3.1. Characterization of Prepared Chitosan Films
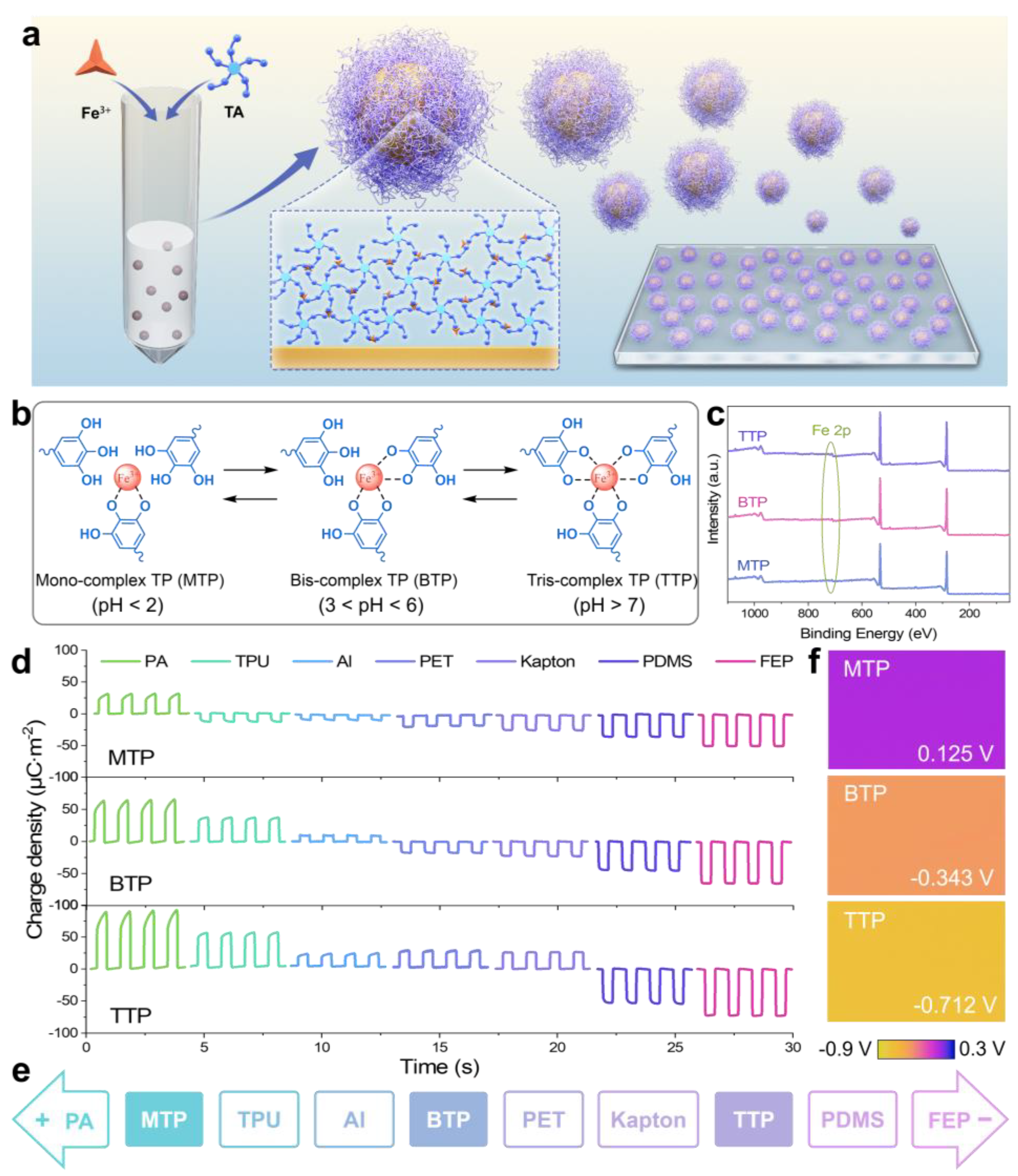
The proposed rapid preparation strategy offered a simple and efficient approach to producing TA-Fe
3+ complex-coated particle (TP)-deposited films in two steps (
Figure 1a). First, Fe
3+ and TA were sequentially added to a PS suspension at a specific ratio and thoroughly mixed. This mixture formed TA-Fe
3+ complexes that coated the PS particles (
D = 0.5 μm), creating core–shell structures. These particles were then deposited onto an ITO/PET substrate that had been soaked in a solution of FeCl
3·6H
2O, resulting in TP films. Remarkably, these processes were completed within 1.2 min, demonstrating high time efficiency (
Figure S2). Additionally, the complex state between TA and Fe
3+ was highly dependent on the pH of the solution, which affected the deprotonation of phenolic -OH groups in TA and their ability to bind with Fe
3+. As shown in
Figure 1b, TA, a polyphenolic compound, could form a mono-, bis-, or tris-complex state with Fe
3+. At pH < 3, the protonation of -OH groups inhibited coordination, favoring a mono-complex state. As the pH rose to between 3 and 6, deprotonation allowed for bis-complex formation, while at pH > 7, the tris-type complex dominated. These complex states significantly affected the key properties of the films, such as surface chemical characteristics, electron affinity, and stability, which in turn influenced their triboelectric performance.
XPS was employed to characterize the surface elemental composition of the mono-complex TP (MTP), bis-complex TP (BTP), and tris-complex TP (TTP) films. As shown in
Figure 1c, the appearance of a characteristic Fe 2p peak at approximately 711 eV confirmed the successful incorporation of Fe
3+ into these TPs. To elucidate the triboelectric performance, a 2 × 2 cm
2 TENG was fabricated using TP films paired with common triboelectric materials, including polyamide (PA), thermoplastic polyurethane (TPU), aluminum (Al), polyethylene terephthalate (PET), commercial polyimide (Kapton), polytetrafluoroethylene (PDMS), and fluorinated ethylene propylene (FEP). As shown in
Figure 1d, the relative positive or negative charge signals indicate the triboelectric charging behavior of TP upon contact with different materials. When the charge signal was positive, TP accepted electrons (
Figure S3a); conversely, when it was negative, it donated electrons (
Figure S3b). Therefore, the triboelectric polarity of three TPs were reflected in the positions of the triboelectric series (
Figure 1e). MTP was located between TPU and PA, BTP between PET and Al, and TTP between Kapton and PDMS. MTP exhibited positive triboelectric polarity, indicating its tendency to donate electrons, while BTP showed intermediate polarity, and TTP displayed the most negative polarity, demonstrating its strong electron-withdrawing capability. The maximum charge transfer densities also varied significantly, with MTP reaching 31.7 μC·m
−2 when paired with FEP, BTP achieving 63.4 μC·m
−2 when paired with PA, and TTP exhibiting the highest output of 92.5 μC·m
−2 when paired with PA.
To understand the underlying factors influencing these triboelectric properties, KPFM was employed to measure the surface potential of three TPs (
Figure 1f). The results show surface potential values of 0.125 V, −0.343 V, and −0.712 V for MTP, BTP, and TTP, respectively. A lower surface potential corresponds to a higher work function, indicating a material’s stronger ability to accept electrons. This trend aligned with the observed triboelectric series positions, where TTP, with the lowest surface potential, exhibited the highest electron affinity and most negative polarity, followed by BTP and MTP. The variation in work function and electron affinity across the three complexes further emphasizes their differences in electron-accepting ability and triboelectric polarity. MTP, with fewer coordinated -OH groups, retained a higher density of uncoordinated electron-donating sites, resulting in its positive triboelectric charging behavior. In contrast, the higher degree of cross-linking in BTP and TTP led to the delocalization of electron density and a reduction in free -OH groups, enhancing their electron affinity and resulting in negative charging behavior. The strong electron-withdrawing behavior of TTP made it particularly suitable for applications requiring high charge transfer efficiency.
3.2. Triboelectric Properties and Influence of TA
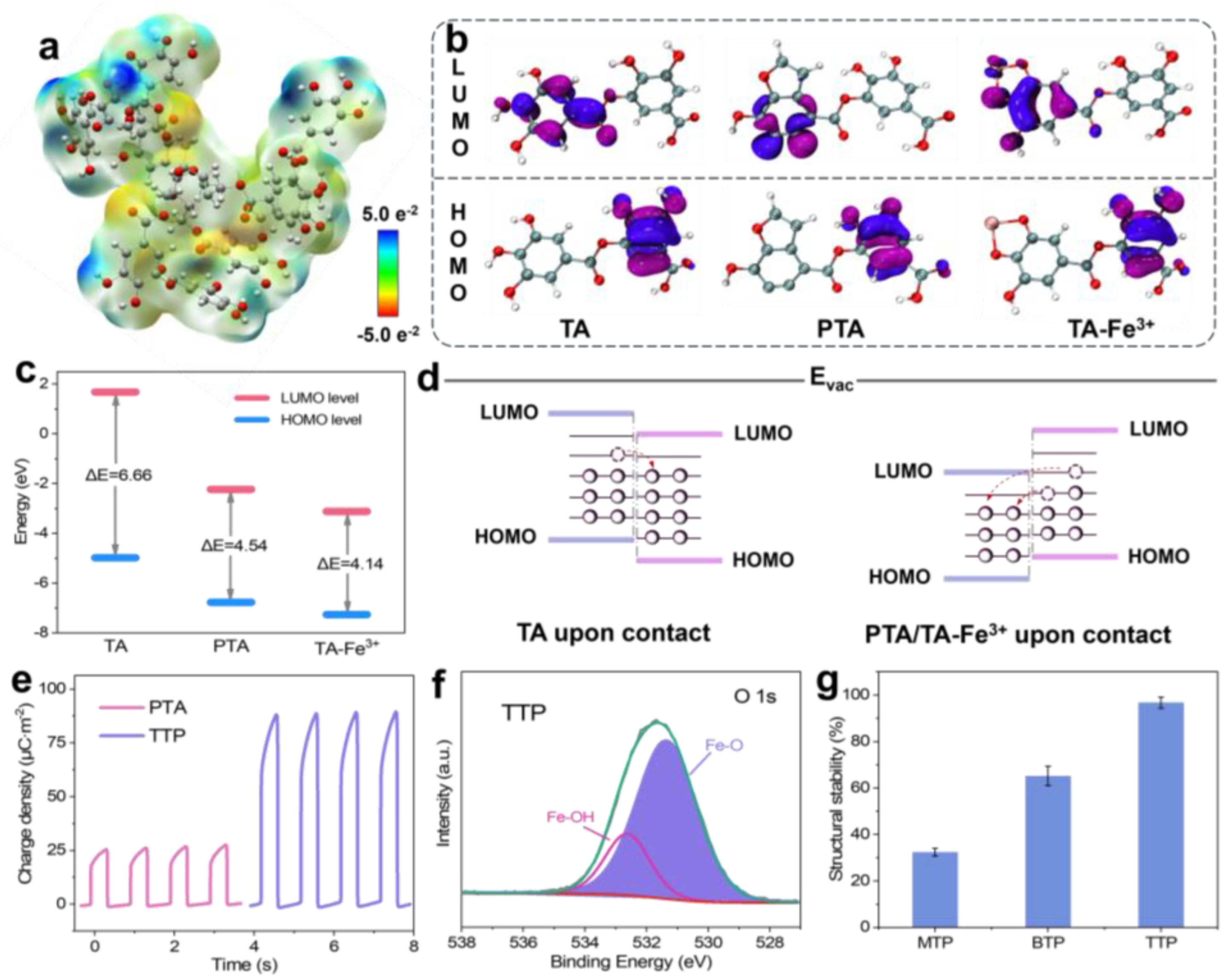
To further investigate the impact of complex states between TA and Fe
3+ on the electron density and triboelectric properties of TPs, density functional theory (DFT) was used to calculate the electrostatic potential map of TA. As shown in
Figure 2a, the blue area represents positive potential, and the red area represents negative potential, corresponding to the electron-deficient region and the electron-rich region, respectively. Notably, the -OH groups exhibited electron-donating ability, while the phenyl groups demonstrated relatively weak electron-withdrawing ability. In the mono-complex state, each Fe
3+ coordinated with a single TA molecule, resulting in a less cross-linked network with abundant uncoordinated phenolic -OH groups. These uncoordinated -OH groups retained their strong electron-donating ability, making the material more prone to losing electrons. Consequently, MTP exhibited positive triboelectric charging behavior. As the coordination progressed to the tris-complex state, each Fe
3+ coordinated with three TA molecules. This resulted in a densely cross-linked network that delocalized electron density and reduced the availability of free -OH groups, restricting their electron-donating capabilities. This delocalization enhanced the material’s electron affinity, making it more inclined to accept electrons during triboelectric interactions. Therefore, TTP exhibited negative triboelectric charging behavior.
In the absence of Fe
3+, deprotonated TA undergoes oxidative self-polymerization to form poly(tannic acid) (PTA), as illustrated in
Figure S4. In this process, the deprotonated TA molecules transformed into quinone structures, which then polymerized [
34,
35]. Further energy level analysis (
Figure 2b) showed the lowest unoccupied molecular orbital (LUMO) and the highest occupied molecular orbital (HOMO) energy level of TA, PTA, and TA-Fe
3+ calculated by DFT simulation. Specifically, all calculations were performed using Gaussian 09 software with the B3LYP functional and the 6-31G(d) basis set. Notably, the LUMO level of TA-Fe
3+ was lower (−3.12 eV) than that of TA (1.68 eV) and PTA (−2.23 eV) (
Figure 2c). The LUMO, which is the electron acceptor orbital of materials, has a lower energy level, indicating higher electron affinity and facilitating electron transfer from materials with a higher HOMO level upon contact [
23,
36,
37,
38]. In triboelectric terms, electrons preferentially transferred from the pair material (e.g., PA) to PTA or TTP, as depicted in
Figure 2d. Using paper as a supporting substrate, it was soaked in TA solution and then dried to obtain the triboelectric film of PTA. Triboelectric tests demonstrated that PTA shifted the triboelectric polarity of paper toward a negative state (
Figure S5), indicating the successful preparation of PTA and its electron-withdrawing behavior. However, compared to PTA, TTP exhibited stronger electron-withdrawing capability and higher triboelectric output performance (
Figure 2e). These results indicate that the introduction of Fe
3+ not only restricted the mobility of electron-donating -OH groups by forming coordination bonds but also lowered the LUMO energy level, resulting in TTP’s negative tendency that has stronger electron affinity.
In addition to influencing triboelectric properties, the complex morphology formed by Fe
3+ and TA significantly enhanced the structural stability of TTP. From the deconvoluted O 1s XPS spectra (
Figure 2f;
Figure S6), peaks appearing at approximately 531.2 eV and 533.0 eV were assigned to Fe-O and Fe-OH species, respectively. The Fe-O peak arose from the coordination between TA and Fe
3+, as previously reported [
33,
39]. A higher proportion of Fe-O groups indicated a more stable complex state because these bonds strengthened the network structure of the material. In the case of TTP, with a 72.3% Fe-O proportion (
Table S1), the material exhibited enhanced stability due to the densely cross-linked network. To evaluate this stability, materials deposited with the three types of complexes are immersed in water for 2 h. The intensity of the ultraviolet absorption peak at 276 nm in the aqueous solution was measured to determine the amount of material that had dissolved or detached from the substrate. By comparing the absorbance with that of a standard solution, structural stability was calculated accordingly. The characteristic absorption curves of standard solutions at different concentrations are presented in
Figure S7. As shown in
Figure 2g, TTP demonstrated a stability of approximately 96.8%, which signifies exceptional stability. This high stability was attributed to the higher density of the cross-linked network in TTP, which resisted dissolution and degradation in aqueous environments. Therefore, the complexation between TA and Fe
3+ played a crucial role in determining the triboelectric properties and structural stability of TPs. Progressing from a mono- to tris-complex state enhanced cross-linking and increased electron affinity, inducing a shift from positive to negative triboelectric charging behavior. Additionally, a cyclic durability test was conducted to assess the long-term electrical stability of the three TP-based TENGs. As shown in
Figure S8, each TENG endured 2500 continuous contact–separation cycles under 1 Hz, during which the output signals remained effectively unchanged. These results confirm the robustness and practical reliability of the TENGs under prolonged operation.
However, the long-term stability of TA-Fe3+ complexes under ambient or humid conditions may be affected due to the reversible nature of phenolic–metal coordination, potential hydrolysis, or competitive binding with environmental ions, leading to degraded triboelectric performance. To address this, future improvements may be achieved through cross-linking stabilization or surface encapsulation strategies to enhance environmental durability. Moreover, although the TP method allows rapid and uniform film formation at the laboratory scale, scaling it up to large-area or continuous production may pose challenges in ensuring consistent particle distribution, coordination control, and film adhesion. As a potential solution, the TP process could be integrated with scalable techniques such as spray coating or 3D bioprinting to facilitate large-area production and versatile device integration.
3.3. Micromorphology and High Performance of TTP
The surface morphology of TPs with different complex states was also characterized by utilizing SEM (
Figure 3a). It was shown that BTP and TTP were completely coated with the TA-Fe
3+ films, whereas MTP exhibited incomplete coverage, suggesting a weaker binding affinity in the mono-complex state. This incomplete surface was correlated with the relatively poorer triboelectric performance observed in MTP. Moreover, BTP and TTP had distinctly thicker films coupled with a denser cross-linked network. TEM confirmed the formation of a continuous TA-Fe
3+ film fully enveloping the surface of TTP (
Figure S9), verifying the successful creation of the coating layer essential for surface integrity and functionality. Additionally, the thickness of TA-Fe
3+ films was determined by direct measurement on the high-resolution TEM images of TPs, as illustrated in
Figure 3b and
Figure S10. The measured thicknesses were 9.0 ± 2.3 nm, 18.7 ± 3.2 nm, and 23.6 ± 1.7 nm for MTP, BTP, and TTP, respectively (
Figure 3c). To explore the effect of the Fe
3+-to-TA ratio on film thickness, the FeCl
3·6H
2O concentration was varied, resulting in an increase in film thickness from 13.8 ± 1.6 nm to 23.6 ± 1.7 nm (
Figure 3d). Saturation was reached at three molar equivalents of TA (2.0 mg mL
−1 [FeCl
3·6H
2O]), beyond which a further increase in Fe
3+ did not increase the thickness. Energy-dispersive X-ray spectroscopy (EDS) further demonstrated the uniform dispersion of Fe
3+ on the TP surfaces (
Figure S11). All three TPs exhibited a comparable Fe
3+ content, at approximately 0.4%, indicating consistent metal incorporation across the complexes.
TTPs were fabricated using PS particles of various sizes (0.1 μm, 0.2 μm, 0.5 μm, 1 μm, and 2 μm) for comparative analysis, with the triboelectric performance evaluated through contact with PA. Among these, the 0.5 μm diameter particle used in this study exhibited the highest charge transfer of 92.5 μC·m
−2 (
Figure 3e). This optimized performance may be attributed to balanced coated area, density, and suitable particle size, which enhance contact area and charge induction during triboelectric interactions. The electrical output properties of the TTP-PA pair were analyzed across various frequencies and areas, as illustrated in
Figure S12. The short-circuit current density (
) increased steadily from 8.6 mA·m
−2 to 22.7 mA·m
−2 as the frequency rose from 1 Hz to 3 Hz, attributed to a higher rate of charge transfer events. Additionally, the open-circuit voltage (
) increased with larger contact areas, reaching output voltages of 104 V to 157 V for contact areas of 1 × 1 cm
2 to 3 × 3 cm
2, respectively. Building on this high performance, the relationship between the instantaneous peak power density and external resistance of the TTP-TENG was examined under 1 Hz (
Figure 3f), with the circuit configuration illustrated in
Figure S13. In this setup, the TENG was directly connected to the external load, and the output voltage (
) across the load was monitored using an oscilloscope. It should be noted that this measured voltage represents the load voltage rather than the open-circuit voltage. The results show a maximum instantaneous power density of 533.7 mW·m
−2 under an external resistance of 40 MΩ. To demonstrate the availability of the TENG, the device was integrated with a full-wave bridge rectifier to charge various aluminum electrolytic capacitors (the circuit diagram is illustrated in
Figure S14). As shown in
Figure 3g, a 2 × 2 cm
2 TENG operating at a frequency of 2 Hz charged a 0.47 μF capacitor to 5 V in 15 s and another 0.47 μF capacitor in 62 s. Additionally, a 1.0 μF capacitor reached 4 V in 100 s. These results demonstrate the outstanding capability of TTP for efficient energy harvesting and storage, suggesting its potential for practical applications in low-power electronic devices.
The potential of TTP paired with other biomaterials to construct bio-TENGs was also explored to advance sustainable green energy harvesting technologies. Three bio-based films, including gelatin, chitosan, and sodium alginate, were employed to make contact with TTP, and their effectiveness regarding triboelectric performance was evaluated, leveraging their known positive triboelectric polarity [
40]. Among the three bio-TENGs, the TTP–chitosan pair exhibited the highest transfer charge density of 37.5 μC·m
−2, surpassing both the gelatin and sodium alginate counterparts (
Figure 3h). This enhanced performance may be attributed to chitosan’s unique amine groups, which facilitate more efficient charge transfer and accumulation during contact electrifications. Further analysis revealed that the TTP–gelatin, TTP–chitosan, and TTP–sodium alginate pairs achieved power outputs of 74.5 mW·m
−2, 228.9 mW·m
−2, and 175.2 mW·m
−2, respectively (
Figure 3i). The TTP–chitosan pair exhibited the highest power output, aligning with charge density tests. To demonstrate availability, a 5 × 5 cm
2 TTP–chitosan TENG was constructed and successfully powered 120 LEDs connected in series (
Figure 3j). This demonstration validated the laboratory-scale performance and feasibility of integrating bio-TENGs into practical energy solutions. The biological advantages of biomaterial-based components enhance their appeal, making bio-TENGs ideal for green energy and medical applications that require compatibility with living tissues and minimal environmental impact.
3.4. Rapid TP Preparation with High Time Efficiency
In the quest for advancing TENG technologies, fast preparation emerges as a critical factor that can significantly impact the practicality of TENGs. To demonstrate the advantages of the TP method in terms of time efficiency, the LbL method was utilized for comparison purposes. Leveraging the strong surface affinity of the phenolic groups, TA-Fe
3+ films were directly deposited onto ITO/PET substrates through sequential immersion. The preparation process for the cyclic coating method is schematically illustrated in
Figure S15, while the growth process of the coating films is shown in
Figure 4a. In this method, the substrate was alternately immersed in Fe
3+ and TA solutions for several minutes per cycle, repeatedly producing TA-Fe
3+ films with varying thicknesses. The SEM images (
Figure 4b) indicate that the coating surface remained compact and continuous for up to eight cycles. Beyond twelve cycles, the top layer became porous and uneven, with visible particulate matter, indicating a deterioration in structural integrity.
An XPS analysis (
Figure S16) suggested that the LbL films possessed a comparable surface chemical composition between TP films. As shown in
Figure 4c, ellipsometry measurements revealed that the film thickness increased significantly during the initial cycles, with each modification adding approximately 15 nm, reaching a total thickness of about 125 nm after eight cycles. After that, the structure exhibited increased porosity, which diminished the efficiency of subsequent layering and adversely affected film quality. Furthermore, PA was employed to make contact with LbL film to characterize its triboelectric performance. As shown in
Figure 4d, increasing the coating thickness enhanced the transfer charge density, reaching approximately 45.6 μC·m
−2 at the eighth cycle. However, exceeding eight cycles led to performance declines due to increased looseness and porosity in the upper layers. During contact–separation processes, loose particles could detach and adhere to the counter material, negatively impacting output performance and stability. Structural stability tests (
Figure 4e) confirmed that it was significantly decreased after eight cycles, as more unstable complexes on the surface became prone to detachment and dissolution in water, reducing stability and durability. Consequently, an eight-cycle number yielded good structural stability and optimal output performance for the LbL method.
To evaluate the triboelectric performance of the TP strategy against the LbL method, the triboelectric outputs of both TP and LbL (eight cycles) films across three complex states were compared, alongside pristine ITO/PET and PS-modified ITO/PET films (
Figure 4f). Notably, films prepared using the TP method in each complex state demonstrated significantly higher output performance than those prepared using the LbL method, with TTP achieving the highest transfer charge density. Although both methods produced films with similar chemical compositions, which exhibited the same triboelectric polarity, the TP method outperformed the LbL method in triboelectric performance. This was attributed to the fact that triboelectric performance is affected not only by the difference in triboelectric polarity but also by the internal dielectric properties of the materials [
16,
27]. LbL films were limited to increasing the coating thickness beyond 150 nm without compromising structural stability, which prevented them from achieving ideal dielectric properties. In contrast, the TP method achieved optimal dielectric properties and stability by selecting appropriate PS particle sizes, resulting in superior triboelectric output.
The TP method significantly enhanced producing efficiency, reducing the preparation time from approximately 40 min with the LbL method to just 1.2 min (
Figure 4g). This comparison reflects the theoretical minimum preparation time for each method, calculated based on standardized procedural steps and expected operation durations. This substantial reduction in time enhances productivity and lowers operational costs, highlighting its practical advantages. Moreover, the TP method rapidly produced stable, high-performance films, enabling innovative applications in sustainable energy harvesting and wearable electronics. Importantly, by tuning the polyphenol or metal ion, the TP strategy can be extended to diverse polyphenol–metal coordination systems, thereby enabling precise customization of the resulting complexes’ triboelectric properties. The compositional diversity revealed in this study offers a promising route for tailoring triboelectric biomaterials to specific applications and underpins the creation of multifunctional sensing platforms for varied physiological and environmental monitoring demands. Moreover, the intrinsic biocompatibility and microporous coating architecture of these coordination systems facilitate their integration with triboelectric and microelectronic devices, enabling stimuli-responsive in vivo drug delivery with exact spatial and temporal control and providing minimally invasive platforms for advanced therapeutic interventions and real-time physiological monitoring.
3.5. Application of TTP on pH and Ion Sensitivity
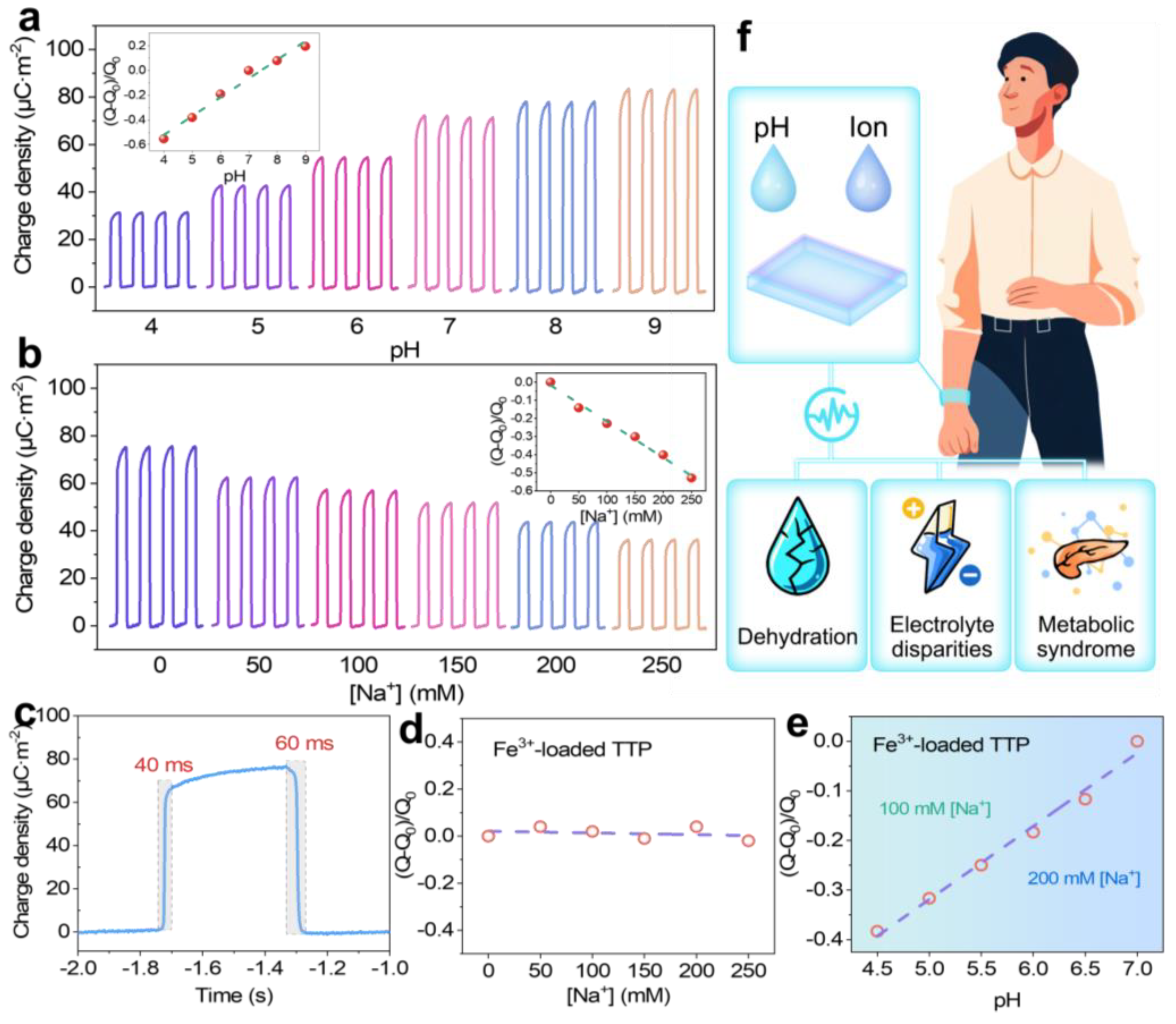
In addition to its bio-TENG application, the significant pH sensitivity of TTP makes it an effective and versatile pH sensor. This sensitivity arises from the protonation and deprotonation of the phenolic -OH groups in TA, which alter the material’s triboelectric polarity under different pH conditions. To evaluate this property, TTP was wetted with solutions of varying pH levels, and the transfer charge was measured after the films dried. As shown in
Figure 5a, the transfer charge gradually increased as the pH increased from 4 to 9. This linear increase indicated a direct correlation between the pH level and the electrical signal generated by TTP, demonstrating their potential for pH sensing applications. Moreover, TTP also functioned effectively as an ion sensor due to its ability to interact with various ionic species. Specifically, it displayed a linear response to Na
+ concentrations ranging from 0 to 250 mM, as illustrated in
Figure 5b. This concentration range encompassed the typical Na
+ levels found in human sweat [
41], making TTP particularly suitable for monitoring electrolyte balance in physiological conditions. The response times of the sensor were approximately 40 ms and 60 ms, respectively, during the contact and separation process (
Figure 5c). This rapid response is crucial for applications requiring immediate feedback, such as athletic performance monitoring or medical diagnostics. However, the dual sensitivity of TTP to both pH and ionic concentration posed challenges when attempting to measure one parameter in environments where both variables fluctuate simultaneously, such as human sweat. In such complex environments, it became difficult to isolate the influence of pH from that of ion concentration on the sensor’s electrical output, potentially leading to inaccurate readings.
To address the challenge of ion interference in pH sensing, a modified version of the TTP sensor was developed by saturating its surfaces with Fe
3+, resulting in Fe
3+-loaded TTP. This strategic modification was designed to eliminate the confounding effects of Na
+ while preserving the sensor’s sensitivity to pH changes. The modification process involved coordinating Fe
3+ with the TA molecules, occupying the binding sites that would typically interact with Na
+. Thus, the Fe
3+-loaded TTP sensor remained selective to pH variations, unaffected by Na
+ concentration changes, as demonstrated in
Figure 5d. Human sweat typically maintains a pH range between 4.5 and 7.0, categorizing it as slightly acidic [
42]. The Fe
3+-loaded TTP sensor could effectively detect pH changes within this range.
Figure 5e demonstrated that the sensor maintained consistent pH sensitivity across varying concentrations of Na
+, ensuring reliable performance in real-world conditions where electrolyte concentrations can fluctuate significantly. Through a thoughtful and integrated design, the pH and ion sensors developed in this study offer the potential to create highly efficient sweat sensors essential for health monitoring. Changes in sweat can serve as indicators of dehydration levels, electrolyte disparities, or metabolic syndrome like diabetes (
Figure 5f). The accurate TTP sensor enables early detection and management of these conditions. It is simple: a flexible design fits into wearables such as smart textiles or wristbands, allowing for non-invasive, continuous monitoring while enhancing comfort and user compliance.