CO2 Geothermal Power Generation: Laboratory Experiment on the Interaction Between Carbonated Water and Rishiri Island Basalt in the Vicinity of Injection Wells
Abstract
1. Introduction
2. Materials and Methods
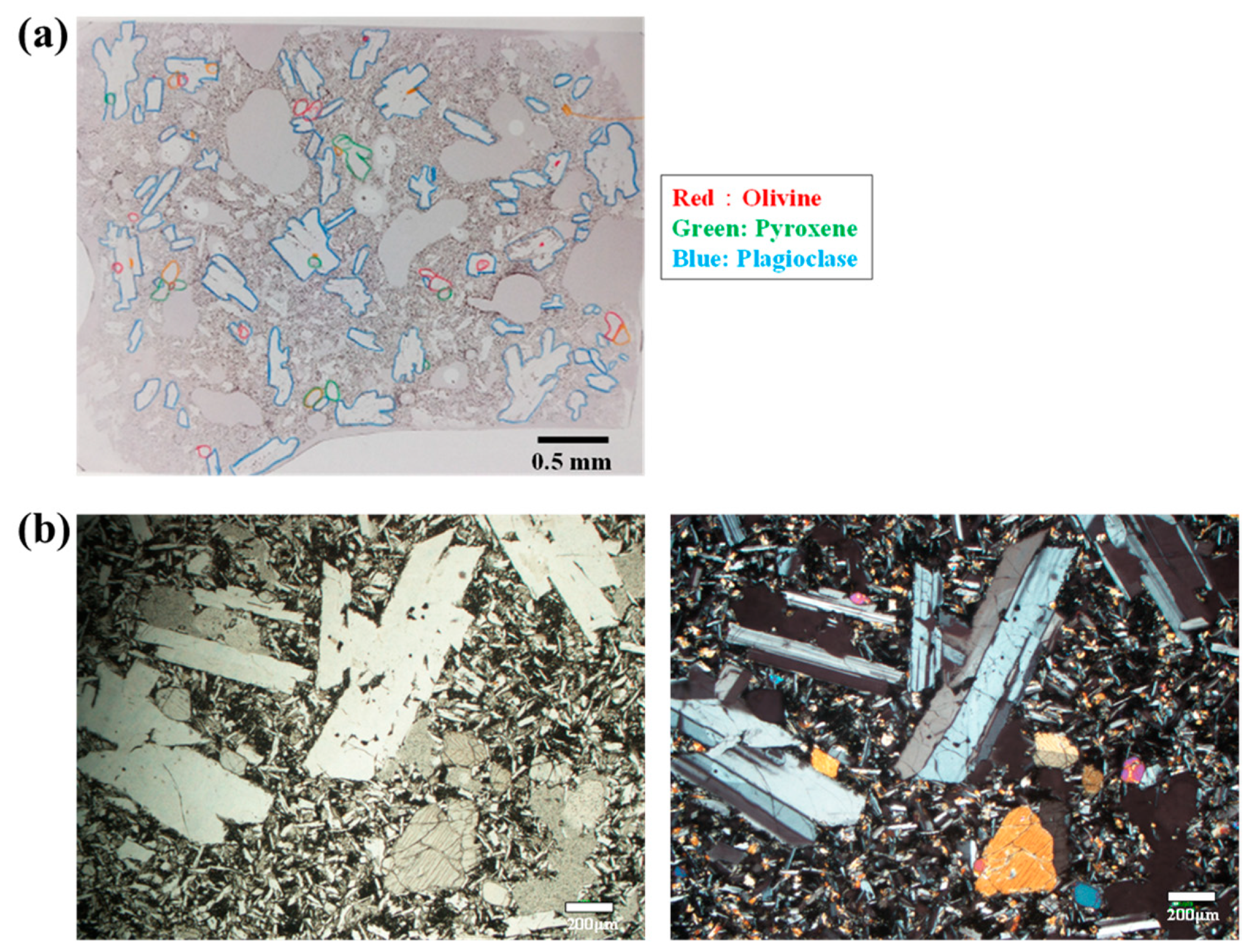
2.1. Rock and Test Equipment
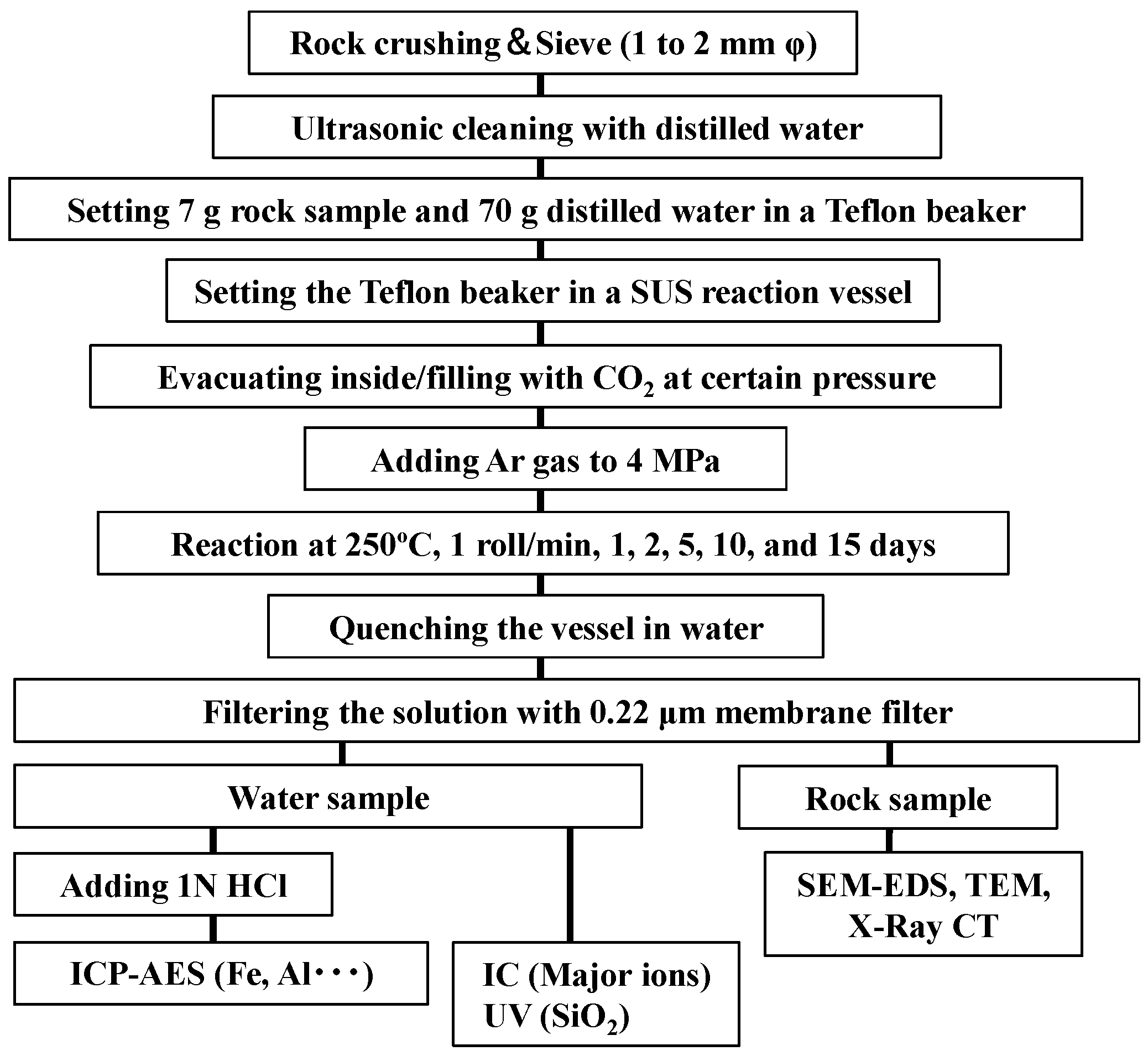
2.2. Test Conditions and Procedures
2.3. Analytical Methods
2.3.1. Chemical Analysis of the Solutions
2.3.2. Analysis of the Rock Sample
3. Results and Discussion
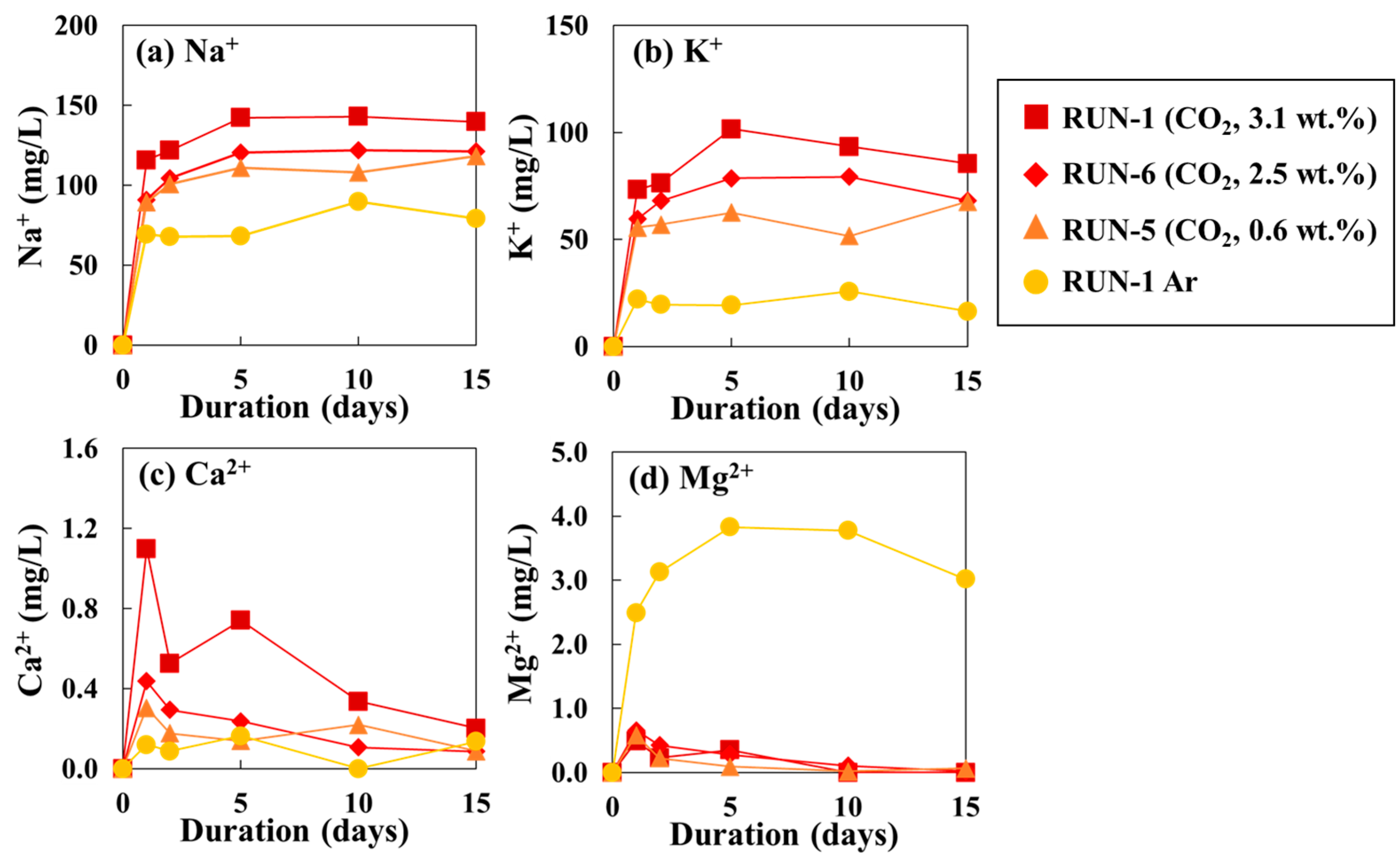
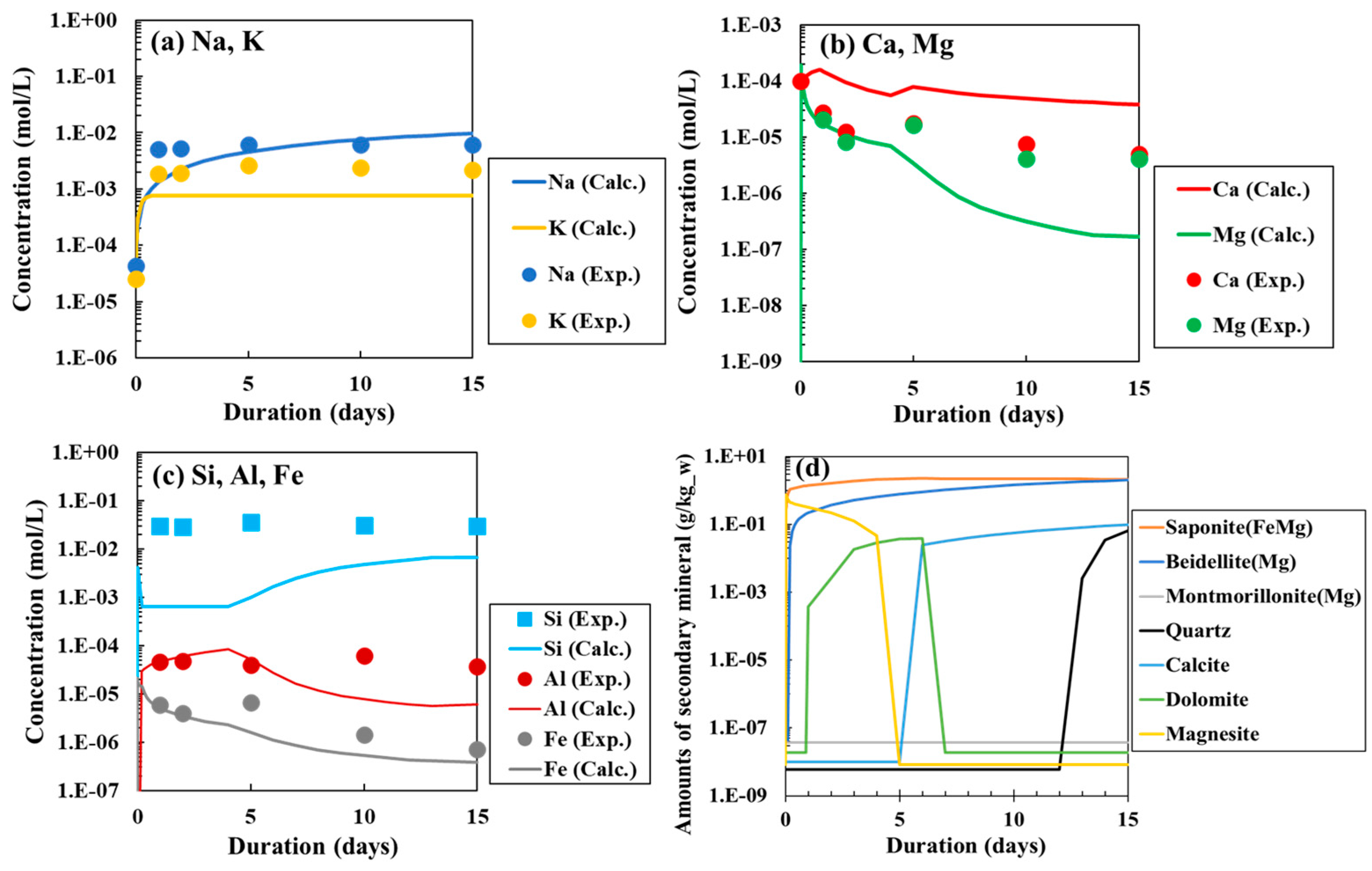
3.1. Chemical Composition of the Reaction Water
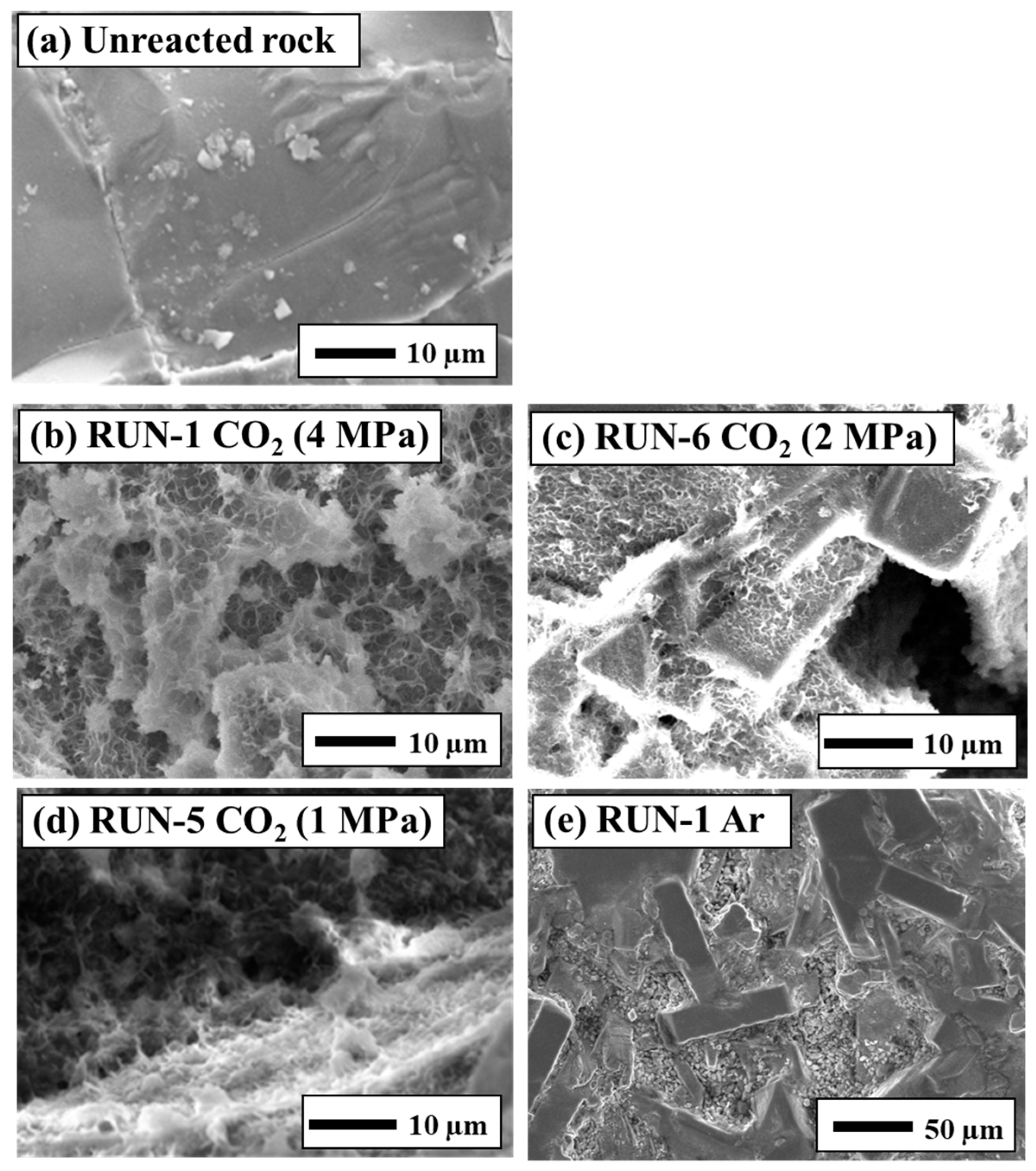
3.2. Surface Observation of Rocks
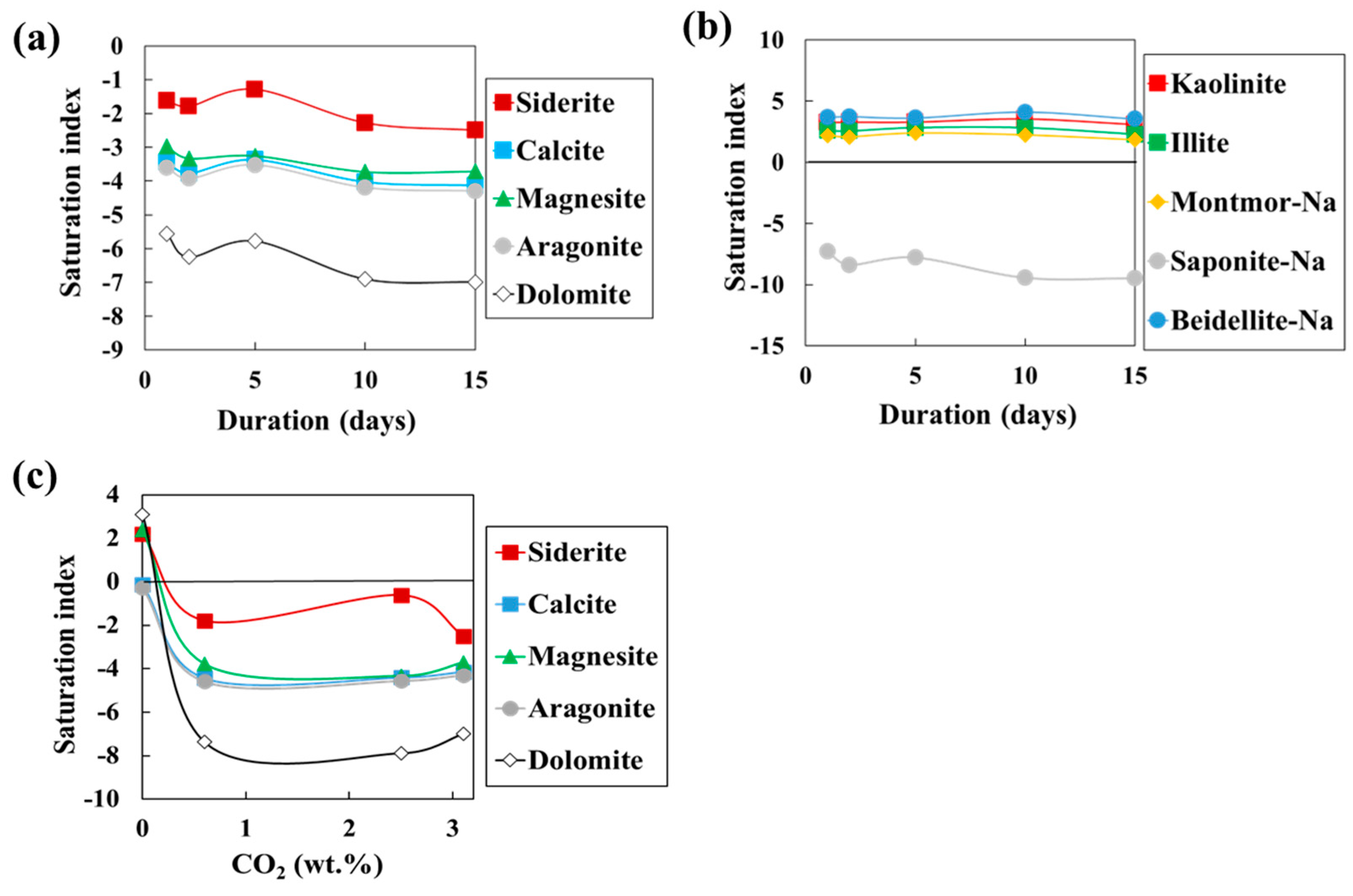
3.3. Investigation of Saturation Degree of Secondary Minerals Using PHREEQC
3.4. Approximation of Laboratory Test Results by PHREEQC Using Kinetic Model
3.5. Behavior of the Carbonated Water in the CO2 Geothermal Power Generation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| (a) Run No. | Gas | Duration (days) | pH | EC | Na | K | Ca | Mg | Fe | Al | SiO2 | Cl | SO4 | HCO3 | pH * | CO2 * | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mS/m | mg/L | wt. % | ||||||||||||||||||||||
| RUN-1 (2022/1/13–2/4) | CO2 | 1 | 7.1 | 67.8 | 116 | 73 | 1.1 | 0.49 | 0.33 | 1.2 | 846 | 5.5 | 3.5 | 333 | 4.1 | 3.1 | ||||||||
| 2 | 7.5 | 69.8 | 122 | 76 | 0.5 | 0.23 | 0.22 | 1.3 | 821 | 9.2 | 4.2 | 591 | 4.1 | 3.1 | ||||||||||
| 5 | 8.8 | 86.8 | 142 | 102 | 0.7 | 0.35 | 0.37 | 1.1 | 1000 | 9.5 | 17.5 | 591 | 4.2 | 3.1 | ||||||||||
| 10 | 7.9 | 83.9 | 143 | 93 | 0.3 | <0.1 | 0.08 | 1.7 | 856 | 10.7 | 4.1 | 625 | 4.0 | 3.1 | ||||||||||
| 15 | 7.6 | 81.1 | 140 | 86 | 0.2 | <0.1 | 0.04 | 1.0 | 849 | 9.6 | 5.7 | 640 | 4.1 | 3.1 | ||||||||||
| Ar | 1 | 8.7 | 32.6 | 65 | 25 | 0.4 | 2.52 | 1.29 | 21.4 | 690 | 9.9 | 5.0 | 166 | 8.1 | - | |||||||||
| 1 | 8.3 | 33.1 | 69 | 22 | 0.1 | 2.49 | 1.37 | 17.1 | 714 | 7.4 | 4.1 | 180 | 8.2 | - | ||||||||||
| 2 | 8.4 | 32.9 | 68 | 20 | 0.1 | 3.13 | 1.47 | 19.3 | 726 | 6.3 | 4.9 | 182 | 8.2 | - | ||||||||||
| 5 | 8.5 | 33.0 | 68 | 19 | 0.2 | 3.83 | 1.55 | 19.2 | 748 | 6.7 | 4.1 | 195 | 8.2 | - | ||||||||||
| 10 | 9.2 | 35.6 | 90 | 26 | <0.1 | 3.78 | 1.29 | 16.9 | 793 | 7.0 | 4.0 | 178 | 8.3 | - | ||||||||||
| 15 | 9.4 | 35.0 | 79 | 16 | 0.1 | 3.02 | 1.80 | 18.3 | 899 | 7.7 | 4.0 | 191 | 8.2 | - | ||||||||||
| RUN-2 (2022/7/11–7/22) | CO2 | 1 | 6.9 | 67.5 | 117 | 74 | 1.4 | 0.67 | <1.0 | 4.6 | 925 | 5.9 | 6.7 | 387 | 4.0 | 3.1 | ||||||||
| 2 | 8.6 | 70.9 | 132 | 81 | 0.8 | 0.40 | <1.0 | 2.1 | 982 | 5.9 | 3.8 | 416 | 4.0 | 3.1 | ||||||||||
| 5 | 7.5 | 83.5 | 156 | 96 | 0.4 | 0.26 | <1.0 | 3.3 | 1034 | 8.8 | 7.2 | 473 | 4.0 | 3.1 | ||||||||||
| 10 | 8.4 | 83.1 | 165 | 99 | 0.4 | 0.21 | <1.0 | 1.4 | 1040 | 7.9 | 3.9 | 488 | 4.1 | 3.1 | ||||||||||
| Ar | 1 | 8.8 | 28.2 | 61 | 19 | <0.1 | 2.28 | 1.20 | 26.8 | 684 | 4.0 | 2.9 | 160 | 8.1 | - | |||||||||
| 2 | 8.9 | 30.8 | 71 | 19 | <0.1 | 2.87 | 1.24 | 23.8 | 762 | 5.1 | 4.0 | 171 | 8.1 | - | ||||||||||
| 5 | 9.2 | 32.5 | 80 | 18 | <0.1 | 3.06 | 1.21 | 20.8 | 829 | 5.9 | 2.8 | 183 | 8.2 | - | ||||||||||
| 10 | 9.2 | 34.0 | 81 | 16 | <0.1 | 3.31 | 0.73 | 23.7 | 864 | 6.7 | 1.6 | 181 | 8.2 | - | ||||||||||
| RUN-5 (2023/5/9–5/25) | CO2 | 1 | 7.8 | 51.2 | 89 | 56 | 0.3 | 0.58 | 0.29 | 2.4 | 775 | 7.2 | 4.4 | 265 | 4.4 | 0.6 | ||||||||
| 2 | 8.1 | 54.1 | 101 | 57 | 0.2 | 0.22 | 0.62 | 2.8 | 842 | 7.0 | 3.2 | 276 | 4.3 | 0.6 | ||||||||||
| 5 | 8.3 | 63.4 | 111 | 63 | 0.1 | 0.09 | 0.27 | 3.1 | 913 | 7.7 | 3.7 | 305 | 4.3 | 0.6 | ||||||||||
| 10 | 8.2 | 58.0 | 108 | 52 | 0.2 | 0.02 | 0.35 | 4.7 | 910 | 7.3 | 3.5 | 292 | 4.2 | 0.6 | ||||||||||
| 15 | 8.5 | 68.3 | 118 | 68 | 0.1 | 0.06 | 0.18 | 2.3 | 946 | 9.2 | 5.1 | 327 | 4.4 | 0.6 | ||||||||||
| RUN-6 (2023/5/9–5/26) | CO2 | 1 | 7.8 | 53.5 | 91 | 60 | 0.4 | 0.66 | 0.63 | 2.2 | 779 | 5.8 | 3.1 | 268 | 4.2 | 1.3 | ||||||||
| 2 | 7.9 | 62.3 | 104 | 68 | 0.3 | 0.43 | 0.62 | 2.1 | 863 | 6.3 | 3.2 | 310 | 4.2 | 1.3 | ||||||||||
| 5 | 8.3 | 71.5 | 120 | 79 | 0.2 | 0.28 | 0.36 | 2.0 | 952 | 7.5 | 3.7 | 354 | 4.2 | 1.3 | ||||||||||
| 10 | 8.1 | 72.6 | 122 | 79 | 0.1 | 0.11 | 0.00 | 0.4 | 938 | 8.3 | 3.5 | 356 | 4.3 | 1.3 | ||||||||||
| 15 | 9.3 | 66.7 | 121 | 68 | 0.1 | 0.02 | 2.78 | 2.4 | 986 | 9.2 | 3.5 | 305 | 4.3 | 1.3 | ||||||||||
| (b) Run No. | Gas | Duration (days) | Mn | Cd | B | Cr | Cu | Mo | Ni | Pb | ||||||||||||||
| mg/L | ||||||||||||||||||||||||
| RUN-2 (2022/7/11–7/22) | CO2 | 1 | <1.0 | <1.0 | 16.1 | <1.0 | <1.0 | 1.8 | <1.0 | <1.0 | ||||||||||||||
| 2 | <1.0 | <1.0 | 6.0 | <1.0 | <1.0 | 0.9 | <1.0 | <1.0 | ||||||||||||||||
| 5 | <1.0 | <1.0 | 8.0 | <1.0 | <1.0 | 0.8 | <1.0 | <1.0 | ||||||||||||||||
| 10 | <1.0 | <1.0 | 6.7 | <1.0 | <1.0 | 0.6 | <1.0 | <1.0 | ||||||||||||||||
| Ar | 1 | <1.0 | <1.0 | 14.8 | <1.0 | <1.0 | 0.5 | <1.0 | <1.0 | |||||||||||||||
| 2 | <1.0 | <1.0 | 14.5 | <1.0 | <1.0 | 0.5 | <1.0 | <1.0 | ||||||||||||||||
| 5 | <1.0 | <1.0 | 7.5 | <1.0 | <1.0 | 0.4 | <1.0 | <1.0 | ||||||||||||||||
| 10 | <1.0 | <1.0 | 18.4 | <1.0 | <1.0 | 0.4 | <1.0 | <1.0 | ||||||||||||||||
| RUN NO | Gas | Duration | Calcite | Siderite | Magnesite | Aragonite | Dolomite | Dawsonite | Kaolinite | Montmor-Na | Illite | Nontronite-Na | Scolecite | Saponite-Na |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NO.1 | CO2 | 1 | −3.4 | −1.6 | −3.0 | −3.6 | −5.5 | −4.9 | 3.2 | 2.3 | 2.7 | 14 | 0.3 | −6.6 |
| 2 | −3.7 | −1.8 | −3.3 | −3.9 | −6.2 | −4.8 | 3.3 | 2.2 | 2.7 | 14 | 0.0 | −7.7 | ||
| 5 | −3.3 | −1.2 | −3.3 | −3.4 | −5.7 | −4.7 | 3.2 | 2.5 | 2.9 | 15 | 0.5 | −7.1 | ||
| 10 | −4.0 | −2.3 | −3.7 | −4.2 | −6.9 | −4.7 | 3.5 | 2.3 | 2.9 | 13 | 0.0 | −8.8 | ||
| 15 | −4.1 | −2.5 | −3.7 | −4.2 | −7.0 | −4.9 | 3.1 | 1.9 | 2.4 | 12 | −0.6 | −8.8 | ||
| NO.1 | Ar | 1 | - | - | - | - | - | - | −0.6 | 3.0 | 2.5 | 26 | 4.1 | 20 |
| 2 | - | - | - | - | - | - | −0.9 | 2.8 | 2.3 | 26 | 3.4 | 21 | ||
| 5 | - | - | - | - | - | - | −0.7 | 3.0 | 2.5 | 26 | 3.5 | 21 | ||
| 10 | - | - | - | - | - | - | −0.7 | 3.1 | 2.5 | 27 | 3.8 | 22 | ||
| 15 | - | - | - | - | - | - | −1.1 | 2.9 | 2.3 | 26 | 3.4 | 23 | ||
| NO.2 | CO2 | 1 | −3.5 | −3.3 | −3.1 | −3.6 | −5.7 | −4.4 | 4.5 | 3.4 | 4.1 | 10 | 1.5 | −6.6 |
| 2 | −3.6 | −3.2 | −3.1 | −3.8 | −5.9 | −4.6 | 3.8 | 2.9 | 3.5 | 10 | 0.7 | −6.7 | ||
| 5 | −3.9 | −3.2 | −3.4 | −4.1 | −6.5 | −4.4 | 4.3 | 3.2 | 4.0 | 11 | 0.9 | −7.5 | ||
| 10 | −3.9 | −3.2 | −3.4 | −4.0 | −6.4 | −4.7 | 3.5 | 2.7 | 3.1 | 11 | 0.2 | −7.4 | ||
| NO.2 | Ar | 1 | - | - | - | - | - | - | −0.3 | 3.2 | 2.8 | 26 | 3.8 | 20 |
| 2 | - | - | - | - | - | - | −0.5 | 3.3 | 2.8 | 26 | 3.8 | 21 | ||
| 5 | - | - | - | - | - | - | −0.6 | 3.3 | 2.7 | 26 | 3.7 | 22 | ||
| 10 | - | - | - | - | - | - | −0.4 | 3.5 | 2.9 | 26 | 3.9 | 22 | ||
| NO.5 | CO2 | 1 | −4.0 | −1.7 | −3.1 | −4.2 | −6.3 | −5.2 | 3.7 | 2.8 | 3.5 | 15 | 0.8 | −4.8 |
| 2 | −4.3 | −1.5 | −3.6 | −4.4 | −7.0 | −5.1 | 3.9 | 2.9 | 3.6 | 16 | 0.8 | −6.1 | ||
| 5 | −4.6 | −1.8 | −4.0 | −4.8 | −7.7 | −5.0 | 4.1 | 3.0 | 3.8 | 15 | 0.6 | −7.1 | ||
| 10 | −4.5 | −2.0 | −4.9 | −4.7 | −8.5 | −4.9 | 4.4 | 3.0 | 3.9 | 15 | 1.1 | −10 | ||
| 15 | −4.4 | −1.8 | −3.9 | −4.6 | −7.4 | −5.0 | 3.8 | 2.9 | 3.6 | 15 | 0.6 | −6.8 | ||
| NO.6 | CO2 | 1 | −3.9 | −1.4 | −2.9 | −4.0 | −5.9 | −5.0 | 3.6 | 2.7 | 3.3 | 15 | 0.5 | −5.2 |
| 2 | −4.0 | −1.4 | −3.1 | −4.1 | −6.3 | −4.9 | 3.7 | 2.8 | 3.4 | 16 | 0.5 | −5.8 | ||
| 5 | −4.1 | −1.5 | −3.3 | −4.3 | −6.6 | −4.9 | 3.7 | 2.9 | 3.5 | 15 | 0.5 | −6.0 | ||
| 10 | −4.3 | −2.0 | −3.6 | −4.4 | −7.1 | −5.5 | 2.3 | 1.6 | 1.8 | 14 | −1.1 | −7.3 | ||
| 15 | −4.3 | −0.5 | −4.3 | −4.5 | −7.8 | −4.7 | 3.9 | 2.8 | 3.4 | 18 | 0.5 | −9.2 |
| (a) Species | Concentration (mol/kg_H2O) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | Charge | |||||||||||||||
| Ca2+ | 1.0 × 10−4 | |||||||||||||||
| Mg2+ | 1 × 10−20 | |||||||||||||||
| Na+ | 4.30 × 10−5 | |||||||||||||||
| K+ | 2.50 × 10−5 | |||||||||||||||
| Fe2+ | 1 × 10−20 | |||||||||||||||
| SiO2(aq) | 1 × 10−20 | |||||||||||||||
| HCO3− | pCO2 = 4 MPa (equilibrated) | |||||||||||||||
| Al3+ | 1 × 10−20 | |||||||||||||||
| (b) | Mineral | Formula | Primary Mineral | |||||||||||||
| Silicates | Albite | NaAlSi3O8 | ✔ | |||||||||||||
| Anorthite | Ca(Al2Si2)O8 | ✔ | ||||||||||||||
| Forsterite | Mg2SiO4 | ✔ | ||||||||||||||
| K-Feldspar | K(AlSi3)O8 | ✔ | ||||||||||||||
| Paragonite (=Glass part) | NaAl2(AlSi3)O10(OH)2 | ✔ | ||||||||||||||
| Fayalite | Fe2SiO4 | ✔ | ||||||||||||||
| Saponite(FeMg) | Mg0.17Mg2FeAl0.34Si3.66O10(OH)2:5.039H2O | |||||||||||||||
| Beidellite(Mg) | Mg0.3Mg0.6Al1.4Si4O10(OH)2:5.129H2O | |||||||||||||||
| Montmorillonite(Mg) | Mg0.3Mg0.6Al1.4Si4O10(OH)2 | |||||||||||||||
| Oxides | Quartz | SiO2 | ||||||||||||||
| Carbonates | Calcite | CaCO3 | ||||||||||||||
| Dolomite | CaMg(CO3)2 | |||||||||||||||
| Magnesite | MgCO3 | |||||||||||||||
| (c) | Initial | Neutral | Acid | Base | ||||||||||||
| Primary | Weight (g) | Weight percent (%) | Radius of mineral grain (m) | Initial surface area (m2/m3) | logK | K (mol/m2/s) | EA | logK | K (mol/m2/s) | EA | n | logK | K (mol/m2/s) | EA | n | |
| Albite | NaAlSi3O8 | 0.483 | 2.3 * | 1 × 10−6 | 1 × 106 * | −12.56 | 2.75 × 10−13 | 69.8 | −10.16 | 6.92 × 10−11 | 65 | 0.457 | −15.6 | 2.51 × 10−16 | 71 | −0.572 |
| Anorthite | Ca(Al2Si2)O8 | 0.77 | 11.1 | 1 × 10−6 | 1 × 105 | −9.12 | 7.59 × 10−10 | 17.8 | −3.5 | 3.16 × 10−4 | 16.6 | 1.411 | - | - | - | - |
| Fayalite | Fe2SiO4 | 0.049 | 0.7 | Equilibrium | ||||||||||||
| Forsterite | Mg2SiO4 | 0.049 | 0.7 | 1 × 10−6 | 1 × 105 | −10.64 | 2.29 × 10−11 | 79 | −6.85 | 1.41 × 10−7 | 67.2 | 0.47 | - | - | - | - |
| K-Feldspar | K(AlSi3)O8 | 0.014 | 1.0 * | 1 × 10−6 | 1 × 108 * | −12.41 | 3.89 × 10−13 | 38 | −10.06 | 8.71 × 10−11 | 51.7 | 0.5 | −21.2 | 6.31 × 10−22 | 94.1 | −0.823 |
| Paragonite | NaAl2(AlSi3)O10(OH)2 | 5.6 | 80 | 1 × 10−6 | 1 × 105 | −13 | 1.00 × 10−13 | 22 | - | - | - | - | - | - | - | - |
| (d) | Si-Based Reactive Minerals | State | (Solid Phase⇔ Liquid Phase) | Reaction-Dominant Mineral | ||||||||||||
| Primary mineral | Albite | Dissolution | 2.87 × 10−2 | ◎ | ||||||||||||
| Anorthite | 2.41 × 10−3 | |||||||||||||||
| Forsterite | 4.97 × 10−3 | |||||||||||||||
| Microcline | 2.23 × 10−3 | |||||||||||||||
| Paragonite | 1.08 × 10−6 | |||||||||||||||
| Fayalite | 2.10 × 10−3 | |||||||||||||||
| Secondary minaeral | Quartz | Precipitation | 7.84 × 10−4 | |||||||||||||
| Saponite(FeMg) | 1.54 × 10−2 | ◎ | ||||||||||||||
| Beidellite(Mg) | 1.76 × 10−2 | ◎ | ||||||||||||||
References
- Ministry of Economy, Trade, and Industry. Japan’s Newest “Strategic Energy Plan” Toward Carbon Neutrality by 2050. Available online: https://www.enecho.meti.go.jp/en/category/special/article/detail_168.html (accessed on 6 February 2025).
- Ministry of the Environment. 2050 Zero Carbon Cities in Japan. Available online: https://www.env.go.jp/en/earth/cc/2050_zero_carbon_cities_in_japan.html (accessed on 6 February 2025).
- Muraoka, H.; Sakaguchi, K.; Komazawa, M.; Sasaki, S. Assessment of Hydrothermal Resource Potentials in Japan. In Proceedings of the Geothermal Research Society of Japan, Kanazawa, Japan, 30 October–1 November 2008. [Google Scholar]
- Kaieda, H.; Kiho, K.; Motojima, I. Multiple Fracture Creation for Hot Dry Rock Development. Trends Geophys. 1993, 2, 127–139. [Google Scholar]
- Kaieda, H.; Yamamoto, T.; Kiho, K.; Kitano, K.; Hori, Y.; Ito, H.; Eguchi, Y.; Fujimitsu, Y.; Motojima, I. Development of Reservoir Construction Methods for Hot Dry Rock Geothermal Power-Two Stage Reservoir Creation and Its Evaluation at Ogachi; Central Research Institute of Electric Power Industry: Tokyo, Japan, 2000. [Google Scholar]
- Further Promotion of Geothermal Development. Available online: https://www.jogmec.go.jp/english/carbonneutral/carbonneutral_15_00006.html (accessed on 6 February 2025).
- Terai, A.; Ichinohe, T.; Tosha, T. Carbon Recycles CO2 Geothermal Power Generation Technology: (1) Project Overview (A22)(Abs.). In Proceedings of the Annual Meeting, Geothermal Research Society of Japan, Sendai, Japan, 26–29 October 2001. [Google Scholar]
- Brown, D.W. Hot Dry Rock Geothermal Energy Concept Utilizing Supercritical CO2 Instead of Water. In Proceedings of the Twenty-Fifth Workshop Geotherm Reservoir Engineering, Stanford, CA, USA, 24–26 January 2000; p. SGP-TR-165. [Google Scholar]
- Cabeza, L.F.; de Gracia, A.; Fernández, A.I.; Farid, M.M. Supercritical CO2 as Heat Transfer Fluid: A Review. Appl. Therm. Eng. 2017, 125, 799–810. [Google Scholar] [CrossRef]
- Okamoto, K.; Ota, J.; Sakurai, K.; Madarame, H. Transient Velocity Distributions for the Supercritical Carbon Dioxide Forced Convection Heat Transfer. J. Nucl. Sci. Technol. 2003, 40, 763–767. [Google Scholar] [CrossRef]
- Pajak, L.; Sowiżdżał, A.; Gładysz, P.; Tomaszewska, B.; Miecznik, M.; Andresen, T.; Frengstad, B.S.; Chmielowska, A. Multi-Criteria Studies and Assessment Supporting the Selection of Locations and Technologies Used in CO2-EGS Systems. Energies 2021, 14, 7683. [Google Scholar] [CrossRef]
- Zhao, W.; Yuan, Y.; Jing, T.; Zhong, C.; Wei, S.; Yin, Y.; Zhao, D.; Yuan, H.; Zheng, J.; Wang, S. Heat Production Performance from an Enhanced Geothermal System (EGS) Using CO2 as the Working Fluid. Energies 2023, 16, 7202. [Google Scholar] [CrossRef]
- Cong, L.; Lu, S.; Jiang, P.; Zheng, T.; Yu, Z.; Lü, X. Research Progress on CO2 as Geothermal Working Fluid: A Review. Energies 2024, 17, 5415. [Google Scholar] [CrossRef]
- Gładysz, P.; Pająk, L.; Andresen, T.; Strojny, M.; Sowiżdżał, A. Process Modeling and Optimization of Supercritical Carbon Dioxide-Enhanced Geothermal Systems in Poland. Energies 2024, 17, 3769. [Google Scholar] [CrossRef]
- Li, Q.; Li, Q.; Cao, H.; Wu, J.; Wang, F.; Wang, Y. The Crack Propagation Behaviour of CO2 Fracturing Fluid in Unconventional Low Permeability Reservoirs: Factor Analysis and Mechanism Revelation. Processes 2025, 13, 159. [Google Scholar] [CrossRef]
- Nianyin, L.; Jiajie, Y.; Chao, W.; Suiwang, Z.; Xiangke, L.; Jia, K.; Yuan, W.; Yinhong, D. Fracturing Technology with Carbon Dioxide: A Review. J. Pet. Sci. Eng. 2021, 205, 108793. [Google Scholar] [CrossRef]
- Aradóttir, E.S.P.; Sonnenthal, E.L.; Björnsson, G.; Jónsson, H. Multidimensional Reactive Transport Modeling of CO2 Mineral Sequestration in Basalts at the Hellisheidi Geothermal Field, Iceland. Int. J. Greenh. Gas. Control. 2012, 9, 24–40. [Google Scholar] [CrossRef]
- Ueda, A.; Kato, K.; Ohsumi, T.; Yajima, T.; Ito, H.; Kaieda, H.; Metcalfe, R.; Takase, H. Experimental Studies of CO2-Rock Interaction at Elevated Temperatures under Hydrothermal Conditions. Geochem. J. 2005, 39, 417–425. [Google Scholar] [CrossRef]
- Itoi, R.; Maekawa, H.; Fukuda, M.; Jinno, K.; Hatakenaka, K.; Yokoyama, T.; Shimizu, S. Study on Decrease of Reservoir Permeability Due to Deposition of Silica Dissolved in Reinjection Water. J. Geotherm. Res. Soc. Jpn. 1986, 8, 229–241. [Google Scholar]
- Gysi, A.P.; Stefánsson, A. Mineralogical Aspects of CO2 Sequestration during Hydrothermal Basalt Alteration-An Experimental Study at 75 to 250 °C and Elevated pCO2. Chem. Geol. 2012, 306–307, 146–159. [Google Scholar] [CrossRef]
- Gysi, A.P.; Stefánsson, A. Experiments and Geochemical Modeling of CO2 Sequestration during Hydrothermal Basalt Alteration. Chem. Geol. 2012, 306–307, 10–28. [Google Scholar] [CrossRef]
- Kumar, A.; Shrivastava, J.P.; Pathak, V. Mineral Carbonation Reactions under Water-Saturated, Hydrothermal-like Conditions and Numerical Simulations of CO2 Sequestration in Tholeiitic Basalt of the Eastern Deccan Volcanic Province, India. Appl. Geochem. 2017, 84, 87–104. [Google Scholar] [CrossRef]
- Chiba, H. Attainment of Solution and Gas Equilibrium in Japanese Geothermal Systems. Geochem. J. 1991, 25, 335–355. [Google Scholar] [CrossRef]
- Ueda, A.; Ajima, S.; Yamamoto, M. Isotopic Study of Carbonate Minerals from the Sumikawa Geothermal Area and Its Application to Water Movement. J. Geotherm. Res. Soc. Jpn. 2001, 23, 181–196. [Google Scholar] [CrossRef]
- Taniuchi, H. Recent Petrological Studies at Rishiri Volcano. Rishiri Stud. 2021, 40, 33–43. [Google Scholar]
- Yang, H.; Mishima, T.; Katazakai, S.; Kagabu, M. Analytical Approach Using a Chemical Equilibrium Formula and Geochemical Modeling for Alkalinity Measurements of Small Natural Water Samples. Appl. Geochem. 2023, 148, 105535. [Google Scholar] [CrossRef]
- Shibuya, T.; Yoshizaki, M.; Masaki, Y.; Suzuki, K.; Takai, K.; Russell, M.J. Reactions between Basalt and CO2-Rich Seawater at 250 and 350 °C, 500bars: Implications for the CO2 Sequestration into the Modern Oceanic Crust and the Composition of Hydrothermal Vent Fluid in the CO2-Rich Early Ocean. Chem. Geol. 2013, 359, 1–9. [Google Scholar] [CrossRef]
- Fournier, R.O. Chemical Geothermometers and Mixing Models for Geothermal Systems. Geothermics 1977, 5, 41–50. [Google Scholar] [CrossRef]
- Tonani, F.B. Some Remarks on the Application of Geochemical Techniques in Geothermal Exploration. In Proceedings of the 2nd International Seminar on the Results of EC Geothermal Energy Research, Ljubljana, Slovenia, 4–6 March 1980; pp. 428–443. [Google Scholar]
- Truesdell, A. Summary of Section Iii: Geochemical Techniques in Exploration. In Proceedings of the 2nd U.N. Symposium on the Development and Use of Geothermal Resources, Berkeley, CA, USA, 20–29 May 1975. [Google Scholar]
- Arnórsson, S.; Gunnlaugsson, E.; Svavarsson, H. The Chemistry of Geothermal Waters in Iceland. II. Mineral Equilibria and Independent Variables Controlling Water Compositions. Geochim. Cosmochim. Acta 1983, 47, 547–566. [Google Scholar] [CrossRef]
- Nieva, D.; Nieva, R. Developments in Geothermal Energy in Mexico—Part Twelve. A Cationic Geothermometer for Prospecting of Geothermal Resources. Heat. Recovery Syst. CHP 1987, 7, 243–258. [Google Scholar] [CrossRef]
- Giggenbach, W.F. Geothermal Solute Equilibria. Derivation of Na-K-Mg-Ca Geoindicators. Geochim. Cosmochim. Acta 1988, 52, 2749–2765. [Google Scholar] [CrossRef]
- Erdogan, Y.; Aksu, M.; Demirbaş, A.; Abali, Y. Analyses of Boronic Ores and Sludges and Solubilities of Boron Minerals in CO2-Saturated Water. Resour. Conserv. Recycl. 1998, 24, 275–283. [Google Scholar] [CrossRef]
- Budak, A.; Gönen, M. Extraction of Boric Acid from Colemanite Mineral by Supercritical Carbon Dioxide. J. Supercrit. Fluids 2014, 92, 183–189. [Google Scholar] [CrossRef]
- Ratouis, T.M.P.; Snæbjörnsdóttir, S.; Voigt, M.J.; Sigfússon, B.; Gunnarsson, G.; Aradóttir, E.S.; Hjörleifsdóttir, V. Carbfix2: A Transport Model of Long-Term CO2 and H2S Injection into Basaltic Rocks at Hellisheidi, SW-Iceland. Int. J. Greenh. Gas. Control. 2022, 114, 103586. [Google Scholar] [CrossRef]
- Parkhust, D.L.; Appelo, C.A.J. User’s Guide to PHREEQC (Ver2)-A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations. U.S. Geol. Surv. 1999, 99–4259, 312. [Google Scholar] [CrossRef]
- Xu, T.; Sonnenthal, E.; Spycher, N.; Pruess, K. TOUGHREACT-A Simulation Program for Non-Isothermal Multiphase Reactive Geochemical Transport in Variably Saturated Geologic Media: Applications to Geothermal Injectivity and CO2 Geological Sequestration. Comput. Geosci. 2006, 32, 145–165. [Google Scholar] [CrossRef]
- Lo Ré, C.; Kaszuba, J.P.; Moore, J.N.; McPherson, B.J. Fluid-Rock Interactions in CO2-Saturated, Granite-Hosted Geothermal Systems: Implications for Natural and Engineered Systems from Geochemical Experiments and Models. Geochim. Cosmochim. Acta 2014, 141, 160–178. [Google Scholar] [CrossRef]
- Hellevang, H.; Haile, B.G.; Tetteh, A. Experimental Study to Better Understand Factors Affecting the CO2 Mineral Trapping Potential of Basalt. Greenh. Gases Sci. Technol. 2017, 7, 143–157. [Google Scholar] [CrossRef]
- Marieni, C.; Voigt, M.; Clark, D.E.; Gíslason, S.R.; Oelkers, E.H. Mineralization Potential of Water-Dissolved CO2 and H2S Injected into Basalts as Function of Temperature: Freshwater versus Seawater. Int. J. Greenh. Gas. Control. 2021, 109, 103357. [Google Scholar] [CrossRef]
- Aradóttir, E.S.P.; Sonnenthal, E.L.; Jónsson, H. Development and Evaluation of a Thermodynamic Dataset for Phases of Interest in CO2 Mineral Sequestration in Basaltic Rocks. Chem. Geol. 2012, 304–305, 26–38. [Google Scholar] [CrossRef]
- Aradóttir, E.S.P.; Sigfússon, B.; Sonnenthal, E.L.; Björnsson, G.; Jónsson, H. Dynamics of Basaltic Glass Dissolution-Capturing Microscopic Effects in Continuum Scale Models. Geochim. Cosmochim. Acta 2013, 121, 311–327. [Google Scholar] [CrossRef]
- Pollyea, R.M.; Rimstidt, J.D. Rate Equations for Modeling Carbon Dioxide Sequestration in Basalt. Appl. Geochem. 2017, 81, 53–62. [Google Scholar] [CrossRef]
- Gysi, A.P.; Stefánsson, A. CO2-Water-Basalt Interaction. Numerical Simulation of Low Temperature CO2 Sequestration into Basalts. Geochim. Cosmochim. Acta 2011, 75, 4728–4751. [Google Scholar] [CrossRef]
- Hangx, S.J.T.; Spiers, C.J. Reaction of Plagioclase Feldspars with CO2 under Hydrothermal Conditions. Chem. Geol. 2009, 265, 88–98. [Google Scholar] [CrossRef]
- Kuroda, Y.; Yamada, Y.; Ueda, A.; Matsuoka, T.; Yamada, N. Experimental Research of Plagioclase (Rock)-Gas-Water Interaction at Hyrrothrmal Conditions for CO2 Mineralization. J. Mineral. Petrol. Sci. 2009, 38, 111–121. [Google Scholar] [CrossRef][Green Version]
- Oochi, R.; Aoyama, K.; Ueda, A. Zero-Emission Geothermal Power Generation: Experimental Study on Carbonate Mineralization through CO2-Andesitic Pyroclastic Rock Interaction at Oku-Aizu Geothermal Plant. J. Energy Power Technol. 2020, 2. [Google Scholar] [CrossRef]
- Mao, D.; Zhang, J.; Hoshino, Y.; Satake, S.; Yang, H.; Kuramitz, H.; Ueda, A.; Terai, A. Geochemical Study of Fluid Origin and Caprock Formation with Carbonate Mineral Precipitation in the Okuaizu Geothermal Area. Geothermics 2025, 127, 103242. [Google Scholar] [CrossRef]


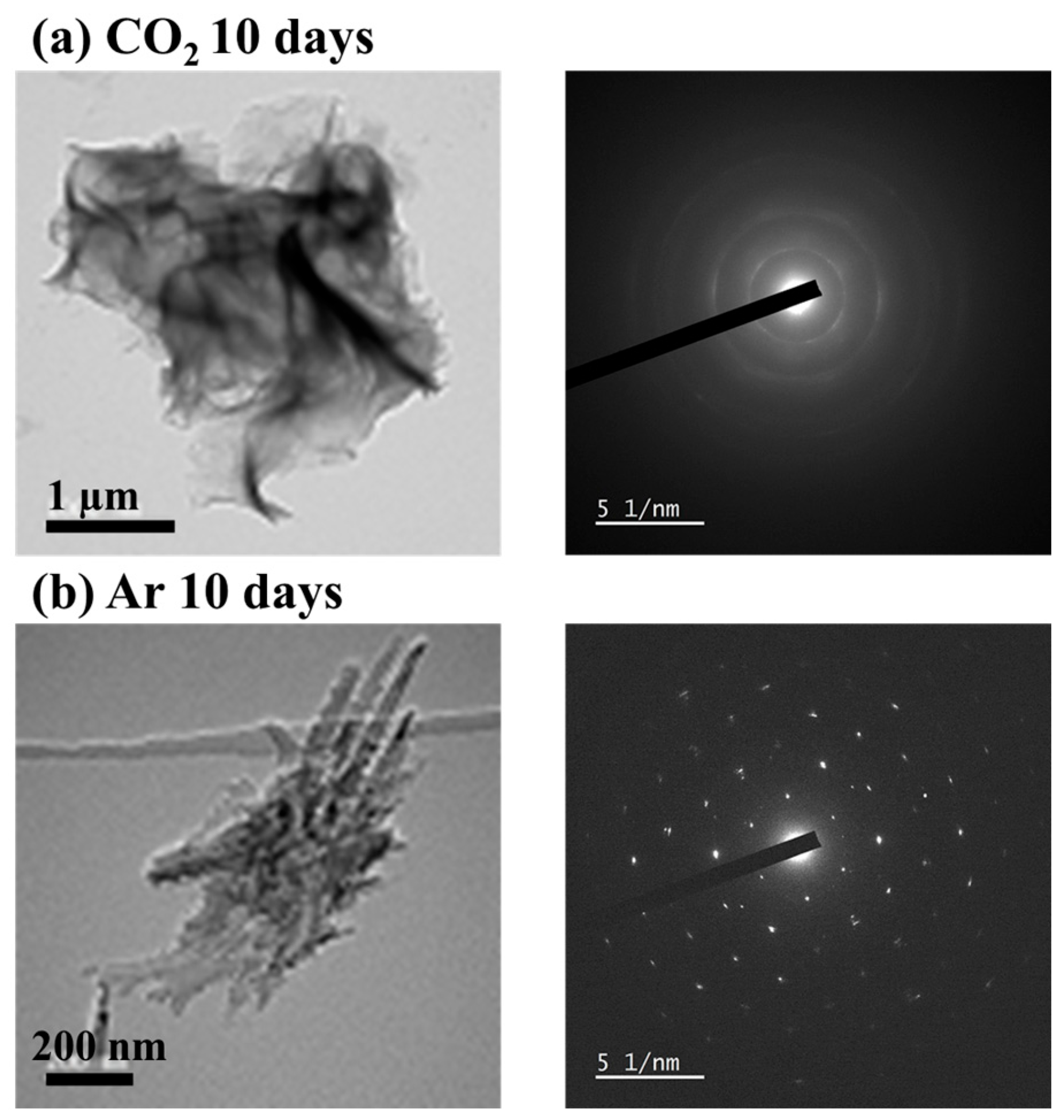

| Component | wt. % |
|---|---|
| SiO2 | 50.2 |
| TiO2 | 1.1 |
| Al2O3 | 14.8 |
| CaO | 9.0 |
| MgO | 3.4 |
| MnO | 0.18 |
| Total Fe as Fe2O3 | 16.1 |
| Na2O | 3.3 |
| K2O | 0.61 |
| P2O5 | 0.22 |
| H2O+ | <0.1 |
| H2O− | 0.20 |
| (a) Olivine | (b) Clinopyroxene | (c) Plagioclase | ||||||
|---|---|---|---|---|---|---|---|---|
| Core 1 | Core 2 | Core | Rim | Core | Rim | |||
| Element | Cations per 4 Oxygens | Element | Cations per 6 Oxygens | Element | Cations per 8 Oxygens | |||
| Si | 0.99 | 0.99 | Si | 1.94 | 1.93 | Si | 2.39 | 2.47 |
| Ti | 0.00 | 0.00 | Ti | 0.02 | 0.02 | Ti | 0.00 | 0.00 |
| Al | 0.00 | 0.00 | Al | 0.07 | 0.10 | Al | 1.58 | 1.50 |
| Cr | 0.00 | 0.00 | Cr | 0.02 | 0.01 | Fe | 0.02 | 0.02 |
| Fe | 0.51 | 0.51 | Fe | 0.21 | 0.22 | Mn | 0.01 | 0.01 |
| Mn | 0.01 | 0.01 | Mn | 0.01 | 0.01 | Ca | 0.62 | 0.54 |
| Ni | 0.00 | 0.00 | Mg | 0.92 | 0.89 | Na | 0.39 | 0.44 |
| Mg | 1.48 | 1.49 | Ca | 0.80 | 0.82 | K | 0.01 | 0.01 |
| Ca | 0.01 | 0.01 | Na | 0.03 | 0.03 | |||
| Total cation | 3.00 | 3.01 | Total cation | 4.01 | 4.01 | Total cation | 5.02 | 5.00 |
| mol % | mol % | mol % | ||||||
| Forsterite | 74.3 | 74.5 | Wollastonite | 41.3 | 42.4 | Anorthite | 61.0 | 54.5 |
| Fayalite | 25.7 | 25.5 | Enstatite | 47.6 | 46.2 | Albite | 38.0 | 44.3 |
| Ferrosilite | 11.1 | 11.3 | Orthoclase | 1.0 | 1.2 | |||
| RUN No. | Gas | Initial CO2 Pressure at 25 °C (MPa) | Initial Total Pressure at 25 °C (MPa) | Total Pressure at 250 °C (MPa) | CO2 (wt. %) at 250 °C | Duration (Days) |
|---|---|---|---|---|---|---|
| 1 | CO2 or Ar | 4 | 4 | 10 | 3.1 | 1–15 |
| 0 | 0 | |||||
| 2 | 4 | 3.1 | 1–10 | |||
| 0 | 0 | |||||
| 5 | 1 | 0.6 | 1–15 | |||
| 6 | 2 | 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satake, S.; Yang, H.; Mori, K.; Hoshino, Y.; Ueda, A.; Kuramitz, H.; Masuoka, K.; Enomoto, H.; Terai, A. CO2 Geothermal Power Generation: Laboratory Experiment on the Interaction Between Carbonated Water and Rishiri Island Basalt in the Vicinity of Injection Wells. Energies 2025, 18, 2251. https://doi.org/10.3390/en18092251
Satake S, Yang H, Mori K, Hoshino Y, Ueda A, Kuramitz H, Masuoka K, Enomoto H, Terai A. CO2 Geothermal Power Generation: Laboratory Experiment on the Interaction Between Carbonated Water and Rishiri Island Basalt in the Vicinity of Injection Wells. Energies. 2025; 18(9):2251. https://doi.org/10.3390/en18092251
Chicago/Turabian StyleSatake, Sakurako, Heejun Yang, Koji Mori, Yukiko Hoshino, Akira Ueda, Hideki Kuramitz, Kentaro Masuoka, Hisako Enomoto, and Amane Terai. 2025. "CO2 Geothermal Power Generation: Laboratory Experiment on the Interaction Between Carbonated Water and Rishiri Island Basalt in the Vicinity of Injection Wells" Energies 18, no. 9: 2251. https://doi.org/10.3390/en18092251
APA StyleSatake, S., Yang, H., Mori, K., Hoshino, Y., Ueda, A., Kuramitz, H., Masuoka, K., Enomoto, H., & Terai, A. (2025). CO2 Geothermal Power Generation: Laboratory Experiment on the Interaction Between Carbonated Water and Rishiri Island Basalt in the Vicinity of Injection Wells. Energies, 18(9), 2251. https://doi.org/10.3390/en18092251






