Advances and Challenges in Biohydrogen Production by Photosynthetic Microorganisms
Abstract
1. Introduction
1.1. Microalgae and Cyanobacteria
1.1.1. Microalgal Strains
Nutritional Strategies for Production and Improvement of H2 in Microalgal Strains
| Microalgal Strain | Main Characteristics | H2 Production Methods | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| Chlamydomonas reinhardtii | Model organism for H2 production | Direct pathway (PSII) and indirect pathway (carbohydrate catabolism) | Easy to cultivate and manipulate | O2-sensitive; requires sulfur deprivation | [7,8,9] |
| Chlorella vulgaris | High ability to produce H2 from carbohydrates | Carbohydrate catabolism, production in both light and dark | High biomass and H2 production | Requires sugar-containing media. Augmented risk of contamination | [7,11] |
| Scenedesmus obliquus | Increased H2 production under potassium deprivation | PSII-independent pathway | Higher production without O₂ generation | Requires specific deprivation conditions | [7,12,13,14] |
| Synechocystis PCC 6803 | Model cyanobacterium, genetically manipulable | Indirect light-driven and anaerobic metabolism production | Acclimatable to various environmental conditions | Limited production in some mutations. High risk of grazing by Poterioochromonas | [15] |
1.1.2. H2 Production in the Cyanobacterium Synechosyctis PCC 6803
| Microalgae | Growth Conditions | H2 Production Conditions | H2 Production | References |
|---|---|---|---|---|
| Closterium moniliferum AARL G041 | Jaworski’s medium (JM), autotrophically, 30.8 μE/m2/s, 25 °C | Sulfur-free JM medium (JM-S), 54 μE/m2/s 25 °C, under Ar, 60 mL vial serum bottles | 0.38 mmol/h/mg (Chl) | [7] |
| Chlorella vulgaris | Artificial wastewater medium, immobilized, 140 μE/m2/s, 25 ± 1 °C | Wastewater medium 10 g/L glucose, sulfur deprivation, 140 μE/m2/s, purple light, 25 ± 1 °C, under N2 atmosphere, pH 8 | 1.63 mL/L/h (or 39.18 mL/L/day) | [19] |
| Scenedesmus obliquus | Artificial wastewater medium, immobilized, 140 μE/m2/s, 25 ± 1 °C | Wastewater medium 10 g/L glucose, sulfur deprivation, 140 μE/m2/s, purple light, 25 ± 1 °C, under N2 atmosphere, pH 8 | 8.53 mL/L/h (or 204.8 mL/L/day) | [19] |
| Chlorella pyrenoidosa | Tris-acetate-phosphate (TAP) medium þ 10 mM NaHCO3 (TCP medium), immobilized, pH 7, 180 ± 10 μE/m2/s, 28 °C, a 3.925 L airlift PBR | TCP medium þ injection of 10 mM DCMU (3-(3,4-dichlorophenyl)-1,1-dimethylurea), after 9 h injection, under N2, under 24 h darkness then 180 ± 10 μE/m2/s, 28 °C, anaerobic bottles with 75 mL TCP medium | 93.86 mL/L | [20] |
| Tetraspora sp. CU2551 | Tris-acetate-phosphate (TAP) medium, immobilized, pH 7.2, 29 μE/m2/s, 36 °C, 125 mL Erlenmeyer flasks | Sulfur-free TAP medium (TAP-S), under air, pH 7.2, 29 μE/m2/s, 36 °C, 100 mL gas-tight vials | 0.182 ± 0.020 mmol/h/mg dry wt | [21] |
| Chlorella lewinii KU201 | Tris-acetate-phosphate (TAP) medium 0.7 mM NH4Cl, 25 °C, 5 μE/m2/s, 14:10 h light/dark cycle, pH 7.3 | Sulfur-free TAP medium (TAP-S) 0.7 mM NH4Cl, 25 °C, 35 μE/m2/s, pH 7.3, under Ar, 650 mL bioreactors | 13.03 mL/L | [22] |
| Chlorella sp. KU209 | Tris-acetate-phosphate (TAP) medium þ 0.7 mM NH4Cl, 25 °C, 35 μE/m2/s, 14:10 h light/dark cycle, pH 7.3 | Sulfur-free TAP medium (TAP-S) þ 0.7 mM NH4Cl, 25 °C, 35 μE/m2/s, pH 7.3, under Ar, 650 mL bioreactors | 12.67 mL/L | [22] |
| Chlorella sorokiniana | KU204 tris-acetate-phosphate (TAP) medium 0.7 mM NH4Cl, 25 °C, 35 μE/m2/s, 14:10 h light/dark cycle, pH 7.3 | Sulfur-free TAP medium (TAP-S) 0.7 mM NH4Cl, 25 °C, 35 μE/m2/s, pH 7.3, under Ar, 650 mL bioreactors | Maximum H2 1.30 mL/L/h, total H2 89.64 mL/L | [22] |
| Cyanobacteria | Growth Conditions | H2 Production Conditions | H2 Production | References |
|---|---|---|---|---|
| Immobilized Synechocystis PCC 6803 | BG11 medium, 28 °C, 70 μE/m2/s, 97% air 3% CO2, Glass tube (400 mL) | Nitrogen-free medium BG110-Tris, 60 μE/m2/s, 28 °C, under N2 atmosphere, serum vials (60 mL) | Total H2 5.80 ± 0.14 mL or maximum H2 5.73 ± 0.69 mL/mg cells | [23] |
| Synechocystis sp. PCC 6803 | BG11 medium, 28 °C, 70 μE/m2/s, 97% air 3% CO2, Glass tube (400 mL) | BG11 medium, 28 °C, 70 μE/m2/s, use of oxygen absorber to create anaerobiosis | 3–5 mL/g/day (direct biophotolysis) | Faraloni et al. (unpublished) |
| Fischerella muscicola | BG11 medium, pH 7.5, bubbling with air, 50 μE/m2/s, 30 °C | Sulfur- and nitrogen-free BG11 medium (BG110-s medium) 0.1% glucose, under Ar, optimal pH 7.5, 25 °C, 250 μE/m2/s, 20 mL vial | 0.35 ± 0.08 mmol/g(Chl)/h | [24] |
| Nostoc calcicola | BG11 medium, pH 7.5, bubbling with air, 50 μE/m2/s, 30 °C | Nitrate-free BG11 medium (BG110 medium) 0.1% glucose, under Ar, optimal pH 7.5, 25 °C, 250 μE/m2/s 20 mL vial | 0.09 ± 0.01 mmol/g(Chl)/h | [24] |
| Scytonema bohneri | BG11 medium, pH 7.5, bubbling with air, 50 μE/m2/s, 30 °C | Nitrate-free BG11 medium (BG110 medium) 0.3% glucose, under Ar, optimal pH 7.5, 25 °C, 250 μE/m2/s, 20 mL vial | 0.09 ± 0.01 mmol/mg(Chl)/h | [24] |
| Tolypothrix distorta | BG11 medium, pH 7.5, bubbling with air, 50 μE/m2/s, 30 °C | Nitrate-free BG11 medium (BG110 medium) 0.1% glucose, under Ar, optimal pH 7.5, 25 °C, 250 μE/m2/s, 20 mL vial | 0.21 ± 0.05 mmol/mg(Chl)/h | [24] |
| Geitlerinema sp. RMK-SH10 | ASN III medium, 30 μE/m2/s, 30 °C Nitrogen-free ASN IIIeN medium 0.2 M NaCl, 18.9 mmol C-atom/L glucose 0.1 mM Ni2, 30 °C | Under dark and Ar atmosphere, a 10 mL gas-tight vial | 0.271 mmol/h/mg dry wt | [25] |
| Anabaena sp. PCC 7120 | DHup 8-times diluted Allen and Arnon medium (AA/8), 30 μE/m2/s, 27 °C, 99% air 1% CO2 | A total of 8-times diluted Allen and Arnon medium without nitrogen (AA/8-N), 290 and 340 μE/m2/s, Ar with 5% CO2, and 3.3% N2, pH 8.2, 22 °C, plastic bags (1 L) | 0.86 mL/h/L or 20.6 mL/day/L or 33.2 mL/L/during 5 days | [26] |
1.2. Enzymes
2. Strategies to Optimize H2 Production
2.1. Genetic Engineering
2.2. Co-Cultivation with Bacteria
2.3. Use of Wastewater
2.4. Light Utilization
2.5. Challenges in Scaling Up of Photobiological H2 Production
3. Potential Use of Microalgae for H2 Production as By-Products
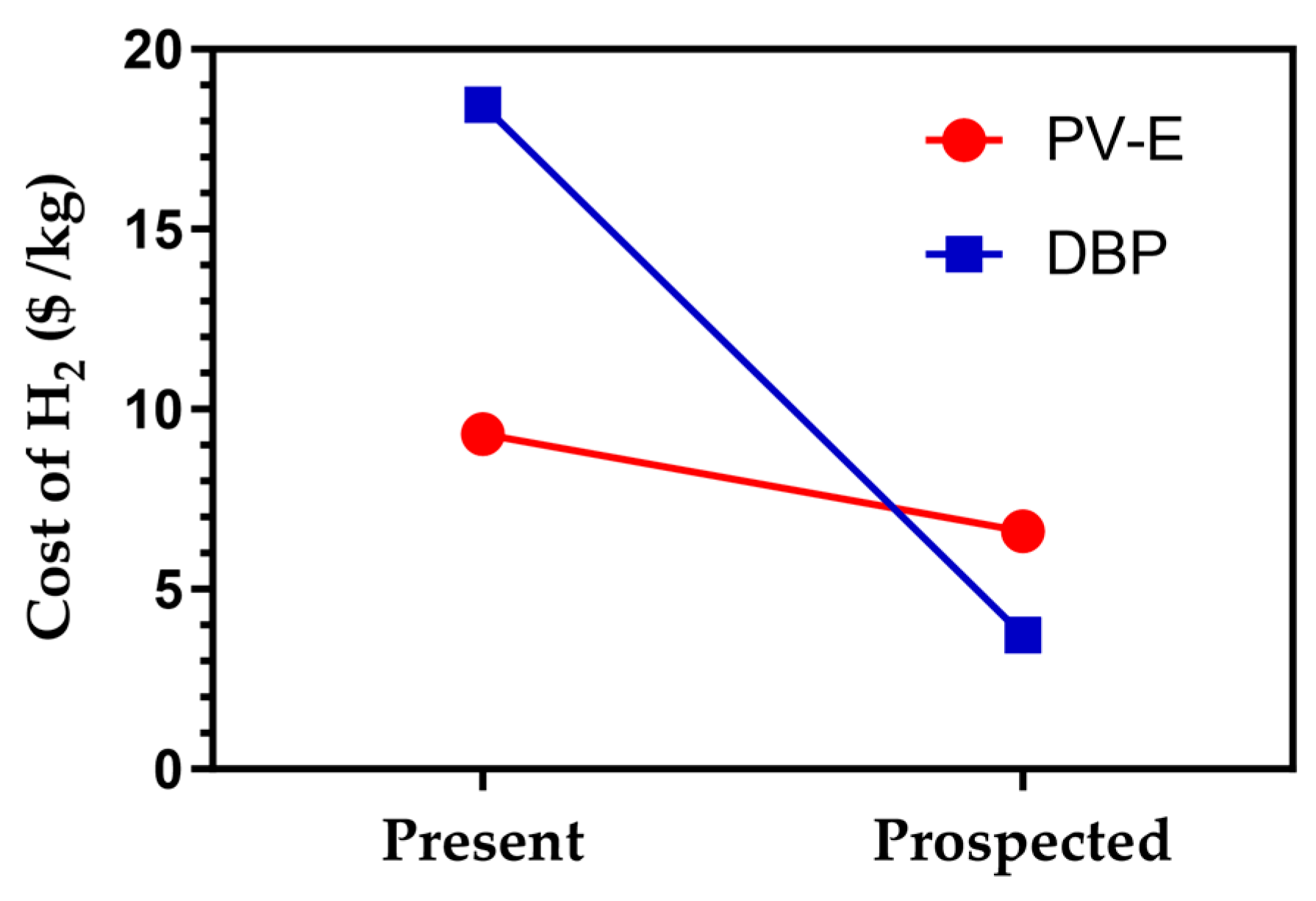
4. Solar H2 Production Cost
5. Challenges and Perspectives
6. Purple Non-Sulfur Photosynthetic Bacteria for Photofermentation
Optimization of the Photofermentation Process with Immobilization
| PBR Type (Volume) | Strain | Carbon Source (g/L) | Rate (mL/L/h) | Reference |
|---|---|---|---|---|
| Schott bottle (100 mL) | R. sphaeroides NCIMB8253 | Palm Oil Mill Effluent/pulp and paper mill effluent | 64.9 | [97] |
| Serological bottles (120 mL) | R. capsulatus ATCC 17015 | Acetate (1.0), Butyrate (11.6), Propionate (1.76) | 19.67 | [119] |
| Cylindrical glass (220 mL) | Rhodopseudomonas sp. | Acetate (4.0) | 19.6 | [90] |
| Glass bottle (260 mL) | R. palustris PB-Z | Glucose (12.6) | 78.7 | [120] |
| Flat panel (4.0 L) | R. capsulatus hup- | Sugar Beet Thick Juice | 25.01 | [121] |
| Tubular (50 L) | R. palustris 42OL | Malate (4.0) | 27.2 | [122] |
| Tubular (70 L) | R. sphaeroides HY01 | Glucose (5.4) | 37.6 | [123] |
| Tubular (20 L) | R. capsulatus YO3 | Molasses | 15.45 | [100] |
| PBR Type (Volume) | Strain | Carbon Source (g/L) | Matrix | Rate (mL/L/h) | Reference |
|---|---|---|---|---|---|
| Roux bottle (200 mL) | Rhodobacter capsulatus YO3 | Acetate (3.6) | Agar | 48.9 | [110] |
| Flat panel (1400 mL) | Rhodobacter capsulatus YO3 | Acetate (3.6) | Agar | 31.2 | [111] |
| Roux bottle (200 mL) | Rhodobacter capsulatus DSM 1710 | Acetate (3.6) | Agar | 18.6 | [110] |
| Flat panel (1400 mL) | Rhodobacter capsulatus DSM 1710 | Acetate (3.6) | Agar | 18.0 | [111] |
| Cylindrical glass bottle (200 mL) | Rhodopseudomonas sp. S16-VOGS3 | Acetate (2.0) | Ca-alginate | 14.96 | [91] |
| Cylindrical glass bottle (200 mL) | Rhodopseudomonas sp. | Acetate (2.0) | Ca-alginate | 10.2 | [99] |
| Flat Roux glass bottle (600 mL) | Rhodopseudomonas sp. S16-VOGS3 | Acetate (2.0) | Ca-alginate | 2.58 | [91] |
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dowaidar, M. Microbial pathways for sustainable hydrogen production. Int. J. Hydrogen Energy 2025, in press. [Google Scholar] [CrossRef]
- Caserta, G.; Lorent, C.; Pelmenschikov, V.; Schoknecht, J.; Yoda, Y.; Hildebrandt, P.; Cramer, S.P.; Zebger, I.; Lenz, O. In vitro assembly as a tool to investigate catalytic intermediates of [NiFe]-hydrogenase. ACS Catal. 2020, 10, 13890–13894. [Google Scholar] [CrossRef] [PubMed]
- Pagnier, A.; Balci, B.; Shepard, E.M.; Broderick, W.E.; Broderick, J.B. [FeFe]-hydrogenase in vitro maturation. Angew. Chem. Int. Ed. Engl. 2022, 61, e202212074. [Google Scholar] [CrossRef] [PubMed]
- Ghirardi, M.L. Implementation of photobiological H2 production: The O2 sensitivity of hydrogenases. Photosynth. Res. 2015, 5, 383–393. [Google Scholar] [CrossRef]
- Yadav, S.; Haas, R.; Birsen Boydas, E.; Roemelt, M.; Happe, T.; Apfel, U.P.; Stripp, S.T. Oxygen sensitivity of [FeFe]-hydrogenase: A comparative study of active site mimics inside vs. outside the enzyme. Phys. Chem. Chem. Phys. 2024, 26, 19105–19116. [Google Scholar] [CrossRef]
- Wulff, P.; Day, C.C.; Sargent, F.; Armstrong, F.A. How oxygen reacts with oxygen-tolerant respiratory [NiFe]-hydrogenases. Proc. Natl. Acad. Sci. USA 2014, 111, 6606–6611. [Google Scholar] [CrossRef]
- Duangjan, K.; Nakkhunthod, W.; Pekkoh, J.; Pumas, C. Comparison of hydrogen production in microalgae under autotrophic and mixotrophic media. Bot. Lith. 2017, 23, 169–177. [Google Scholar] [CrossRef]
- Faraloni, C.; Torzillo, G. Xanthophyll cycle induction by anaerobic conditions under low light in Chlamydomonas reinhardtii. J. Appl. Phycol. 2013, 25, 1457–1471. [Google Scholar] [CrossRef]
- Torzillo, G.; Scoma, A.; Faraloni, C.; Giannelli, L. Advances in the biotechnology of hydrogen production with the microalga Chlamydomonas reinhardtii. Crit. Rev. Biotechnol. 2014, 35, 485–496. [Google Scholar] [CrossRef]
- Eroglu, E.; Melis, A. Microalgal hydrogen production research. Int. J. Hydrogen Energy 2016, 41, 12772–12798. [Google Scholar] [CrossRef]
- Touloupakis, E.; Faraloni, C.; Silva Benavides, A.M.; Masojídek, J.; Torzillo, G. Sustained photobiological hydrogen production by Chlorella vulgaris without nutrient starvation. Int. J. Hydrogen Energy 2021, 46, 3684–3694. [Google Scholar] [CrossRef]
- Papazi, A.; Gjindali, A.I.; Kastanaki, E.; Assimakopoulos, K.; Stamatakis, K.; Kotzabasis, K. Potassium deficiency, a “smart” cellular switch for sustained high yield hydrogen production by the green alga Scenedesmus obliquus. Int. J. Hydrogen Energy 2014, 39, 19452–19464. [Google Scholar] [CrossRef]
- Papazi, A.; Karamanli, M.; Kotzabasis, K. Comparative biodegradation of all chlorinated phenols by the microalga Scenedesmus obliquus—The biodegradation strategy of microalgae. J. Biotechnol. 2019, 296, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Lestari, K.R.; Hidayanti, F.; Darusalam, U. Hydrogen production by algae Scenedesmus sp. biomass through photosynthesis process. Int. J. Sci. Soc. 2022, 4, 3. [Google Scholar] [CrossRef]
- Touloupakis, E.; Silva Benavides, A.M.; Cicchi, B.; Torzillo, G. Growth and hydrogen production of outdoor cultures of Synechocystis PCC 6803. Algal Res. 2016, 16, 78–85. [Google Scholar] [CrossRef]
- Opel, F.; Itzenhäuser, M.A.; Wehner, I.; Lupacchini, S.; Lauterbach, L.; Lenz, O.; Klähn, S. Toward a synthetic hydrogen sensor in cyanobacteria: Functional production of an oxygen-tolerant regulatory hydrogenase in Synechocystis sp. PCC 6803. Front. Microbiol. 2023, 14, 1122078. [Google Scholar] [CrossRef]
- Touloupakis, E.; Cicchi, B.; Silva Benavides, A.M.; Torzillo, G. Effect of high pH on growth of Synechocystis sp. PCC 6803 cultures and their contamination by golden algae (Poterioochromonas sp.). Appl. Microbiol. Biotechnol. 2016, 100, 1333–1341. [Google Scholar] [CrossRef]
- Akiyama, M.; Osanai, T. Regulation of organic acid and hydrogen production by NADH/NAD+ ratio in Synechocystis sp. PCC 6803. Front. Microbiol. 2024, 14, 1332449. [Google Scholar] [CrossRef]
- Ruiz-Marin, A.; Canedo-Lopez, Y.; Chavez-Fuentes, P. Biohydrogen production by Chlorella vulgaris and Scenedesmus obliquus immobilized cultivated in artificial wastewater under different light quality. Amb. Express 2020, 10, 191. [Google Scholar] [CrossRef]
- Liu, R.; Qin, S.; Li, W. Phycocyanin: Anti-inflammatory effect and mechanism. Biomed. Pharmacother. 2022, 153, 113362. [Google Scholar] [CrossRef]
- Maswanna, T.; Phunpruch, S.; Lindblad, P.; Maneeruttanarungroj, C. Enhanced hydrogen production by optimization of immobilized cells of the green alga Tetraspora sp. CU2551 grown under anaerobic condition. Biomass Bioenerg. 2018, 111, 88–95. [Google Scholar] [CrossRef]
- Pongpadung, P.; Liu, J.; Yokthongwattana, K.; Techapinyawat, S.; Juntawong, N. Screening for hydrogen-producing strains of green microalgae in phosphorus or sulphur deprived medium under nitrogen limitation. Sci. Asia 2015, 41, 97–107. [Google Scholar] [CrossRef]
- Touloupakis, E.; Rontogiannis, G.; Silva Benavides, A.M.; Cicchi, B.; Ghanotakis, D.F.; Torzillo, G. Hydrogen production by immobilized Synechocystis sp. PCC 6803. Int. J. Hydrogen Energy 2016, 41, 15181–15186. [Google Scholar] [CrossRef]
- Yodsang, P.; Raksajit, W.; Aro, E.-M.; Maenpaa, P.; Incharoensakdi, A. Factors affecting photobiological hydrogen production in five filamentous cyanobacteria from Thailand. Photosynthetica 2018, 56, 334–341. [Google Scholar] [CrossRef]
- Tinpranee, N.; Incharoensakdi, A.; Phunpruch, S. Screening cyanobacteria from marine coastal waters of Thailand for biohydrogen production. J. Appl. Phycol. 2018, 30, 3471–3481. [Google Scholar] [CrossRef]
- Shastik, E.; Romanova, A.; Laurinavichene, T.; Petushkova, E.; Sakurai, H.; Tsygankov, A. Plastic bags as simple photobioreactors for cyanobacterial hydrogen production outdoors in Moscow region. Int. J. Energy Environ. Eng. 2020, 11, 1–8. [Google Scholar] [CrossRef]
- Greening, C.; Biswas, A.; Carere, C.R.; Jackson, C.J.; Taylor, M.C.; Stott, M.B.; Cook, G.M.; Morales, S.E. Genomic and metagenomic surveys of hydrogenase distribution indicate H2 is a widely utilised energy source for microbial growth and survival. ISME J. 2016, 10, 761–777. [Google Scholar] [CrossRef]
- Lenz, O.; Lauterbach, L.; Frielingsdorf, S. Chapter Five—O2-tolerant [NiFe]-hydrogenases of Ralstonia eutropha H16: Physiology, molecular biology, purification, and biochemical analysis. In Methods in Enzymology; Fraser, A., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 117–151. [Google Scholar]
- Schumann, C.; Fernández Méndez, J.; Berggren, G.; Lindblad, P. Novel concepts and engineering strategies for heterologous expression of efficient hydrogenases in photosynthetic microorganisms. Front. Microbiol. 2023, 14, 1179607. [Google Scholar] [CrossRef]
- Gutekunst, K.; Hoffmann, D.; Westernströer, U.; Schulz, R.; Garbe-Schönberg, D.; Appel, J. In-vivo turnover frequency of the cyanobacterial NiFe-hydrogenase during photohydrogen production outperforms in-vitro systems. Sci. Rep. 2018, 8, 6083. [Google Scholar] [CrossRef]
- Gupta, R.; Pa, A.K. Bioinspired photo-driven hydrogen evolution systems based on hydrogenases and their mimics. Sustain. Energy Fuels 2024, 8, 4709. [Google Scholar] [CrossRef]
- Seefeldt, L.C.; Yang, Z.Y.; Lukoyanov, D.A.; Harris, D.F.; Dean, D.R.; Raugei, S.; Hoffman, B.M. Reduction of substrates by nitrogenases. Chem. Rev. 2020, 120, 5082–5106. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.W.; Syn, J.W.; Hsieh, C.H.; Huang, C.C.; Chien, L.F. Genetically engineered hydrogenases promote biophotocatalysis-mediated H2 production in the green alga Chlorella sp. DT. Int. J. Hydrogen Energy 2019, 44, 2533–2545. [Google Scholar] [CrossRef]
- Goswami, R.K.; Mehariya, S.; Obulisamy, P.K.; Verma, P. Advanced microalgae-based renewable biohydrogen production systems: A review. Bioresour. Technol. 2021, 320, 124301. [Google Scholar] [CrossRef] [PubMed]
- Adamu, H.; Bello, U.; Yuguda, A.U.; Tafida, U.I.; Jalam, A.M.; Sabo, A.; Qamar, M. Production processes, techno-economic and policy challenges of bioenergy production from fruit and vegetable wastes. Renew. Sustain. Energy Rev. 2023, 186, 113686. [Google Scholar] [CrossRef]
- Sun, J.; Yang, P.; Li, N.; Zhao, M.; Zhang, X.; Zhang, Y.; Yuan, Y.; Lu, X.; Lu, X. Extraction of photosynthetic electron from mixed photosynthetic consortium of bacteria and algae towards sustainable bioelectrical energy harvesting. Electrochim. Acta 2020, 336, 135710. [Google Scholar] [CrossRef]
- Taikhao, S.; Phunpruch, S. Biomass and biohydrogen production by unicellular green alga Chlorella vulgaris var. vulgaris TISTR 8261 using frozen food industrial wastewater. Asia-Pac. J. Sci. Technol. 2022, 27, APST-27-01-19. [Google Scholar]
- Faraloni, C.; Ena, A.; Pintucci, C.; Torzillo, G. Enhanced hydrogen production by means of sulfur-deprived Chlamydomonas reinhardtii cultures grown in pre-treated olive-mill wastewater. Int. J. Hydrogen Energy 2011, 36, 5920–5931. [Google Scholar] [CrossRef]
- Anwar, M.; Lou, S.; Chen, L.; Li, H.; Hu, Z. Recent advancement and strategy on bio-hydrogen production from photosynthetic microalgae. Bioresour. Technol. 2019, 292, 121972. [Google Scholar] [CrossRef]
- Oncel, S.; Vardar Sukan, F. Effect of light intensity and the light: Dark cycles on the long term hydrogen production of Chlamydomonas reinhardtii by batch cultures. Biomass Bioenerg. 2011, 35, 1066–1074. [Google Scholar] [CrossRef]
- Redding, K.E.; Appel, J.; Boehm, M.; Schuhmann, W.; Nowaczyk, M.M.; Yacoby, I.; Gutekunst, K. Advances and challenges in photosynthetic hydrogen production. Trends Biotechnol. 2022, 40, 1313–1325. [Google Scholar] [CrossRef]
- Marco, P.; Elman, T.; Yacoby, I. Binding of ferredoxin NADP(+) oxidoreductase (FNR) to plant photosystem I. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Torzillo, G.; Seibert, M. Hydrogen production by Chlamydomonas reinhardtii. In Handbook of Microalgal Culture; Wiley–Blackwell: Hoboken, NJ, USA, 2013; pp. 417–432. [Google Scholar]
- Jaramillo, A.; Satta, A.; Pinto, F.; Faraloni, C.; Chini Zittelli, G.; Silva Benavides, A.M.; Torzillo, G.; Schumann, C.; Fernandez Mendez, J.; Berggren, G.; et al. Outlook on synthetic biology-driven hydrogen production: Lessons from algal photosynthesis applied to cyanobacteria. Energy Fuels 2025, 31, 4987–5006. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Chen, X.; Wu, S.; Wang, Q. Co-cultivation of Chlamydomonas reinhardtii with Azotobacter chroococcum improved H2 production. Biotechnol. Lett. 2017, 39, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, D.; Wang, Q.; Wu, S. Improved hydrogen production and biomass through the co-cultivation of Chlamydomonas reinhardtii and Bradyrhizobium japonicum. Int. J. Hydrogen Energy 2016, 41, 9276–9283. [Google Scholar] [CrossRef]
- Fakhimi, N.; Tavakoli, O. Improving hydrogen production using co-cultivation of bacteria with Chlamydomonas reinhardtii microalga. Mater. Sci. Energy Technol. 2019, 2, 1–7. [Google Scholar] [CrossRef]
- Ban, S.; Lin, W.; Wu, F.; Luo, J. Algal-bacterial cooperation improvesalgal photolysis-mediated hydrogen production. Bioresour. Technol. 2018, 251, 350–357. [Google Scholar] [CrossRef]
- Karchiyappan, T.; Ettiyagounder, P.; Selvaraj, P.S.; Ramanujam, K. Advancements in green hydrogen (GH2) recovery from industrial wastewater: A comprehensive review. Desalin. Water Treat. 2025, 321, 100966. [Google Scholar]
- Mus, F.; Dubini, A.; Seibert, M.; Posewitz, M.C.; Grossman, A.R. Anaerobic acclimation in Chlamydomonas reinhardtii: Anoxic gene expression, hydrogenase induction, and metabolic pathways. J. Biol. Chem. 2007, 282, 25475–25486. [Google Scholar] [CrossRef]
- Zarei, Z.; Malekshahi, P.; Morowvat, M.H.; Trzcinski, A.P. A review of bioreactor configurations for hydrogen production by cyanobacteria and microalgae. Int. J. Hydrogen Energy 2024, 49, 472–495. [Google Scholar] [CrossRef]
- Frowijn, L.S.F.; van Sark, W.G.J.H.M. Analysis of photon-driven solar-to-hydrogen production methods in the Netherlands. Sustain. Energy Technol. Assess. 2021, 48, 101631. [Google Scholar] [CrossRef]
- Melis, A.; Zhang, L.; Forestier, M.; Ghirardi, M.L.; Seibert, M. Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green alga Chlamydomonas reinhardtii. Plant Physiol. 2000, 122, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Torzillo, G.; Zittelli, G.C.; Cicchi, B.; Diano, M.; Parente, M.; Silva Benavides, A.M.; Esposito, S.; Touloupakis, E. Effect of plate distance on light conversion efficiency of a Synechocystis culture grown outdoors in a multiplate photobioreactor. Sci. Total Environ. 2022, 842, 156840. [Google Scholar] [CrossRef] [PubMed]
- Torri, C.; Samorì, C.; Adamiano, A.; Fabbri, D.; Faraloni, C.; Torzillo, G. Preliminary investigation on the production of fuels and bio-char from Chlamydomonas reinhardtii biomass residue after bio-hydrogen production. Bioresour. Technol. 2011, 102, 8707–8713. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Nagarajan, D.; Zhang, Q.; Chang, J.S.; Lee, D.J. Heterotrophic cultivation of microalgae for pigment production: A review. Biotechnol. Adv. 2018, 36, 5467. [Google Scholar] [CrossRef]
- Serra, A.T.; Silva, S.D.; Pleno de Gouveia, L.; Alexandre, A.M.R.C.; Pereira, C.V.; Pereira, A.B.; Partidário, A.C.; Silva, N.E.; Bohn, T.; Gonçalves, V.S.S.; et al. A single dose of marine Chlorella vulgaris increases plasma concentrations of lutein, β-carotene and zeaxanthin in healthy male volunteers. Antioxidants 2021, 10, 1164. [Google Scholar] [CrossRef]
- Bernaerts, T.M.M.; Gheysen, L.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. The potential of microalgae and their biopolymers as structuring ingredients in food: A review. Biotechnol. Adv. 2019, 37, 107419. [Google Scholar] [CrossRef]
- Niccolai, A.; Zittelli, G.C.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae of interest as food source: Biochemical composition and digestibility. Algal Res. 2019, 42, 101617. [Google Scholar] [CrossRef]
- Wild, K.J.; Steinga, H.; Rodehutscord, M. Variability in nutrient composition and in vitro crude protein digestibility of 16 microalgae products. J. Anim. Physiol. Anim. Nutr. 2018, 1020, 13061319. [Google Scholar] [CrossRef]
- Galasso, C.; Gentile, A.; Orefice, I.; Ianora, A.; Bruno, A.; Noonan, D.M.; Sansone, C.; Albini, A.; Brunet, C. Microalgal derivatives as potential nutraceutical and food supplements for human health: A focus on cancer prevention and interception. Nutrients 2019, 11, 1226. [Google Scholar] [CrossRef]
- Sadeghi, S.; Jalili, H.; Ranaei Siadat, S.O.; Sedighi, M. Anticancer and antibacterial properties in peptide fractions from hydrolyzed spirulina protein. J. Agric. Sci. Technol. 2018, 20, 673683. [Google Scholar]
- Simsa, R.; Yuen, J.; Stout, A.; Rubio, N.; Fogelstrand, P.; Kaplan, D.L. Extracellular heme proteins influence bovine myosatellite cell proliferation and the color of cell-based meat. Foods 2019, 8, 521. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, G.; Sampathkumar, P.; Kavisri, M.; Moovendhan, M. Extraction and characterization of phycocyanin from Spirulina platensis and evaluation of its anticancer, antidiabetic and antiinflammatory effect. Int. J. Biol. Macromol. 2020, 153, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. The bioactivities of phycocyanobilin from Spirulina. J. Immunol. Res. 2022, 2022, 4008991. [Google Scholar] [CrossRef] [PubMed]
- Pentón-Rol, G.; Marín-Prida, J.; McCarty, M.F. C-Phycocyanin-derived phycocyanobilin as a potential nutraceutical approach for major neurodegenerative disorders and COVID-19- induced damage to the nervous system. Curr. Neuropharmacol. 2021, 19, 2250–2275. [Google Scholar] [CrossRef]
- Garcia-Pliego, E.; Franco-Colin, M.; Rojas-Franco, P.; Blas-Valdivia, V.; Serrano-Contreras, J.I.; Pentón-Rol, G.; Cano-Europa, E. Phycocyanobilin is the molecule responsible for the nephroprotective action of phycocyanin in acute kidney injury caused by mercury. Food Funct. 2021, 12, 2985–2994. [Google Scholar] [CrossRef]
- Gdara, N.B.; Belgacem, A.; Khemiri, I.; Mannai, S.; Bitri, L. Protective effects of phycocyanin on ischemia/reperfusion liver injuries. Biomed. Pharmacother. 2018, 102, 196–202. [Google Scholar] [CrossRef]
- Xie, Y.; Li, W.; Zhu, L.; Zhai, S.; Qin, S.; Du, Z. Effects of phycocyanin in modulating the intestinal microbiota of mice. Microbiologyopen 2019, 8, e00825. [Google Scholar] [CrossRef]
- Yang, F.H.; Dong, X.L.; Liu, G.X.; Teng, L.; Wang, L.; Zhu, F.; Xu, F.H.; Yang, Y.F.; Cao, C.; Chen, G.; et al. The protective effect of C-phycocyanin in male mouse reproductive system. Food Funct. 2022, 13, 2631–2646. [Google Scholar] [CrossRef]
- Touloupakis, E.; Cicchi, B.; Torzillo, G. A bioenergetic assessment of photosynthetic growth of Synechocystis sp. PCC 6803 in continuous cultures. Biotechnol. Biofuels 2015, 8, 133. [Google Scholar] [CrossRef]
- Kumar, P.; Saravanan, P.; Omer, S.N.; Rajeshkannan, R.; Kumar, S.V. Enhanced biohydrogen yield through cyanobacterial engineering: A detailed review. Biomass Convers. Biorefin. 2025, in press. [Google Scholar] [CrossRef]
- Skovgaard, J.; van Asselt, H. The politics of fossil fuel subsidies and their reform: Implications for climate change mitigation. Wiley Interdiscip. Rev. Clim. Change 2019, 10, e581. [Google Scholar] [CrossRef]
- Jewell, J.; McCollum, D.; Emmerling, J.; Bertram, C.; Gernaat, D.E.H.J.; Krey, V.; Paroussos, L.; Berger, L.; Fragkiadakis, K.; Keppo, I.; et al. Limited emission reductions from fuel subsidy removal exceptin energy-exporting regions. Nature 2018, 554, 229–233. [Google Scholar] [CrossRef]
- Ambec, S.; Crampes, C. Decarbonizing electricity generation with intermittent sources of energy. J. Assoc. Environ. Resour. Econ. 2019, 6, 1105–1134. [Google Scholar] [CrossRef]
- Nicodemus, J.H. Technological learning and the future of solar H2: A component learning comparison of solar thermochemical cycles and electrolysis with solar PV. Energ. Policy 2018, 120, 100–109. [Google Scholar] [CrossRef]
- Joy, J.; Mathew, J.; George, S.C. Nanomaterials for photoelectrochemical water splitting–review. Int. J. Hydrogen Energy 2018, 43, 4804–4817. [Google Scholar] [CrossRef]
- Liao, C.; Que, L.; Fu, Z.; Deng, P.; Li, A.; Wang, X.; Cheng, L. Reseatch status of electrolytic preparation of rare Earth metals and alloys in fluoride meltem salt sysem. A minireview of China. Metals 2024, 14, 407. [Google Scholar] [CrossRef]
- Dhar, K.; Venkateswarlu, K.; Megharaj, M. Anoxygenic phototrophic purple non-sulfur bacteria: Tool for bioremediation of hazardous environmental pollutants. World J. Microbiol. Biotechnol. 2023, 39, 283. [Google Scholar] [CrossRef]
- Imhoff, J.F. Diversity of anaerobic anoxygenic phototrophic purple bacteria. In Modern Topics in the Phototrophic Prokaryotes; Hallenbeck, P., Ed.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Reungsang, A.; Zhong, N.; Yang, Y.; Sittijunda, S.; Xia, A.; Liao, Q. Hydrogen from photo fermentation. In Bioreactors for Microbial Biomass and Energy Conversion. Green Energy and Technology; Liao, Q., Chang, J., Herrmann, C., Xia, A., Eds.; Springer: Singapore, 2018. [Google Scholar]
- Haq, I.U.; Christensen, A.; Fixen, K.R. Evolution of Rhodopseudomonas palustris to degrade halogenated aromatic compounds involves changes in pathway regulation and enzyme specificity. Appl. Environ. Microbiol. 2024, 90, e0210423. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, G.; He, S.; Zhao, R.; Zhu, D. Purple non-sulfur bacteria technology: A promising and potential approach for wastewater treatment and bioresources recovery. World J. Microbiol. Biotechnol. 2021, 37, 161. [Google Scholar] [CrossRef]
- Budiman, P.M.; Wu, T.Y. Role of chemicals addition in affecting biohydrogen production through photofermentation. Energy Convers. Manag. 2018, 165, 509–527. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Aghbashlo, M.; Tabatabaei, M.; Younesi, H.; Najafpour, G. Energy analysis of biohydrogen production from various carbon sources via anaerobic photosynthetic bacteria (Rhodospirillum rubrum). Energy 2015, 93, 730–739. [Google Scholar] [CrossRef]
- Adessi, A.; Concato, M.; Sanchini, A.; Rossi, F.; De Philippis, R. Hydrogen production under salt stress conditions by a freshwater Rhodopseudomonas palustris strain. Appl. Microbiol. Biotechnol. 2016, 100, 2917–2926. [Google Scholar] [CrossRef] [PubMed]
- Al-Mohammedawi, H.H.; Znad, H.; Eroglu, E. Synergistic effects and optimization of photo-fermentative hydrogen production of Rhodobacter sphaeroides DSM 158. Int. J. Hydrogen Energy 2018, 43, 15823–15834. [Google Scholar] [CrossRef]
- Cai, J.; Guan, Y.; Jia, T.; Yang, J.; Hu, Y.; Li, P.; Duan, Y.; Zhang, L.; Yu, P. Hydrogen production from high slat medium by co-culture of Rhodovulum sulfidophilum and dark fermentative microflora. Int. J. Hydrogen Energy 2018, 43, 10959–10966. [Google Scholar] [CrossRef]
- Tiang, M.F.; Fitri Hanipa, M.A.; Abdul, P.M.; Jahim, J.M.d.; Mahmod, S.S.; Takriff, M.S.; Lay, C.H.; Reungsang, A.; Wu, S.Y. Recent advanced biotechnological strategies to enhance photo-fermentative biohydrogen production by purple non-sulphur bacteria: An overview. Int. J. Hydrogen Energy 2020, 45, 13211–13230. [Google Scholar] [CrossRef]
- Touloupakis, E.; Poloniataki, E.G.; Ghanotakis, D.F.; Carlozzi, P. Production of biohydrogen and/or poly-β-hydroxybutyrate by Rhodopseudomonas sp. using various carbon sources as substrate. Appl. Biochem. Biotechnol. 2021, 193, 307–318. [Google Scholar] [CrossRef]
- Moia, I.C.; Kanaropoulou, A.; Ghanotakis, D.F.; Carlozzi, P.; Touloupakis, E. Photofermentative hydrogen production by immobilized Rhodopseudomonas sp. S16-VOGS3 cells in photobioreactors. Energy Rev. 2024, 3, 100055. [Google Scholar] [CrossRef]
- Ghosh, S.; Dairkee, U.K.; Chowdhury, R.; Bhattacharya, P. Hydrogen from food processing wastes via photofermentation using Purple Non-sulfur Bacteria (PNSB)—A review. Energy Convers. Manag. 2017, 141, 299–314. [Google Scholar] [CrossRef]
- Ghosh, S. Retrofitting nanotechnology into photofermentation: State-of-the-art and prospect analysis for ameliorated production of clean hydrogen. Fuel 2024, 359, 130374. [Google Scholar] [CrossRef]
- Putatunda, C.; Behl, M.; Solanki, P.; Sharma, S.; Bhatia, S.K.; Walia, A.; Bhatia, R.K. Current challenges and future technology in photofermentation-driven biohydrogen production by utilizing algae and bacteria. Int. J. Hydrogen Energy 2023, 48, 21088–21109. [Google Scholar] [CrossRef]
- Goveas, L.C.; Nayak, S.; Kumar, P.S.; Vinayagam, R.; Selvaraj, R.; Rangasamy, G. Recent advances in fermentative biohydrogen production. Int. J. Hydrogen Energy 2024, 54, 200–217. [Google Scholar] [CrossRef]
- Agyekum, E.B.; Odoi-Yorke, F. Review of over two decades of research on dark and photo fermentation for biohydrogen production—A combination of traditional, systematic, and bibliometric approaches. Int. J. Hydrogen Energy 2024, 91, 1149–1169. [Google Scholar] [CrossRef]
- Budiman, P.M.; Wu, T.Y.; Ramanan, R.N.; Jahim, J.M. Improvement of biohydrogen production through combined reuses of palm oil mill effluent together with pulp and paper mill effluent in photofermentation. Energ. Fuel 2015, 29, 5816–5824. [Google Scholar] [CrossRef]
- Hakobyan, L.; Gabrielyan, L.; Trchounian, A. Biohydrogen by Rhodobacter sphaeroides during photo-fermentation: Mixed vs. sole carbon sources enhance bacterial growth and H2 production. Int. J. Hydrogen Energy 2019, 44, 674–679. [Google Scholar] [CrossRef]
- Touloupakis, E.; Chatziathanasiou, A.; Ghanotakis, D.F.; Carlozzi, P.; Pecorini, I. Hydrogen production by immobilized Rhodopseudomonas sp. cells in calcium alginate beads. Energies 2022, 15, 8355. [Google Scholar] [CrossRef]
- Oflaz, F.B.; Koku, H. Pilot-scale outdoor photofermentative hydrogen production from molasses using pH control. Int. J. Hydrogen Energy 2021, 46, 29160–29172. [Google Scholar] [CrossRef]
- Policastro, G.; Cesaro, A.; Fabbricino, M. Photo-fermentative hydrogen production from cheese whey: Engineering of a mixed culture process in a semi-continuous, tubular photo-bioreactor. Int. J. Hydrogen Energy 2023, 48, 21038–21054. [Google Scholar] [CrossRef]
- Hay, J.X.W.; Wu, T.Y.; Juan, J.C.; Jahim, J.M. Effect of adding brewery wastewater to pulp and paper mill effluent to enhance the photofermentation process: Wastewater characteristics, biohydrogen production, overall performance, and kinetic modeling. Environ. Sci. Pollut. Res. 2017, 24, 10354–10363. [Google Scholar] [CrossRef]
- Policastro, G.; Carraturo, F.; Compagnone, M.; Guida, M.; Fabbricino, M. Enhancing hydrogen production from winery wastewater through fermentative microbial culture selection. Bioresour. Technol. Rep. 2022, 19, 101196. [Google Scholar] [CrossRef]
- Allegue, L.D.; Puyol, D.; Melero, J.A. Food waste valorization by purple phototrophic bacteria and anaerobic digestion after thermal hydrolysis. Biomass Bioenerg. 2020, 142, 105803. [Google Scholar] [CrossRef]
- Smith, S.C.; Toledo-Alarcón, J.; Schiappacasse, M.C.; Tapia-Venegas, E. Enrichment of a mixed culture of purple non-sulfur bacteria for hydrogen production from organic acids. Sustainability 2023, 15, 16607. [Google Scholar] [CrossRef]
- Chandra, R.; Nikhil, G.N.; Mohan, S.V. Single-stage operation of hybrid dark-photo fermentation to enhance biohydrogen production through regulation of system redox condition: Evaluation with real-field wastewater. Int. J. Mol. Sci. 2015, 1, 9540–9556. [Google Scholar] [CrossRef] [PubMed]
- Laurinavichene, T.; Tsygankov, A. Different types of H2 photoproduction by starch-utilizing co-cultures of Clostridium butyricum and Rhodobacter sphaeroides. Int. J. Hydrogen Energy 2016, 41, 13419–13425. [Google Scholar] [CrossRef]
- Scognamiglio, V.; Giardi, M.T.; Zappi, D.; Touloupakis, E.; Antonacci, A. Photoautotrophs-bacteria co-cultures: Advances, challenges and applications. Materials 2021, 14, 3027. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tabatabaei, M.; Li, F.; Ho, S.H. A review of green biohydrogen production using anoxygenic photosynthetic bacteria for hydrogen economy: Challenges and opportunities. Int. J. Hydrogen Energy 2024, 54, 218–238. [Google Scholar] [CrossRef]
- Elkahlout, K.; Alipour, S.; Eroglu, I.; Gunduz, U.; Yucel, M. Long-term biological hydrogen production by agar immobilized Rhodobacter capsulatus in a sequential batch photobioreactor. Bioproc. Biosyst. Eng. 2017, 40, 589–599. [Google Scholar] [CrossRef]
- Elkahlout, K.; Sagir, E.; Alipour, S.; Koku, H.; Gunduz, U.; Eroglu, I.; Yucel, M. Long-term stable hydrogen production from acetate using immobilized Rhodobacter capsulatus in a panel photobioreactor. Int. J. Hydrogen Energy 2018, 44, 18801–18810. [Google Scholar] [CrossRef]
- Wang, Y.; Tahir, N.; Cao, W.; Zhang, Q.; Lee, D.J. Grid columnar flat panel photobioreactor with immobilized photosynthetic bacteria for continuous photofermentative hydrogen production. Bioresour. Technol. 2019, 291, 121806. [Google Scholar]
- Ross, B.S.; Pott, R.W.M. Hydrogen production by immobilized Rhodopseudomonas palustris in packed or fluidized bed photobioreactor systems. Int. J. Hydrogen Energy 2021, 46, 1715–1727. [Google Scholar] [CrossRef]
- Sagir, E.; Alipour, S. Photofermentative hydrogen production by immobilized photosynthetic bacteria: Current perspectives and challenges. Renew. Sustain. Energy Rev. 2021, 141, 110796. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, G.; Zhang, Q.; Lee, D.; Zhang, Z.; Li, Y.; Li, P.; Hu, J.; Yan, B. Photosynthetic hydrogen production by alginate immobilized bacterial consortium. Bioresour. Technol. 2017, 236, 44–48. [Google Scholar] [CrossRef] [PubMed]
- du Toit, J.-P.; Pott, R.W.M. Transparent polyvinyl-alcohol cryogel as immobilisation matrix for continuous biohydrogen production by phototrophic bacteria. Biotechnol. Biofuels 2020, 13, 105. [Google Scholar] [CrossRef] [PubMed]
- Mabutyana, L.; Pott, R.W.M. Photo-fermentative hydrogen production by Rhodopseudomonas palustris CGA009 in the presence of inhibitory compounds. Int. J. Hydrogen Energy 2021, 46, 29088–29099. [Google Scholar] [CrossRef]
- Sağır, E.; Yucel, M.; Hallenbeck, P.C. Demonstration and optimization of sequential microaerobic dark- and photo-fermentation biohydrogen production by immobilized Rhodobacter capsulatus JP91. Bioresour. Technol. 2018, 250, 43–52. [Google Scholar] [CrossRef]
- Montiel, C.V.; Le, B.S.; Revah, S.; Morales, M. Effect of light-dark cycles on hydrogen and poly-β-hydroxybutyrate production by a photoheterotrophic culture and Rhodobacter capsulatus using a dark fermentation effluent as substrate. Bioresour. Technol. 2017, 226, 238–246. [Google Scholar] [CrossRef]
- Sun, M.L.Y.; Liu, Y. A new hydrogen-producing strain and its characterization of hydrogen production. Appl. Biochem. Biotechnol. 2015, 177, 1676–1689. [Google Scholar] [CrossRef]
- Özkan, E.; Uyar, B.; Özgür, E.; Yücel, M.; Eroglu, I.; Gündüz, U. Photofermentative hydrogen production using dark fermentation effluent of sugar beet thick juice in outdoor conditions. Int. J. Hydrogen Energy 2012, 37, 2044–2049. [Google Scholar] [CrossRef]
- Adessi, A.; Torzillo, G.; Baccetti, E.; De Philippis, R. Sustained outdoor H2 production with Rhodopseudomonas palustris cultures in a 50 L tubular photobioreactor. Int. J. Hydrogen Energy 2012, 37, 8840–8849. [Google Scholar] [CrossRef]
- Ren, C.; Zhang, S.; Li, Q.; Jiang, Q.; Li, Y.; Gao, Z.; Cao, W.; Guo, L. Pilot composite tubular bioreactor for outdoor photo-fermentation hydrogen production: From batch to continuous operation. Bioresour. Technol. 2024, 401, 130705. [Google Scholar] [CrossRef]
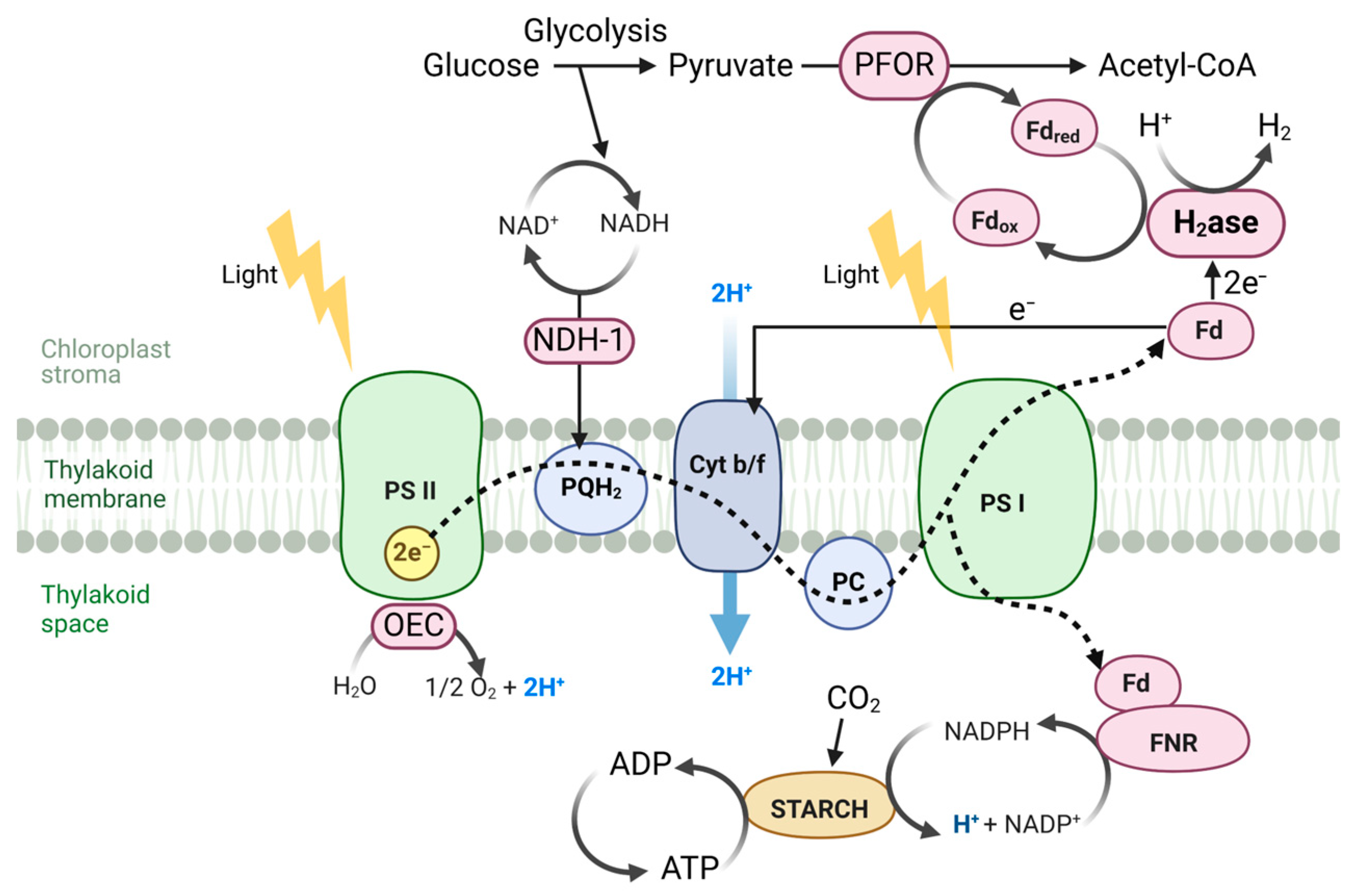



| Enzyme Type | Characteristics | H2 Production Mechanism | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| [FeFe]-Hydrogenase | Found in microalgae; highly active but O2-sensitive | Catalyzes H2 production at rates of ~104 H2/s in vitro | High efficiency in H2 production | Highly sensitive to O2, requiring anaerobic conditions | [30] |
| [NiFe]-Hydrogenase | Found in cyanobacteria; more resistant to O2 exposure | Lower H2 production rates (~1000–2000 H2/s) | Some variants can function in oxygenated environments | Lower catalytic efficiency compared to [FeFe]-hydrogenase | [28] |
| Nitrogenase | Converts N₂ into ammonia, producing H2 as a by-product | Requires ATP (16 ATP per H2 with N2, 4 ATP without N2) | Can produce H2 for longer periods in anaerobic conditions | High energy cost due to ATP consumption | [32] |
| Optimization Strategy | Description | Advantages | Challenges | References |
|---|---|---|---|---|
| Genetic Engineering | Modifying microalgae/cyanobacteria for improved H2 output | Can enhance enzyme efficiency and O2 tolerance | Ethical concerns, regulatory restrictions | [33,34] |
| Co-Cultivation with Bacteria | Using bacteria to assist H2 production (e.g., dark fermentation) | Converts biomass into additional H2 | Requires controlled symbiotic relationships | [35,36] |
| Nutrient Starvation | Limiting sulfur, nitrogen, or potassium to enhance H2 yield | Enhances hydrogenase activity, reduces O2 production | Can stress cells, lowering overall biomass production | [19] |
| Wastewater Utilization | Using wastewater as a nutrient source for microalgae | Reduces costs, reuses waste, increases biomass | Variability in wastewater composition | [37,38] |
| Light Optimization | Using specific wavelengths (e.g., purple light) | Increases photosynthetic efficiency and H2 production | Requires precise control of light exposure | [39,40] |
| Solar to H2 Technology | Solar to H2 Efficiency | Material-Catalysts | TRL | Reference |
|---|---|---|---|---|
| PV Alkaline Electrolysis | 10–12.3% | Material: Perovskite + Alkaline Catalysts: a multilayer anode nickel–iron hydroxide (NiFe) electrocatalyst layer coated on a nickel sulfide (NiSx) layer formed on porous Ni foam (NiFe/NiSx-Ni). | 9 | [52] |
| Direct Biophotolysis | 1–13.4% | Microalgae: C. reinhardtii; C. reinhardtii D1 mutants; Chlorella sp G-120.; Cyanobacteria; Synechocystis 6803; Synnechocystis mutants Catalysts: Hydrogenases | 4–5 | [11,43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faraloni, C.; Torzillo, G.; Balestra, F.; Moia, I.C.; Zampieri, R.M.; Jiménez-Conejo, N.; Touloupakis, E. Advances and Challenges in Biohydrogen Production by Photosynthetic Microorganisms. Energies 2025, 18, 2319. https://doi.org/10.3390/en18092319
Faraloni C, Torzillo G, Balestra F, Moia IC, Zampieri RM, Jiménez-Conejo N, Touloupakis E. Advances and Challenges in Biohydrogen Production by Photosynthetic Microorganisms. Energies. 2025; 18(9):2319. https://doi.org/10.3390/en18092319
Chicago/Turabian StyleFaraloni, Cecilia, Giuseppe Torzillo, Francesco Balestra, Isabela Calegari Moia, Raffaella Margherita Zampieri, Natalia Jiménez-Conejo, and Eleftherios Touloupakis. 2025. "Advances and Challenges in Biohydrogen Production by Photosynthetic Microorganisms" Energies 18, no. 9: 2319. https://doi.org/10.3390/en18092319
APA StyleFaraloni, C., Torzillo, G., Balestra, F., Moia, I. C., Zampieri, R. M., Jiménez-Conejo, N., & Touloupakis, E. (2025). Advances and Challenges in Biohydrogen Production by Photosynthetic Microorganisms. Energies, 18(9), 2319. https://doi.org/10.3390/en18092319









