Analysis of the Possibility of Using CO2 Capture in a Coal-Fired Power Plant
Abstract
1. Introduction
- Research on CO2 separation is currently being conducted in several directions:
- ✓
- Separation before combustion—pre-combustion;
- ✓
- Separation after combustion—post-combustion;
- ✓
- Combustion in pure oxygen—oxyfuel combustion.
- CO2 separation before combustion—pre-combustion
- ✓
- Processes combined with CO to CO2 conversion;
- ✓
- Processes without CO to CO2 conversion;
- ✓
- Carbon gasification in pure oxygen, Integrated Gasification Combined Cycle (IGCC).
- CO2 separation after combustion—post-combustion
- ➢
- Absorption;
- ➢
- Adsorption;
- ➢
- Membrane;
- ➢
- Cryogenic.
- It must have a high sorption capacity;
- It is required that the process of adsorption be a reversible process;
- It must show a high selectivity relative to the gas separated.
- Pressure swing adsorption (PSA);
- Temperature swing adsorption (TSA);
- Combination of pressure swing adsorption and temperature swing adsorption, i.e., pressure temperature swing adsorption (PTSA);
- Pressure swing adsorption where desorption is carried out under vacuum, i.e., vacuum swing adsorption (VSA) or vacuum pressure swing adsorption (VPSA);
- Adsorption using a low-voltage electrical current passed through the bed during desorption, i.e., electrical swing adsorption (ESA) or electrical thermal swing adsorption (ETSA);
- Rapid pressure swing adsorption (RPSA);
- Ultra-rapid pressure swing adsorption (URPSA).
2. Simulations of a CO2 Capture System
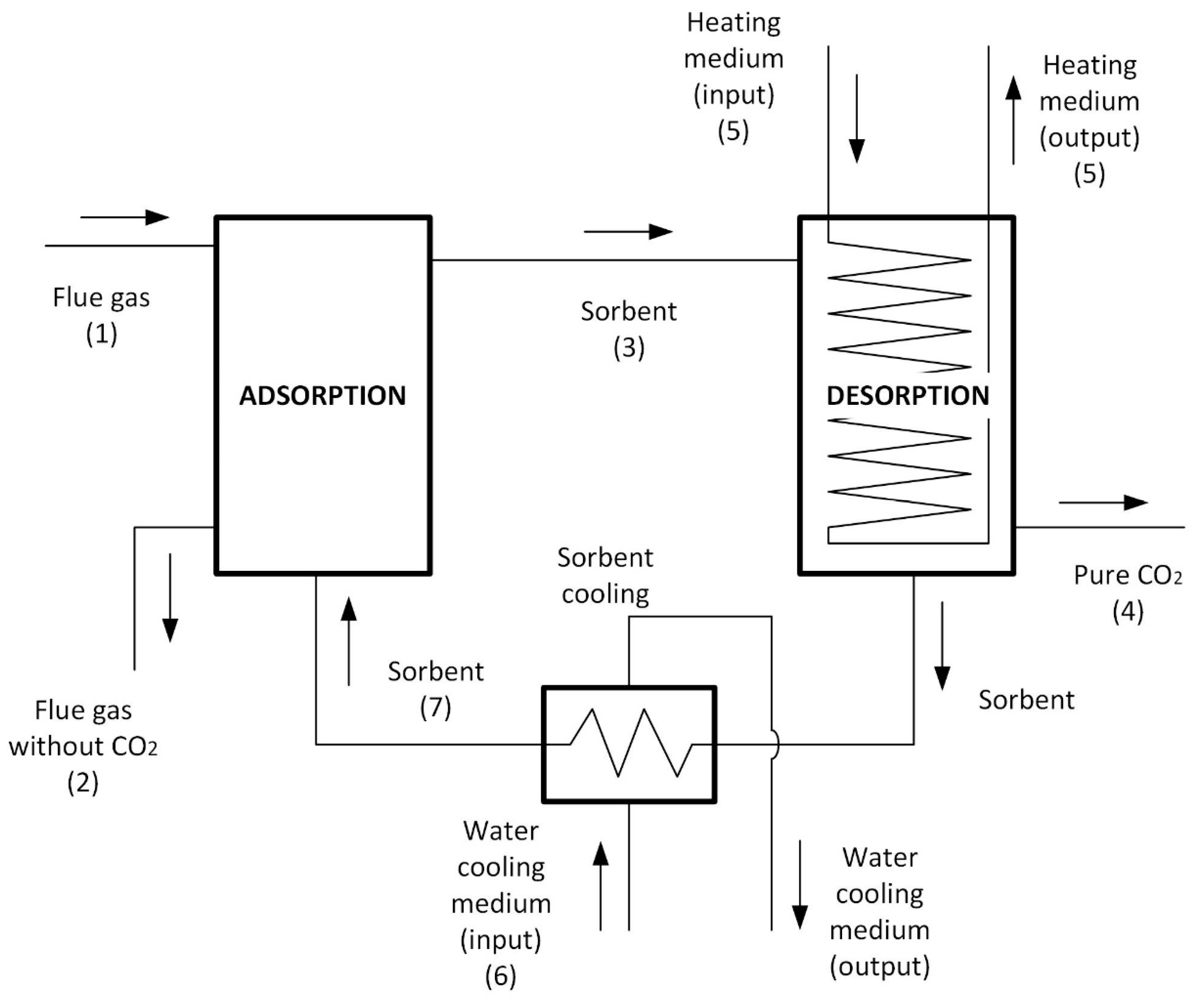
2.1. CO2 Capture Model Based on Adsorption Methods
- Dust removal: A high content of particulate matter in the flue gas may lead to deactivation of adsorption sites (pore blockage), which can significantly disrupt the CO2 adsorption process. Therefore, it is recommended to reduce the dust concentration in the flue gas to a level below 10 mg/Nm3 through effective dedusting.
- Desulfurization: The presence of SO2 at concentrations between 500 and 2000 ppm has been shown to reduce CO2 adsorption capacity on zeolites by approximately 10–15%. Conventional power plants are typically equipped with flue gas desulfurization systems, which lower SO2 levels to meet regulatory standards. According to Polish legislation, SO2 emissions should not exceed 200 mg/Nm3, equivalent to about 80 ppm. At this concentration, the impact of SO2 on the CO2 adsorption process is considered negligible.
- Denitrification: Based on available literature data, the sorption capacity of zeolites for NOx is very low. Therefore, the influence of NOx in the CO2 capture process can be regarded as minimal. It is sufficient for the concentration of NOx in the flue gas entering the separation unit to be reduced to 200 mg/Nm3.
- Dehydration: The presence of water vapour in the flue gas at levels of 0.1–2.5% reduces CO2 adsorption on zeolites to between 5 and 40% of the capacity observed under dry gas conditions. At higher water vapour concentrations (in this case approximately 12%), CO2 adsorption on zeolites can be almost entirely inhibited. Therefore, it is essential to dry the flue gas to the lowest possible humidity level before the adsorption process. This significant reduction in CO2 adsorption is due to the progressive displacement of CO2 molecules by adsorbed water molecules [39,41,44].
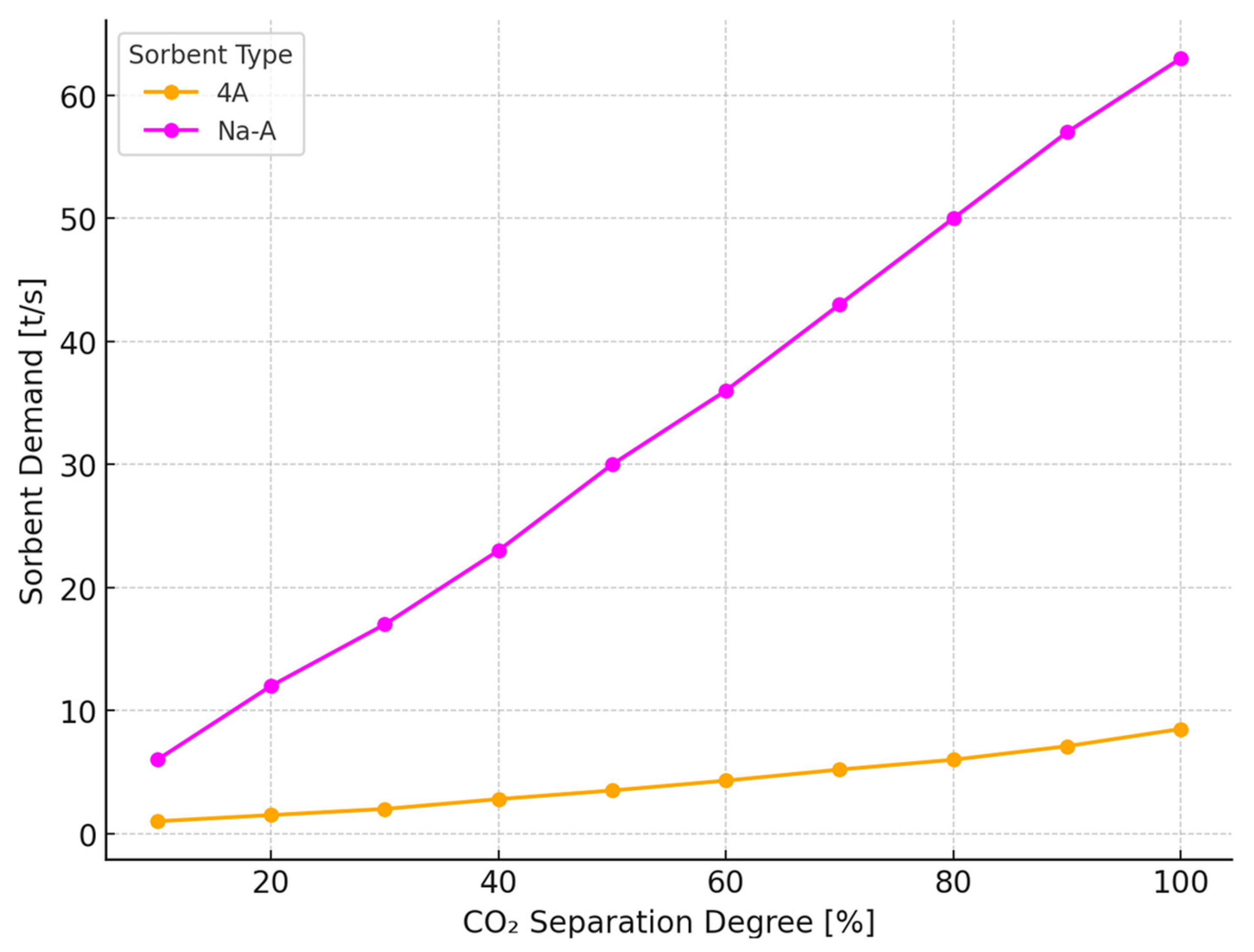
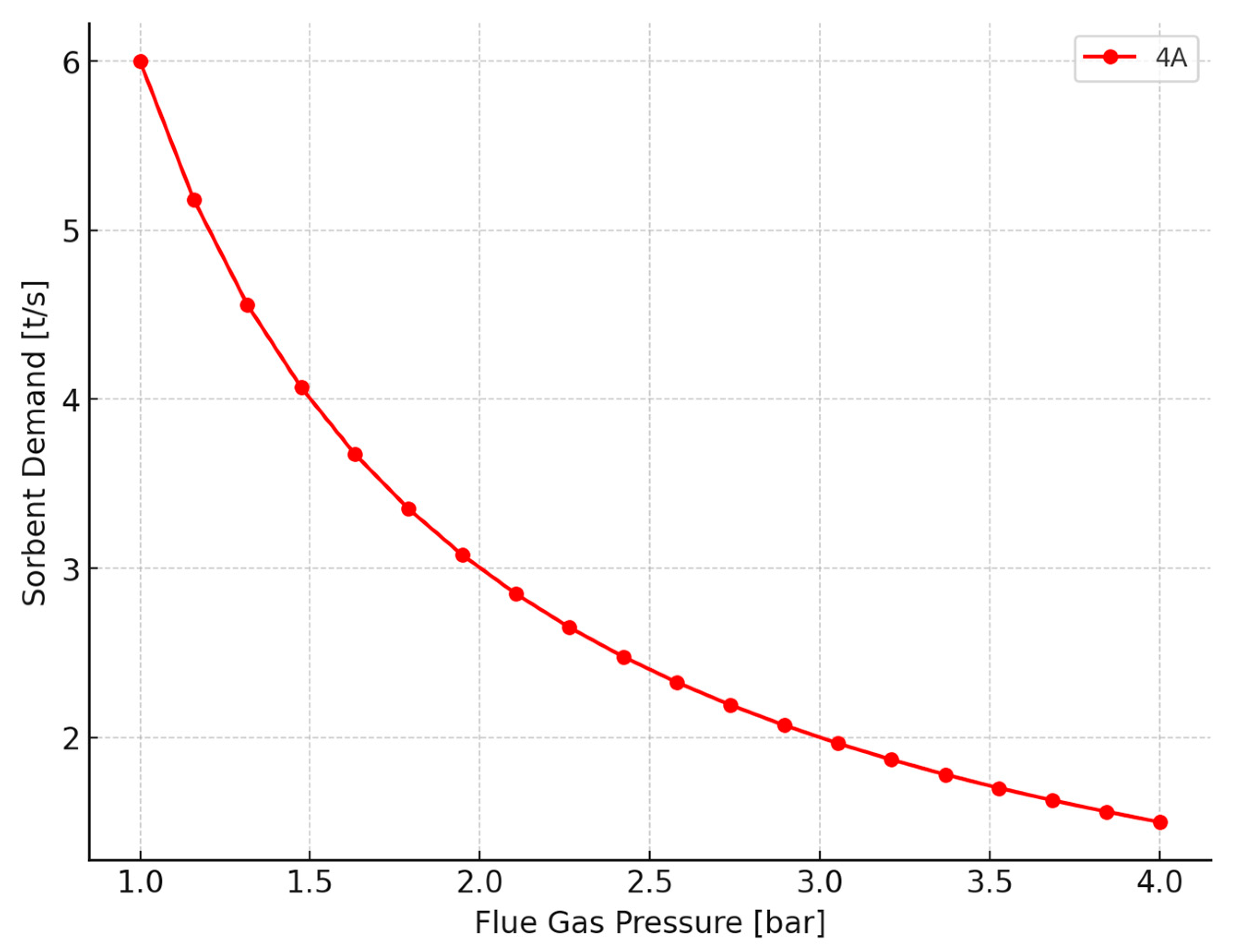
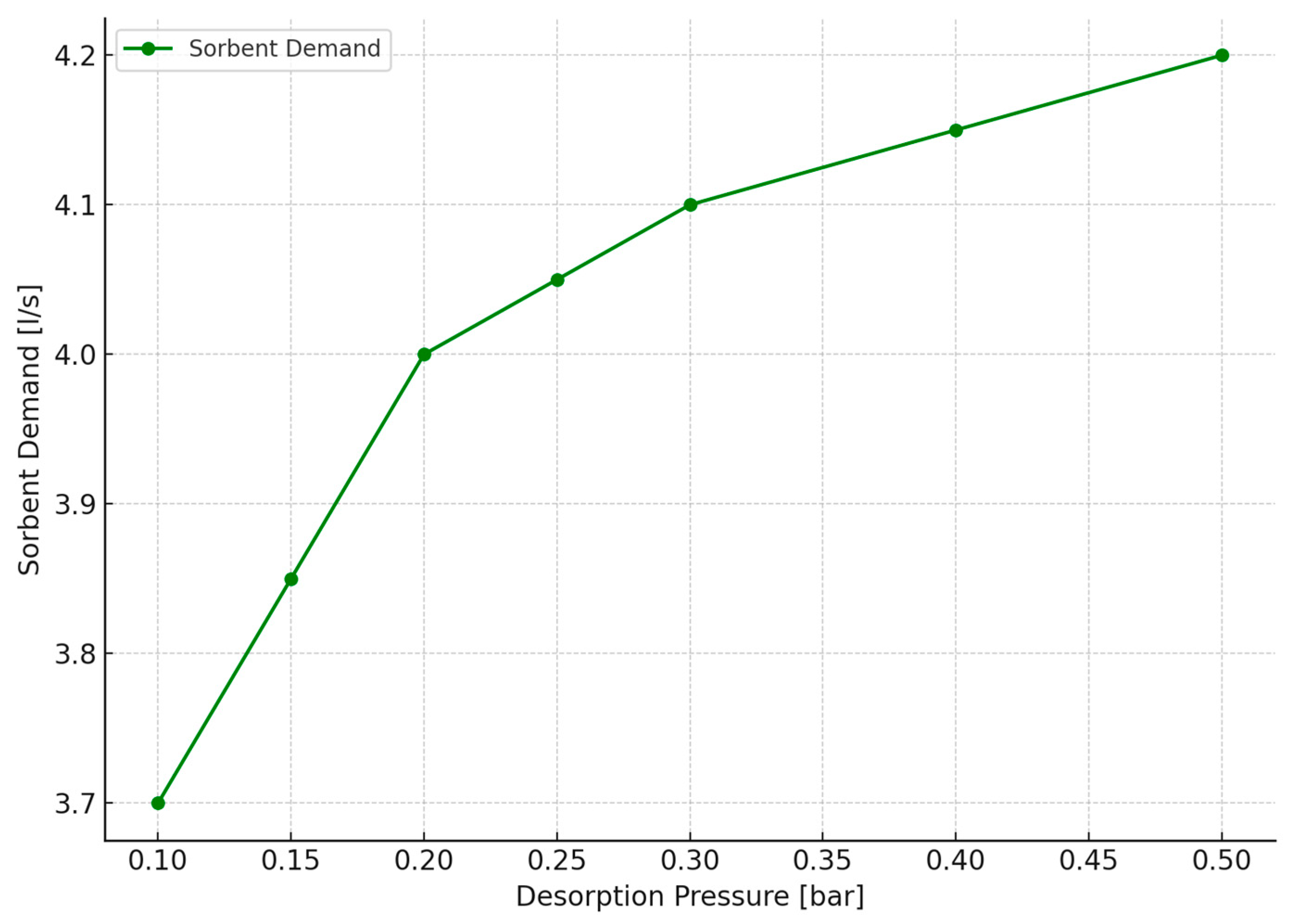
2.2. Analysis of the Operation of the CO2 Separation System of a PTSA Unit
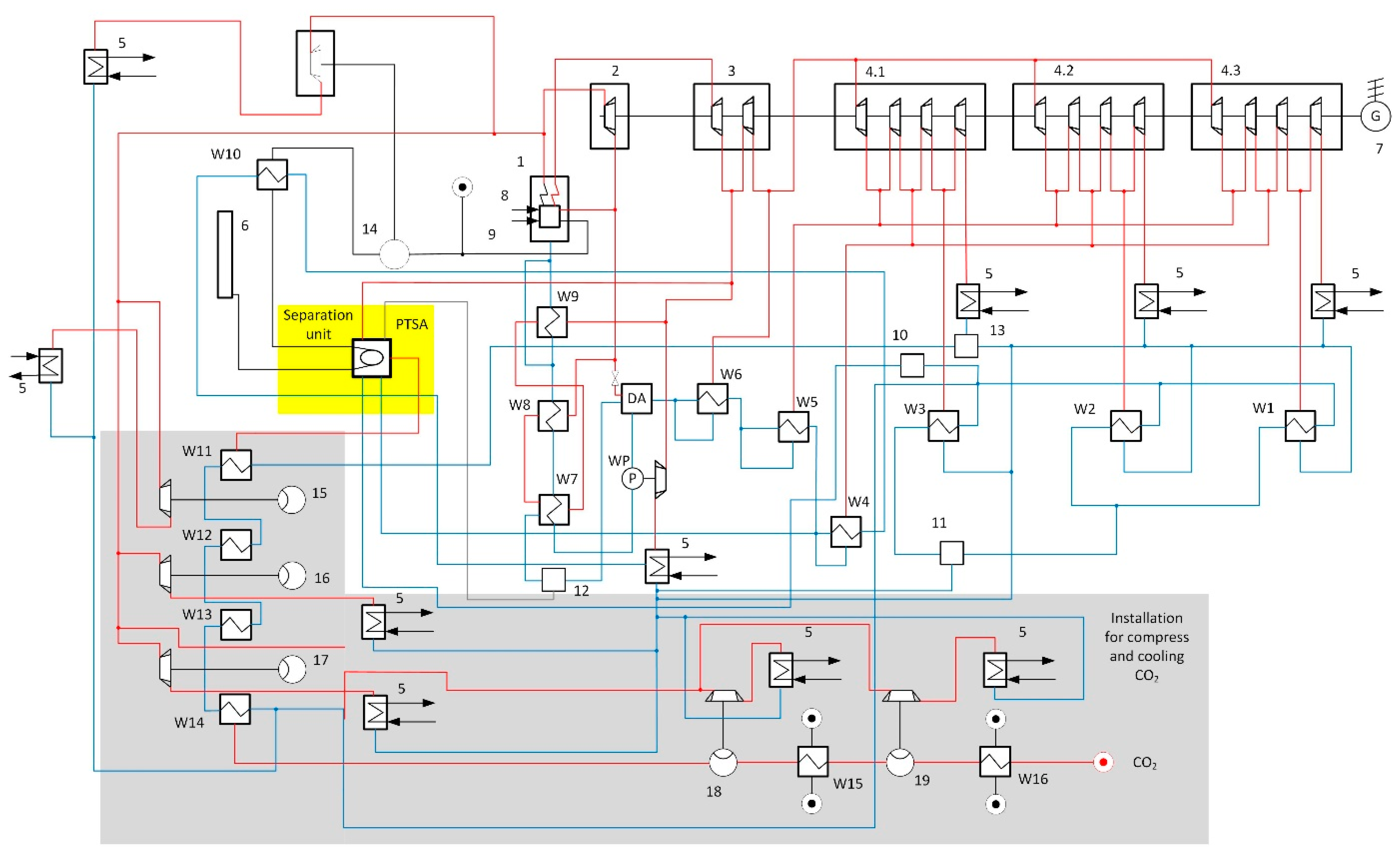
2.3. Analysis of the Integration of the CO2 Capture System into the Thermal Cycle of the Power Unit
- ✓
- Flue gas entering the separation system is free of sulphur and nitrogen oxides;
- ✓
- Flue gas is dry and dedusted;
- ✓
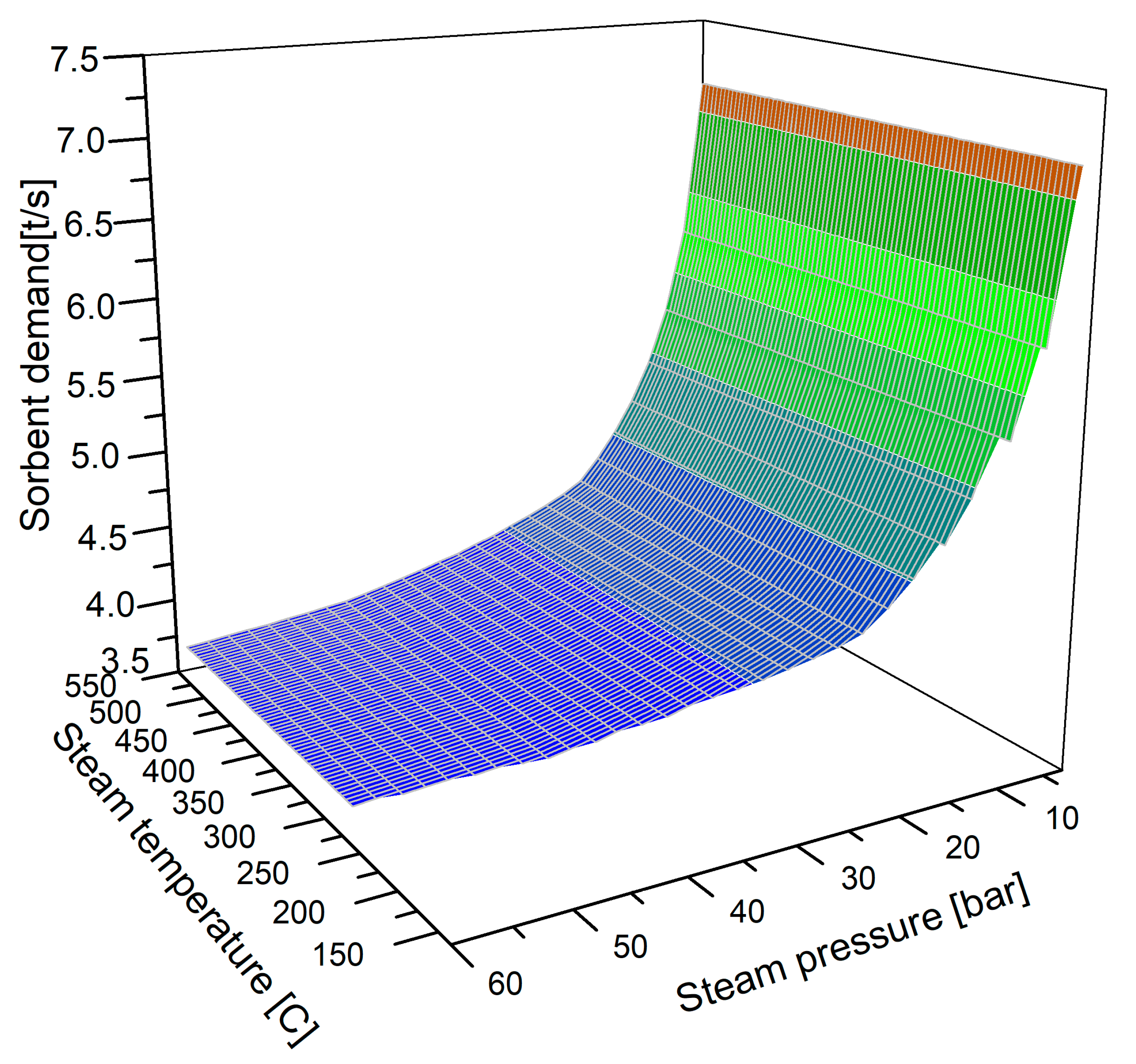
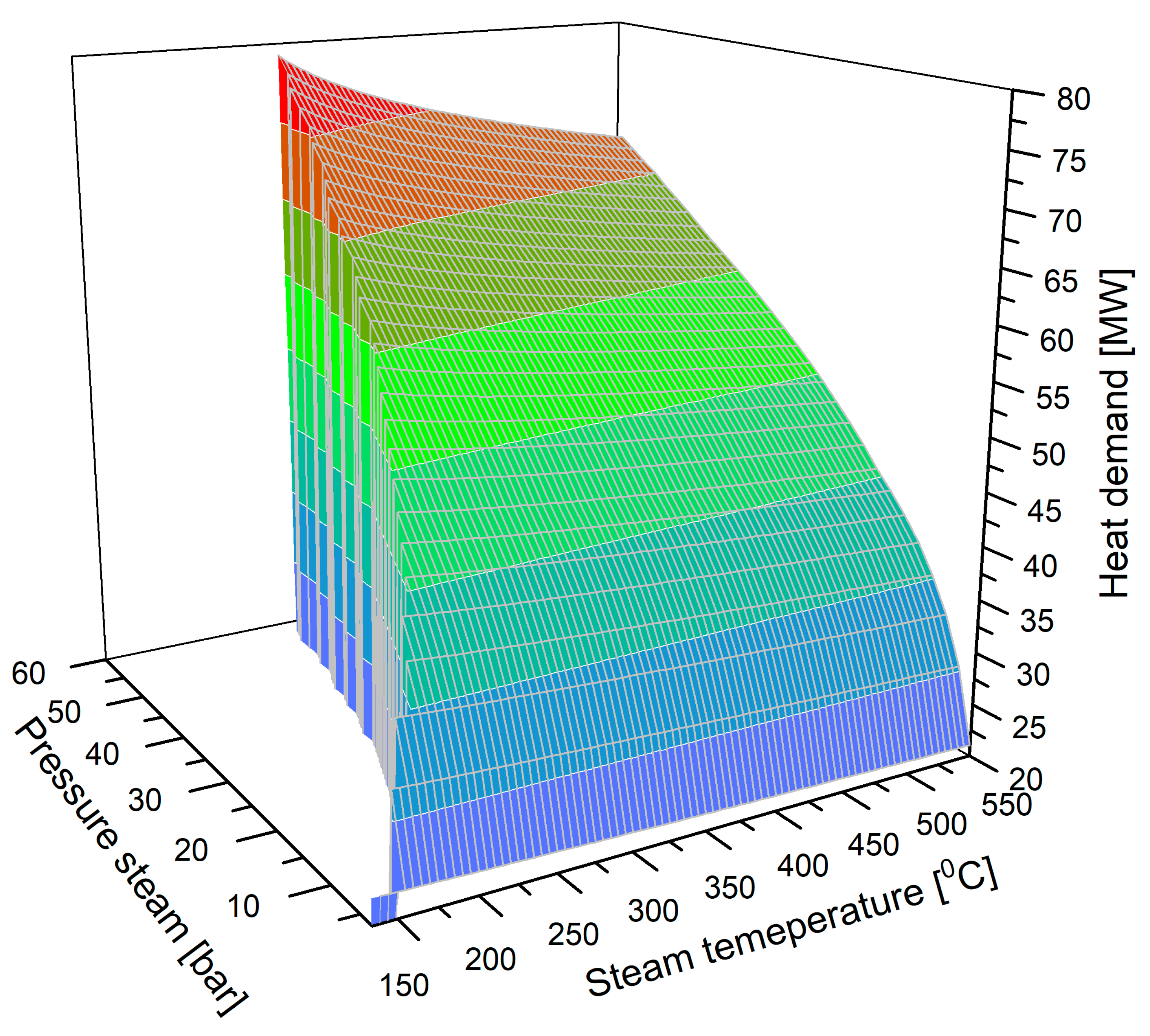
- For zeolite 4A, flue gas is introduced into the separation unit at a pressure of p = 2.0 bar and a temperature of t = 110 °C;
- ✓
- The source of heat required for the regeneration of zeolite 4A is steam with a pressure of p = 20 bar and a temperature in the range t = 200–550 °C;
- ✓
- Compression and cooling of the separated CO2 occurs.
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Symbols | |
| Mass flow [kg/s] | |
| p | Pressure [bar] |
| t | Temperature [°C] |
| h | Entalphy [J/kg] |
| Q | Sorption heat [kJ/kgCO2] |
| Heat transfer [W] | |
| a | Sorption capacity [kg/kg] |
| X | Mass share of the exhaust gas components [kg/kg] |
| Temperature drop [°C] | |
| Pressure drop [bar] | |
| Power plan efficiency [-] | |
| P | Power [W] |
| Subscripts | |
| 1 | Exhaust gas |
| 2 | Clear carbon dioxide |
| 3 | Exhaust gas without CO2 |
| 4 | Sorbent regeneration steam inlet |
| 5 | Sorbent regeneration steam outlet |
| 6 | PTSA cooling water inlet |
| 7 | PTSA cooling water outlet |
| 8 | Sorbent after desorption |
| 9 | Cooled sorbent after desorption |
| 10 | Sorbent before desorption |
| a | Adsorption |
| d | Desorption |
| trans | Heat transferred to the sorbent during adsorption |
| sorb | Sorbent |
| tr1 | Heating the sorbent during desorption |
| tr2 | Heating the CO2 during desorption |
| sp | Exhaust gas |
| g | Sorbent regeneration steam |
| ch | PTSA cooling water |
| el,gross | total electricity |
| fuel | chemical power |
| el,own cons. | electricity consumption of auxiliary equipment |
References
- Filonchyk, M.; Peterson, M.P.; Zhang, L.; Hurynovich, V.; He, Y. Greenhouse gases emissions and global climate change: Examining the influence of CO2, CH4, and N2O. Sci. Total Environ. 2024, 935, 173359. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, R.W.; Gudadhe, M.D.; Bhagat, S.L. Carbon dioxide capture, tolerance and sequestration using microalgae—A review. Int. J. Pharm. Chem. Biol. Sci. 2016, 6, 345–349. [Google Scholar]
- Tarkowski, R.; Uliasz-Misiak, B.; Tarkowski, P. Storage of hydrogen, natural gas, and carbon dioxide—Geological and legal conditions. Int. J. Hydrogen Energy 2021, 46, 20010–20022. [Google Scholar] [CrossRef]
- IPCC SRCCS. Special Report of the Intergovernmental Panel of Climate Change on Carbon Capture and Storage; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Kotowicz, J.; Janusz, K. Methods for reducing CO2 emissions from energy processes. Energy Market. 2007, 41, 171–192, ISSN 1425-5960. [Google Scholar]
- Prajapati, M.; Thesia, D.; Thesia, V.; Rakholia, R.; Tailor, J.; Patel, A.; Pardiwala, J.M.; Shah, M. Carbon capture, utilization, and storage (CCUS): A critical review towards carbon neutrality in India. Case Stud. Chem. Environ. Eng. 2024, 10, 100770. [Google Scholar] [CrossRef]
- Construction of 833 MW Power Unit at BOT-Elektrownia Bełchatów S.A.: Summary of the Environmental Impact Statement; 2006. Available online: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://www.ebrd.com/english/pages/project/eia/25438e.pdf&ved=2ahUKEwiyhbyKrf-MAxW3KhAIHc32D8EQFnoECBAQAQ&usg=AOvVaw3SgGxsFAmTlmHgERaNTgzL (accessed on 1 January 2006).
- Dreszer, K.; Solny, L.W. Reduction of CO2 emissions in the energy sector: Possible technology pathways. Energy Policy. 2008, 11, 117–129. [Google Scholar]
- CO2Europipe. Towards a Transport Infrastructure for Large-Scale CCS in European Collaborative Project; D4.4.2 WP4.4 Report, Belchatow Power Plant—Test Case; CO2Europipe: Utrecht, The Netherlands, 2009; Available online: https://www.co2europipe.eu/Publications/D4.4.2%20-%20Belchatow%20Power%20Plant%20-%20test%20case.pdf (accessed on 1 January 2025).
- Our World in Data. CO2 and Greenhouse Gas Emissions. Available online: https://ourworldindata.org/co2-and-greenhouse-gas-emissions (accessed on 1 January 2025).
- Davoodi, S.; Al-Shargabi, M.; Wood, D.A.; Rukavishnikov, V.S.; Minaev, K.M. Review of technological progress in carbon dioxide capture, storage, and utilization. J. Nat. Gas Sci. Eng. 2023, 117, 205070. [Google Scholar] [CrossRef]
- IPCC. Summary for policymakers. In Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; pp. 1–34. [Google Scholar] [CrossRef]
- McLaughlin, H.; Littlefield, A.A.; Menefee, M.; Kinzer, A.; Hull, T.; Sovacool, B.K.; Bazilian, M.D.; Kim, J.; Griffiths, S. Carbon capture utilization and storage in review: Sociotechnical implications for a carbon reliant world. Renew. Sustain. Energy Rev. 2023, 177, 113215. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Mazurkiewicz, M. Methods of CO2 separation and capture. Energy Policy 2005, 8, 527–538. [Google Scholar]
- Czakiert, T.; Nowak, W.; Bis, Z. Combustion in oxygen-modified atmospheres as a development direction for CWF boilers. Energetics Probl. Power Eng. Fuel Energy Econ. 2008, 10, 713–718, ISSN 0013-7294. [Google Scholar]
- Alami, A.H.; Alasad, S.; Ali, M.; Alshamsi, M. Investigating algae for CO2 capture and accumulation and simultaneous production of biomass for biodiesel production. Sci. Total Environ. 2021, 759, 143529. [Google Scholar] [CrossRef]
- Lu, G.; Wang, Z.; Bhatti, U.H.; Fan, X. Recent progress in carbon dioxide capture technologies: A review. Clean Energy Sci. Technol. 2023, 1, 32. [Google Scholar] [CrossRef]
- Warych, J. Gas Cleaning Processes: Design and Computational Problems; Warsaw University of Technology Press: Warsaw, Poland, 2000; p. 466. ISBN 8372071128. [Google Scholar]
- Cavalcante, C.L. Industrial adsorption separation processes: Fundamentals, modeling, and applications. Lat. Am. Appl. Res. 2000, 30, 357–364. [Google Scholar]
- Feron, P. Absorption-Based Post-Combustion Capture of Carbon Dioxide; Woodhead Publishing: Cambridge, UK, 2016. [Google Scholar]
- Xu, X.; Chunshan, S.C.; Miller, G.B.; Scaroni, W.A. Adsorption separation of carbon dioxide from flue gas of natural gas-fired boiler by a novel nanoporous molecular basket adsorbent. Fuel Process. Technol. 2005, 86, 1457–1472. [Google Scholar] [CrossRef]
- Hong, W.Y. A techno-economic review on carbon capture, utilisation and storage systems for achieving a net-zero CO2 emissions future. Carbon Capture Sci. Technol. 2022, 3, 100044. [Google Scholar] [CrossRef]
- Göttlicher, G.; Pruschek, R. Comparison of CO₂ removal systems for fossil-fuelled power plant processes. Energy Convers. Manag. 1997, 38, S173–S178. [Google Scholar] [CrossRef]
- Chmielniak, T.; Ziębik, A. (Eds.) Thermal Cycles of Supercritical Coal-Fired Units: Collective Work; Silesian University of Technology Press: Gliwice, Poland, 2010; p. 458. ISBN 978-83-7335-680-1. [Google Scholar]
- Gomes, V.G.; Yee, K.W.K. Pressure swing adsorption for carbon dioxide sequestration from exhaust gases. Sep. Purif. Technol. 2002, 28, 161–171. [Google Scholar] [CrossRef]
- Agarwal, A. Advanced Strategies for Optimal Design and Operation of Pressure Swing Adsorption Processes. Ph.D. Thesis, Carnegie Mellon University, Pittsburgh, PA, USA, 2010. Available online: https://numero.cheme.cmu.edu/content/thesis/sree_thesis.pdf (accessed on 1 January 2011).
- Majchrzak-Kucęba, I.; Nowak, W. Studies of CO2 removal from flue gases using various types of zeolites. In Proceedings of the 2nd International Conference on Contemporary Problems of Thermal Engineering, Gliwice, Poland, 23–26 September 2004. [Google Scholar]
- Ahmad, M.; Dutta, A. Recent advances in pressure swing adsorption for carbon dioxide capture. Renew. Sustain. Energy Rev. 2023, 182, 113417. [Google Scholar] [CrossRef]
- Chiang, A.S.T.; Hong, M.C. Radial flow rapid pressure swing adsorption. Adsorption 1995, 1, 153–164. [Google Scholar] [CrossRef]
- Suzuki, M.; Susuk, T.; Sakoda, A.; Izumi, J. Piston-driven ultra-rapid pressure swing adsorption. Adsorption 1996, 2, 111. [Google Scholar] [CrossRef]
- Grande, C.A.; Ribeiro, R.P.P.L.; Rodrigues, A.E. Electric Swing Adsorption for CO2 removal from flue gases. Int. J. Greenh. Gas Control. 2008, 2, 194–202. [Google Scholar] [CrossRef]
- Chevrel, C.; de Joannis, P.; Castel, C.; Authier, O. Modeling of CO2 capture by electro-swing reactive adsorption from low-concentration streams. Clean Technol. 2025, 7, 18. [Google Scholar] [CrossRef]
- Ishibashi, M.; Otake, K.; Kanamori, S. Study on CO2 removal technology from flue gas of thermal power plants by physical adsorption methods. Greenh. Gas Control Technol. 1999, 37, 929–933. [Google Scholar]
- European Commission. Proposal for a Decision of the European Parliament and of the Council on the Effort of Member States to Reduce Their Greenhouse Gas Emissions to Meet the Community’s Greenhouse Gas Emission Reduction Commitments Up to 2020. COM(2008) 17 Final, Brussels, Belgium, 2008. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:52008PC0017 (accessed on 1 January 2008).
- Warych, J. Gas Cleaning: Processes and Equipment; Scientific and Technical Publishers: Warsaw, Poland, 1998; ISBN 8320423058. [Google Scholar]
- Riboldi, L.; Bolland, O. Evaluating Pressure Swing Adsorption as a CO2 separation technique in coal-fired power plants. Int. J. Greenh. Gas Control 2015, 39, 1–16. [Google Scholar] [CrossRef]
- Wawryńczak, D.; Majchrzak-Kucęba, I.; Srokosz, K.; Kozak, M.; Nowak, W.; Zdeb, J.; Smółka, W.; Zajchowski, A. The Pilot Dual-Reflux Vacuum Pressure Swing Adsorption Unit for CO2 Capture from Flue Gas. Sep. Purif. Technol. 2019, 209, 560–570. [Google Scholar] [CrossRef]
- US. Department of Energy, National Energy Technology Laboratory (NETL). Cost and Performance Baseline for Fossil Energy Plants. Volume 1: Bituminous Coal and Natural Gas to Electricity; DOE/NETL-2023/4320; NETL: Pittsburgh, PA, USA, 2022.
- Sztekler, K.; Kalawa, W.; Nowak, W.; Stefański, S.; Krzywański, J.; Grabowska, K.; Mika, Ł. Possibility of use adsorption chillers for increase efficiency in conventional power Plant. In Proceedings of the EPJ Web of Conferences, Venice, Italy, 13–17 May 2019; Volume 213, p. 02082. [Google Scholar] [CrossRef]
- Majchrzak-Kucęba, I. Study on the Removal and Utilization of Carbon Dioxide from Boiler Flue Gases Using Zeolites. Ph.D. Thesis, Czestochowa University of Technology, Czestochowa, Poland, 26 April 2001. [Google Scholar]
- Wawryńczak, D.; Majchrzak-Kucęba, I.; Nowak, W. Possibilities of Using the Adsorption Method for CO2 Removal from Boiler Flue Gases. Sci. Pap. Rzesz. Univ. Technol. Mech. 2014, 86, 285–293. [Google Scholar]
- Mulgundmath, V.; Tezel, F.H. Optimisation of carbon dioxide recovery from flue gas in a TPSA system. Adsorption 2010, 16, 587–598. [Google Scholar] [CrossRef]
- Suchecki, T. Zeolites from Fly Ash: Production and Applications in Environmental Engineering; Ossolineum Publishing House: Wrocław, Poland, 2005. [Google Scholar]
- Majchrzak, A. Testing and Optimization of Solid Sorbents for CO2 Capture from Flue Gas. Ph.D. Thesis, AGH University of Science and Technology, Kraków, Poland, 2016. [Google Scholar]



| Adsorbent | Characteristics | Application |
|---|---|---|
| Activated carbon | Hydrophobic properties; adsorption of organic compounds from air and water; low cost compared to other adsorbents; difficulty in regeneration; attrition; blocking pores. | Removal of organic pollutants |
| Carbon molecular sieves (CMSs) | Separation based on different particle diffusivity; selective adsorption of O2 only. | Production of N2 from air |
| Silica gel | Hydrophilic adsorbent with high capacity; high sorption efficiency; incapable of removing trace amounts of water. | Gas drying, removal of hydrocarbons to a low extent |
| Activated alumina | Hydrophilic adsorbent with high capacity; high sorption efficiency; does not remove trace amounts of water. | Gas drying |
| Zeolite molecular sieves (ZMSs) | Hydrophilic properties; polar; regular pores; high adsorption selectivity; separation based on polarity and spatial structure of compounds. | Separation of air components and other mixtures, dewatering |
| Silicalite | Hydrophobic adsorbent with adsorption characteristics similar to activated carbon; burns more easily than activated carbon and is more costly in comparison therewith. | Removal of organic compounds from gases |
| Adsorbent polymers | Ordinary styrene–divinylbenzene copolymers; have good mechanical properties, minimising abrasion and erosion; more costly compared to activated carbon. | Removal of organic compounds from gases |
| Non-regenerative adsorbents | Selectively reactive; able to remove trace contaminants; economic use at low contaminant concentrations and removal of less than 4 kg/hour. | Removal of contaminants with low concentrations, such as H2S, SO2, and others from gases |
| Biosorbents | Biochemically active material deposited on a porous support; no regeneration is necessary when using this adsorbent; low sorption capacity compared with other sorbents. | Removal of organic compounds from gases |
| Power output PG | 830 | MW |
| Fresh steam pressure | 266 | bar |
| Fresh steam temperature | 554 | °C |
| Fresh steam mass flow rate | 625 | kg/s |
| Reheated steam pressure | 54 | bar |
| Reheated steam temperature | 582 | °C |
| Efficiency of the reference cycle | 45.1 | % |
| Feed water temperature | 275 | °C |
| Mass flow rate of boiler flue gas | 1000 | kg/s |
| Mass flow rate of CO2 | 170 | kg/s |
| Conditions of CO2 separation | Sorbent |
| Zeolite 4A | |
| Selected parameters of the flue gas | p = 2.0 bar |
| t = 110 °C | |
| Selected parameters of the heating steam | p = 20 bar |
| t = 200–550 °C | |
| Pressure of desorption | p = 0.15 bar |
| Parameters | Zeolite 4A |
| Power input to the flue gas compressor | 110 MW |
| Demand for the sorbent | 4200 kg/s |
| Adsorption parameters | p = 2.0 bar t = 110 °C |
| Desorption parameters | p = 0.15 bar t = 212 °C |
| Thermal power for the desorption process [MWt] | 50 MWt |
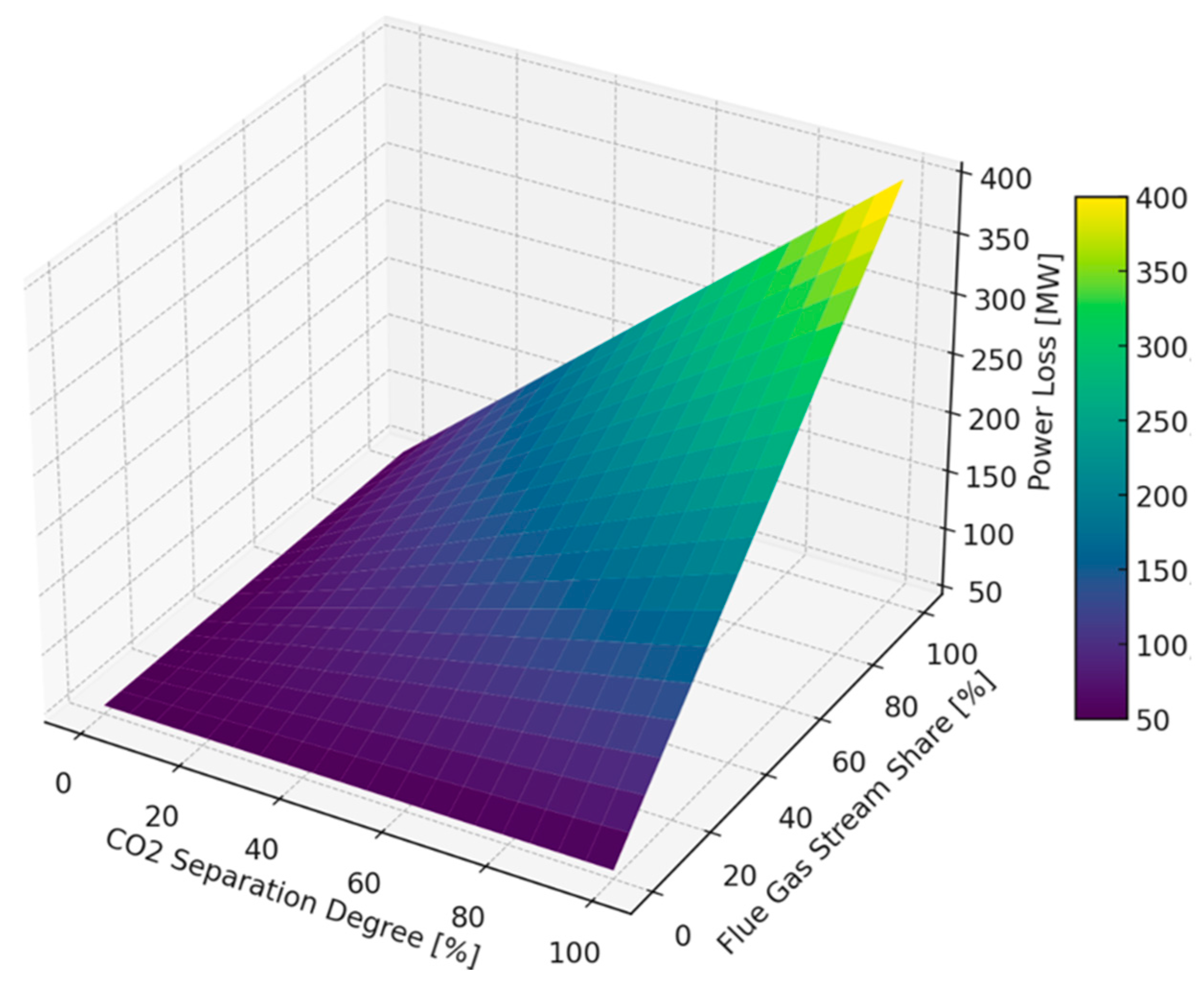
| Total decrease in power output of the unit | 358 MWe |
| Total decrease in cycle efficiency | 15.4% |
| Power demand for CO2 compressors | 90 MW |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mika, Ł.; Sztekler, K. Analysis of the Possibility of Using CO2 Capture in a Coal-Fired Power Plant. Energies 2025, 18, 2387. https://doi.org/10.3390/en18092387
Mika Ł, Sztekler K. Analysis of the Possibility of Using CO2 Capture in a Coal-Fired Power Plant. Energies. 2025; 18(9):2387. https://doi.org/10.3390/en18092387
Chicago/Turabian StyleMika, Łukasz, and Karol Sztekler. 2025. "Analysis of the Possibility of Using CO2 Capture in a Coal-Fired Power Plant" Energies 18, no. 9: 2387. https://doi.org/10.3390/en18092387
APA StyleMika, Ł., & Sztekler, K. (2025). Analysis of the Possibility of Using CO2 Capture in a Coal-Fired Power Plant. Energies, 18(9), 2387. https://doi.org/10.3390/en18092387





