Scholar] [CrossRef]
- Crnomarkovic, N.; Repic, B.; Mladenovic, R.; Neskovic, O.; Veljkovic, M. Experimental investigation of role of steam in entrained flow coal gasification. Fuel 2007, 86, 194–202. [Google Scholar] [CrossRef]
- Cousins, A.; Paterson, N.; Dugwell, D.R.; Kandiyoti, R. An investigation of the reactivity of chars formed in fluidized bed gasifiers: The effect of reactionconditions and particle size on coal char reactivity. Energy Fuels 2006, 20, 2489–2497. [Google Scholar] [CrossRef]
- Liu, H.; Kaneko, M.; Luo, C.H.; Kato, S.; Kojima, T. Effect of pyrolysis time on the gasification reactivity of char with CO2 at elevated temperatures. Fuel 2004, 83, 1055–1061. [Google Scholar] [CrossRef]
- Guo, X.; Tay, H.L.; Zhang, S.; Li, C.Z. Changes in char structure during the gasification of a Victorian brown coal in steam and oxygen at 800 °C. Energy Fuels 2008, 22, 4034–4038. [Google Scholar] [CrossRef]
- Li, X.T.; Grace, J.R.; Lima, C.J. Biomass gasification in a circulating fluidized bed. Biomass Bioenergy 2004, 26, 171–193. [Google Scholar] [CrossRef]
- Encinar, J.M.; Gonza´lez, J.F.; Gonza´lez, J. Fixed-bed pyrolysis of Cynara cardunculus L. Product yields and compositions. Fuel Process. Technol. 2000, 68, 209–222. [Google Scholar] [CrossRef]
- Encinar, J.M.; Gonza´lez, J.F.; Gonza´lez, J. Steam gasification of Cynara cardunculus L. Influence of variables. Fuel Process. Technol. 2002, 75, 27–43. [Google Scholar] [CrossRef]
- Dyk, J.C.; Keyser, M.J.; Coertzen, M. Syngas production from South African coal sources using Sasol-Lurgi gasifiers. Int. J. Coal Geol. 2006, 65, 243–253. [Google Scholar] [CrossRef]
- Boon, J.J. Cellulose char structure: A combined analytical Py-GC-MS, FTIR, and NMR study. Carbohydr. Res. 1994, 262, 27. [Google Scholar] [CrossRef]
- Annual Book of ASTM Standards; American Society of Testing Materials: Philadelphia, PA, USA, 1993; Volume 1994, pp. D3172–D3189, Section 5.
- Devi, T.G.; Kannan, M.P. Gasification of biomass chars in air—Effect of heat treatment temperature. Energy Fuels 2000, 14, 127–130. [Google Scholar] [CrossRef]
- Mansary, K.G.; Ghaly, A.E.; Al-Taweel, A.M.; hamdullahpur, F.; Ugursal, V.I. Air gasification of rice husk in a dual distributor type fluidized bed gasifier. Biomass Bioenergy 1999, 4, 315–332. [Google Scholar] [CrossRef]
- Dalai, A.K.; Chaudhari, S.; Bakhshi, N.N. Production of hydrogen and/or syngas (H2 + CO) via steam gasification of biomass-derived chars. Energy Fuels 2003, 17, 1062–1067. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mohd Salleh, M.A.; Kisiki, N.H.; Yusuf, H.M.; Ab Karim Ghani, W.A.W. Gasification of Biochar from Empty Fruit Bunch in a Fluidized Bed Reactor. Energies 2010, 3, 1344-1352. https://doi.org/10.3390/en3071344
Mohd Salleh MA, Kisiki NH, Yusuf HM, Ab Karim Ghani WAW. Gasification of Biochar from Empty Fruit Bunch in a Fluidized Bed Reactor. Energies. 2010; 3(7):1344-1352. https://doi.org/10.3390/en3071344
Chicago/Turabian StyleMohd Salleh, M. A., Nsamba Hussein Kisiki, H. M. Yusuf, and W. A. Wan Ab Karim Ghani. 2010. "Gasification of Biochar from Empty Fruit Bunch in a Fluidized Bed Reactor" Energies 3, no. 7: 1344-1352. https://doi.org/10.3390/en3071344
APA StyleMohd Salleh, M. A., Kisiki, N. H., Yusuf, H. M., & Ab Karim Ghani, W. A. W. (2010). Gasification of Biochar from Empty Fruit Bunch in a Fluidized Bed Reactor. Energies, 3(7), 1344-1352. https://doi.org/10.3390/en3071344




