1. Introduction
Recently, research efforts have been focused on exploring new alternative energy sources that are both sustainable and environmentally friendly. Towards this end, residual lipid-based feedstocks are being explored for biofuel production under the circular economy concept [
1,
2]. More specifically, biofuels can be derived from various feedstocks, including energy crops, agricultural and forest residues, and organic wastes [
3]. In this context, WCOs are the residues generated from food cooking processes in food industries, restaurants, fast food establishments, and households, and they can be utilized for energy production [
4].
Globally, it is estimated that approximately 17 million tons of WCOs are generated annually. For instance, China is reported to produce 4–8 million tons per year, while Europe generates approximately 1 million tons. Alarmingly, about 60% of the total volume of WCOs is discarded, often in environmentally harmful ways, leading to serious environmental problems [
5,
6].
Therefore, it is evident that WCOs constitute a highly available feedstock that could be valorized via technological schemes for biofuels while mitigating the environmental risks associated with their improper disposal.
The composition of WCOs depends on the chemical changes that occur during the cooking process, as well as the type of the parent oils. Edible oils are primarily composed of triglycerides, which constitute over 99% of their structure, along with smaller quantities of free fatty acids (FFAs), carotenoids, sterols, hydrocarbons, trace elements, and other compounds. Nevertheless, due to their origin, WCOs are highly heterogeneous and are often degraded, rendering a pretreatment step necessary prior to their conversion to fuels.
In particular, the degradation products that are present in WCOs include polymers, compounds that are oxidized, volatile organics, peroxides, and fatty acids. Furthermore, these degradation products create issues since fatty acids and water contribute to saponification, reduce catalyst efficiency, and cause separation challenges [
7]. In addition, WCOs are characterized by high acidity, which results from frying and extended storage that needs to be decreased prior to the conversion step. Specifically, acidity reduction is a significant challenge for the pretreatment process, as it affects the subsequent conversion processes. According to M.U.H. Suzihaque et al. 2022, in order for WCOs to be converted into biodiesel via transesterification without pretreatment, the FFA content should be lower than 2%. Otherwise, pretreatment is required before conversion into biofuel [
8].
Moreover, while WCOs constitute a promising feedstock for biofuels and aviation fuels, pretreatment, including filtration and cleaning, is compulsory [
9,
10]. Based on the composition and properties of the WCOs, filtration or centrifugation is often necessary to remove food residues and suspended solids. Τhen, drying is applied to reduce the water content before proceeding with the WCOs’ pretreatment. Pretreatment methods are divided into physical and chemical processes, including esterification, glycerolysis, and adsorption, reducing FFA levels to 0.5–1%. Particularly, chemical treatments such as esterification and physical processes such as adsorption have been utilized for WCOs with high FFAs [
11].
In the literature, some studies have investigated a pretreatment method of WCOs that is commonly referred to as chemical refining, which is composed of multiple steps (reduction of phospholipid content, neutralization, bleaching, odor reduction). This method is particularly advantageous because it minimizes oil loss during treatment [
12]. More specifically, one of the stages of chemical refining is neutralization, which is targeted at reducing the levels of FFAs to improve the lipids’ quality for further processing.
As aforementioned, acidity is a critical property of the produced biofuels, and the pretreatment process specifically aims to reduce the high acidity of lipid-based feedstocks. Therefore, various methods are available to lower the acidity of WCOs, which are classified into chemical and physical processes. Chemical methods include neutralization, bioprocessing, and esterification, while physical methods involve solvent extraction, adsorption, and distillation. A short description of these methods follows.
Esterification applies to any oil quality, delivering high yields and possible integration with transesterification. Alternatively, according to Samya Elias et al. 2020, WCOs’ acidity can be reduced through simultaneous esterification–transesterification with the use of bifunctional catalysts and enzymes [
13]. Another method, glycerolysis, also known as glycerin esterification, reduces the amount of FFAs in degraded oils without the need for vacuum stripping, methanol, or acid. More specifically, glycerin and FFAs combine to generate acylglycerols (glycerides) and water when glycerin is added to degraded oil at about 238 °C. Through base-catalyzed transesterification, these glycerides can then be directly converted to biodiesel [
14]. However, these processes present several disadvantages, including high production costs, the generation of waste and byproducts, equipment corrosion due to the use of acidic catalysts, and low catalyst recovery rates.
Neutralization can be applied to oils of any quality and requires low energy consumption. It exhibits high selectivity in acid removal. Nevertheless, this method has some drawbacks, including high consumption of oil and water during the washing process as well as the formation of soaps and wastewater, which require further treatment.
Acidity reduction through distillation is straightforward, generates no byproducts, and involves low water consumption. On the other hand, it demands high energy input and extreme conditions of temperature and pressure. Additionally, this method is ineffective at removing phospholipids and polymers contained in the oil.
Adsorption is a method utilized for oils of varying quality, with advantages including low energy consumption and minimal wastewater production. Sri Rahayu et al. 2018 examined bagasse as a bio-adsorbent and found that the type of the adsorbent notably affects the reduction of acidity in WCOs [
15]. Nonetheless, its disadvantages include high consumption of adsorbent materials, the generation of solid waste, low retention rates, and the need for a variety of adsorbents to achieve high efficiency.
Solvent extraction avoids the generation of byproducts and allows for solvent recovery. Still, the development of such a method is costly, and its effectiveness in reducing acidity is incomplete. Although it is environmentally friendly, it is not considered suitable for short-chain fatty acids and is associated with high production costs [
7].
Intensified technologies (membrane reactors, microwaves, microreactors, etc.) that have shown FFA reduction <1 wt% can also be implemented; nonetheless, their relatively high cost limits their selection [
11].
Based on the above, these methods have strengths and limitations that should be thoroughly considered based on the specific requirements of the WCO pretreatment process and the subsequent conversion process. Nevertheless, the necessity arises for a simple process with few steps that will improve the quality of the lipids.
The present work explores the pretreatment of degraded WCOs for renewable fuels, aiming to improve their quality via a three-step process consisting of neutralization, washing of soaps, and drying. The novelty of this work is the investigation of a systematic and straightforward pretreatment process with high yield without producing significant amounts of waste, examining WCOs of different origins and quality.
2. Materials and Methods
WCOs with different origins (e.g., households, restaurants) and properties (acidity, moisture content) were utilized in this study.
Table 1 presents the properties of the utilized WCOs prior to pretreatment. The moisture content and the acidity were determined according to the Karl Fischer method (ASTM D 1744 [
16]) and the EN 14104 method [
17], respectively. The saponification content was measured using the method AOCS Cc 17-95 [
18].
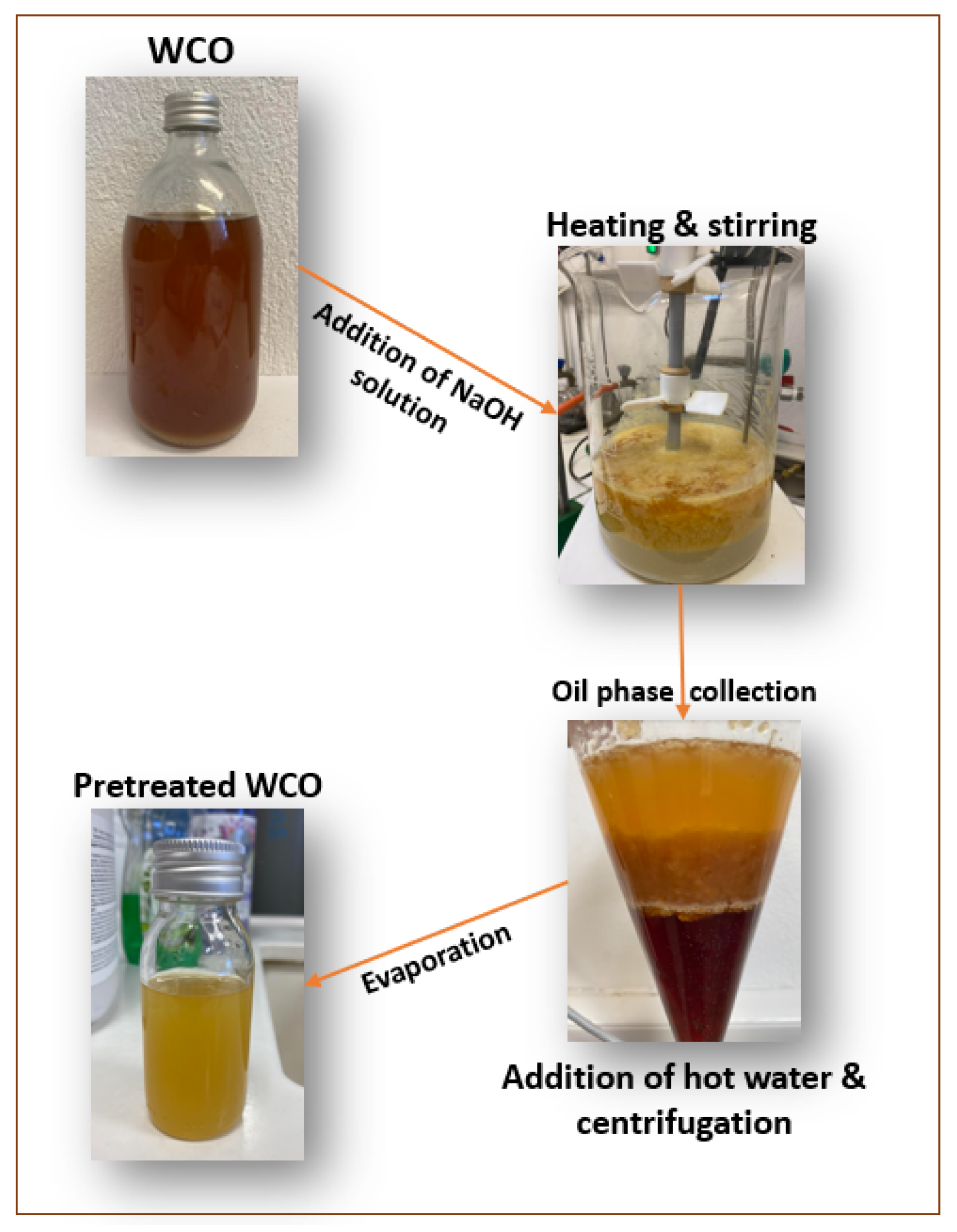
The experimental procedure is schematically depicted in
Figure 1, representing the main steps.
The tested process included three steps, as mentioned above. Firstly, the neutralization step enables the separation of FFAs from the WCOs by reacting them with a base, resulting in soap formation [
19]. The process primarily involves the removal of FFAs and is controlled by the chemical removal of FFAs through a chemical reaction in an alkaline environment (using NaOH, which neutralizes the FFAs by converting them into sodium salts (soaps) and removes any residual pigments). For this purpose, a 10 wt% aqueous NaOH solution was prepared and added to the WCO. The mixture is gently stirred under heating for 2 h, allowing aggregates of the suspended soaps to form. Afterward, it is left to rest for a few hours to enable the soaps to settle.
During the neutralization reaction, soaps are formed. The triglycerides of the WCOs consist of FFAs and glycerol. The functional group of the FFAs is an acidic hydrogen ion (H+), which is central to the neutralization reaction’s mechanism. Upon reacting with NaOH, the acidic H+ group of the free fatty acid binds with the OH- group of the base, producing water and soap.
The soaps formed during the reaction are considered undesirable byproducts and must be effectively removed. Their removal is further facilitated by the difference in specific gravity between the soap and the oil and some additional steps. After saponification, the subsequent stages involve centrifugation of the oil phase to remove the soap content, followed by washing the collected oil phase with 10% hot water to eliminate any soap residues, combined with centrifugation (1500 rpm for 8 min). The following step involves collecting the oil from the centrifugation tubes and then drying it in a rotary evaporator to reduce moisture content. Each pretreatment experiment was repeated three times.
As previously reported, the acidity analysis was conducted according to the EN 14104 method, which involves titration with a standard potassium hydroxide (KOH) solution in ethanol. The endpoint of the volumetric analysis was determined using phenolphthalein as an indicator. Specifically, a 1:1 mixture of ethyl ether and 95% ethanol, a 0.1% (w/v) phenolphthalein solution in ethanol, and a 0.1 mol/L KOH solution in ethanol were used.
For the experimental procedure, 25 g of the oil sample was weighed into a 250 mL conical flask. The ethyl ether–ethanol mixture, containing phenolphthalein and a few drops of the standard KOH solution added just before use, was then introduced. Titration was performed with the standard KOH solution under continuous stirring until the endpoint was reached, indicated by a color change from yellow to pink. The volume of KOH solution consumed (in mL) was recorded, and the acid number (expressed in mg KOH/g) was calculated
Soap content (measured in ppm) was determined according to the AOCS Cc 17-95 method, generated after the neutralization reaction. The reagents used included a 2% aqueous acetone solution, approximately 0.01 N hydrochloric acid (HCl), a 1% aqueous bromophenol blue indicator solution, and approximately 0.01 N sodium hydroxide (NaOH).
For the experiments, a control solution (A) was first prepared by adding 0.5 mL of bromophenol blue indicator to 100 mL of the acetone–water solution in a 500 mL volumetric flask. The solution was then titrated with 0.01 N HCl until it turned yellow. Next, 40 g of the oil sample was weighed into a 250 mL conical flask, followed by the addition of 1 mL of water. The mixture was heated in a water bath while stirring vigorously. After that, 50 mL of control solution (A) was added, and heating and stirring were continued. The solution was then allowed to settle into two layers. If soap was present, the upper layer appeared green to blue.
A blank sample (without soap) was titrated, and the volume of HCl consumed (mLb) was recorded. The sample was then titrated with 0.01 N HCl until the green/blue color changed to yellow. Titration continued until the yellow color persisted even after vigorous shaking, and the total volume of acid consumed (mL) was recorded. If the soap concentration exceeded 0.05%, a 4 g sample was analyzed. The soap content in ppm was then calculated.
3. Results
Figure 2 presents the analysis results of the pretreated WCOs; it can be observed that all monitored properties were reduced, even for WCO4, a degraded sample with high acidity, and pieces of solid materials (that were removed via filtration). It is important to note that WCO4 contains a significant amount of unsaturated bonds, leading to an increase in acidity (with the number of double bonds). As a result, during the neutralization stage, a substantial amount of soap was produced, preventing the separation of the individual phases, including the oil phase that was further processed.
More particularly, the analyses of the WCOs after their pretreatment showed a significant reduction in acidity, saponification, and moisture content. Specifically, the acidity of the samples was found to be zero after the neutralization reaction, as the FFAs were converted into soaps (
Figure 2a). It is worth emphasizing that in WCO4, which had the highest acidity, the acidity was zero after the neutralization reaction. This demonstrates that the experimental procedure is both applicable and highly effective, even for WCOs with high acidity. Regarding the moisture content, after the neutralization reaction, a reduction was achieved through centrifugation, the addition of warm water, and evaporation. The reduction in moisture during the pretreatment was evident across all samples (
Figure 2b). However, during the reaction, the moisture content varied due to its expected modification as soaps and water were formed.
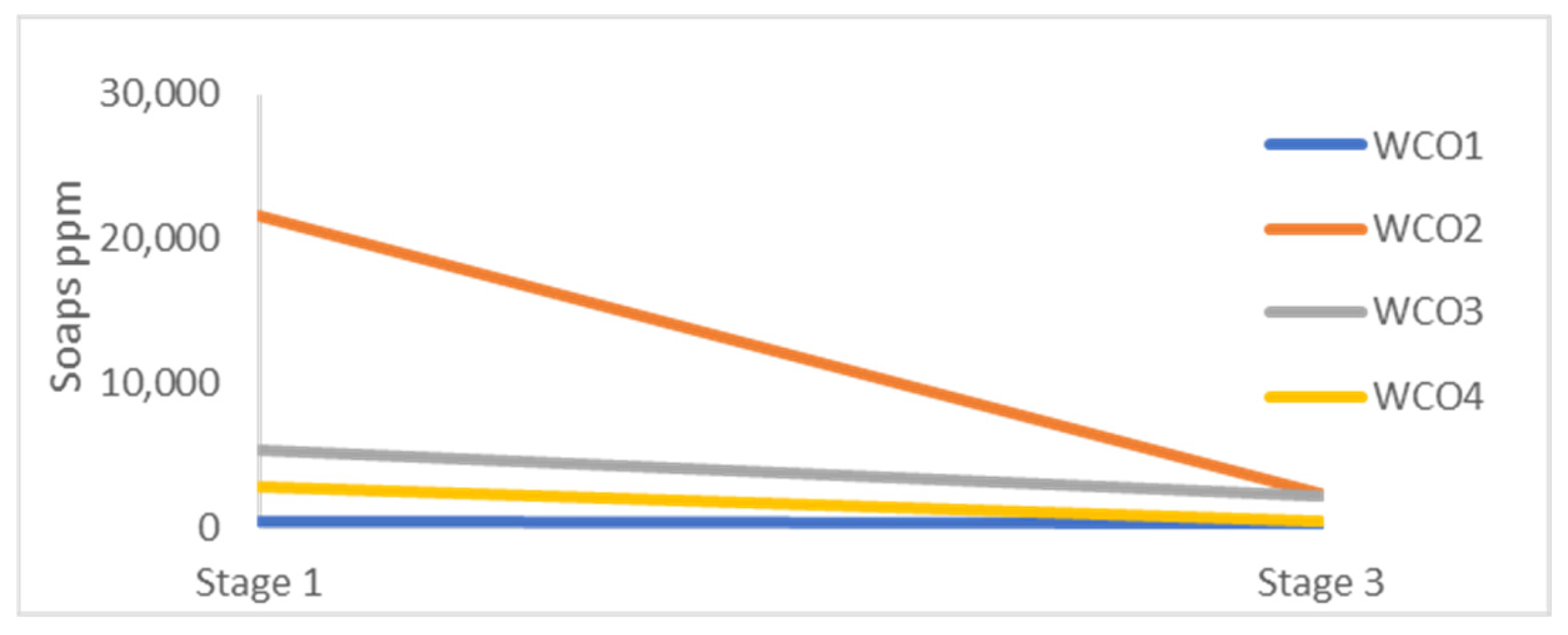
Furthermore, according to Tatiana Siska Wardani et al. 2020, the products of the neutralization reaction are liquid soaps and contribute to the acidity reduction [
20]. These components are also decreased during the centrifugation steps, as presented in
Figure 3. This suggests that the centrifugation process effectively removed a significant amount of soaps, contributing to the overall reduction of acidity and moisture content. These findings highlight the efficacy of the examined combined pretreatment process in improving the degraded lipids’ quality, particularly by reducing the high acidity and moisture levels, while satisfactorily removing the solid impurities.
Accordingly, these results confirm that the suggested pretreatment method—filtration, neutralization, and centrifugation—is effective in lowering the acidity, saponification, and moisture content of the degraded WCO samples, leading to purified and refined feedstocks that could potentially be further upgraded with milder conditions for biofuel production. Firstly, neutralization lowers the FFA content of the WCOs and reduces the overall acidity of the oils. Secondly, washing and soap removal help to eliminate impurities and residual soaps which can disrupt the biodiesel production process by affecting catalyst activity or causing undesired side reactions. The purification of oils at this stage will lead to the production of fuels with minimal contamination [
11].
Lastly, the drying step removes the excess of moisture, which is crucial because water can reduce biofuel yields and lead to undesirable byproducts. By ensuring that the oil has a low moisture content, this step stabilizes the process and prevents side reactions, leading to more efficient biofuel production [
11]. Moreover, the examined process appears to be particularly significant in cases of highly contaminated lipids, which require multiple pretreatment steps in order to improve their quality prior their conversion to biofuels.