Energy Potential of Zea mays Grown in Cadmium-Contaminated Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. The Scope of the Study
2.2. Vegetation Experiment
2.3. Laboratory Analysis Methodology
2.4. Preparation of Results
- The heating value of the dry matter of shoots and leaves of Zea mays is expressed as Hv (MJ kg−1) and is calculated as follows: × 0.0244, where Q—heat of combustion of the dry matter of shoots and leaves of Zea mays; MC—moisture content of the biomass (%); and 0.0244—correction coefficient for the enthalpy of water evaporation (MJ kg−1).
- The energy yield of the dry matter of shoots and leaves of Zea mays is expressed as YEP (kJ) and is calculated as follows: YEP = Hv × Y, where Hv—the heating value of the dry matter of the shoots and leaves of Zea mays (MJ kg−1); and Y—the dry matter of the shoots and leaves of Zea mays produced from 1 kg of soil.
- Cadmium uptake by Zea mays (U) is expressed as the sum of the uptake by the leaves (UA) and by the roots (UR) of the plants: U = UA + UR, where UA = YA × CdA and UR = YR × CdR. Cadmium uptake by Zea mays expressed in μg Cd per pot. YA indicates shoots and leaves yield; YR—root yield; CdA—cadmium content in shoots and leaves of Zm; and CdR—cadmium content in the roots of Zm.
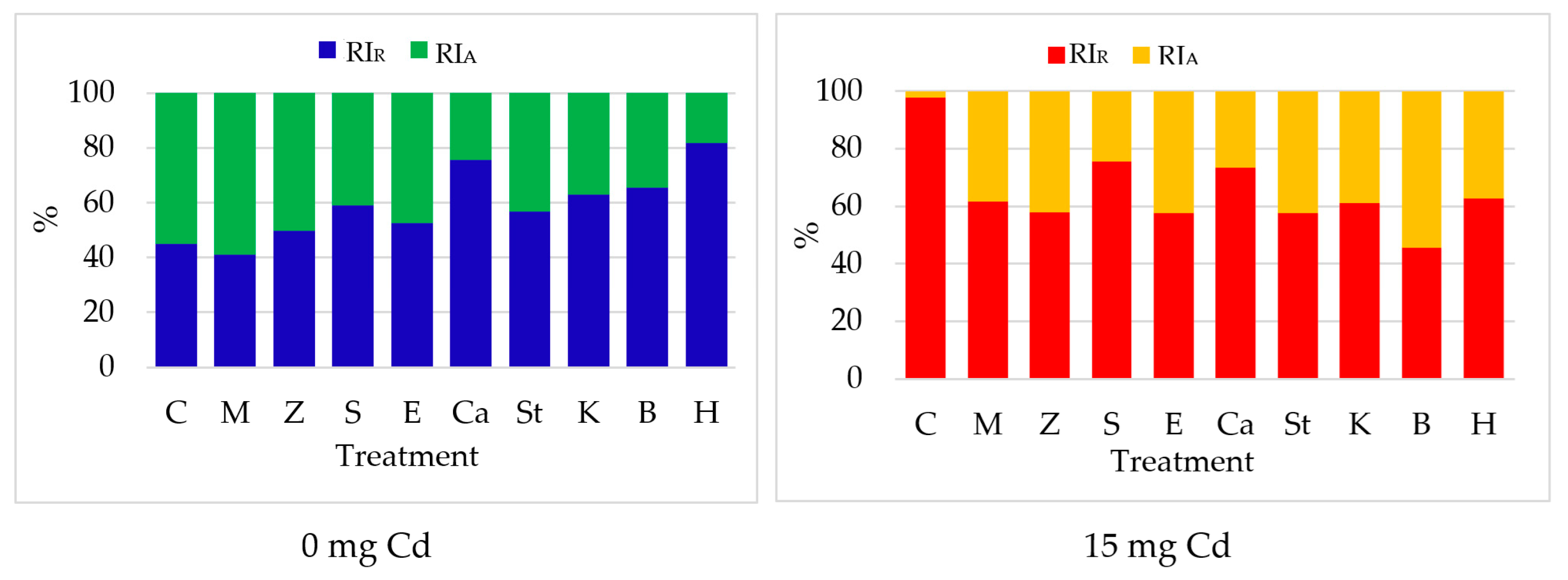
- The indices of cadmium uptake in shoots and leaves (RIA) and in roots (RIR) are calculated according to the formulas: %; %.
- The cadmium bioaccumulation index in shoots and leaves (BAFA) and in roots (BAFR) of Zea mays was calculated according to the following equation: and , where CdA denotes the cadmium content of the shoots and leaves of Zm; CdR denotes the cadmium content of the roots of Zm; and CdS is the cadmium content of the soil, of course, in comparable units.
3. Results
3.1. Impact of Cadmium on Zea mays Biomass
3.1.1. Yield and SPAD
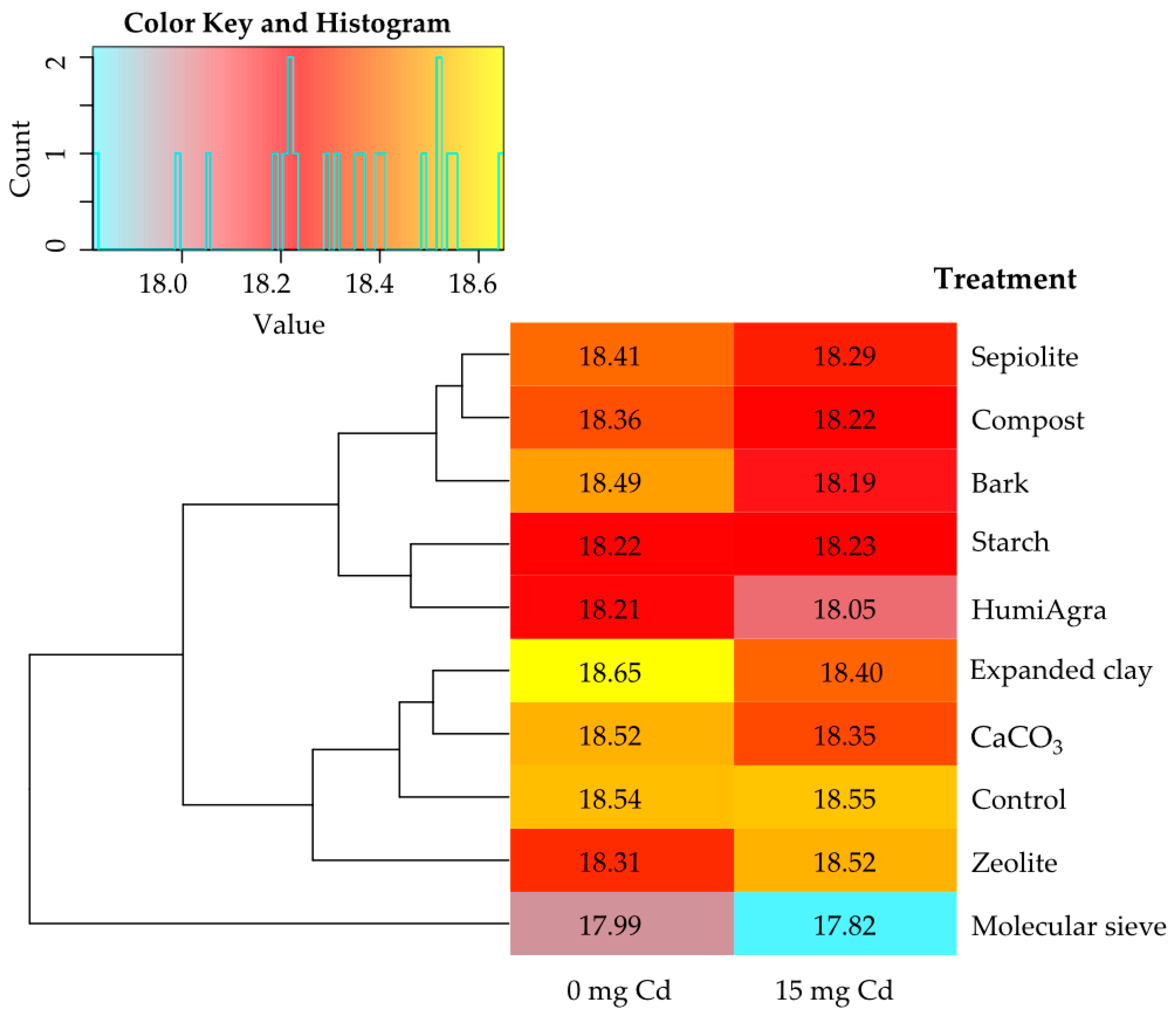
3.1.2. The Energy Value of Zea mays
3.1.3. Cadmium Content of Zea mays
3.1.4. Interdependencies Between Quantitative Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Saleem, M. Possibility of Utilizing Agriculture Biomass as a Renewable and Sustainable Future Energy Source. Heliyon 2022, 8, e08905. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, T.; Islam, T.; Nandi, A.; Anik, M.A.A.M.; Hossain, M.S.; Hasan, M.K.; Hossain, M.S. Biomass-Derived Carbon Materials for Sustainable Energy Applications: A Comprehensive Review. Sustain. Energy Fuels 2025, 9, 693–723. [Google Scholar] [CrossRef]
- Nurmustaqimah; Jamilatun, S.; Rahayu, A.; Hakika, D.C.; Muthadin, A.S.; Taufiqurahman, M.A. Heavy Metal Phytoremediation: Plant Hyperaccumulators and Clean Strategies for the Environment. Indones. J. Chem. Eng. 2024, 2, 9–21. [Google Scholar] [CrossRef]
- Ren, C.; Xiao, J.-H.; Li, J.-T.; Du, Q.-Q.; Zhu, L.-W.; Wang, H.; Zhu, R.-Z.; Zhao, H.-Y. Accumulation and Transport Characteristics of Cd, Pb, Zn, and As in Different Maize Varieties. Huan Jing Ke Xue 2022, 43, 4232–4252. [Google Scholar] [CrossRef]
- Devi, P.; Kaur, A.; Kumar, P. Chapter 15—Phytoremediation of Metal Contaminated Soil Using Energy Crops: Soil Health Maintenance along with Biofuel Production. In Bioremediation of Emerging Contaminants from Soils; Kumar, P., Srivastav, A.L., Chaudhary, V., van Hullebusch, E.D., Busquets, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 307–333. ISBN 978-0-443-13993-2. [Google Scholar]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. Calorific Value of Zea mays Biomass Derived from Soil Contaminated with Chromium (VI) Disrupting the Soil’s Biochemical Properties. Energies 2023, 16, 3788. [Google Scholar] [CrossRef]
- Khan, A.H.A.; Kiyani, A.; Santiago-Herrera, M.; Ibáñez, J.; Yousaf, S.; Iqbal, M.; Martel-Martín, S.; Barros, R. Sustainability of Phytoremediation: Post-Harvest Stratagems and Economic Opportunities for the Produced Metals Contaminated Biomass. J. Environ. Manag. 2023, 326, 116700. [Google Scholar] [CrossRef] [PubMed]
- Mocek-Płóciniak, A.; Mencel, J.; Zakrzewski, W.; Roszkowski, S. Phytoremediation as an Effective Remedy for Removing Trace Elements from Ecosystems. Plants 2023, 12, 1653. [Google Scholar] [CrossRef]
- Panoutsou, C.; Giarola, S.; Ibrahim, D.; Verzandvoort, S.; Elbersen, B.; Sandford, C.; Malins, C.; Politi, M.; Vourliotakis, G.; Zita, V.E.; et al. Opportunities for Low Indirect Land Use Biomass for Biofuels in Europe. Appl. Sci. 2022, 12, 4623. [Google Scholar] [CrossRef]
- European Union Directive (EU) 2023/2413 of the European Parliament and of the Council of 18 October 2023 Amending Directive (EU) 2018/2001, Regulation (EU) 2018/1999 and Directive 98/70/EC as Regards the Promotion of Energy from Renewable Sources, and Repealing Council Directive (EU) 2015/652. Available online: https://eur-lex.europa.eu/eli/dir/2023/2413/oj/eng (accessed on 19 March 2025).
- Sumfleth, B.; Majer, S.; Thrän, D. A Review of Trade-Offs in Low ILUC-Risk Certification for Biofuels—Towards an Integrated Assessment Framework. Sustainability 2023, 15, 16303. [Google Scholar] [CrossRef]
- Sandford, C.; Malins, C.; Vourliotakis, G.; Panoutsou, C. ‘Low ILUC-Risk’ as a Sustainability Standard for Biofuels in the EU. Energies 2024, 17, 2365. [Google Scholar] [CrossRef]
- Meers, E.; Van Slycken, S.; Adriaensen, K.; Ruttens, A.; Vangronsveld, J.; Du Laing, G.; Witters, N.; Thewys, T.; Tack, F.M.G. The Use of Bio-Energy Crops (Zea mays) for ‘Phytoattenuation’ of Heavy Metals on Moderately Contaminated Soils: A Field Experiment. Chemosphere 2010, 78, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Varalakshmi, S.; Singh, N.K.; Pareek, N.; Senthilkumar, V. Assessing Potential of Teosinte in Diversification of Maize Germplasm for Kernel Protein. Genet. Resour. Crop Evol. 2025, 72, 1013–1026. [Google Scholar] [CrossRef]
- Syarifuddin, S.; Suryani, S.; Tahir, D. Global Advances and Innovations in Bacteria-Based Biosorption for Heavy Metal Remediation: A Bibliometric and Analytical Perspective. Integr. Environ. Assess. Manag. 2025, 21, 507–525. [Google Scholar] [CrossRef]
- Directorate-General for Internal Market, Industry, Entrepreneurship and SMEs (European Commission); Grohol, M.; Veeh, C. Study on the Critical Raw Materials for the EU 2023: Final Report; Publications Office of the European Union: Luxembourg, 2023; ISBN 978-92-68-00414-2. [Google Scholar]
- da Silva, D.B.; Pianovski, M.A.D.; Filho, N.P.d.C. Environmental Pollution and Cancer. J. Pediatr. 2025, 101, S18–S26. [Google Scholar] [CrossRef]
- Sattar, S.; Yahya, M.; Aslam, S.; Hussain, R.; Shah, S.M.M.; Rauf, Z.; Zamir, A.; Ullah, R.; Shahzad, A. Environmental Occurrence, Hazards, and Remediation Strategies for the Removal of Cadmium from the Polluted Environment. Results Eng. 2025, 25, 104322. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E. Trophic Transfer, Bioaccumulation, and Biomagnification of Non-Essential Hazardous Heavy Metals and Metalloids in Food Chains/Webs—Concepts and Implications for Wildlife and Human Health. Hum. Ecol. Risk Assess. 2019, 25, 1353–1376. [Google Scholar] [CrossRef]
- Bouida, L.; Rafatullah, M.; Kerrouche, A.; Qutob, M.; Alosaimi, A.M.; Alorfi, H.S.; Hussein, M.A. A Review on Cadmium and Lead Contamination: Sources, Fate, Mechanism, Health Effects and Remediation Methods. Water 2022, 14, 3432. [Google Scholar] [CrossRef]
- Wild, C.; Weiderpass, E.; Stewart, B. World Cancer Report: Cancer Research for Cancer Prevention; IARC: Lyon, France, 2020; ISBN 978-92-832-0447-3. [Google Scholar]
- Bhattacharya, S. Protective Role of the Essential Trace Elements in the Obviation of Cadmium Toxicity: Glimpses of Mechanisms. Biol. Trace Elem. Res. 2022, 200, 2239–2246. [Google Scholar] [CrossRef]
- Wang, H.; Gan, X.; Tang, Y. Mechanisms of Heavy Metal Cadmium (Cd)-Induced Malignancy. Biol. Trace Elem. Res. 2025, 203, 608–623. [Google Scholar] [CrossRef]
- Rasin, P.; Ashwathi, A.V.; Basheer, S.M.; Haribabu, J.; Santibanez, J.F.; Garrote, C.A.; Arulraj, A.; Mangalaraja, R.V. Exposure to Cadmium and Its Impacts on Human Health: A Short Review. J. Hazard. Mater. Adv. 2025, 17, 100608. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Z.; Song, W.; Hong, D.; Huang, L.; Li, Y. A Review on Cadmium Exposure in the Population and Intervention Strategies Against Cadmium Toxicity. Bull. Environ. Contam. Toxicol. 2021, 106, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Zheng, W. Cadmium Exposure: Mechanisms and Pathways of Toxicity and Implications for Human Health. Toxics 2024, 12, 388. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Xi, S. The Effects of Heavy Metals on Human Metabolism. Toxicol. Mech. Methods 2020, 30, 167–176. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, B.; Zhao, Y.; Zhang, S.; Duan, X.; Liu, H.; Meng, L. Visual Analysis of Research Progress on the Impact of Cadmium Stress on Horticultural Plants over 25 Years. Horticulturae 2025, 11, 28. [Google Scholar] [CrossRef]
- Elik, Ü.; Gül, Z. Accumulation Potential of Lead and Cadmium Metals in Maize (Zea mays L.) and Effects on Physiological-Morphological Characteristics. Life 2025, 15, 310. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. Evaluation of the Usefulness of Sorbents in the Remediation of Soil Exposed to the Pressure of Cadmium and Cobalt. Materials 2022, 15, 5738. [Google Scholar] [CrossRef]
- Zaborowska, M.; Wyszkowska, J.; Kucharski, J. Maintenance of Soil Homeostasis under Exposure to Cadmium. Commun. Soil Sci. Plant Anal. 2015, 46, 2051–2069. [Google Scholar] [CrossRef]
- Salam, L.B.; Obayori, O.S.; Ilori, M.O.; Amund, O.O. Effects of Cadmium Perturbation on the Microbial Community Structure and Heavy Metal Resistome of a Tropical Agricultural Soil. Bioresour. Bioprocess. 2020, 7, 25. [Google Scholar] [CrossRef]
- Rathored, J.; Malode, U.; Shende, S.; Kalode, D. Toxic Effect of Heavy Metal Poisoning on Living Organisms in Water and Health Risk: A Central India Study. Geomicrobiol. J. 2025, 42, 1–9. [Google Scholar] [CrossRef]
- Kraiem, K.; Bessadok, S.; Tabassi, D.; Fernandez, D.; Jaouani, A. Enhanced Pollutant Removal Efficiency in a Long-Term Integrated Constructed Wetland System Using Cork and Date Palm by-Products as Biomaterials. Int. J. Environ. Sci. Technol. 2025. [Google Scholar] [CrossRef]
- Irfan, M.; Liu, X.; Hussain, K.; Mushtaq, S.; Cabrera, J.; Zhang, P. The Global Research Trend on Cadmium in Freshwater: A Bibliometric Review. Environ. Sci. Pollut. Res. 2023, 30, 71585–71598. [Google Scholar] [CrossRef]
- Barkhordari, M.S.; Qi, C. Prediction of Zinc, Cadmium, and Arsenic in European Soils Using Multi-End Machine Learning Models. J. Hazard. Mater. 2025, 490, 137800. [Google Scholar] [CrossRef]
- Zheng, Y.; Xiao, C.; Chi, R. Remediation of Soil Cadmium Pollution by Biomineralization Using Microbial-Induced Precipitation: A Review. World J. Microbiol. Biotechnol. 2021, 37, 208. [Google Scholar] [CrossRef]
- Zhu, X.; Tu, C.; Zhou, J.; Yang, S.; Li, Y.; Wu, L.; Newman, L.A.; Luo, Y. Cadmium Phytoextraction by Sedum alfredii and Sedum plumbizincicola: Mechanisms, Challenges and Prospects. Int. J. Phytoremediation 2025, 27, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yan, L.; Chen, X.; Lam, S.S.; Rinklebe, J.; Yu, Q.; Yang, Y.; Peng, W.; Sonne, C. Phytoremediation of Cadmium from Soil, Air and Water. Chemosphere 2023, 320, 138058. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Shinde, A.; Aeron, V.; Verma, A.; Arif, N.S. Genetic Engineering of Plants for Phytoremediation: Advances and Challenges. J. Plant Biochem. Biotechnol. 2023, 32, 12–30. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Yusof, M.L.M.; Ghosh, S.; Chen, Z. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Tennakoon, A.; Galahitigama, H.; Samarakoon, S.M.A.B.K.; Perera, I.J.J.U.N.; Thakshila, G.P.G.I.; Thiruketheeswaranathan, S.; Roshana, M.R.; Sandamal, S.; Sewwandi, G.P.G.S.M.; Bellanthudawa, B.K.A. Remediating Contaminated Environmental Systems: The Role of Plants in Cadmium Removal. Int. J. Phytoremediation 2025, 27, 896–915. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kumar, T.; Das, D.K.; Mittal, A.; Verma, N. Vinod Phytoremediation of Heavy Metals in Soil—Concepts, Advancements, and Future Directions. J. Soil Sci. Plant Nutr. 2025, 25, 1253–1280. [Google Scholar] [CrossRef]
- Saeng-ngam, S.; Jampasri, K. Phytostabilization of Soils Contaminated with Cadmium by Peristrophe bivalvis. Bull. Environ. Contam. Toxicol. 2024, 114, 14. [Google Scholar] [CrossRef]
- Zhou, T.; Huang, H.; Mu, T.; Wang, Y.; Zhou, J.; Li, X.; Wu, L.; Christie, P. Does Phytoextraction with Sedum plumbizincicola Increase Cadmium Leaching from Polluted Agricultural Soil? Int. J. Phytoremediation 2024, 26, 241–249. [Google Scholar] [CrossRef]
- Zeng, P.; Guo, Z.; Xiao, X.; Cao, X.; Peng, C. Response to Cadmium and Phytostabilization Potential of Platycladus orientalis in Contaminated Soil. Int. J. Phytoremediation 2018, 20, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Bakshe, P.; Jugade, R. Phytostabilization and Rhizofiltration of Toxic Heavy Metals by Heavy Metal Accumulator Plants for Sustainable Management of Contaminated Industrial Sites: A Comprehensive Review. J. Hazard. Mater. Adv. 2023, 10, 100293. [Google Scholar] [CrossRef]
- Panda, A.; Fatnani, D.; Parida, A.K. Uptake, Impact, Adaptive Mechanisms, and Phytoremediation of Heavy Metals by Plants: Role of Transporters in Heavy Metal Sequestration. Plant Physiol. Biochem. 2025, 221, 109578. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.K.; Kumar, N.; Singh, N.P.; Santal, A.R. Phytoremediation Technologies and Their Mechanism for Removal of Heavy Metal from Contaminated Soil: An Approach for a Sustainable Environment. Front. Plant Sci. 2023, 14, 1076876. [Google Scholar] [CrossRef]
- Musa, I.O.; Ijah, U.J.J.; Abioye, O.P.; Abdulsalam, M.; Pal, S.K.; Abamhekhelu, I.A.; Maude, A.M.; Yusuf, O.O.; Akande, S.A. Phytoremediation of Heavy Metal-Contaminated Soil. In Eco-Restoration of Polluted Environment; CRC Press: Boca Raton, FL, USA, 2024; ISBN 978-1-003-42339-3. [Google Scholar]
- Wyszkowska, J.; Boros-Lajszner, E.; Kucharski, J. Calorific Value of Festuca Rubra Biomass in the Phytostabilization of Soil Contaminated with Nickel, Cobalt and Cadmium Which Disrupt the Microbiological and Biochemical Properties of Soil. Energies 2022, 15, 3445. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ok, Y.S.; Zia-ur-Rehman, M.; Abbas, Z.; Hannan, F. Use of Maize (Zea mays L.) for Phytomanagement of Cd-Contaminated Soils: A Critical Review. Environ. Geochem. Health 2017, 39, 259–277. [Google Scholar] [CrossRef]
- Adiloğlu, S.; Göker, M. Phytoremediation: Elimination of Hexavalent Chromium Heavy Metal Using Corn (Zea mays L.). Cereal Res. Commun. 2021, 49, 65–72. [Google Scholar] [CrossRef]
- FAO. World Food and Agriculture—Statistical Yearbook 2024; FAO: Rome, Italy, 2024; ISBN 978-92-5-139255-3. [Google Scholar]
- FAO. Agricultural Production Statistics 2000–2022; FAOSTAT Analytical Briefs, 79; CC9205EN/1/12.23; FAO: Rome, Italy; Available online: https://doi.org/10.4060/cc9205en (accessed on 28 April 2025).
- Boros-Lajszner, E.; Wyszkowska, J.; Kucharski, J. Effect of Ash from Salix viminalis on the Biomass and Heating Value of Zea mays and on the Biochemical and Physicochemical Properties of Soils. Energies 2023, 16, 8037. [Google Scholar] [CrossRef]
- Konieczna, A.; Roman, K.; Roman, M.; Śliwiński, D.; Roman, M. Energy Efficiency of Maize Production Technology: Evidence from Polish Farms. Energies 2021, 14, 170. [Google Scholar] [CrossRef]
- Menardo, S.; Airoldi, G.; Cacciatore, V.; Balsari, P. Potential Biogas and Methane Yield of Maize Stover Fractions and Evaluation of Some Possible Stover Harvest Chains. Biosyst. Eng. 2015, 129, 352–359. [Google Scholar] [CrossRef]
- Amon, T.; Amon, B.; Kryvoruchko, V.; Machmüller, A.; Hopfner-Sixt, K.; Bodiroza, V.; Hrbek, R.; Friedel, J.; Pötsch, E.; Wagentristl, H.; et al. Methane Production through Anaerobic Digestion of Various Energy Crops Grown in Sustainable Crop Rotations. Bioresour. Technol. 2007, 98, 3204–3212. [Google Scholar] [CrossRef]
- Schulz, V.S.; Munz, S.; Stolzenburg, K.; Hartung, J.; Weisenburger, S.; Mastel, K.; Möller, K.; Claupein, W.; Graeff-Hönninger, S. Biomass and Biogas Yield of Maize (Zea mays L.) Grown under Artificial Shading. Agriculture 2018, 8, 178. [Google Scholar] [CrossRef]
- Cao, J.; Tan, Y.; Zhang, C. Combined Approaches for the Remediation of Cadmium- and Arsenic-Contaminated Soil: Phytoremediation and Stabilization Strategies. Appl. Sci. 2024, 14, 7144. [Google Scholar] [CrossRef]
- Luo, N.; Zhang, X.J.; Gong, L.F.; Yang, L.Q.; Yao, Q.; Song, J.F. Review of Remediation Methods for Soil Contaminated with Cadmium. Eurasian Soil Sci. 2025, 58, 3. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Kucharski, M.; Kucharski, J. Effect of Cadmium, Copper and Zinc on Plants, Soil Microorganisms and Soil Enzymes. J. Elem. 2013, 18, 769–796. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Boros-Lajszner, E.; Borowik, A.; Kucharski, J. The Role of Cellulose in Microbial Diversity Changes in the Soil Contaminated with Cadmium. Sustainability 2022, 14, 14242. [Google Scholar] [CrossRef]
- Huang, T. Imran Mitigating Cadmium Contamination in Soil Using Biochar, Sulfur-Modified Biochar, and Other Organic Amendments. Int. J. Phytoremediation 2025, 27, 874–887. [Google Scholar] [CrossRef]
- Petruzzelli, G.; Grifoni, M.; Barbafieri, M.; Rosellini, I.; Pedron, F. Sorption: Release Processes in Soil—The Basis of Phytoremediation Efficiency. In Phytoremediation: Management of Environmental Contaminants, 6; Ansari, A.A., Gill, S.S., Gill, R., Lanza, G.R., Newman, L., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 91–112. ISBN 978-3-319-99651-6. [Google Scholar]
- Barbafieri, M.; Pedron, F.; Petruzzelli, G.; Rosellini, I.; Franchi, E.; Bagatin, R.; Vocciante, M. Assisted Phytoremediation of a Multi-Contaminated Soil: Investigation on Arsenic and Lead Combined Mobilization and Removal. J. Environ. Manag. 2017, 203, 316–329. [Google Scholar] [CrossRef]
- Costa, T.B.; Matias, P.M.C.; Sharma, M.; Murtinho, D.; Rosa, D.S.; Valente, A.J.M. Recent Advances on Starch-Based Adsorbents for Heavy Metal and Emerging Pollutant Remediation. Polymers 2025, 17, 15. [Google Scholar] [CrossRef]
- Starch Europe EU Starch Market Data. Available online: https://starch.eu/the-european-starch-industry/ (accessed on 28 April 2025).
- Rosen, V.; Chen, Y. Effects of Compost Application on Soil Vulnerability to Heavy Metal Pollution. Environ. Sci. Pollut. Res. 2018, 25, 35221–35231. [Google Scholar] [CrossRef] [PubMed]
- Junior, A.C.G.; Strey, L.; Lindino, C.A.; Nacke, H.; Schwantes, D.; Seidel, E.P. Applicability of the Pinus bark (Pinus elliottii) for the adsorption of toxic heavy metals from aqueous solutions. Acta Sci. Technol. 2012, 34, 79–87. [Google Scholar] [CrossRef]
- Piccolo, A.; Spaccini, R.; De Martino, A.; Scognamiglio, F.; di Meo, V. Soil Washing with Solutions of Humic Substances from Manure Compost Removes Heavy Metal Contaminants as a Function of Humic Molecular Composition. Chemosphere 2019, 225, 150–156. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Xu, L.; Wu, D.; Li, Q.; Ai, Y.; Liu, W.; Li, D.; Zhou, Y.; Zhang, B.; et al. EDTA Functionalized Mg/Al Hydroxides Modified Biochar for Pb(II) and Cd(II) Removal: Adsorption Performance and Mechanism. Sep. Purif. Technol. 2024, 335, 126199. [Google Scholar] [CrossRef]
- Zhang, D.; Zhu, L.; Ma, T.; Liang, X.; Sun, N.; Liu, F. Process Optimization and Performance Characterization of Preparing 4A Molecular Sieves from Coal Gangue. Symmetry 2025, 17, 603. [Google Scholar] [CrossRef]
- Eroglu, N.; Emekci, M.; Athanassiou, C.G. Applications of Natural Zeolites on Agriculture and Food Production. J. Sci. Food Agric. 2017, 97, 3487–3499. [Google Scholar] [CrossRef]
- Możdżeń, K.; Barabasz-Krasny, B.; Kviatková, T.; Zandi, P.; Turisová, I. Effect of Sorbent Additives to Copper-Contaminated Soils on Seed Germination and Early Growth of Grass Seedlings. Molecules 2021, 26, 5449. [Google Scholar] [CrossRef]
- Song, N.; Hursthouse, A.; McLellan, I.; Wang, Z. Treatment of Environmental Contamination Using Sepiolite: Current Approaches and Future Potential. Environ. Geochem. Health 2021, 43, 2679–2697. [Google Scholar] [CrossRef]
- MarketResearch. Available online: https://www.verifiedmarketresearch.com/product/lightweight-expanded-clay-aggregate-sales-market/?utm_source=chatgpt.com (accessed on 28 April 2025).
- Malakootian, M.; Nouri, J.; Hossaini, H. Removal of Heavy Metals from Paint Industry’s Wastewater Using Leca as an Available Adsorbent. Int. J. Environ. Sci. Technol. 2009, 6, 183–190. [Google Scholar] [CrossRef]
- Alghamdi, A.G.; Alasmary, Z. Fate and Transport of Lead and Copper in Calcareous Soil. Sustainability 2023, 15, 775. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. The Usability of Sorbents in Restoring Enzymatic Activity in Soils Polluted with Petroleum-Derived Products. Materials 2023, 16, 3738. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Wyszkowska, J.; Kordala, N.; Zaborowska, M. Molecular Sieve, Halloysite, Sepiolite and Expanded Clay as a Tool in Reducing the Content of Trace Elements in Helianthus annuus L. on Copper-Contaminated Soil. Materials 2023, 16, 1827. [Google Scholar] [CrossRef]
- Boros-Lajszner, E.; Wyszkowska, J.; Kucharski, J. Use of a Zeolite and Molecular Sieve to Restore Homeostasis of Soil Contaminated with Cobalt. Minerals 2020, 10, 53. [Google Scholar] [CrossRef]
- Boros-Lajszner, E.; Wyszkowska, J.; Kucharski, J. Use of Zeolite to Neutralise Nickel in a Soil Environment. Environ. Monit. Assess. 2017, 190, 54. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Kucharski, J. The Role of Grass Compost and Zea mays in Alleviating Toxic Effects of Tetracycline on the Soil Bacteria Community. Int. J. Environ. Res. Public Health 2022, 19, 7357. [Google Scholar] [CrossRef] [PubMed]
- Zaborowska, M.; Wyszkowska, J.; Borowik, A.; Kucharski, J. Evaluation of the Effectiveness of Innovative Sorbents in Restoring Enzymatic Activity of Soil Contaminated with Bisphenol A (BPA). Molecules 2024, 29, 3113. [Google Scholar] [CrossRef]
- PN-EN 14780: 2017; Solid Biofuels—Sample Preparation. Polish Standardization Committee: Warsaw, Poland, 2020.
- PN-EN ISO 18125: 2017-07; Solid Biofuels—Determination of Calorific Value. Polish Standardization Committee: Warsaw, Poland, 2017.
- TIBCO Software Inc. Statistica, Version 13; Data Analysis Software System; Tibco Software Inc.: Palo Alto, CA, USA. Available online: https://Statistica.Io (accessed on 10 January 2025).
- RStudio. Available online: https://support.posit.co/hc/en-us/articles/206212048-Citing-RStudio (accessed on 10 January 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.r-project.org (accessed on 10 January 2025).
- Warnes, G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Huber, W.; Liaw, A.; Lumley, T.; Maechler, M.; Magnusson, M.; Moeller, S.; et al. Gplots: Various R Programming Tools for Plotting Data, Version 3.20.0; R Package; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://rdrr.io/cran/gplots/ (accessed on 5 December 2024).
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An Information Aesthetic for Comparative Genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Gómez, X.; Bernal, M.P.; Zárate, P.P.; Álvarez-Robles, M.J.; González, R.; Clemente, R. Thermal Evaluation of Plant Biomass from the Phytostabilisation of Soils Contaminated by Potentially Toxic Elements. Chemosphere 2023, 342, 140116. [Google Scholar] [CrossRef]
- Zeng, P.; He, S.; He, L.; Yang, M.; Zhu, X.; Wu, M. Screening of Maize Varieties with High Biomass and Low Accumulation of Pb and Cd around Lead and Zinc Smelting Enterprises: Field Experiment. Agriculture 2024, 14, 423. [Google Scholar] [CrossRef]
- Boros-Lajszner, E.; Wyszkowska, J.; Kucharski, J. Phytoremediation of Soil Contaminated with Nickel, Cadmium and Cobalt. Int. J. Phytoremediation 2021, 23, 252–262. [Google Scholar] [CrossRef]
- Raza, A.; Habib, M.; Kakavand, S.N.; Zahid, Z.; Zahra, N.; Sharif, R.; Hasanuzzaman, M. Phytoremediation of Cadmium: Physiological, Biochemical, and Molecular Mechanisms. Biology 2020, 9, 177. [Google Scholar] [CrossRef]
- de Araújo, R.P.; de Almeida, A.-A.F.; Pereira, L.S.; Mangabeira, P.A.; Souza, J.O.; Pirovani, C.P.; Ahnert, D.; Baligar, V.C. Photosynthetic, Antioxidative, Molecular and Ultrastructural Responses of Young Cacao Plants to Cd Toxicity in the Soil. Ecotoxicol. Environ. Saf. 2017, 144, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Ali, S.; Hameed, A.; Bharwana, S.A.; Rizwan, M.; Ishaque, W.; Farid, M.; Mahmood, K.; Iqbal, Z. Cadmium Stress in Cotton Seedlings: Physiological, Photosynthesis and Oxidative Damages Alleviated by Glycinebetaine. S. Afr. J. Bot. 2016, 104, 61–68. [Google Scholar] [CrossRef]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium Toxicity in Plants: Impacts and Remediation Strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef]
- Karalija, E.; Parić, A. Antioxidant Defense: A Key Mechanism of Cadmium Tolerance. In Cadmium Toxicity Mitigation; Jha, A.K., Kumar, N., Eds.; Springer Nature Switzerland: Cham, Switzerland, 2024; pp. 195–214. ISBN 978-3-031-47390-6. [Google Scholar]
- Abbas, T.; Rizwan, M.; Ali, S.; Adrees, M.; Zia-ur-Rehman, M.; Qayyum, M.F.; Ok, Y.S.; Murtaza, G. Effect of Biochar on Alleviation of Cadmium Toxicity in Wheat (Triticum aestivum L.) Grown on Cd-Contaminated Saline Soil. Environ. Sci. Pollut. Res. 2018, 25, 25668–25680. [Google Scholar] [CrossRef]
- Bao, C.; Cao, Y.; Zhao, L.; Li, X.; Zhang, J.; Mao, C. Biofuel Production from Phytoremediated Biomass via Various Conversion Routes: A Review. Energies 2025, 18, 822. [Google Scholar] [CrossRef]
- Wei, K.; Guo, T. The Changes of Tolerance, Accumulation and Oxidative Stress Response to Cadmium in Tobacco Caused by Introducing Datura stramonium L. Genes. Agronomy 2023, 13, 882. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Zaborowska, M.; Kucharski, J. Energy Quality of Corn Biomass from Gasoline-Contaminated Soils Remediated with Sorbents. Energies 2024, 17, 5322. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Zaborowska, M.; Kucharski, J. Soil Enzyme Response and Calorific Value of Zea mays Used for the Phytoremediation of Soils Contaminated with Diesel Oil. Energies 2024, 17, 2552. [Google Scholar] [CrossRef]
- Boros-Lajszner, E.; Wyszkowska, J.; Borowik, A.; Kucharski, J. Energetic Value of Elymus elongatus L. and Zea mays L. Grown on Soil Polluted with Ni2+, Co2+, Cd2+, and Sensitivity of Rhizospheric Bacteria to Heavy Metals. Energies 2021, 14, 4903. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Boros-Lajszner, E.; Kucharski, J. The Impact of Soil Contamination with Lead on the Biomass of Maize Intended for Energy Purposes, and the Biochemical and Physicochemical Properties of the Soil. Energies 2024, 17, 1156. [Google Scholar] [CrossRef]
- Boros-Lajszner, E.; Wyszkowska, J.; Kucharski, J. The Effect of Carpinus betulus Ash on the Maize as an Energy Crop and the Enzymatic Soil Properties. Energies 2024, 17, 3031. [Google Scholar] [CrossRef]
- Shah, T.M.; Khan, A.H.; Nicholls, C.; Sohoo, I.; Otterpohl, R. Using Landfill Sites and Marginal Lands for Socio-Economically Sustainable Biomass Production through Cultivation of Non-Food Energy Crops: An Analysis Focused on South Asia and Europe. Sustainability 2023, 15, 4923. [Google Scholar] [CrossRef]
- Pulighe, G.; Bonati, G.; Colangeli, M.; Morese, M.M.; Traverso, L.; Lupia, F.; Khawaja, C.; Janssen, R.; Fava, F. Ongoing and Emerging Issues for Sustainable Bioenergy Production on Marginal Lands in the Mediterranean Regions. Renew. Sustain. Energy Rev. 2019, 103, 58–70. [Google Scholar] [CrossRef]
- Amabogha, O.N.; Garelick, H.; Jones, H.; Purchase, D. Combining Phytoremediation with Bioenergy Production: Developing a Multi-Criteria Decision Matrix for Plant Species Selection. Environ. Sci. Pollut. Res. 2023, 30, 40698–40711. [Google Scholar] [CrossRef]
- Sameena, P.P.; Puthur, J.T. Heavy Metal Phytoremediation by Bioenergy Plants and Associated Tolerance Mechanisms. Soil Sediment. Contam. Int. J. 2021, 30, 253–274. [Google Scholar] [CrossRef]
- Wijekoon, W.; Priyashantha, H.; Gajanayake, P.; Manage, P.; Liyanage, C.; Jayarathna, S.; Kumarasinghe, U. Review and Prospects of Phytoremediation: Harnessing Biofuel-Producing Plants for Environmental Remediation. Sustainability 2025, 17, 822. [Google Scholar] [CrossRef]
- Jankowski, K.J.; Dubis, B.; Sokólski, M.M.; Załuski, D.; Bórawski, P.; Szempliński, W. Productivity and Energy Balance of Maize and Sorghum Grown for Biogas in a Large-Area Farm in Poland: An 11-Year Field Experiment. Ind. Crops Prod. 2020, 148, 112326. [Google Scholar] [CrossRef]
- Mazurkiewicz, J.; Marczuk, A.; Pochwatka, P.; Kujawa, S. Maize Straw as a Valuable Energetic Material for Biogas Plant Feeding. Materials 2019, 12, 3848. [Google Scholar] [CrossRef]
- Abbas, S.; Javed, M.T.; Ali, Q.; Akram, M.S.; Tanwir, K.; Ali, S.; Chaudhary, H.J.; Iqbal, N. Elucidating Cd-Mediated Distinct Rhizospheric and in Planta Ionomic and Physio-Biochemical Responses of Two Contrasting Zea mays L. Cultivars. Physiol. Mol. Biol. Plants 2021, 27, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Yang, Y.; Zeng, H.; Chen, Y.; Zeng, Q. Variations in Iron Plaque, Root Morphology and Metal Bioavailability Response to Seedling Establishment Methods and Their Impacts on Cd and Pb Accumulation and Translocation in Rice (Oryza Sativa L.). J. Hazard. Mater. 2020, 384, 121343. [Google Scholar] [CrossRef]
- Basu, S.; Priyadarshini, P.; Prasad, R.; Kumar, G. Effects of Microbial Signaling in Plant Growth and Development. In Beneficial Microorganisms in Agriculture; Prasad, R., Zhang, S.-H., Eds.; Springer Nature: Singapore, 2022; pp. 329–348. ISBN 978-981-19-0733-3. [Google Scholar]
- Tian, B.; Yang, Y.; Chen, A.; Peng, L.; Deng, X.; Yang, Y.; Zeng, Q.; Luo, S. Long-Term Straw Removal and Double-Cropping System Reduce Soil Cadmium Content and Uptake in Rice: A Four-Year Field Analysis. J. Environ. Sci. 2025, 152, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, L.; Ippolito, J.A.; Xing, W.; Wang, Y.; Wang, Y.; Cheng, Y.; Qiu, K. Heavy Metal Distribution in Wheat Plant Components Following Foliar Cd Application. Chemosphere 2023, 322, 138177. [Google Scholar] [CrossRef]
- Lin, L.; Wu, X.; Deng, X.; Lin, Z.; Liu, C.; Zhang, J.; He, T.; Yi, Y.; Liu, H.; Wang, Y.; et al. Mechanisms of Low Cadmium Accumulation in Crops: A Comprehensive Overview from Rhizosphere Soil to Edible Parts. Environ. Res. 2024, 245, 118054. [Google Scholar] [CrossRef]
- Kanwal, F.; Riaz, A.; Ali, S.; Zhang, G. NRAMPs and Manganese: Magic Keys to Reduce Cadmium Toxicity and Accumulation in Plants. Sci. Total Environ. 2024, 921, 171005. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Long, L.; Chen, A.; Dong, X.; Liu, Z.; Chen, L.; Wang, J.; Yuan, L. Tonoplast-Localized Transporter ZmNRAMP2 Confers Root-to-Shoot Translocation of Manganese in Maize. Plant Physiol. 2022, 190, 2601–2616. [Google Scholar] [CrossRef]
- Haque, A.F.M.M.; Gohari, G.; El-Shehawi, A.M.; Dutta, A.K.; Elseehy, M.M.; Kabir, A.H. Genome-Wide Identification, Characterization and Expression Profiles of Heavy Metal ATPase 3 (HMA3) in Plants. J. King Saud Univ. Sci. 2022, 34, 101730. [Google Scholar] [CrossRef]
- Cadar, O.; Stupar, Z.; Senila, M.; Levei, L.; Moldovan, A.; Becze, A.; Ozunu, A.; Levei, E.A. Zeolites Reduce the Transfer of Potentially Toxic Elements from Soil to Leafy Vegetables. Materials 2022, 15, 5657. [Google Scholar] [CrossRef]
- Amoah-Antwi, C.; Kwiatkowska-Malina, J.; Szara, E.; Thornton, S.; Fenton, O.; Malina, G. Efficacy of Woodchip Biochar and Brown Coal Waste as Stable Sorbents for Abatement of Bioavailable Cadmium, Lead and Zinc in Soil. Water Air Soil Pollut. 2020, 231, 515. [Google Scholar] [CrossRef]
- Lwin, C.S.; Seo, B.-H.; Kim, H.-U.; Owens, G.; Kim, K.-R. Application of Soil Amendments to Contaminated Soils for Heavy Metal Immobilization and Improved Soil Quality—A Critical Review. J. Soil Sci. Plant Nutr. 2018, 64, 156–167. [Google Scholar] [CrossRef]
- Kordala, N.; Wyszkowski, M. Zeolite Properties, Methods of Synthesis, and Selected Applications. Molecules 2024, 29, 1069. [Google Scholar] [CrossRef]
- Kohli, D.; Garg, S.; Jana, A. Synthesis of Cross-Linked Starch Based Polymers for Sorption of Organic Pollutants from Aqueous Solutions. Indian Chem. Eng. 2012, 54, 210–222. [Google Scholar] [CrossRef]
- Dou, J.; Wang, J.; Hietala, S.; Evtuguin, D.V.; Vuorinen, T.; Zhao, J. Structural Features of Lignin–Hemicellulose–Pectin (LHP) Orchestrate a Tailored Enzyme Cocktail for Potential Applications in Bark Biorefineries. Green Chem. 2023, 25, 5661–5678. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Xu, H.; Zhao, W.; Feng, G.; Xiao, C. Green Synthesis of Coal Gangue-Derived NaX Zeolite for Enhanced Adsorption of Cu2+ and CO2. Materials 2025, 18, 1443. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef]
- Huang, M.; Zhu, Y.; Li, Z.; Huang, B.; Luo, N.; Liu, C.; Zeng, G. Compost as a Soil Amendment to Remediate Heavy Metal-Contaminated Agricultural Soil: Mechanisms, Efficacy, Problems, and Strategies. Water Air Soil Pollut. 2016, 227, 359. [Google Scholar] [CrossRef]
- Tang, Y.; Pan, J.; Li, B.; Zhao, S.; Zhang, L. Residual and Ecological Risk Assessment of Heavy Metals in Fly Ash from Co-Combustion of Excess Sludge and Coal. Sci. Rep. 2021, 11, 2499. [Google Scholar] [CrossRef]







| Type | Abbreviation | Characteristics |
|---|---|---|
| Soil | ||
| Sandy loam | SL | Content (%): sand (0.05–2 mm)—63.61, silt (0.002–0.05 mm)—32.68, clay (<0.002 mm)—3.71. Content (g kg−1 of soil dry matter): Corg—10.00 and Ntotal—0.83. Content (mg kg−1 of soil dry matter): Cd2+—0.142. pH in 1 mol KCl dm−3—4.40, pH in H2O dm−3—5.52. Hydrolytic acidity—26.10 mM(+) kg−1 d.m. of soil, exchangeable base cations—63.60 mM(+) kg−1 d.m. of soil, cation exchange capacity—89.70 mM(+) kg−1 d.m. of soil, alkaline cation saturation—70.90%. |
| Plant | ||
| Zea mays | Zm | Variety DS1897B. It is a crop suitable for grain, silage, and energy purposes. It is a plant adapted to existence in various conditions. It tolerates both low-fertility and high-fertility soils well. |
| Mineral substances for biomass production enhancement | ||
| Molecular sieve | MS | Silosiv A3 is a white, hydrated aluminosilicate characterized by a micropore diameter of 0.3 nm and a pHKCl value of 8.5. Its maximum volatile content at 950 °C does not exceed 2.5%. Manufacturer: Sylosiv, (Columbia, MD, USA). |
| Zeolite Bio.Zeo.S.01 | Z | Aluminosilicate mineral of volcanic origin containing 60% clinoptilolite, 33% Si, 3.26% Al, 1.17% K, 2.44% Ca, 0.56% Mg, 0.52% Fe. Manufacturer: BioDrain (Rzeszow, Poland). |
| Sepiolite | S | Hydrated magnesium silicate (Mg4 [Si6O15(OH)2] × 6H2O), having a pore diameter of 1.4 nm, fibrous texture, and a pH KCl = 7.1. Manufacturer: Sepiolsa Minersa Group (Guadalajara, Spain). |
| Expanded clay | E | A lightweight ceramic aggregate formed by firing clay at a temperature of about 1200 °C, with a particle size of 75 to 710 μm, and a pHKCl 7.1. Manufacturer: GardenGURU, Piła, Poland. |
| Calcium carbonate | Ca | White powder (CaCO3) with a molar mass of 100.09 g mol−1. Manufacturer: Chempur (Piekary Śląskie, Poland). |
| Organic substances for biomass production enhancement | ||
| Starch | St | White powder (C6H10O5) with a molar mass of 162.1 g mol−1, and solubility in water of 50 g dm−3 (90 °C, pH 6.0–7.5). Manufacturer: Chempur (Piekary Śląskie, Poland). |
| Compost | K | Compost prepared from composted grasses. pH in 1 mol KCl dm−3—6.1. Content (g kg−1 of soil dry matter): Corg—146.61, Ntotal—20.18. Content (mg kg−1 of soil dry matter): P—3.41, K—9.25, and Mg—5.69. |
| Fermented bark | B | Fermented bark of coniferous trees with bark flake size of 20–50 mm. Content of dry matter ≥30%, organic matter ≥50%, and pH H2O ≤6.0. Manufacturer: Athena Bio-Produkty Sp. z o.o. (Golczewo, Poland). |
| HumiAgra | H | Ecological product (powder) of dark brown color, pH 8–10. Contains 90% humic acids, with a humic-to-fulvic acid ratio of 1:1, 8% K2O, and 3% S. Manufacturer: AgraPlant (Kielce, Poland). |
| Treatment | Dose Cd, mg kg−1 | ||
|---|---|---|---|
| 0 | 15 | Average | |
| Shoots and leaves | |||
| Control | 12.43 ± 0.23 a | 12.45 ± 0.71 a | 12.44 A |
| Molecular sieve | 12.20 ± 0.60 a | 12.55 ± 0.73 a | 12.38 A |
| Zeolite | 12.29 ± 0.54 a | 12.73 ± 0.45 a | 12.51 A |
| Sepiolite | 12.43 ± 0.33 a | 12.62 ± 1.01 a | 12.53 A |
| Expanded clay | 12.45 ± 0.25 a | 12.55 ± 0.58 a | 12.50 A |
| Calcium carbonate | 12.46 ± 0.42 a | 12.23 ± 0.82 a | 12.37 A |
| Starch | 12.50 ± 0.71 a | 12.76 ± 0.83 a | 12.63 A |
| Compost | 12.48 ± 0.12 a | 12.66 ± 0.44 a | 12.58 A |
| Fermented bark | 12.22 ± 0.19 a | 12.91 ± 0.98 a | 12.58 A |
| HumiAgra | 12.11 ± 0.40 a | 12.61 ± 0.42 a | 12.36 A |
| Average | 12.36 I | 12.61 I | |
| Source of variation (F statistic and probability level): Cd dose: F—15,187.19 (p < 0.01); Treatment: F—315.07 (p < 0.01); Cd dose × Treatment: F—48.71 (p < 0.01) | |||
| Roots | |||
| Control | 12.13 ± 0.56 a | 11.41 ± 0.87 a | 11.77 A |
| Molecular sieve | 10.68 ± 0.35 a | 11.04 ± 0.75 a | 10.86 A |
| Zeolite | 11.96 ± 0.72 a | 11.68 ± 0.07 a | 11.82 A |
| Sepiolite | 11.65 ± 0.48 a | 11.53 ± 0.91 a | 11.59 A |
| Expanded clay | 11.48 ± 0.37 a | 11.93 ± 0.94 a | 11.71 A |
| Calcium carbonate | 11.91 ± 0.87 a | 11.48 ± 0.68 a | 11.69 A |
| Starch | 11.76 ± 0.50 a | 11.53 ± 0.79 a | 11.65 A |
| Compost | 11.60 ± 0.68 a | 11.71 ± 1.02 a | 11.66 A |
| Fermented bark | 11.58 ± 0.80 a | 11.46 ± 0.81 a | 11.52 A |
| HumiAgra | 12.30 ± 0.87 a | 11.74 ± 0.88 a | 12.02 A |
| Average | 11.71 I | 11.55 I | |
| Source of variation (F statistic and probability level): Cd dose: F—2605.51 (p < 0.01); Treatment: F—24.95 (p < 0.01); Cd dose × Treatment: F—16.72 (p < 0.01) | |||
| Treatment | Dose Cd, mg kg−1 | ||
|---|---|---|---|
| 0 | 15 | Average | |
| Control | 35.61 ± 1.91 a–c | 32.02 ± 2.00 c–g | 33.82 AB |
| Molecular sieve | 35.90 ± 0.69 a–c | 32.09 ±1.53 b–g | 34.00 A |
| Zeolite | 35.08 ± 2.12 a–d | 32.63 ± 0.50 b–f | 33.86 A |
| Sepiolite | 37.82 ±2.29 a | 34.97 ± 2.38 a–e | 36.40 A |
| Expanded clay | 27.48 ± 1.55 gh | 30.53 ± 1.72 d–g | 29.01 CD |
| Calcium carbonate | 32.08 ± 1.95 b–g | 29.70 ± 089 f–h | 30.89 BC |
| Starch | 28.21 ± 2.91 f–h | 25.24 ± 1.13 h | 26.72 D |
| Compost | 36.78 ± 2.74 ab | 30.64 ± 1.44 d–g | 33.71 AB |
| Fermented bark | 30.05 ± 1.74 fg | 28.94 ± 0.94 f–h | 29.49 CD |
| HumiAgra | 30.30 ± 0.85 efg | 29.29 ± 2.03 f–h | 29.80 C |
| Average | 32.93 I | 30.61 II | |
| Source of variation (F statistic and probability level): Cd dose: F—33.65 (p < 0.01); Treatment: F—22.59 (p < 0.01); Cd dose × Treatment: F—3.54 (p < 0.01) | |||
| Treatment | Dose Cd, mg kg−1 | ||
|---|---|---|---|
| 0 | 15 | Average | |
| Soil | |||
| Control | 0.115 ± 0.002 d | 14.029 ± 0.489 b | 7.072 C |
| Molecular sieve | 1.619 ± 0.115 c | 14.160 ± 0.577 b | 7.890 AB |
| Zeolite | 0.276 ± 0.012 d | 14.395 ± 0.157 b | 7.336 BC |
| Sepiolite | 0.228 ± 0.026 d | 14.510 ± 0.349 b | 7.369 BC |
| Expanded clay | 0.211 ± 0.016 d | 14.874 ± 0.605 b | 7.543 A–C |
| Calcium carbonate | 0.225 ± 0.005 d | 14.456 ± 0.247 b | 7.341 BC |
| Starch | 0.222 ± 0.036 d | 14.238 ± 0.159 b | 7.230 C |
| Compost | 0.189 ± 0.012 d | 15.980 ± 1.155 a | 8.085 A |
| Fermented bark | 0.194 ± 0.006 d | 14.931 ± 0.297 b | 7.563 A–C |
| HumiAgra | 0.165 ± 0.007 d | 14.663 ± 0.491 b | 7.414 BC |
| Average | 0.344 II | 14.624 I | |
| Source of variation (F statistic and probability level): Cd dose: F—28,615.14 (p < 0.01); Treatment: F—5.18 (p < 0.01); Cd dose × Treatment: F—9.27 (p < 0.01) | |||
| Shoots and leaves | |||
| Control | 0.015 ± 0.001 l | 0.030 ± 0.004 i | 0.023 I |
| Molecular sieve | 0.011 ± 0.000 jk | 0.094 ± 0.001 h | 0.053 H |
| Zeolite | 0.010 ± 0.000 jk | 0.718 ± 0.001 b | 0.364 B |
| Sepiolite | 0.010 ± 0.001 jk | 0.378 ± 0.001 f | 0.194 F |
| Expanded clay | 0.009 ± 0.001 kl | 0.572 ± 0.005 c | 0.291 C |
| Calcium carbonate | 0.003 ± 0.000 l | 0.305 ± 0.001 g | 0.154 G |
| Starch | 0.010 ± 0.001 jk | 0.833 ± 0.007 a | 0.422 A |
| Compost | 0.005 ± 0.001 kl | 0.445 ± 0.009 e | 0.225 E |
| Fermented bark | 0.008 ± 0.001 kl | 0.829 ± 0.002 a | 0.419 A |
| HumiAgra | 0.003 ± 0.001 l | 0.474 ± 0.002 d | 0.239 D |
| Average | 0.008 II | 0.468 I | |
| Source of variation (F statistic and probability level): Cd dose: F—621,495.70 (p < 0.01); Treatment: F—22,934.70 (p < 0.01); Cd dose × Treatment: F—23,196.00 (p < 0.01) | |||
| Roots | |||
| Control | 0.050 ± 0.004 h | 5.512 ± 0.096 a | 2.781 A |
| Molecular sieve | 0.037 ± 0.008 h | 0.743 ± 0.016 g | 0.390 G |
| Zeolite | 0.034 ± 0.002 h | 3.170 ± 0.007 c | 1.602 C |
| Sepiolite | 0.051 ± 0.001 h | 2.893 ± 0.005 e | 1.472 E |
| Expanded clay | 0.043 ± 0.002 h | 3.535 ± 0.008 b | 1.789 B |
| Calcium carbonate | 0.034 ± 0.001 h | 2.012 ± 0.004 f | 1.023 F |
| Starch | 0.052 ± 0.002 h | 3.569 ± 0.009 b | 1.811 B |
| Compost | 0.039 ± 0.012 h | 2.884 ± 0.079 e | 1.462 E |
| Fermented bark | 0.060 ± 0.002 h | 3.001 ± 0.009 d | 1.531 D |
| HumiAgra | 0.056 ± 0.003 h | 3.003 ± 0.012 d | 1.530 D |
| Average | 0.050 II | 3.030 I | |
| Source of variation (F statistic and probability level): Cd dose: F—152,384.40 (p < 0.01); Treatment: F—2489.80 (p < 0.01); Cd dose × Treatment: F—2457.70 (p < 0.01) | |||
| Treatment | Dose Cd, mg kg−1 | ||
|---|---|---|---|
| 0 | 15 | Average | |
| Shoots and leaves | |||
| Control | 0.130 ± 0.009 a | 0.002 ± 0.000 l | 0.066 A |
| Molecular sieve | 0.007 ± 0.001 l | 0.007 ± 0.001 l | 0.007 H |
| Zeolite | 0.036 ± 0.002 fg | 0.050 ± 0.001 cd | 0.043 CD |
| Sepiolite | 0.044 ± 0.005 d–f | 0.026 ± 0.001 h–j | 0.035 E |
| Expanded clay | 0.043 ± 0.003 d–f | 0.038 ± 0.002 fg | 0.041 D |
| Calcium carbonate | 0.013 ± 0.002 k | 0.021 ± 0.001 i–k | 0.017 G |
| Starch | 0.045 ± 0.004 d–f | 0.059 ± 0.001 b | 0.052 B |
| Compost | 0.026 ± 0.002 h–j | 0.028 ± 0.002 hi | 0.027 F |
| Fermented bark | 0.041 ± 0.003 ef | 0.056 ± 0.001 bc | 0.048 C |
| HumiAgra | 0.018 ± 0.001 jk | 0.032 ± 0.001 gh | 0.025 F |
| Average | 0.040 I | 0.032 II | |
| Source of variation (F statistic and probability level): Cd dose: F—173.17 (p < 0.01); Treatment: F—287.80 (p < 0.01); Cd dose × Treatment: F—428.28 (p < 0.01) | |||
| Roots | |||
| Control | 0.435 ± 0.032 a | 0.393 ± 0.017 b | 0.414 A |
| Molecular sieve | 0.023 ± 0.002 j | 0.052 ± 0.003 j | 0.038 H |
| Zeolite | 0.123 ± 0.005 i | 0.220 ± 0.003 d–g | 0.172 F |
| Sepiolite | 0.224 ± 0.026 d–f | 0.199 ± 0.005 fg | 0.212 DE |
| Expanded clay | 0.204 ± 0.016 e–g | 0.238 ± 0.010 de | 0.221 D |
| Calcium carbonate | 0.151 ± 0.003 hi | 0.139 ± 0.003 i | 0.145 G |
| Starch | 0.234 ± 0.027 de | 0.251 ± 0.002 d | 0.242 C |
| Compost | 0.206 ± 0.010 e–g | 0.180 ± 0.009 gh | 0.193 EF |
| Fermented bark | 0.309 ± 0.010 c | 0.201 ± 0.004 fg | 0.255 BC |
| HumiAgra | 0.339 ± 0.015 c | 0.205 ± 0.007 e–g | 0.272 B |
| Average | 0.225 I | 0.208 II | |
| Source of variation (F statistic and probability level): Cd dose: F—29.71 (p < 0.01); Treatment: F—399.78 (p < 0.01); Cd dose × Treatment: F—79.62 (p < 0.01) | |||
| Development Stage of Zea mays | Type of Contamination | Dose per kg of Soil d.m. | Heating Value | References |
|---|---|---|---|---|
| BBCH 51 phase | Cr6+ | 0 mg 60 mg | 14.80 14.85 | [6] |
| BBCH 51 phase | Unleaded gasoline 95 | 0 cm3 24 cm3 | 15.12 15.34 | [106] |
| BBCH 51 phase | Diesel oil | 0 cm3 24 cm3 | 15.12 15.04 | [107] |
| BBCH 39 phase | Ni2+ | 0 mg 400 mg | 14.79 14.95 | [108] |
| BBCH 39 phase | Co2+ | 0 mg 80 mg | 14.79 14.91 | [108] |
| BBCH 39 phase | Pb2+ | 0 mg 800 mg | 16.48 16.54 | [109] |
| BBCH 39 phase | Ash from Salix viminalis | 0 mg 20 g | 16.50 15.93 | [56] |
| BBCH 39 phase | Ash from Carpinus betulus | 0 mg 20 g | 16.29 16.07 | [110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borowik, A.; Wyszkowska, J.; Zaborowska, M.; Kucharski, J. Energy Potential of Zea mays Grown in Cadmium-Contaminated Soil. Energies 2025, 18, 2402. https://doi.org/10.3390/en18092402
Borowik A, Wyszkowska J, Zaborowska M, Kucharski J. Energy Potential of Zea mays Grown in Cadmium-Contaminated Soil. Energies. 2025; 18(9):2402. https://doi.org/10.3390/en18092402
Chicago/Turabian StyleBorowik, Agata, Jadwiga Wyszkowska, Magdalena Zaborowska, and Jan Kucharski. 2025. "Energy Potential of Zea mays Grown in Cadmium-Contaminated Soil" Energies 18, no. 9: 2402. https://doi.org/10.3390/en18092402
APA StyleBorowik, A., Wyszkowska, J., Zaborowska, M., & Kucharski, J. (2025). Energy Potential of Zea mays Grown in Cadmium-Contaminated Soil. Energies, 18(9), 2402. https://doi.org/10.3390/en18092402










