Abstract
Progressing soil degradation worldwide is a complex socio-environmental threat. Implementing environmental policies and actions such as the Sustainable Development Goals, the European Green Deal, and the Renewable Energy Directive III regarding environmental protection aims to protect, conserve, and enhance the EU’s natural capital, focusing on soil protection. As assumed in the Green Deal, the European economy has to be turned into a resource-efficient and green economy with zero net emission of greenhouse gases. Since soil quality strongly influences all ecosystem elements, soil remediation is increasingly promoted as a sustainable option to enhance soil quality and, at the same time, help achieve overarching goals set out in European climate law. Biomass in phytoremediation is particularly important in regenerative agriculture, as it emphasizes improving soil quality, increasing biodiversity, and sequestering carbon. Selected plants and microbes can clean degraded agricultural areas, removing heavy metals and pesticides, thus lowering soil toxicity and improving food and feed security. Moreover, the post-phytoremediation biomass can be processed into biofuels or bioproducts, supporting the circular economy. This article summarizes the role of plants and microbial biomass in the struggle to achieve EU environmental goals, enabling the regeneration of degraded ecosystems while supporting sustainable development in agriculture.
1. Introduction
Physical, chemical, and biological soil degradation poses one of the most critical threats to all ecosystems and public health [1]. The Food and Agriculture Organization of the United Nations defined soil degradation as a ‘change in soil health status resulting in a diminished capacity of the ecosystem to provide goods and services for its beneficiaries [2]. Soil degradation results in biodiversity loss, organic matter, lower fertility and productivity, water and air pollution, salinity, and structure decline [3]. Soils with low fertility have lower abilities to sequester carbon and, in effect, emit higher CO2 amounts, influencing climate change [4].
The problem of soil degradation worldwide increases due to human activity, intensive industry development, poorly managed agriculture, illegal waste disposal and storage, and intensive mining [5]. Due to this result, soils are increasingly contaminated with various types of pollutants such as chemicals, medicine, heavy metals (mainly lead, cadmium, mercury), polycyclic aromatic hydrocarbons (PAHs), pesticides, fertilizers and their contamination, etc. [6]. In particular, exploiting mineral resources generates waste and pollutes the soil with heavy metals, sulfates, and other toxic substances [7]. In the last few decades, there has been a deep concern about emerging contaminants (ECs) and micro- and nano-pollutants. Since 2021, 17,000 articles have been published on this aspect, emphasizing its importance and threats. The United States Environmental Protection Agency (US EPA), World Health Organization (WHO), and global environmental policy together emphasize the need for soil remediation and the removal of existing contaminants [8,9]. These actions aim to improve soil quality, protect the environment and climate, enhance food security, safeguard public health, and promote biodiversity [10,11].
This article provides a comprehensive analysis of the latest global knowledge on the role of phytoremediation in the context of legal regulations and environmental policies. The paper analyzes the mechanisms of using plant biomass and microorganisms for the remediation of degraded ecosystems and their impact on soil quality, biodiversity, carbon sequestration, and energy production. The importance of phytoremediation in achieving international environmental goals such as the Paris Agreement, the European Green Deal, and the Renewable Energy Directive III (RED III) is highlighted. Particular attention is paid to the role of biomass in regenerative agriculture and its potential for the production of biofuels and bioproducts, supporting the circular economy. The paper provides a synthetic review of current research, technologies, and challenges related to the implementation of phytoremediation on a global role in environmental and climate protection and carbon sequestration.
2. Soil Degradation: Problem Scale
Soil is the most crucial medium for crops. Essential changes in matter occur in the soil, and the circulation of matter in nature begins and ends in the soil [12]. Good-quality soil is both a determinant and guarantor of healthy, safe food [13]. Unfortunately, despite increasing knowledge about soil environmental protection and ongoing technological progress, the share of degraded soils continues to rise each year [14]. This trend is primarily the result of the irrational management of soil resources, widespread industrialization, and climate change [15]. The declining fertility of soil due to degradation is reducing the area of land suitable for agriculture, thereby intensifying global food security challenges. Therefore, soil degradation and especially soil pollution remains a major global issue [16].
Because of strong interconnections among ecosystem components, contaminated soil can lead to the contamination of groundwater, surface water, and air [17]. Substances that remain in the soil have dangerous effects on both human and animal health. Studies estimate that environmental pollution (water, soil, and air) is responsible for approximately 9 million deaths annually [18]. Such alarming data necessitate more intensive efforts to ensure a toxic-free environment. A report on the state of soils in Europe, published in 2024, revealed troubling statistics [19]. It indicated that annual soil erosion in Europe reaches 1 trillion tons, with 60–70% of soils degraded and subjected to one or more degrading factors [20]. Of these, 24% of soils are affected by water erosion, primarily in agricultural areas. Additionally, nutrient imbalances (particularly involving nitrogen) have been observed in 74% of soils. Soil degradation is also closely correlated with the decline in organic carbon content. The report notes that approximately 70 million tons of organic carbon were lost over 9 years across the EU and Great Britain. According to recent research, as much as 62% of soils in the European Union are classified as unhealthy [21]. Data from [22] indicate that, in the case of forests, 64% of soils are unhealthy, while only 36% are considered healthy (Figure 1).
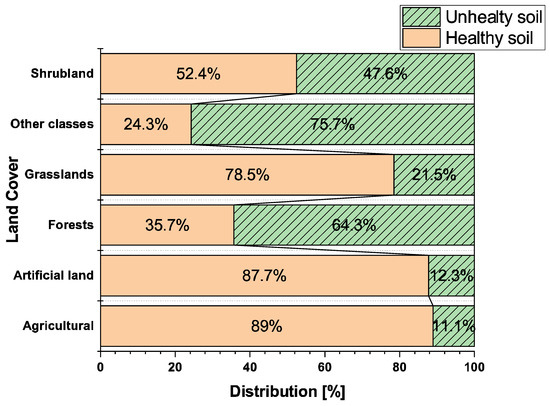
Figure 1.
Distribution of healthy and unhealthy soils per Corine land cover class. Reproduced with permission of [21,22].
Strategies for Preventing and Restoring Soil Degradation
Protecting soil, a valuable resource, requires understanding the causes and effects of soil degradation and implementing effective corrective measures [23,24]. Soil degradation refers to the loss of natural physical, chemical, biological, and ecological properties, which may result from either natural processes or human activities. Many factors contribute to soil degradation [25], including the initial condition of the soil, which largely determines its future trajectory. Other influences include the type of stress to which the soil is subjected, how the soil responds to stress factors, and the overall impact on natural resources. A quick response to soil quality regression is essential to ensure that ecosystems can continue to provide services and products [26]. Rapid action is extremely important in ensuring environmental safety and access to natural resources for future generations [27]. As part of the EU Soil Strategy for 2030, continuous monitoring of soil conditions is being carried out. The strategy’s objectives include ensuring good soil quality and promoting biodiversity. Promoting agricultural practices that protect soils, such as limiting the use of pesticides, rational use of fertilizers, and the sustainability of farming practices, also contributes to soil protection. These actions consistently aim to reduce the proportion of degraded soils and limit excessive CO2 emissions from soils by intensifying carbon sequestration. The goals set by the EU Soil Strategy for 2030 can be achieved using biotechnological methods. The use of living organisms, such as plants and soil microflora, can help combat ongoing degradation and assist in the restoration of degraded soils [28,29].
3. Phytoremediation as a Strategy for Soil Restoration
3.1. Types and Mechanisms of Phytoremediation in Soil Decontamination
Soil decontamination can be effectively achieved using phytoremediation techniques. This method allows for soil renewal and reclamation as a standalone approach and can also complement other remediation methods [30]. It facilitates the removal, immobilization, or stabilization of contaminants, thereby mitigating soil toxicity [31]. The mechanisms governing a given phytoremediation process depend on the plant species, type of contamination, and characteristics of the soil medium [32]. The diversity of these mechanisms has led to the classification of different types of phytoremediation.
One such method is phytostabilization, which limits the mobility of contaminants but does not result in their removal from the soil. In this process, inorganic contaminants are precipitated or immobilized at the soil surface, on the root surface, or within tissues [33]. Plants used in phytostabilization typically have extensive root systems and a large rhizosphere surface area. Grasses such as fescue (Festuca sp.) and ryegrass (Lolium sp.) are common examples [34]. Phytostabilization is particularly effective for stabilizing unstable substrates in waste dumps and other post-industrial sites [35].
Another method is phytoextraction, in which plants absorb pollutants through their roots and accumulate them in above-ground parts, effectively removing them from the soil [36]. This technique is particularly useful for extracting heavy metals and radioactive substances. It requires the use of plants with high biomass yield and resistance to relatively high concentrations of pollutants, known as hyperaccumulators [37].
Phytovolatilization is a technique that uses plants to transform pollutants into vapor. It involves several chemical transformations of pollutants with the participation of the plant [38]. A characteristic plant used in this technique is Brassica juncea, known for transforming selenium into dimethyl diselenide or selenomethionine [39]. This process reduces selenium toxicity by 500–700 times [40]. Phytovolatilization can also be used to remove mercury by converting it into elemental mercury, which is less toxic [38].
Lastly, phytodegradation is used to remove organic pollutants and is mainly applied to soils contaminated with petroleum derivatives, aromatic hydrocarbons, or surfactants [41]. The degradation of organic pollutants is dependent on the plant’s ability to produce specific endogenous lytic enzymes. The activity of enzymes that break down organic compounds has also been identified in the surrounding soil medium [42]. In some cases, pollutants accumulated by plants may be decomposed and released as CO2 and H2O. Phytodegradation is currently a significant focus of research, especially in the context of removing emerging pollutants [43].
3.2. Limitations of Phytoremediation in Soil Remediation in Real-World Conditions
Phytoremediation, by its nature, is limited by the relatively long time required to achieve remediation effects, a restricted depth of action [44], seasonal dependence [45], and reduced effectiveness at very high contaminant concentrations [36]. The overall effectiveness of this technique is constrained by several factors that must be critically examined, especially under natural conditions. Phytoextraction is highly effective for heavy metals, but its success depends on the bioavailability of metals in the soil, which can be significantly influenced by factors such as pH, soil texture, and organic matter content [46]. Additionally, the capacity of plant biomass to absorb contaminants is limited, meaning that in heavily polluted areas, phytoextraction may not provide adequate remediation within a practical timeframe. Phytodegradation also faces challenges in degrading organic pollutants, as it relies heavily on microbial activity in the rhizosphere. This activity is influenced by soil temperature, humidity, and nutrient availability. In environments with low microbial diversity or high toxicity, the effectiveness of phytodegradation is reduced, potentially leading to incomplete contaminant breakdown [41,42]. Phytovolatilization involves the uptake of volatile pollutants by plants, which are then released into the atmosphere. Although useful for specific pollutants such as volatile organic compounds, this technique can contribute to the dispersion of contaminants into the air, potentially causing secondary pollution or air quality concerns [47]. Its effectiveness is also influenced by plant species and environmental factors such as temperature and humidity, which can vary significantly across regions. Phyto-stabilization, while effective in preventing the spread of contaminants, immobilizes them in the soil rather than removing them. This limits the potential for complete site cleanup. Moreover, the stability of immobilized contaminants can be compromised by environmental changes such as soil erosion or flooding, which may remobilize previously stabilized contaminants [48]. In addition to these technical limitations, the success of phytoremediation is also affected by environmental factors such as soil depth, climate, and the presence of competing vegetation [30]. In some cases, plant growth may be stunted by extreme weather events, or plants may fail to develop strong root systems in infertile or highly compacted soils, further diminishing their capacity to remediate pollution [49].
Beyond soil purification, phytoremediation contributes to erosion control and prevents the dispersion of pollutants into aquatic and atmospheric environments [50]. Given its versatility, phytoremediation can be integrated with other remediation methods and has low infrastructure requirements compared to chemical soil treatment as it does not necessitate the construction of complex treatment facilities. Additionally, phytoremediation offers further benefits, such as promoting biodiversity, in contrast to conventional remediation methods. It also provides an opportunity to revitalize extensively degraded areas, with the added advantage of being widely accepted by society due to its environmentally friendly and aesthetically pleasing nature [51]. A schematic presentation of the role of phytoremediation in environmental protection on both local and global scales is shown in Figure 2.
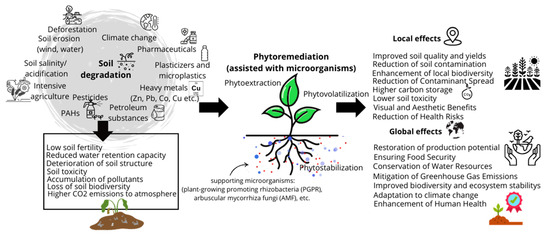
Figure 2.
General scheme of phytoremediation in environmental protection: a local and global perspective.
3.3. Plants in Phytoremediation
Plants employed in phytoremediation share several common characteristics, with rapid growth and substantial biomass production being among the key factors determining their suitability for such applications. High biomass output is particularly significant for effective phytoremediation, as the amount of plant organic matter directly influences the capacity of plants to remove, accumulate, and stabilize contaminants within the soil matrix [52]. This directly contributes to increased efficiency in soil remediation [38]. Plants must also demonstrate high tolerance to the specific contaminants present in a given area [53]. Another crucial determinant of plant suitability is their ability to accumulate significant concentrations of pollutants—such as heavy metals—within their tissues. This trait is especially evident in metallophytes and hyperaccumulator species, which enable the permanent removal of contaminants from the soil through biomass harvesting [54]. Additionally, a plant’s effectiveness in phytoremediation is influenced by its capacity to metabolize or degrade pollutants, thereby contributing to the detoxification and restoration of contaminated environments. Some plants can degrade organic contaminants, such as pesticides or petroleum derivatives, for example, grasses and legumes [55]. Plants with large, extensive root systems provide a greater surface area for contact between roots and pollutants enhancing the phytoremediation process in the rhizosphere [50]. This feature also allows for the purification of deeper soil layers and even groundwater [30]. Given the specificity of polluted areas, plants used in phytoremediation should have low habitat requirements, making them suitable for use in degraded and nutrient-poor regions [56]. Plants such as miscanthus and mustard have low habitat requirements, enabling them to be used for land reclamation [57]. The perfect solution seems to be using plants with a high calorific value. Collecting plant biomass after phytoremediation makes it possible to use it for energy production (for instance, poplar) [58].
The diversity of available plant species allows for the selection of the most effective plants and those best adapted for the removal of specific soil contaminants. This enables the adaptation of phytoremediation methods to particular environmental conditions and types of pollution. Phytoremediation is frequently in the reclamation of post-industrial or post-mining areas where relatively high concentrations of heavy metals are often found. Commonly used plants for heavy metal removal include poplar (Populus sp.) [59], willow (Salix sp.) [60], and mustard (Sinapis sp.) [61]. Table 1 presents selected phytoremediation plants along with the types of contaminants against which they are effective. It includes conventional pollutants such as heavy metals and petroleum hydrocarbons, as well as substances that have recently garnered growing interest among scientists and decision-makers—the so-called emerging contaminants. This group includes chemical compounds not yet subject to detailed legal regulation but potentially posing serious threats to human health and ecosystem function. An increasing number of studies are now focusing on the use of green technologies, including phytoremediation, to eliminate these persistent and difficult-to-remove pollutants from soil. Such developments may represent a breakthrough in the sustainable management of contaminated areas. The applicability of selected plants for the phytoremediation of specific environmental pollutants is summarized in Table 1.

Table 1.
Selected phytoremediation plants and their applications in selected pollutant removal.
3.4. Root-Zone Interactions and Soil Stabilization
The root zone is the critical environment for the processes driving phytoremediation and serves as the focal point for soil remediation mechanisms [30]. Within this zone, dynamic interactions occur between plant roots, soil microflora, and contaminants, facilitating the transformation, accumulation, or stabilization of pollutants in the soil. Plants actively respond to environmental changes by releasing root secretions [79]. Their presence makes the root zone a hot spot for dynamic changes within the plant–soil microflora–soil system. The composition of these secretions is complex and includes diffusates, exudates, and excretions. Root exudates consist of mucus, colloids, and lysates—products of root cell biometabolism [80]. By modifying the soil’s chemical, physical, and biological properties, these exudates play a key role in regulating the microecological function of the rhizosphere [81]. Their composition and quality are strictly dependent on the plant species [80]. Root exudates are pivotal in transforming toxic substances, thereby reducing soil toxicity. Furthermore, the release of various chemical compounds, such as amino acids, into the root zone creates a favorable habitat for diverse soil microorganisms—including bacteria, fungi, and protozoa—which further contribute to the remediation process [82]. These microorganisms, through the enzymes they synthesize, can degrade pollutants, directly enhancing the effectiveness of remediation. In some plants, especially hyperaccumulators, the root zone also serves as a site for contaminant accumulation [83]. Additionally, chemical secretion from plants can promote soil aggregation, facilitating pollutant immobilization and offering protection in areas susceptible to erosion. The extensive root networks developed by plants stabilize the soil and improve its permeability, looseness, ventilation, water management, and nutrient availability (for instance, altering the surface charge of absorbents) [80]. This complex network of interactions improves the living conditions for soil microflora, favoring its development and degradation activity of pollutants present in the soil. The root zone of plants can also limit the mobility of contaminants in the soil, which makes them less available to other organisms, thus limiting soil toxicity and affecting biodiversity.
3.5. Microorganisms as Allies of Plants in Phytoremediation
Microorganisms also play an important role in the phytoremediation process. Due to the complexity of plant–soil microflora interactions, microorganisms support plants at various stages of pollutant detoxification and/or accumulation [84]. Microbiota assist in both the mobilization and immobilization of contaminants. By synthesizing enzymes, they increase the bioavailability of pollutants to plants, while reducing their harmful effects [42]. The indigenous soil microflora is particularly important in the phytoremediation of contaminated soils. These microorganisms support plants in nutrient uptake, regulate plant growth, protect against pathogens, and can even collaborate with plants in the detoxification of pollutants [85]. Some microorganisms are capable of degrading organic compounds, including hazardous petroleum substances and aromatic hydrocarbons [86]. Recent studies also highlight the potential of microorganisms to remove emerging contaminants [87]. Examples of microorganisms capable of breaking down specific pollutants are provided in Table 2. Given the capabilities of microorganisms, an integrated approach that combines their assistance with phytoremediation enables the effective reduction of pollutant toxicity. Microorganisms can convert contaminants into completely non-toxic substances and, in some cases, remove them permanently from the environment [30]. For this reason, applying microorganisms to the soil in the form of “soil vaccines” through bioaugmentation is increasingly seen as a necessary, biological, and safe alternative to synthetic soil fertilizers. Their use also reduces the need for artificial fertilizers [88]. This approach supports the Strategic Plan stemming from the European Green Deal, which includes a goal to reduce fertilizer use by 50% [9].

Table 2.
Microorganisms as a key for emerging contaminants degradation with applicability for assisted phytoremediation.
3.5.1. Plant-Growth-Promoting Rhizobacteria
A significant group supporting plants in phytoremediation is plant-growth-promoting rhizobacteria (PGPR). These bacteria inhabit the root zone of plants, enhance the availability of essential plant nutrients, and alleviate plant stress caused by soil contaminants [123]. Their presence significantly supports root development, and through an extensive root system, plants can more effectively absorb contaminants from the soil [124]. This is particularly important as plants can act only on the bioavailable fractions of harmful pollutants and are ineffective against nonbioavailable forms [125]. PGPR can produce organic acids (e.g., acetic acid) and chelators that increase the bioavailability of metals, including heavy metals. Compounds such as ACC deaminase (e.g., Pseudomonas koreensis S2CB45) [126], phytohormones (e.g., indole-3-acetic acid, IAA), and phosphate-solubilizing agents are all known to enhance plant growth. Moreover, the production of plant hormones by rhizobacteria contributes to pollutant accumulation in plant tissues [127]. Through the synthesis of siderophores, biosurfactants, and exopolymers, PGPR aid in the removal of metals from soil by respective metal complexes. Consequently, PGPR are widely employed in remediating heavy-metal-contaminated soils [128]. Additionally, nitrogen-fixing bacteria such as Rhizobium, Sinorhizobium, Azospirillum, Azotobacter, Bradyrhizobium, Burkholderia, Herbaspirillum significantly increase plant biomass and are especially important in agricultural soils, where biological nitrogen fixation reduces dependence on artificial fertilizers [129,130]. Furthermore, bacteria capable of degrading xenobiotic substances—including Pseudomonas aeruginosa, Burkholderia gladioli, and P. pseudoalcaligenes—are considered promising agents in phytoremediation. Other strains, such as Bacillus spp., Ciceribacter azotifigens, and Serratia marcescens, have been shown to degrade persistent pesticides effectively [131,132].
3.5.2. Arbuscular Mycorrhizal Fungi
Fungi also play a vital role in environmental purification. A notable group is arbuscular mycorrhizal fungi (AMF), which act as natural bioaccelerators by interacting with phytoremediation plants to support ecological restoration processes [133]. The glomalin produced by AMF (e.g., Glomus, Rhizophagus spp.) improves soil structure, while mycelial hyphae increase soil porosity and act as a structural scaffold, stabilizing the soil matrix. AMFs are recognized as essential agents in the remediation of heavy-metal-contaminated soils [134]. Successful removal of aromatic hydrocarbons has also been demonstrated using Salix viminalis L. supported by AMF [135]. The release of organic acids by AMF enhances pollutant degradation and uptake in contaminated soils [136]. For example, Glomus etunicatum has been shown to increase the accumulation of dichlorodiphenyltrichloroetane (DDT) in phytoremediation involving alfalfa [137]. Other observed benefits of AMF include faster biomass production in host plants, which can directly shorten the duration of remediation efforts [138]. Moreover, through interactions with soil bacteria, AMF accelerate contaminant uptake and enhance bioavailability—effects documented in soils contaminated with hydrocarbons [139].
The properties of the soil, along with the type and concentration of pollutants and the plant species used in phytoremediation, strictly determine the effect of AMF [50]. It has been observed that the chemical composition of root exudates—including flavonoid content—also influences AMF–plant interactions. These factors directly affect the efficiency of pollutant uptake by plants from the soil. For example, the use of Sorghum bicolor in phytoremediation, when supported by bioaugmentation with Claroideoglomus etunicatum (BEG168), resulted in a fourfold increase in the accumulation of molybdenum (Mo) from the soil [140]. Research by Meyer et al. [141] investigated the cultivation of fragrant vetiver (Chrysopogon zizanioides) on substrates containing mine waste and found that the application of AMF species—including Acaulospora colombiana, A. morrowiae, A. scrobiculata, and Gigaspora margarita—increased the accumulation of both heavy metals and biogenic elements (e.g., phosphorus) in the plant’s root tissues. Other studies demonstrated that the application of Glomus mosseae reduced heavy-metal-induced stress in Trifolium repens (white clover). In addition, AMF–plant mycorrhizal associations have been shown to enhance plant biomass. For instance, arbuscular mycorrhiza between Trifolium repens L. and G. mosseae and G. claroideum effectively reduced stress caused by the pathogenic fungus Pythium ultimum. This was accompanied by a reduced production of stress-related flavonoids—only two out of eight tested were produced or derived from glycosides in response to pathogen infection [142]. Further research on the same plant species found that inoculation with Funneliformis mosseae and Paraglomus occultum improved the plant’s tolerance to drought stress by enhancing proline content, soluble protein levels, flavonoid production, and nutrient uptake [143]. Interestingly AMF strains have also been shown to support plants in adapting to climate change. One study reported that a vaccine based on AMF reduced the negative impact of acidic water on Lupinus angustifolius grown on sterilized mining waste. In addition to these effects, AMF influences root metabolism. A study by Wang et al. [144] showed that inoculation of Medicago sativa Glomus mosseae increased the expression of 739 metabolites. It was indicated as a key factor in modifying the soil microbiome in this context.
3.5.3. Soil Microbial Interactions
The co-presence of PGPR and AMF induces interactions along the microflora–plant axis, which may be manifest as synergistic between the microorganisms and the host plant [145]. It has been reported that AMF and PGPR complement each other by enhancing surface absorption and facilitating the uptake of P and N [146]. Furthermore, AMF have been shown to alter root exudate composition, which, in turn, influences the microbial community in the root zone, often increasing the abundance of beneficial microorganisms. For example, the AMF Claroideoglomus tunicate not only improved the bioavailability of K, P, Ca, and Mg, but also increased the abundance of microbial genera such as Planomicrobium, Lysobacter, Saccharothrix, Agrococcus, Microbacterium, Streptomyces, and Penicillum [147]. In a cucumber monoculture, the application of arbuscular fungi with an organic substrate positively affected the root-zone microbiome and improved soil quality [148]. Mishra et al. [146] investigated the synergistic effect of AMF and PGPR on the phytoremediation of iron-contaminated soils. Three AMF genera—Glomus, Acaulospora, and Scutellospora—and four PGPR genera—Streptomyces, Azotobacter, Pseudomonas, and Paenibacillus—were examined, using Pennisetum glaucum and Sorghum bicolor as host plants. Enhanced Fe3+ removal was observed in samples inoculated with the AMF and PGPR consortium, showing removal efficiencies of 60.35% and 64.85% in roots and 50% and 35.55% in shoots for P. glaucum with mycorrhizal colonization at 47%. The study demonstrated that these microbial consortia significantly enhanced iron accumulation.
Colonization of the root zone by microorganisms is influenced by multiple factors, including soil hydrological conditions. Monokrousos et al. [149] suggested that AMF may exert a greater influence on the rhizosphere microbiome in dry soils than in wet soils. Their study, conducted on Festuca pratensis, Dactylis glomerata, and their mixture, involved inoculation with Rhizophagus irregularis. It was found that in wet soils, the microbial composition was more strongly influenced by the inoculum itself, while in dry soils, soil type played a larger role in shaping the microbial community. These findings suggest that the success of bioaugmentation using AMF and PGPR may vary depending on the soil’s water regime. Given the stronger microbiome impact of AMF in dry soils, bioaugmentation strategies could be particularly advantageous in arid regions or adjusted according to meteorological conditions. A relatively new approach is the use of ectomycorrhiza associated with bacteria (EMAB), which harnesses the synergy between ectomycorrhizal fungi and bacteria. Though a distinct type of mycorrhiza from AMF, EMAB has been proposed as a promising method for accelerating plant-assisted remediation. EMAB has been shown to increase ectomycorrhiza formation, promote plant growth, and enhance the accumulation of heavy metals such as Zn and Cd [150]. Additionally, bacteria have been observed to facilitate root colonization by ectomycorrhizal fungi, further improving the effectiveness of phytoextraction [151]. In summary, the synergy of plant-associated microorganisms—including PGPR, AMF, and EMAB—is a critical factor influencing the overall effectiveness of phytoremediation.
4. Role of Assisted Phytoremediation Towards Environmental Policies
4.1. Plant and Microbial Biomass Towards Achieving Sustainable Development Goals
The synergy of actions aimed at restoring natural environments is one of the key strategies for achieving the Sustainable Development Goals (SDGs)—the global objectives of the United Nations [152]. Emphasizing ecosystem services, biodiversity restoration, natural resource protection, and appropriate soil management are among the primary factors that support the achievement of the Sustainable Development Goals (SDGs) (Figure 3) [153]. Soil remediation, by reducing toxicity and increasing land suitable for agriculture, contributes directly to SDG 1: No Poverty and SDG 2: Zero Hunger [154]. Enhanced soil quality and reduced contamination levels lead to higher crop yields, greater food production, and improved food security—all essential for ensuring access to adequate, healthy food and supporting public health. Moreover, the removal of soil contaminants, including emerging pollutants, improves sanitary and hygienic conditions and positively impacts groundwater quality, supporting the achievement of SDG 6: Clean Water and Sanitation [155]. Reclaimed soils with appropriate structure, aggregation, and enzymatic activity also function as effective filters for rainwater and precipitation [156]. Soils contaminated with pesticides, heavy metals, micropollutants, pharmaceuticals, plasticizers, and other substances can serve as sources of pollution for groundwater and surface water bodies [157]. In this context, the biomass of plants and microorganisms used in assisted phytoremediation plays a vital role in preventing the migration of pollutants into other components of the ecosystem [158].
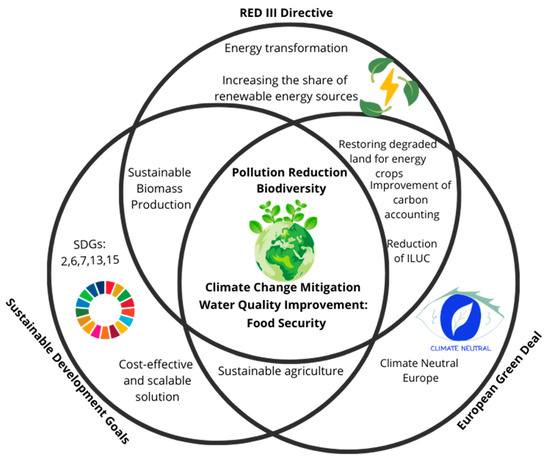
Figure 3.
Role of phytoremediation in achieving the Sustainable Development Goals, the European Green Deal, and the Renewable Energy Directive (RED) III.
Improving soil quality can also contribute to achieving SDG 12: Responsible Consumption and Production [159]. Sustainable agricultural practices, including regenerative agriculture, help achieve this goal by reducing pressure on the soil, enhancing the efficiency of natural resource use, and promoting more balanced agricultural production [160]. In addition, the use of plant and microbial biomass in phytoremediation, alongside soil quality improvement, can play a significant role in climate protection and the achievement of SDG 13: Climate Action [161].
Increasing soil fertility—and in extreme cases, even restoring soil ecosystem services—can enhance carbon sequestration [14]. This is especially important for climate protection and the reduction in CO2 emissions into the atmosphere. Degraded soils are major contributors to CO2 emissions [162]. Through phytoremediation, especially when involving plant and microbial biomass, soil remediation reintroduces organic matter into poor and contaminated soil. Enhancing soil fertility parameters in this way intensifies the carbon sequestration process, supporting long-term environmental sustainability [163].
AMF can play a crucially important role in this context, particularly through the production of glomalin, which improves soil structure, stabilizes organic matter, and enhances carbon sequestration [164]. Increasing the stability of organic carbon in the soil contributes to a net reduction in CO2 emissions, directly supporting climate protection. Furthermore, microbial-assisted phytoremediation promotes biodiversity, aligning with SDG 15: Life on Land [165]. Goal 15 emphasizes the protection of sustainable terrestrial ecosystems and the preservation of microbial biodiversity [152].
Enhancing soil quality, preventing and mitigating erosion and degradation, and removing contaminants through phytoremediation—supported by plant and microbial biomass—are key strategies for restoring degraded terrestrial ecosystems [56]. These efforts help protect biodiversity by creating new ecological habitats for soil microflora, fauna, plants, and animals. In doing so, they support the recovery of terrestrial ecosystems and their essential functions and align with the goals of the EU Biodiversity Strategy for 2030 [166]. Although many of these objectives overlap, it is important to emphasize the vital role of plant biomass and microorganisms involved in the restoration and purification of soils. Their use contributes to the creation and regeneration of productive, stable ecosystems that are more resilient to climate change, including extreme events such as droughts and floods.
4.2. Assisted Phytoremediation Towards the Green Deal
The European Green Deal (EGD) is a European directive aimed at protecting the environment and climate to achieve climate neutrality by 2050 [9]. In this context, several initiatives have been proposed, focusing primarily on reducing CO2 emissions, limiting global warming to a maximum of +1.5 °C compared to pre-industrial levels, promoting sustainable agriculture, and protecting biodiversity [167].
Given ongoing climate change, the protection of soil resources has become especially important. Degraded soils are known to emit significantly more CO2 into the atmosphere than healthy soils [4]. Therefore, improving soil function through phytoremediation, particularly when assisted by microorganisms, can help reduce CO2 emissions by stabilizing and storing organic carbon in the soil [168]. Phytoremediation also supports broader nature restoration goals and contributes to creating a healthy environment for future generations—one of the EGD’s specific objectives (Figure 3) [169]. It can play a significant role in rehabilitating difficult areas such as postmining, metallurgical, or landfill sites [170]. Numerous studies have highlighted the potential of plant and microbial biomass to restore soil ecosystem services and meet several of the EGD’s key targets [171,172]. Additionally, improving soil quality contributes to increased crop yields by reducing the need for pesticide use [173]. Moreover, supporting phytoremediation with microorganisms makes microbiological vaccines act as a natural booster, protecting against biotic and abiotic stress and eliminating the use of pesticides and their contamination of soils [174]. Moreover, the plant biomass produced through phytoremediation can serve as a renewable resource for biofuels, supporting green chemistry through the production of bioethanol, biodiesel, biogas, pellets, and briquettes [175]. Biomass can also be used for electricity generation (e.g., from energy willow or giant miscanthus) [175]. Some plants, such as willow, can also serve as a raw material in paper production [45], contributing to the circular economy, another major objective of the EGD.
4.3. Plant and Microbial Biomass Towards Directive Renewable Energy Directive III
The Renewable Energy Directive III (RED III), which entered into force on 19 November 2023, is a key element of European climate policy, applying to the energy, heating, industry, and transport sectors [176]. It provides a strong legal framework that is highly significant for the energy sector and environmental protection. One of its central goals is the promotion of renewable energy, with an ambitious target of achieving a 42.5% share of renewable energy in the European Union’s overall energy consumption by 2030 [177]. RED III aims to increase the role of green energy in the economies of EU Member States, thereby supporting the implementation of the Fit For 55 package [178]. The directive focuses on transitioning energy systems toward renewable sources while also emphasizing the increased role of biofuel components.
RED III also introduces new regulations for the transport sector, mandating a minimum share of 5.5% for advanced biofuels and renewable fuels of non-biological origin (e.g., hydrogen and its derivatives) by 2030 [179]. In this context, phytoremediation can serve as a valuable source of fuel biocomponents [180]. The plant biomass collected after soil remediation may be used for energy purposes, contributing to the production of advanced biofuels, and supporting RED III targets in the transport sector (Figure 3) [181]. Biomass produced in phytoremediation can be combusted or processed in biogas installations, helping to increase the share of renewable energy sources (RESs) [181]. Using advanced technologies, this biomass can also be transformed into hydrogen or other synthetic fuels [182]. Additionally, RED III introduces sustainability criteria for biomass use, particularly aimed at forest and soil protection. In this regard, post-remediation biomass, when converted into value-added products such as biocomposites or fertilizers [175], supports the implementation of RED III objectives. Phytoremediation of degraded areas also offers a solution for growing energy crops on noncompetitive lands, avoiding conflicts with agricultural or forested areas. Focusing energy crop cultivation on degraded or marginal lands helps to limit indirect land use change, thereby preserving agriculturally productive areas for food crops and ensuring a sustainable food supply. In summary, phytoremediation—through the efficient conversion of biomass into energy or value-added products—has significant potential to contribute to Europe’s sustainable energy transformation under RED III.
4.4. Waste-Free Phytoremediation and Phycoremediation Towards a Circular Economy and RED III
The integration of phytoremediation with a zero-waste industry approach enables the recovery of valuable raw materials while simultaneously protecting the natural environment [183]. Innovative strategies combining phytoremediation and mycoremediation have been described, allowing for the effective degradation of complex contaminants and supporting the broader goal of zero-waste remediation [184]. In this context, “zero-waste” refers to designing the entire process to minimize or eliminate waste generation. Tan et al. [185] emphasize that the management of post-remediation biomass can yield significant economic value. Techniques such as pyrolysis and composting offer not only solutions to biomass disposal but also avenues for generating economic benefits [186]. Other researchers have highlighted the industrial potential of fiber plants used in phytoremediation, such as hemp and flax. Owing to their extensive root systems and ability to penetrate deep into the soil, these plants are especially useful for reclaiming contaminated soils.
Studies by Placido and Lee [187] showed that the concentrations of heavy metals in hemp fibers grown in Cd-contaminated (11 mg kg−1) and Pb-contaminated (664 mg kg−1) soils remained significantly below the permissible limits for textile product safety [188]. Several safe technologies have been proposed to obtain raw materials for textile production and to develop composite materials for the automotive and construction industries. Despite these applications, the primary use of phytoremediation biomass remains energy production, including the generation of biogas, bioethanol, biodiesel, and biochar. Additionally, contaminated plant biomass, once processed and purified of pollutants, can also be repurposed for sustainable building materials such as hempcrete and biocomposite panels. The proper processing of phytoremediation biomass is critical for enabling its further applications and ensuring alignment with the zero-waste approach.
Another innovative approach is phycoremediation, where algae serve as the primary agents of remediation. Their abilities in absorption, accumulation, biomineralization, and biosorption of contaminants make them highly effective in environmental purification, particularly in aquatic ecosystems. Integrating zero-waste principles into phycoremediation allows the resulting algal biomass to be used for energy production (e.g., biofuels) [189] as well as for animal feed due to its richness in proteins, vitamins, and minerals [190]. However, thorough toxicological studies must be conducted before feed application to ensure safety, especially in environments with elevated biogenic element concentrations [191]. Furthermore, biorefineries, as integrated facilities that combine multiple processes, allow for the comprehensive conversion of microalgal biomass into proteins and organic fertilizers, potentially reducing the need for chemical fertilizers [192].
Zero-waste approaches applied to the phytoremediation of heavy-metal-contaminated sites also open the door to phytomining—the recovery of metals from plant biomass. This concept supports domestic supply chains of critical minerals (e.g., Ni, Zn), offering a more environmentally sustainable alternative to traditional mining methods [193]. Although phytomining has been recognized as a promising technique for decades, its large-scale application remains limited. Nonetheless, its economic potential makes it an increasingly attractive approach [194]. Akinbile et al. [195] noted that phytomining is still not capable of fully replacing conventional metal extraction methods. However, Kumar and Singh [196] proposed an innovative direction for phytomining, utilizing leachates, sediments, and wastewater derived from e-waste processing. They suggested that implementing hydroponic baths with hyperaccumulator plants could not only improve metal recovery but also serve as a valuable complementary technique in e-waste recycling, offering a low environmental impact solution.
Zero-waste remediation enables a closed-loop system in both production and environmental protection, reducing the exploitation of natural resources and promoting a sustainable approach to environmental purification—aligned with the waste minimization principles outlined in the RED III Directive.
5. Phytoremediation in Regenerative Agriculture
It has been estimated that global agriculture is responsible for approximately 25% of annual anthropogenic greenhouse gas (GHG) emissions. Moreover, agricultural production accounts for nearly one-third of terrestrial acidification [197]. This is largely due to agriculture being the most extensive form of land use on Earth [198]. Intensive agricultural practices, driven by growing food demand, inefficient soil resource management, and excessive fertilization, significantly degrade soil quality and productivity [199]. Improving soil quality and enhancing biodiversity through the use of plants and microorganisms represents a key ecological pillar of regenerative agriculture [200]. Phytoremediation plants can be incorporated as cover crops to provide permanent vegetation cover, supporting soil structure and ecosystem resilience [201]. Soil ecosystem services and safety can also be improved through adherence to the “Farm to Fork” strategy, which aims to protect food from soil contaminants and pesticide residues.
Regenerative agriculture further integrates agroforestry practices—combining crops with trees—to improve soil quality and reduce the environmental impact of agriculture [202]. One promising approach is the use of phytoremediation plants between tree alleys in fruit orchards (e.g., apple orchards, pear orchards, etc.) [203]. This method, combined with plant biomass and microorganisms, can help remove contaminants from soil and reduce their presence in food products. Moreover, improving soil quality, increasing nutrient availability, and modifying the soil microbiome can lead to reduced reliance on fertilizers and pesticides [50]. The application of microbiological vaccines, especially those based on AMF, can further contribute to carbon sequestration in agricultural soils [204]. Thus, phytoremediation can serve as a valuable complementary technique in the implementation of regenerative agriculture.
6. Future Perspectives
6.1. Advancements in Genetic Engineering for Enhanced Phytoremediation and Soil Restoration
Despite numerous studies on microorganism–plant interactions in the phytoremediation process, a comprehensive approach that considers multi-level interactions between the entire soil microbial community and remediating plants is still lacking. A deeper understanding of these mechanisms and interactions can shed new light on ways to remove contaminants from soil and could facilitate planning an effective phytoremediation process. A thorough understanding of the mechanisms governing the relationships between the entire community of microorganisms and remediating plants may be possible thanks to molecular genetics [205]. Furthermore, detailed knowledge of substrate preferences and metabolic profiles of microorganisms will allow for more targeted and efficient implementation of assisted phytoremediation and can also form the basis for the genetic modification of plants and/or microorganisms [206]. The development of genetic engineering tools, particularly those focused on gene expression responsible for contaminant accumulation/degradation by plants, as well as genes that enhance plant resistance to abiotic stress, holds the potential to significantly improve the efficiency of soil remediation [207]. Systems based on the cooperation of genetically modified plants and/or microorganisms, or programming them for more effective uptake and degradation of pollutants, could greatly accelerate land restoration, contributing to the achievement of European environmental and climate protection goals. However, the use of GMOs is accompanied by concerns regarding societal acceptance and ethical considerations [208]. Nonetheless, genetic engineering technologies—when applied to develop modified plants and microorganisms as catch crops in regenerative agriculture—can significantly improve the quality of agricultural soils, reduce the use of artificial fertilizers and pesticides, enhance crop safety, and support the protection of biodiversity.
6.2. Integration of Phytoremediation and Nanotechnology for Soil Regeneration
A modern and promising approach to soil regeneration is the integration of phytoremediation with nanotechnology. Due to their high surface-to-volume ratio, high reactivity, and ability to interact with pollutants at the molecular level, nanoparticles can significantly enhance phytoremediation efficiency [209]. Nanotechnology is an emerging and innovative field within environmental remediation, offering novel opportunities to improve the effectiveness and precision of phytoremediation strategies. The combination of microorganism-assisted phytoremediation with the application of nanoparticles has shown promise [210]. While this concept has been tested primarily in the context of heavy metal pollution, ongoing research is expanding its application to include organic pollutants and emerging contaminants [209]. Studies indicate that nano-phytoremediation can improve plant health and reduce the effects of soil toxicity. Yasin et al. [210] reported that nanoparticles enhance soil enzymes and increase cadmium absorption. Similarly, Hussain et al. [55] found that using ZnO nanoparticles significantly increased the accumulation of Pb in the roots, stem, and leaves of Persicaria hydropiper L. Other studies have indicated a reduction in plant oxidative stress resulting from the application of nanoparticles [211]. The use of nanoparticles can effectively improve phytoremediation. Still, the optimal dose of nanoparticles should be determined beforehand because a dose that is too high can inhibit plant growth and accumulate heavy metals [55]. Nanoparticles have been shown to enhance the bioavailability of pollutants in the plant–microorganism system, thereby improving the efficiency of pollutant uptake and degradation. Additionally, they can mitigate the toxic effects of these pollutants on plants, further supporting the phytoremediation process [52]. Despite several advantages resulting from the application of nanoparticles for assisted phytoremediation, there is a lack of studies considering the fate of nanoparticles in the environment. However, due to their novelty and limited use time, the potential risks associated with using nanomaterials in this process have not yet been fully identified. Further research is needed to understand better nanotechnology’s long-term impacts on the environment and ecosystems and to develop strategies to minimize risks.
6.3. Potential Gaps in the Literature and Directions for Future Research
Although phytoremediation has been extensively studied in recent years, several gaps remain in our understanding of its full potential and limitations. While some studies emphasize the role of microorganisms in enhancing phytoremediation efficiency, there is a need for more comprehensive studies on how microbial consortia can be tailored to specific contaminants, from the droplet composition to the interactions between microorganisms. Another significant gap in the literature pertains to the long-term effects of phytoremediation on soil health and ecosystem functioning. While most studies have focused on short-term outcomes, such as contaminant removal, the long-term impact of phytoremediation on soil biodiversity, nutrient cycling, and overall soil health remains poorly understood. Therefore, studies investigating soil resilience after remediation and the risks of bioaccumulation in plant and microbial tissues should be prioritized in future research. Understanding these long-term effects is crucial for assessing the sustainability of phytoremediation as an environmental management strategy. Additionally, further investigation into the ecological consequences of prolonged phytoremediation will help guide more effective and sustainable practices. By addressing these gaps, future studies could provide valuable insights into optimizing phytoremediation for long-term soil restoration.
7. Conclusions
Plants and microorganisms form a synergistic foundation that enables comprehensive soil restoration, effective pollutant removal, and the protection of surface and groundwater. This integration enhances agricultural productivity, reduces dependence on pesticides and fertilizers, protects biodiversity, supports climate protection, and improves food safety. When properly selected, plants and microorganisms can effectively remove a wide range of soil contaminants including emerging contaminants of increasing concern. The integration of microorganism-assisted phytoremediation can significantly contribute to achieving global and European environmental goals, addressing climate change, and improving quality of life. By restoring ecosystem functions to degraded soils, this approach strengthens ecological resilience and protects against biotic and abiotic stresses. Furthermore, by supporting green infrastructure and regenerative agriculture, phytoremediation improves the carbon sequestration capacity of soil—helping to mitigate climate change and supporting the objectives of the EDG. With its strong economic, commercial, and practical potential, phytoremediation stands as a powerful tool in achieving both the SDGs and the long-term vision of regenerative agriculture.
Author Contributions
Conceptualization, A.K.; methodology, A.K.; investigation, A.K.; writing—original draft preparation, A.K.; writing—review and editing, R.B.; visualization, A.K.; supervision, R.B.; funding acquisition, R.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Jan Dlugosz University in Czestochowa, grant number SBR/WNSPT/KBBE/18/2024.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rathod, S.V.; Saras, P.; Gondaliya, S.M. Environmental pollution: Threats and challenges for management. In Eco-Restoration of Polluted Environment; Rathod, S.V., Ed.; CRP Press: Boca Raton, FL, USA, 2025; pp. 1–34. [Google Scholar]
- Efthimiou, N. Governance and degradation of soil in the EU. An overview of policies with a focus on soil erosion. Soil Tillage Res. 2025, 245, 106308. [Google Scholar] [CrossRef]
- Tadesse, A.; Hailu, W. Causes and consequences of land degradation in Ethiopia: A review. Int. J. Sci. Qual. Anal. 2024, 10, 10–21. [Google Scholar] [CrossRef]
- Han, H.; Zeeshan, Z.; Talpur, B.A.; Sadiq, T.; Bhatti, U.A.; Awwad, E.M.; Al-Razgan, M.; Ghadi, Y.Y. Studying long term relationship between carbon Emissions, Soil, and climate Change: Insights from a global Earth modeling Framework. Int. J. Appl. Earth Obs. Geoinf. 2024, 130, 103902. [Google Scholar] [CrossRef]
- Inbit, M.J.O.; Kazem, A.A.A.; Hussein, H.H.; Abd AL-kadum mageed Brism, R. The Impact of Human Activities on Environmental Sustainability. J. Med. Genet. Clin. Biol. 2024, 1, 119–141. [Google Scholar] [CrossRef]
- Adepoju, A.O.; Femi-Adepoju, A.; Jalloh, A.; Faeflen, S. Soil pollution and management practices. In Environmental Pollution and Public Health; Frazer-Williams, R., Ogundiran, M.B., Unuabonach, E.I., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 187–236. [Google Scholar] [CrossRef]
- Singh, A.; Shah, S.S.; Sharma, C.; Gupta, V.; Sundramoorthy, A.K.; Kumar, P.; Arya, S. An approach towards different techniques for detection of heavy metal ions and their removal from waste water. J. Environ. Chem. Eng. 2024, 12, 113032. [Google Scholar] [CrossRef]
- Manaswini, G.; Sivagami, K.; Gopalakrishnan, M.; Harshini, P.; Janjoren, D.; Ganesan, S. Biodegradation of Low Molecular Weight Polycyclic Aromatic Hydrocarbons in Soil: Insights into Bacterial Activities and Bioremediation Techniques. Sustain. Chem. Environ. 2024, 7, 100146. [Google Scholar] [CrossRef]
- Fetting, C. The European Green Deal: Our Pact for the Future, European Sustainable Development Network (ESDN) Conference Report; ESDN Office: Vienna, Austria, 2020. [Google Scholar]
- Ige, O.E.; Ojo, F.R.; Onikanni, S.A. Rural and Urban Development: Pathways to Environmental Conservation and Sustainability. In Prospects for Soil Regeneration and Its Impact on Environmental Protection; Springer Nature: Cham, Switzerland, 2024; pp. 307–333. [Google Scholar]
- Kowalska, A.; Grobelak, A. Maximizing soil carbon storage: Leveraging microbial factors and limitations for carbon remediation. In Biotechnology of Emerging Microbes; Sharma, H., Joshi, S.J., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 73–89. [Google Scholar] [CrossRef]
- Regassa, A.; Kibret, K.; Selassie, Y.G.; Kiflu, A.; Tena, W. Soil properties. In The Soils of Ethiopia; Beyene, S., Regassa, A., Mishra, B.B., Haile, M., Eds.; World Soils Book Series; Springer: Cham, Switzerland, 2023; pp. 111–156. [Google Scholar] [CrossRef]
- Kharel, M.; Dahal, B.M.; Raut, N. Good agriculture practices for safe food and sustainable agriculture in Nepal: A review. J. Agric. Food Res. 2022, 10, 100447. [Google Scholar] [CrossRef]
- Mosier, S.; Córdova, S.C.; Robertson, G.P. Restoring soil fertility on degraded lands to meet food, fuel, and climate security needs via perennialization. Front. Sustain. Food Syst. 2021, 5, 706142. [Google Scholar] [CrossRef]
- Bhardwaj, A. The Soils of Black Folk: WEB Du Bois’s Theories of Environmental Racialization. Sociol. Theory 2023, 41, 105–128. [Google Scholar] [CrossRef]
- Sofo, A.; Zanella, A.; Ponge, J.-F. Soil quality and fertility in sustainable agriculture, with a contribution to the biological classification of agricultural soils. Soil Use Manag. 2022, 38, 1085–1112. [Google Scholar] [CrossRef]
- Njoku, V.O.N.; Arinze, C.; Chizoruo, I.F.; Blessing, E.N. A Review: Effects of air, water and land dumpsite on human health and analytical methods for determination of pollutants. Anal. Methods Environ. Chem. J. 2021, 4, 80–106. [Google Scholar] [CrossRef]
- Vieira, D.C.S.; Yunta, F.; Baragaño, D.; Evrard, O.; Reiff, T.; Silva, V.; de la Torre, A.; Zhang, C.; Panagos, P.; Jones, A.; et al. Soil pollution in the European Union—An outlook. Environ. Sci. Policy 2024, 161, 103876. [Google Scholar] [CrossRef]
- European Commission: Joint Research Centre; Akca, E.; Aldrian, U.; Alewell, C.; Anzalone, E.; Arcidiacono, A.; Arias Navarro, C.; Auclerc, A.; Aydinsakir, K.; Ballabio, C.; et al. The State of Soils in Europe; Arias Navarro, C., Baritz, R., Jones, A., Eds.; Publications Office of the European Union: Luxembourg, 2024; Available online: https://data.europa.eu/doi/10.2760/7007291 (accessed on 12 January 2025).
- European Commission. EU Soil Strategy for 2030, COM (2021) 699 Final. 2021. Available online: https://ec.europa.eu/environment/publications/eu-soil-strategy-2030_en (accessed on 15 February 2022).
- Panagos, P.; Borrelli, P.; Jones, A.; Robinson, D.A. A 1 billion euro mission: A Soil Deal for Europe. Eur. J. Soil Sci. 2024, 75, e13466. [Google Scholar] [CrossRef]
- Panagos, P.; Van Liedekerke, M.; Borrelli, P.; Köninger, J.; Ballabio, C.; Orgiazzi, A.; Lugato, E.; Liakos, L.; Hervas, J.; Jones, A.; et al. European Soil Data Centre 2.0: Soil data and knowledge in support of the EU policies. Eur. J. Soil Sci. 2022, 73, e13315. [Google Scholar] [CrossRef]
- Gobinath, R.; Ganapathy, G.P.; Gayathiri, E.; Salunkhe, A.A.; Pourghasemi, H.R. Ecoengineering practices for soil degradation protection of vulnerable hill slopes. In Computers in Earth and Environmental Sciences; Pourghasemi, H.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 255–270. [Google Scholar] [CrossRef]
- Prăvălie, R. Exploring the multiple land degradation pathways across the planet. Earth-Sci. Rev. 2021, 220, 103689. [Google Scholar] [CrossRef]
- Voltr, V.; Menšík, L.; Hlisnikovský, L.; Hruška, M.; Pokorný, E.; Pospíšilová, L. The soil organic matter in connection with soil properties and soil inputs. Agronomy 2021, 11, 779. [Google Scholar] [CrossRef]
- Koval, V.; Mikhno, I.; Udovychenko, I.; Gordiichuk, Y.; Kalina, I. Sustainable natural resource management to ensure strategic environmental development. TEM J. 2021, 3, 1022–1030. [Google Scholar]
- Coban, O.; De Deyn, G.B.; van der Ploeg, M. Soil microbiota as game-changers in restoration of degraded lands. Science 2022, 375, abe0725. [Google Scholar] [CrossRef]
- Pedrinho, A.; Mendes, L.W.; de Araujo Pereira, A.P.; Araujo, A.S.F.; Vaishnav, A.; Karpouzas, D.G.; Singh, B.K. Soil microbial diversity plays an important role in resisting and restoring degraded ecosystems. Plant Soil 2024, 500, 325–349. [Google Scholar] [CrossRef]
- Liu, N.; Zhao, J.; Du, J.; Hou, C.; Zhou, X.; Chen, J.; Zhang, Y. Non-phytoremediation and phytoremediation technologies of integrated remediation for water and soil heavy metal pollution: A comprehensive review. Sci. Total Environ. 2024, 948, 174237. [Google Scholar] [CrossRef]
- Zhu, Y.; Gu, H.; Li, H.; Lam, S.S.; Verma, M.; Ng, H.S.; Sonne, C.; Liew, R.K.; Peng, W. Phytoremediation of contaminants in urban soils: A review. Environ. Chem. Lett. 2024, 22, 355–371. [Google Scholar] [CrossRef]
- Mohanty, C.; Kumar, V.; Bisoi, S.; Joseph, M.A.S.; Das, P.K.; Farzana Ahmad, M.; Selvaray, C.I.; Ratha, B.N.; Nanda, S.; Gangwar, S.P. Ecological implications of chromium-contaminated effluents from Indian tanneries and their phytoremediation: A sustainable approach. Environ. Monit. Assess. 2024, 196, 995. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, S.; Zahoor, I.; Kazerooni, E.A. Phytostabilization of contaminated soils. Int. J. Chem. Biochem. Sci. (IJCBS) 2024, 25, 136–153. [Google Scholar]
- Rabêlo, F.H.S.; Vangronsveld, J.; Baker, A.J.; van der Ent, A.; Alleoni, L.R.F. Are grasses really useful for the phytoremediation of potentially toxic trace elements? A review. Front. Plant Sci. 2021, 12, 778275. [Google Scholar] [CrossRef]
- Krzesłowska, M.; Goliński, P.; Szostek, M.; Mocek-Płóciniak, A.; Drzewiecka, K.; Piechalak, A.; Ilek, A.; Neumann, U.; Timmers, A.C.J.; Budzyńska, S.; et al. Morphology and Physiology of Plants Growing on Highly Polluted Mining Wastes. In Phytoremediation for Environmental Sustainability; Prasad, R., Ed.; Springer Nature: Singapore, 2022; pp. 151–200. [Google Scholar] [CrossRef]
- Gavrilescu, M. Enhancing phytoremediation of soils polluted with heavy metals. Curr. Opin. Biotechnol. 2022, 74, 21–31. [Google Scholar] [CrossRef]
- Nwogwu, N.A.; Ajala, O.A.; Ajibade, F.O.; Ajibade, T.F.; Adelodun, B.; Lasisi, K.H.; Ugya, A.Y.; Kumar, P.; Omotade, I.F.; Babalola, T.E.; et al. Phytoremediation mechanisms of heavy metal removal: A step towards a green and sustainable environment. In Innovative Bio-Based Technologies for Environmental Remediation; Singh, P., Hussain, C.M., Sillanpää, M., Eds.; CRP Press: Boca Raton, FL, USA, 2022; pp. 207–236. [Google Scholar] [CrossRef]
- Sharma, K.; Devi, P.; Dey, S.R.; Kumar, P. Mercury phytovolatilization: An overview of the mechanism and mitigation. In Role of Green Chemistry in Ecosystem Restoration to Achieve Environmental Sustainability; Srivastav, A.L., Grewal, A.S., Tiwari, M., Pham, T.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 325–331. [Google Scholar] [CrossRef]
- Li, S.; Liu, C. Use of selenium accumulators and hyperaccumulators in Se-phytoremediation technologies: Recent progress and future perspectives. In Selenium and Nano-Selenium in Environmental Stress Management and Crop Quality Improvement; Hossain, M.A., Ahammed, G.J., Kolbert, Z., El-Ramady, H., Islam, T., Schiavon, M., Eds.; Springer: Cham, Switzerland, 2022; pp. 365–381. [Google Scholar] [CrossRef]
- Genchi, G.; Lauria, G.; Catalano, A.; Sinicropi, M.S.; Carocci, A. Biological activity of selenium and its impact on human health. Int. J. Mol. Sci. 2023, 24, 2633. [Google Scholar] [CrossRef]
- Abdullah, S.R.S.; Al-Baldawi, I.A.; Almansoory, A.F.; Purwanti, I.F.; Al-Sbani, N.H.; Sharuddin, S.S.N. Plant-assisted remediation of hydrocarbons in water and soil: Application, mechanisms, challenges and opportunities. Chemosphere 2020, 247, 125932. [Google Scholar] [CrossRef]
- Xiang, L.; Harindintwali, J.D.; Wang, F.; Redmile-Gordon, M.; Chang, S.X.; Fu, Y.; He, C.; Muhoza, B.; Brahushi, B.; Bolan, N.; et al. Integrating biochar, bacteria, and plants for sustainable remediation of soils contaminated with organic pollutants. Environ. Sci. Technol. 2022, 56, 16546–16566. [Google Scholar] [CrossRef]
- Nasr, M.; Samy, M. Plant-based adsorbents for emerging pollutants removal: A decade review. In Sustainable Technologies for Remediation of Emerging Pollutants from Aqueous Environment; Dehghani, M.H., Karr, R.R., Tyagi, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 241–262. [Google Scholar] [CrossRef]
- Coughlan, N.E.; Walsh, É.; Ahern, R.; Burnell, G.; O’Mahoney, R.; Kuehnhold, H.; Jansen, M.A.K. Flow Rate and Water Depth Alters Biomass Production and Phytoremediation Capacity of Lemna minor. Plants 2022, 11, 2170. [Google Scholar] [CrossRef]
- Khan, A.H.A.; Kiyani, A.; Mirza, C.R.; Butt, T.A.; Barros, R.; Ali, B.; Iqbal, M.; Yousaf, S. Ornamental plants for the phytoremediation of heavy metals: Present knowledge and future perspectives. Environ. Res. 2021, 195, 110780. [Google Scholar] [CrossRef]
- Phang, L.Y.; Mingyuan, L.; Mohammadi, M.; Tee, C.S.; Yuswan, M.H.; Cheng, W.H.; Lai, K.S. Phytoremediation as a viable ecological and socioeconomic management strategy. Environ. Sci. Pollut. Res. 2024, 31, 50126–50141. [Google Scholar] [CrossRef] [PubMed]
- Garraud, J.; Plihon, H.; Capiaux, H.; Le Guern, C.; Mench, M.; Lebeau, T. Drivers to improve metal (loid) phytoextraction with a focus on microbial degradation of dissolved organic matter in soils. Int. J. Phytoremediation 2024, 26, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Van Le, Q.; Peng, W.; Yang, Y.; Yang, H.; Gu, H.; Lam, S.S.; Sonne, C. A review on phytoremediation of contaminants in air, water and soil. J. Hazard. Mater. 2021, 403, 123658. [Google Scholar] [CrossRef] [PubMed]
- Mensah, A.K.; Amoakwah, E. Soil Biogeochemical Factors Influencing Mobilization of Toxic. In Perspectives and Insights on Soil Contamination and Effective Remediation Techniques; Hakeem, K.R., Ed.; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Mohammed, J.N.; Mohammed, A.; Muhammad, I.L.; Mohammed, S.; Alka, S.; Muhammad, R.G. Role of Plant Growth Promoting Rhizobacteria in Remediation of Fluoride Toxicity. In Fluoride and Fluorocarbon Toxicity; Kumar, N., Ed.; Springer Nature: Singapore, 2024; pp. 331–344. [Google Scholar] [CrossRef]
- Hou, D.; Al-Tabbaa, A.; O’Connor, D.; Hu, Q.; Zhu, Y.G.; Wang, L.; Kirkwood, N.; Ok, Y.S.; Tsang, D.C.W.; Bolan, W.S.; et al. Sustainable remediation and redevelopment of brownfield sites. Nat. Rev. Earth Environ. 2023, 4, 271–286. [Google Scholar] [CrossRef]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, plant selection and enhancement by natural and synthetic agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Kumar, A.; Jigyasu, D.K.; Kumar, A.; Subrahmanyam, G.; Mondal, R.; Shabnam, A.A.; Cabral-Pinto, M.M.S.; Molyan, S.K.; Chaturvedi, A.K.; Gupta, D.K.; et al. Nickel in terrestrial biota: Comprehensive review on contamination, toxicity, tolerance and its remediation approaches. Chemosphere 2021, 275, 129996. [Google Scholar] [CrossRef]
- Memon, A.; Kusur, F.; Memon, M. Metal hyperaccumulator plants and their role in phytoremediation. In Phytoremediation for Environmental Sustainability; Prasad, R., Ed.; Springer Nature: Singapore, 2022; pp. 1–24. [Google Scholar] [CrossRef]
- Hussain, M.M.; Farooqi, Z.U.R.; Mohy-Ud-Din, W.; Younas, F.; Shahzad, M.T.T.; Ghani, M.U.M.U.; Ayub, M.A.; Qadeer, A. Application of bioremediation as sustainable approach to remediate heavy metal and pesticide polluted environments. Plant Environ. 2021, 2, 62–92. [Google Scholar]
- Sekhohola-Dlamini, L.M.; Keshinro, O.M.; Masudi, W.L.; Cowan, A.K. Elaboration of a phytoremediation strategy for successful and sustainable rehabilitation of disturbed and degraded land. Minerals 2022, 12, 111. [Google Scholar] [CrossRef]
- Peco, J.D.; Higueras, P.; Campos, J.A.; Esbrí, J.M.; Moreno, M.M.; Battaglia-Brunet, F.; Sandalio, L.M. Abandoned mine lands reclamation by plant remediation technologies. Sustainability 2021, 13, 6555. [Google Scholar] [CrossRef]
- Amabogha, O.N.; Garelick, H.; Jones, H.; Purchase, D. Combining phytoremediation with bioenergy production: Developing a multi-criteria decision matrix for plant species selection. Environ. Sci. Pollut. Res. 2023, 30, 40698–40711. [Google Scholar] [CrossRef]
- Li, M.; Heng, Q.; Hu, C.; Wang, Z.; Jiang, Y.; Wang, X.; He, X.; Yong, J.W.H.; Dawoud, T.M.; Rahman, S.U.; et al. Phytoremediation efficiency of poplar hybrid varieties with diverse genetic backgrounds in soil contaminated by multiple toxic metals (Cd, Hg, Pb, and As). Ecotoxicol. Environ. Saf. 2024, 283, 116843. [Google Scholar] [CrossRef] [PubMed]
- Bajraktari, D.; Zeneli, L.; Bauer, B. Salix alba phytoremediation potential of heavy metals. Maced. Pharm. Bull. 2022, 68, 89–90. [Google Scholar] [CrossRef]
- Landberg, T.; Greger, M. Phytoremediation using willow in industrial contaminated soil. Sustainability 2022, 14, 8449. [Google Scholar] [CrossRef]
- Novakovskiy, A.B.; Kanev, V.A.; Markarova, M.Y. Long-term dynamics of plant communities after biological remediation of oil-contaminated soils in Far North. Sci. Rep. 2021, 11, 4888. [Google Scholar] [CrossRef]
- Zamani, N.; Sabzalian, M.R.; Afyuni, M. Elevated atmospheric CO2 combined with Epichloë endophyte may improve growth and Cd phytoremediation potential of tall fescue (Festuca arundinacea L.). Environ. Sci. Pollut. Res. 2024, 31, 8164–8185. [Google Scholar] [CrossRef]
- Tatian, M.R.; Tamartash, R.; Agajantabar Ali, H.; Faraji, A. Evaluation of phytoremediation potential of lead and cadmium in rangeland plant species, Dactylis glomerata, Festuca ovina and Medicago sativa. J. Nat. Environ. 2023, 76, 15–28. [Google Scholar] [CrossRef]
- Pusz, A.; Wiśniewska, M.; Rogalski, D. Assessment of the Accumulation Ability of Festuca rubra L. and Alyssum saxatile L. Tested on Soils Contaminated with Zn, Cd, Ni, Pb, Cr, and Cu. Resources 2021, 10, 46. [Google Scholar] [CrossRef]
- Havryliuk, O.; Hovorukha, V.; Bida, I.; Danko, Y.; Gladka, G.; Zakutevsky, O.; Mariychuk, R.; Tashyrev, O. Bioremediation of Copper-and Chromium-Contaminated Soils Using Agrostis capillaris L.; Festuca pratensis Huds., and Poa pratensis L. Mixture of Lawn Grasses. Land 2022, 11, 623. [Google Scholar] [CrossRef]
- Wu, Y.; Trejo, H.X.; Chen, G.; Li, S. Phytoremediation of contaminants of emerging concern from soil with industrial hemp (Cannabis sativa L.): A review. Environ. Dev. Sustain. 2021, 23, 14405–14435. [Google Scholar] [CrossRef]
- Panchenko, L.; Muratova, A.; Turkovskaya, O. Comparison of the phytoremediation potentials of Medicago falcata L. and Medicago sativa L. in aged oil-sludge-contaminated soil. Environ. Sci. Pollut. Res. Int. 2017, 24, 3117–3130. [Google Scholar] [CrossRef]
- Jiao, S.; Hou, X.; Zhao, G.; Feng, Y.; Zhang, S.; Zhang, H.; Liu, J.; Jiang, G. Migration of polycyclic aromatic hydrocarbons in the rhizosphere micro-interface of soil-ryegrass (Lolium perenne L.) system. Sci. Total Environ. 2023, 903, 166299. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, G.B.; Janczak, K.; Richert, A. Combined use of Bacillus strains and Miscanthus for accelerating biodegradation of poly (lactic acid) and poly (ethylene terephthalate). PeerJ 2021, 9, e10957. [Google Scholar] [CrossRef]
- LeFevre, G.H.; Portmann, A.C.; Müller, C.E.; Sattely, E.S.; Luthy, R. Plant assimilation kinetics and metabolism of 2-mercaptobenzothiazole tire rubber vulcanizers by Arabidopsis. Environ. Sci. Technol. 2016, 50, 6762–6771. [Google Scholar] [CrossRef] [PubMed]
- Nassazzi, W.; Bezabhe, Y.H.; Guo, C.; Tapase, S.; Jaffe, B.D.; Key, T.A.; Lai, F.Y.; Jass, J.; Ahrens, L. Role of hormone and microbial amendment in per-and polyfluoroalkyl substances (PFAS) phytoremediation using willow and poplar. Environ. Technol. Innov. 2025, 37, 104048. [Google Scholar] [CrossRef]
- Sharma, N.; Barion, G.; Shrestha, I.; Ebinezer, L.B.; Trentin, A.R.; Vamerali, T.; Mezzalira, G.; Masi, A.; Ghisi, R. Accumulation and effects of perfluoroalkyl substances in three hydroponically grown Salix L. species. Ecotoxicol. Environ. Saf. 2020, 191, 110150. [Google Scholar] [CrossRef]
- Ren, W.; Wang, Y.; Huang, Y.; Liu, F.; Teng, Y. Uptake, translocation and metabolism of di-n-butyl phthalate in alfalfa (Medicago sativa). Sci. Total Environ. 2020, 731, 138974. [Google Scholar] [CrossRef]
- Aioub, A.A.; Fahmy, M.A.; Ammar, E.E.; Maher, M.; Ismail, H.A.; Yue, J.; Zhang, Q.; Abdel-Wahab, S.I.Z. Decontamination of Chlorpyrifos Residue in Soil by Using Mentha piperita (Lamiales: Lamiaceae) for Phytoremediation and Two Bacterial Strains. Toxics 2024, 12, 435. [Google Scholar] [CrossRef]
- Ramadan, M.R.; Aioub, A.A.; Romeh, A.A.; Shalaby, A.A. Phytoremediation of soil and water contaminated with diazinon. Zagazig J. Agric. Res. 2015, 42, 843. [Google Scholar]
- Maheshwari, G.; Setia, K.; Gauba Mathur, S. Exploring phytoremediation potential for estrogen hormone. Int. J. Res. Rev. 2019, 6, 195–202. [Google Scholar]
- Teerakun, M.; Reungsang, A. Determination of plant species for the phytoremediation of carbofuran residue in rice field soils. Songklanakarin J. Sci. Technol. 2025, 27, 967–973. [Google Scholar]
- Wang, N.Q.; Kong, C.H.; Wang, P.; Meiners, S.J. Root exudate signals in plant–plant interactions. Plant Cell Environ. 2021, 44, 1044–1058. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Tang, S.; Dengzeng, Z.; Zhang, D.; Zhang, T.; Ma, X. Root exudates contribute to belowground ecosystem hotspots: A review. Front. Microbiol. 2022, 13, 937940. [Google Scholar] [CrossRef]
- Sun, W.; Li, Q.; Qiao, B.; Jia, K.; Li, C.; Zhao, C. Advances in Plant–Soil Feedback Driven by Root Exudates in Forest Ecosystems. Forests 2024, 15, 515. [Google Scholar] [CrossRef]
- Podar, D.; Maathuis, F.J. The role of roots and rhizosphere in providing tolerance to toxic metals and metalloids. Plant Cell Environ. 2022, 45, 719–736. [Google Scholar] [CrossRef]
- Pasricha, S.; Mathur, V.; Garg, A.; Lenka, S.; Verma, K.; Agarwal, S. Molecular mechanisms underlying heavy metal uptake, translocation and tolerance in hyperaccumulators-an analysis: Heavy metal tolerance in hyperaccumulators. Environ. Chall. 2021, 4, 100197. [Google Scholar] [CrossRef]
- Supreeth, M. Enhanced remediation of pollutants by microorganisms–plant combination. Int. J. Environ. Sci. Technol. 2022, 19, 4587–4598. [Google Scholar] [CrossRef]
- Das, P.P.; Singh, K.R.; Nagpure, G.; Mansoori, A.; Singh, R.P.; Ghazi, I.A.; Kumar, A.; Singh, J. Plant-soil-microbes: A tripartite interaction for nutrient acquisition and better plant growth for sustainable agricultural practices. Environ. Res. 2022, 214, 113821. [Google Scholar] [CrossRef]
- Imam, A.; Suman, S.K.; Kanaujia, P.K.; Ray, A. Biological machinery for polycyclic aromatic hydrocarbons degradation: A review. Bioresour. Technol. 2022, 343, 126121. [Google Scholar] [CrossRef]
- Kristanti, R.A.; Tirtalistyani, R.; Tang, Y.Y.; Thao, N.T.T.; Kasongo, J.; Wijayanti, Y. Phytoremediation mechanism for emerging pollutants: A review. Trop. Aquat. Soil Pollut. 2023, 3, 88–108. [Google Scholar] [CrossRef]
- Grobelak, A.; Całus-Makowska, K.; Jasińska, A.; Klimasz, M.; Wypart-Pawul, A.; Augustajtys, D.; Baor, E.; Sławczyk, D.; Kowalska, A. Environmental Impacts and Contaminants Management in Sewage Sludge-to-Energy and Fertilizer Technologies: Current Trends and Future Directions. Energies 2024, 17, 4983. [Google Scholar] [CrossRef]
- Wang, J.; Ma, W.; Ma, W.; Yao, Z.; Jiang, Y.; Jiang, W.; Xin, F.; Zhang, W.; Jiang, M. Microbial Astaxanthin Synthesis by Komagataella phaffii through Metabolic and Fermentation Engineering. J. Agric. Food Chem. 2025, 73, 1952–1964. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gao, S.; Pan, K.; Liu, H.; Li, Q.; Bai, X.; Zhu, Q.; Chen, Z.; Yan, X.; Hong, Q. Functional analysis, diversity, and distribution of the ean cluster responsible for 17β-estradiol degradation in sphingomonads. Appl. Environ. Microbiol. 2024, 90, e01974-23. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Duan, M.; Zhang, X.; Yang, Z.; Zhuo, R. Bacterial community structure analysis of sludge from Taozi lake and isolation of an efficient 17β-Estradiol (E2) degrading strain Sphingobacterium sp. GEMB-CSS-01. Chemosphere 2024, 355, 141806. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, L.; Hou, J.; Wang, X.; Liu, H.; Zheng, D.; Liang, R. iTRAQ-based quantitative proteomic analysis of the global response to 17β-estradiol in estrogen-degradation strain Pseudomonas putida SJTE-1. Sci. Rep. 2017, 7, 41682. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Gao, H.; Dong, Q.; Su, Y.; Dai, T.; Qin, Z.; Yang, Y.; Gao, Q. Bacteria are better predictive biomarkers of environmental estrogen transmission than fungi. Environ. Pollut. 2022, 298, 118838. [Google Scholar] [CrossRef]
- Daâssi, D.; Alharbi, S.R. Degradation of endocrine-disrupting chemicals in wastewater by new thermophilic fungal isolates and their laccases. 3 Biotech 2023, 13, 26. [Google Scholar] [CrossRef]
- Feng, N.-X.; Feng, Y.-X.; Liang, Q.-F.; Chen, X.; Xiang, L.; Zhao, H.M.; Liu, B.-L.; Cao, G.; Li, Y.-W.; Li, H.; et al. Complete biodegradation of di-n-butyl phthalate (DBP) by a novel Pseudomonas sp. YJB6. Sci. Total Environ. 2021, 761, 143208. [Google Scholar] [CrossRef]
- Wu, X.; Li, J.; Zhou, Z.; Lin, Z.; Pang, S.; Bhatt, P.; Mishra, S.; Chen, S. Environmental occurrence, toxicity concerns, and degradation of diazinon using a microbial system. Front. Microbiol. 2021, 12, 717286. [Google Scholar] [CrossRef]
- Sun, W.; Chen, Y.; Liu, L.; Tang, J.; Chen, J.; Liu, P. Conidia immobilization of T-DNA inserted Trichoderma atroviride mutant AMT-28 with dichlorvos degradation ability and exploration of biodegradation mechanism. Bioresour. Technol. 2010, 101, 9197–9203. [Google Scholar] [CrossRef]
- Parte, S.G.; Mohekar, A.D.; Kharat, A.S. Aerobic dichlorvos degradation by Pseudomonas stutzeri smk: Complete pathway and implications for toxicity in Mus musculus. Iran. J. Microbiol. 2020, 12, 138. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Li, J.; Pang, S.; Mishra, S.; Bhatt, P.; Zeng, D.; Chen, S. Emerging Technologies for Degradation of Dichlorvos: A Review. Int. J. Environ. Res. Public Health 2021, 18, 5789. [Google Scholar] [CrossRef] [PubMed]
- Srinivasulu, M.; Raju, M.N.; Chandra, M.S.; Shankar, P.C.; Rangaswamy, V.; Prasad, R. Microbe-pesticide interactions: Soil enzyme analysis and bacterial degradation of chlorpyrifos. J. Environ. Chem. Ecotoxicol. 2024, 6, 180–191. [Google Scholar] [CrossRef]
- Wepukhulu, M.; Wachira, P.; Huria, N.; Sifuna, P.; Essuman, S.; Asamba, M. Optimization of Growth Conditions for Chlorpyrifos-Degrading Bacteria in Farm Soils in Nakuru County, Kenya. BioMed Res. Int. 2024, 2024, 1611871. [Google Scholar] [CrossRef] [PubMed]
- Salah-Tazdaït, R.; Tazdaït, D. Use of microbial enzymes to degrade pesticide residues in agroecosystems-sustainable practices. In Biotechnology of Emerging Microbes; Sharma, H., Joshi, S.J., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 189–215. [Google Scholar] [CrossRef]
- Bhende, R.S.; Bombaywala, S.; Dafale, N.A. Unleashing potential of Pseudomonas aeruginosa RNC3 and Stenotrophomonas maltophilia RNC7 for chlorpyrifos biodegradation by genome analysis and kinetic studies. J. Hazard. Mater. 2024, 461, 132668. [Google Scholar] [CrossRef]
- Bosu, S.; Rajamohan, N.; Al Salti, S.; Rajasimman, M.; Das, P. Biodegradation of chlorpyrifos pollution from contaminated environment—A review on operating variables and mechanism. Environ. Res. 2024, 248, 118212. [Google Scholar] [CrossRef]
- Kosimov, D.; Zaynitdinova, L.; Mavjudova, A.; Muminov, M.; Shukurov, O. Isolation, Identification and Use of Bacterial Strain Ochrobactrum intermedium PDB-3 for Degradation of the Pesticide Chlorpyrifos. Microbiol. Biotechnol. Lett. 2024, 52, 44–54. [Google Scholar] [CrossRef]
- Pan, Q.; Li, Y.; Zhang, J.; Hu, T.; Hou, Y.; Tang, S. Mechanisms of oxidative response during biodegradation of malathion by S. oneidensis MR-1. Environ. Sci. Pollut. Res. 2024, 31, 16832–16845. [Google Scholar] [CrossRef]
- Dar, M.A.; Pandey, J.; Kaushik, G. Optimizing malathion biodegradation by Bacillus cereus via a design of experiment framework. Bioremediat. J. 2024, 1–19. [Google Scholar] [CrossRef]
- Swathy, K.; Vivekanandhan, P.; Yuvaraj, A.; Sarayut, P.; Kim, J.S.; Krutmuang, P. Biodegradation of pesticide in agricultural soil employing entomopathogenic fungi: Current state of the art and future perspectives. Heliyon 2024, 10, e23406. [Google Scholar] [CrossRef]
- Duc, H.D.; Hung, N.V.; Oanh, N.T. Anaerobic Degradation of Endosulfans by a Mixed Culture of Pseudomonas sp. and Staphylococcus sp. Appl. Biochem. Microbiol. 2021, 57, 327–334. [Google Scholar] [CrossRef]
- Baharudin, N.S.; Ahmad, H.; Hossain, M.S. Understanding the Degradation of Carbofuran in Agricultural Area: A Review of Fate, Metabolites, and Toxicity. Pertanika J. Sci. Technol. 2024, 32, 285–322. [Google Scholar] [CrossRef]
- Ivshina, I.B.; Tyumina, E.A.; Kuzmina, M.V.; Vikhareva, E.V. Features of diclofenac biodegradation by Rhodococcus ruber IEGM 346. Sci. Rep. 2019, 9, 9159. [Google Scholar] [CrossRef] [PubMed]
- Aracagök, Y.D.; Göker, H.; Cihangir, N. Biodegradation of diclofenac with fungal strains. Arch. Environ. Prot. 2018, 44, 55–62. [Google Scholar] [CrossRef]
- Fetyan, N.A.H.; Asair, A.A.; Ismail, I.M.; Elsakhawy, T.A.; Elnagdy, S.M.; Mohamed, M.S.M. Bacterial Degradation of Ibuprofen: Insights into Metabolites, Enzymes, and Environmental Fate Biodegradation of Ibuprofen by Achromobacter Species. Microbiol. Res. 2024, 15, 2298–2315. [Google Scholar] [CrossRef]
- Lara-Moreno, A.; Costa, M.C.; Vargas-Villagomez, A.; Carlier, J.D. New bacterial strains for ibuprofen biodegradation: Drug removal, transformation, and potential catabolic genes. Environ. Microbiol. Rep. 2024, 16, e13320. [Google Scholar] [CrossRef]
- Palma, T.L.; Magno, G.; Costa, M.C. Biodegradation of paracetamol by some gram-positive bacterial isolates. Curr. Microbiol. 2021, 78, 2774–2786. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, J.; Zhou, S.; Lian, M. Microbial degradation of carbamazepine by a newly isolated of Gordonia polyophrenivorans. Environ. Technol. Innov. 2023, 32, 103322. [Google Scholar] [CrossRef]
- Xu, W.-J.; Wan, Q.; Wang, W.-F.; Wang, Y.; Feng, F.-Y.; Cheng, J.-J.; Juan, J.-J.; Yu, X.-Y. Biodegradation of dibutyl phthalate by a novel endophytic Bacillus subtilis strain HB-T2 under in-vitro and in-vivo conditions. Environ. Technol. 2022, 43, 1917–1926. [Google Scholar] [CrossRef]
- Du, H.; Cheng, J.-L.; Li, Z.-Y.; Zhong, H.-N.; Wei, S.; Gu, Y.-J.; Yao, C.-C.; Zhang, M.; Cai, Q.-Y.; Zhao, H.M.; et al. Molecular insights into the catabolism of dibutyl phthalate in Pseudomonas aeruginosa PS1 based on biochemical and multi-omics approaches. Sci. Total Environ. 2024, 926, 171852. [Google Scholar] [CrossRef]
- Hernández-Sánchez, B.; Santacruz-Juárez, E.; Figueroa-Martínez, F.; Castañeda-Antonio, D.; Portillo-Reyes, R.; Viniegra-González, G.; Sánchez, C. A novel and efficient strategy for the biodegradation of di (2-ethylhexyl) phthalate by Fusarium culmorum. Appl. Microbiol. Biotechnol. 2024, 108, 94. [Google Scholar] [CrossRef]
- Patil, R.; Bagde, U.S. Isolation of polyvinyl chloride degrading bacterial strains from environmental samples using enrichment culture technique. Afr. J. Biotechnol. 2012, 11, 7947–7956. [Google Scholar] [CrossRef]
- Giacomucci, L.; Raddadi, N.; Soccio, M.; Lotti, N.; Fava, F. Polyvinyl chloride biodegradation by Pseudomonas citronellolis and Bacillus flexus. New Biotechnol. 2019, 52, 35–41. [Google Scholar] [CrossRef]
- Okal, E.J.; Heng, G.; Magige, E.A.; Khan, S.; Wu, S.; Ge, Z.; Zhang, T.; Mortimer, P.E.; Xu, J. Insights into the mechanisms involved in the fungal degradation of plastics. Ecotoxicol. Environ. Saf. 2023, 262, 115202. [Google Scholar] [CrossRef]
- Zainab, N.; Amna; Khan, A.A.; Azeem, M.A.; Ali, B.; Wang, T.; Shi, F.; Alghanem, S.M.; Hussain Munis, M.F.; Hashem, M.; et al. PGPR-mediated plant growth attributes and metal extraction ability of Sesbania sesban L. in industrially contaminated soils. Agronomy 2021, 11, 1820. [Google Scholar] [CrossRef]
- Naz, M.; Afzal, M.R.; Qi, S.S.; Dai, Z.; Sun, Q.; Du, D. Microbial-assistance and chelation-support techniques promoting phytoremediation under abiotic stresses. Chemosphere 2024, 365, 143397. [Google Scholar] [CrossRef]
- Bhanse, P.; Kumar, M.; Singh, L.; Awasthi, M.K.; Qureshi, A. Role of plant growth-promoting rhizobacteria in boosting the phytoremediation of stressed soils: Opportunities, challenges, and prospects. Chemosphere 2022, 303, 134954. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B.R. Recent advances in bacterial amelioration of plant drought and salt stress. Biology 2022, 11, 437. [Google Scholar] [CrossRef]
- Jan, S.; Bhardwaj, R.; Sharma, N.R.; Singh, R. Unraveling the role of plant growth regulators and plant growth promoting rhizobacteria in phytoremediation. J. Plant Growth Regul. 2024, 43, 2471–2487. [Google Scholar] [CrossRef]
- Montreemuk, J.; Stewart, T.N.; Prapagdee, B. Bacterial-assisted phytoremediation of heavy metals: Concepts, current knowledge, and future directions. Environ. Technol. Innov. 2024, 33, 103488. [Google Scholar] [CrossRef]
- Gómez-Godínez, L.J.; Aguirre-Noyola, J.L.; Martínez-Romero, E.; Arteaga-Garibay, R.I.; Ireta-Moreno, J.; Ruvalcaba-Gómez, J.M. A look at plant-growth-promoting bacteria. Plants 2023, 12, 1668. [Google Scholar] [CrossRef]
- Shah, A.; Nazari, M.; Antar, M.; Msimbira, L.A.; Naamala, J.; Lyu, D.; Rabileh, M.; Zajonc, J.; Smith, D.L. PGPR in agriculture: A sustainable approach to increasing climate change resilience. Front. Sustain. Food Syst. 2021, 5, 667546. [Google Scholar] [CrossRef]
- Shahid, M.; Khan, M.S.; Singh, U.B. Pesticide-tolerant microbial consortia: Potential candidates for remediation/clean-up of pesticide-contaminated agricultural soil. Environ. Res. 2023, 236, 116724. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Singh, U.B. Enhancing Spinach (Spinacia oleracea L.) Resilience in Pesticide-Contaminated Soil: Role of Pesticide-Tolerant Ciceribacter azotifigens and Serratia marcescens in Root Architecture, Leaf Gas Exchange Attributes and Antioxidant Response Restoration. Chemosphere 2024, 361, 142487. [Google Scholar] [CrossRef] [PubMed]
- Hnini, M.; Rabeh, K.; Oubohssaine, M. Interactions between beneficial soil microorganisms (PGPR and AMF) and host plants for environmental restoration: A systematic review. Plant Stress 2024, 11, 100391. [Google Scholar] [CrossRef]
- Tiwari, J.; Ma, Y.; Bauddh, K. Arbuscular mycorrhizal fungi: An ecological accelerator of phytoremediation of metal contaminated soils. Arch. Agron. Soil Sci. 2022, 68, 283–296. [Google Scholar] [CrossRef]
- Salifu, M.; John, M.A.; Abubakar, M.; Bankole, I.A.; Ajayi, N.D.; Amusan, O. Phytoremediation Strategies for Heavy Metal Contamination: A Review on Sustainable Approach for Environmental Restoration. J. Environ. Prot. 2024, 15, 450–474. [Google Scholar] [CrossRef]
- Li, X.; Kang, X.; Zou, J.; Yin, J.; Wang, Y.; Li, A.; Ma, X. Allochthonous arbuscular mycorrhizal fungi promote Salix viminalis L.–mediated phytoremediation of polycyclic aromatic hydrocarbons characterized by increasing the release of organic acids and enzymes in soils. Ecotoxicol. Environ. Saf. 2023, 249, 114461. [Google Scholar] [CrossRef]
- Hu, B.; Hu, S.; Vymazal, J.; Chen, Z. Arbuscular mycorrhizal symbiosis in constructed wetlands with different substrates: Effects on the phytoremediation of ibuprofen and diclofenac. J. Environ. Manag. 2021, 296, 113217. [Google Scholar] [CrossRef]
- Naseer, M.; Zhu, Y.; Li, F.-M.; Yang, Y.-M.; Wang, S.; Xiong, Y.-C. Nano-enabled improvements of growth and colonization rate in wheat inoculated with arbuscular mycorrhizal fungi. Environ. Pollut. 2022, 295, 118724. [Google Scholar] [CrossRef]
- Kebede, G.; Tafese, T.; Abda, E.M.; Kamaraj, M.; Assefa, F. Factors influencing the bacterial bioremediation of hydrocarbon contaminants in the soil: Mechanisms and impacts. J. Chem. 2021, 2021, 9823362. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, J.; Lu, S.; Li, Y.; Wang, F. Arbuscular Mycorrhizal Fungi Improve the Performance of Sweet Sorghum Grown in a Mo-Contaminated Soil. J. Fungi 2020, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Meyer, E.; Londoño, D.M.M.; de Armas, R.D.; Giachini, A.J.; Rossi, M.J.; Stoffel, S.C.G.; Soares, C.R.F.S. Arbuscular mycorrhizal fungi in the growth and extraction of trace elements by Chrysopogon zizanioides (vetiver) in a substrate containing coal mine wastes. Int. J. Phytoremediat. 2017, 19, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, S.C.K.; Understrup, A.; Fomsgaard, I.S.; Mortensen, A.G.; Ravnskov, S. Flavonoids in roots of white clover: Interaction of arbuscular mycorrhizal fungi and a pathogenic fungus. Plant Soil 2008, 302, 33–43. [Google Scholar] [CrossRef]
- Tuo, X.-Q.; He, L.; Zou, Y.-N. Alleviation of drought stress in white clover after inoculation with arbuscular mycorrhizal Fungi. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 45, 220–224. [Google Scholar] [CrossRef]
- Wang, H.-R.; Du, X.-R.; Zhang, Z.-Y.; Feng, F.-J.; Zhang, J.-M. Rhizosphere interface microbiome reassembly by arbuscular mycorrhizal fungi weakens cadmium migration dynamics. iMeta 2023, 2, e133. [Google Scholar] [CrossRef]
- Das, T.; Bhattacharyya, A.; Bhar, A. Linking phyllosphere and rhizosphere microbiome to the plant–insect interplay: The new dimension of tripartite interaction. Physiologia 2023, 3, 129–144. [Google Scholar] [CrossRef]
- Mishra, V.; Gupta, A.; Kaur, P.; Singh, S.; Singh, N.; Gehlot, P.; Singh, J. Synergistic effects of Arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria in bioremediation of iron contaminated soils. Int. J. Phytoremediat. 2016, 18, 697–703. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, Z.; Hao, B.; Diao, F.; Zhang, J.; Bao, Z.; Guo, W. Arbuscular mycorrhizal fungi alter microbiome structure of rhizosphere soil to enhance maize tolerance to La. Ecotoxicol. Environ. Saf. 2021, 212, 111996. [Google Scholar] [CrossRef]
- Ali, A.; Ghani, M.I.; Ding, H.; Iqbal, M.; Cheng, Z.; Cai, Z. Arbuscular mycorrhizal inoculum coupled with organic substrate induces synergistic effects for soil quality changes, and rhizosphere microbiome structure in long-term monocropped cucumber planted soil. Rhizosphere 2021, 20, 100428. [Google Scholar] [CrossRef]
- Monokrousos, N.; Papatheodorou, E.M.; Orfanoudakis, M.; Jones, D.-G.; Scullion, J.; Stamou, G.P. The effects of plant type, AMF inoculation and water regime on rhizosphere microbial communities. Eur. J. Soil Sci. 2020, 71, 265–278. [Google Scholar] [CrossRef]
- Ma, Y.; Ankit; Tiwari, J.; Bauddh, K. Plant-Mycorrhizal Fungi Interactions in Phytoremediation of Geogenic Contaminated Soils. Front. Microbiol. 2022, 13, 843415. [Google Scholar] [CrossRef]
- Ponce-Hernández, A.; Gómez-Rubio, J.A.; Ceballos-Maldonado, J.G.; Martínez-Soto, D.; Márquez-Vega, M.; Hernández-Morales, A.; Maldonado-Miranda, J.J.; Carranza-Álvarez, C. Endophytic Fungi and Bacteria: Enhancement of Heavy Metal Phytoextraction. In Aquatic Contamination: Tolerance and Bioremediation; Bhat, R.A., Dar, G.H., Tonelli, F.M.P., Hamid, S., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2024; pp. 43–59. [Google Scholar] [CrossRef]
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development; A/RES/70/1; United Nations: New York, NY, USA, 2015; Available online: https://sdgs.un.org/2030agenda (accessed on 16 February 2025).
- Yin, C.; Zhao, W.; Pereira, P. Soil conservation service underpins sustainable development goals. Glob. Ecol. Conserv. 2022, 33, e01974. [Google Scholar] [CrossRef]
- Rydz-Żbikowska, A. Implementing Sustainable Development Goals within the COVID–19 Pandemic Future Challenges for the 2030 Agenda. Comp. Econ. Res. Cent. East. Eur. 2022, 25, 135–160. [Google Scholar]
- Sonowal, S.; Nava, A.R.; Joshi, S.J.; Borah, S.N.; Islam, N.F.; Pandit, S.; Prasad, R.; Sarma, H. Biosurfactant-assisted phytoremediation of potentially toxic elements in soil: Green technology for meeting the United Nations Sustainable Development Goals. Pedosphere 2022, 32, 198–210. [Google Scholar] [CrossRef]
- Yang, Q.; Yu, H.; Zhao, Z.; Ju, Z. Effects of Enhanced Phytoremediation Techniques on Soil Aggregate Structure. Agriculture 2024, 14, 1882. [Google Scholar] [CrossRef]
- Ghosh, A.; Manna, M.C.; Jha, S.; Singh, A.K.; Misra, S.; Srivastava, R.C.; Srivastava, P.P.; Laik, R.; Bhattacharyya, R.; Prasad, S.S.; et al. Impact of soil-water contaminants on tropical agriculture, animal and societal environment. Adv. Agron. 2022, 176, 209–274. [Google Scholar] [CrossRef]
- Raklami, A.; Meddich, A.; Oufdou, K.; Baslam, M. Plants—Microorganisms-based bioremediation for heavy metal cleanup: Recent developments, phytoremediation techniques, regulation mechanisms, and molecular responses. Int. J. Mol. Sci. 2022, 23, 5031. [Google Scholar] [CrossRef]
- Mohan, C.; Robinson, J.; Vodwal, L.; Kumari, N. Sustainable Development Goals for addressing environmental challenges. In Green Chemistry Approaches to Environmental Sustainability; Garg, V.K., Mohan, C., Kumari, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 357–374. [Google Scholar] [CrossRef]
- Giller, K.E.; Hijbeek, R.; Andersson, J.A.; Sumberg, J. Regenerative agriculture: An agronomic perspective. Outlook Agric. 2021, 50, 13–25. [Google Scholar] [CrossRef]
- Sayed, E.T.; Abdelkareem, M.A.; Obaideen, K.; Elsaid, K.; Wilberforce, T.; Maghrabie, H.M.; Olabi, A.G. Progress in plant-based bioelectrochemical systems and their connection with sustainable development goals. Carbon Resour. Convers. 2021, 4, 169–183. [Google Scholar] [CrossRef]
- Lal, R.; Monger, C.; Nave, L.; Smith, P. The role of soil in regulation of climate. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2021, 376, 20210084. [Google Scholar] [CrossRef]
- Kowalska, A.; Grobelak, A.; Almås, Å.R.; Singh, B.R. Effect of Biowastes on Soil Remediation, Plant Productivity and Soil Organic Carbon Sequestration: A Review. Energies 2020, 13, 5813. [Google Scholar] [CrossRef]
- Hossain, M.B. Glomalin and contribution of glomalin to carbon sequestration in soil: A review. Turk. J. Agric.-Food Sci. Technol. 2021, 9, 191–196. [Google Scholar] [CrossRef]
- Bodner, G.; Mentler, A.; Keiblinger, K. Plant roots for sustainable soil structure management in cropping systems. In The Root Systems in Sustainable Agricultural Intensification; Rengel, Z., Djalovic, I., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 45–90. [Google Scholar] [CrossRef]
- Köninger, J.; Panagos, P.; Jones, A.; Briones, M.J.I.; Orgiazzi, A. In defence of soil biodiversity: Towards an inclusive protection in the European Union. Biol. Conserv. 2022, 268, 109475. [Google Scholar] [CrossRef]
- Bhatt, R.P. Achievement of SDGS globally in biodiversity conservation and reduction of greenhouse gas emissions by using green energy and maintaining forest cover. GSC Adv. Res. Rev. 2023, 17, 001–021. [Google Scholar] [CrossRef]
- Lei, Y.; Huang, D.; Zhou, W.; Wang, G.; Xiao, R.; Xu, W.; Huan, H.; Li, S.; Shen, L.; Ren, Y.; et al. Combining phytoremediation with carbon-based materials under carbon neutral background: Is it a close step to sustainable restoration? Crit. Rev. Environ. Sci. Technol. 2024, 54, 1070–1091. [Google Scholar] [CrossRef]
- Lee, S.-H.; Park, H.; Kim, J.-G. Current status of and challenges for phytoremediation as a sustainable environmental management plan for abandoned mine areas in Korea. Sustainability 2023, 15, 2761. [Google Scholar] [CrossRef]
- Bandyopadhyay, S. Plant-assisted metal remediation in mine-degraded land: A scientometric review. Int. J. Environ. Sci. Technol. 2022, 19, 8085–8112. [Google Scholar] [CrossRef]
- Liu, H.; Yin, H.; Kong, F.; Middel, A.; Zheng, X.; Huang, J.; Sun, T.; Wang, D.; Lensky, I.M. Change of nutrients, microorganisms, and physical properties of exposed extensive green roof substrate. Sci. Total Environ. 2022, 805, 150344. [Google Scholar] [CrossRef]
- Fagorzi, C. The Green Deal Challenge: Exploiting Biotic Interactions from Bacterial Strains to Communities. Ph.D. Thesis, Università degli Studi di Ferrara, Ferrara, Italy, 2021. [Google Scholar]
- Zhou, W.; Li, M.; Achal, V. A comprehensive review on environmental and human health impacts of chemical pesticide usage. Emerg. Contam. 2024, 11, 100410. [Google Scholar] [CrossRef]
- Singh, R.; Choudhary, P.; Kumar, S.; Daima, H.K. Mechanistic approaches for crosstalk between nanomaterials and plants: Plant immunomodulation, defense mechanisms, stress resilience, toxicity, and perspectives. Environ. Sci. Nano 2024, 11, 2324–2351. [Google Scholar] [CrossRef]
- Ionata, E.; Caputo, E.; Mandrich, L.; Marcolongo, L. Moving towards biofuels and high-value products through phytoremediation and biocatalytic processes. Catalysts 2024, 14, 118. [Google Scholar] [CrossRef]
- Lehnert, W.; Traum, Y. The ‘new’ Renewable Energy Directive (RED III): An overview. Eur. Energy Clim. J. 2024, 12, 40–47. [Google Scholar] [CrossRef]
- Onori, F.; Milletti, T.; Rossi, M.; Comodi, G. Towards the target of the Renewable Energy Directive (RED) III using photovoltaic and batteries: The case study of Italy. J. Phys. Conf. Ser. 2024, 2893, 012004. [Google Scholar] [CrossRef]
- Fermeglia, M.; Perišić, M. Nature-based solution to man-made problems: Fostering the uptake of phytoremediation and low-iluc biofuels in the EU. J. Eur. Environ. Plan. Law 2023, 20, 145–167. [Google Scholar] [CrossRef]
- Migliavacca, G.; Carlini, C.; Domenighini, P.; Zagano, C. Hydrogen: Prospects and Criticalities for Future Development and Analysis of Present EU and National Regulation. Energies 2024, 17, 4827. [Google Scholar] [CrossRef]
- Devi Chinmayee, M.; Swapna, T.S. Application of Biodiesel Plant (Jatropha curcus L.) for Phytoremediation of Heavy Metal Contaminated Soil. In Plant Genetic Resource Utilization. An Appraisal; Swapna, T.S., Suhaa Beevy, S., Radhamany, P.M., Eds.; Department of Botany, University of Kerala: Thiruvananthapuram, India, 2021; pp. 156–163. [Google Scholar]
- Wijekoon, W.; Priyashantha, H.; Gajanayake, P.; Manage, P.; Liyanage, C.; Jayarathna, S.; Kumarasinghe, U. Review and Prospects of Phytoremediation: Harnessing Biofuel-Producing Plants for Environmental Remediation. Sustainability 2025, 17, 822. [Google Scholar] [CrossRef]
- Obiora, N.K.; Ujah, C.O.; Asadu, C.O.; Kolawole, F.O.; Ekwueme, B.N. Production of hydrogen energy from biomass: Prospects and challenges. Green Technol. Sustain. 2024, 2, 100100. [Google Scholar] [CrossRef]
- Poore, J.; Nemecek, T. Reducing food’s environmental impacts through producers and consumers. Science 2018, 360, 987–992. [Google Scholar] [CrossRef]
- Hait, M.; Patel, D.; Izah, S.C. Molecular Techniques and Technologies in Biomonitoring for Environmental Sustainability. In Biomonitoring of Pollutants in the Global South; Izah, S.C., Ogwu, M.C., Hamidifar, H., Eds.; Springer Nature: Singapore, 2024; pp. 605–637. [Google Scholar] [CrossRef]
- Parida, S.K.; Satpathy, A.; Dalai, A.; Kullu, S.; Hota, S.; Mishra, S. Novel Methods and Techniques for the Remediation of Mining Waste Residues. In Sustainable Management of Mining Waste and Tailings; Das, A.P., van Hullebusch, E.D., Akçil, A., Eds.; CRC Press: Boca Raton, FL, USA, 2024; pp. 1–29. [Google Scholar]
- Tan, H.W.; Pang, Y.L.; Lim, S.; Chong, W.C. A state-of-the-art of phytoremediation approach for sustainable management of heavy metals recovery. Environ. Technol. Innov. 2023, 30, 103043. [Google Scholar] [CrossRef]
- Bao, C.; Cao, Y.; Zhao, L.; Li, X.; Zhang, J.; Mao, C. Biofuel Production from Phytoremediated Biomass via Various Conversion Routes: A Review. Energies 2025, 18, 822. [Google Scholar] [CrossRef]
- Placido, D.F.; Lee, C.C. Potential of industrial hemp for phytoremediation of heavy metals. Plants 2022, 11, 595. [Google Scholar] [CrossRef] [PubMed]
- De Vos, B.; Souza, M.F.; Michels, E.; Meers, E. Industrial hemp (Cannabis sativa L.) in a phytoattenuation strategy: Remediation potential of a Cd, Pb and Zn contaminated soil and valorization potential of the fibers for textile production. Ind. Crops Prod. 2022, 178, 114592. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Udayan, A.; Sreekumar, N. Phycoremediation of emerging contaminants and heavy metals from industrial wastewater: A sustainable green approach for bioeconomy. Bioresour. Technol. Rep. 2024, 27, 101920. [Google Scholar] [CrossRef]
- Blanco-Vieites, M.; Álvarez-Gil, M.; Delgado, F.; García-Ruesgas, L.; Rodríguez, E. Livestock wastewater bioremediation through indigenous microalgae culturing as a circular bioeconomy approach as cattle feed. Algal Res. 2024, 78, 103424. [Google Scholar] [CrossRef]
- Singh, A.; Kostova, I. Health effects of heavy metal contaminants Vis-à-Vis microbial response in their bioremediation. Inorganica Chim. Acta 2024, 568, 122068. [Google Scholar] [CrossRef]
- Ezhumalai, G.; Arun, M.; Manavalan, A.; Rajkumar, R.; Heese, K. A holistic approach to circular bioeconomy through the sustainable utilization of microalgal biomass for biofuel and other value-added products. Microb. Ecol. 2024, 87, 61. [Google Scholar] [CrossRef]
- Rabbani, M.; Rabbani, M.T.; Muthoni, F.; Sun, Y.; Vahidi, E. Advancing phytomining: Harnessing plant potential for sustainable rare earth element extraction. Bioresour.Technol. 2024, 401, 130751. [Google Scholar] [CrossRef]
- Kikis, C.; Thalassinos, G.; Antoniadis, V. Soil Phytomining: Recent Developments—A Review. Soil Syst. 2024, 8, 8. [Google Scholar] [CrossRef]
- Akinbile, B.J.; Mbohwa, C. Incorporating hyperaccumulating plants in phytomining, remediation and resource recovery: Recent trends in the African region—A review. RSC Sustain. 2025, 3, 1652–1671. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, V.P. The Potential of Chrysopogon Zizanioides (L.) Nash in Remediation of Heavy. In Proceedings of Indian Geotechnical and Geoenvironmental Engineering Conference (IGGEC); Agnihotri, A.K., Reddy, K.R., Chore, H.S., Eds.; Springer Nature: Singapore, 2021; Volume 2, pp. 63–74. [Google Scholar]
- Campbell, B.M.; Beare, D.J.; Bennett, E.M.; Hall-Spencer, J.M.; Ingram, J.S.; Jaramillo, F.; Ortiz, R.; Ramankutty, N.; Sayer, J.A.; Shindell, D. Agriculture production as a major driver of the Earth system exceeding planetary boundaries. Ecol. Soc. 2017, 22, 8. [Google Scholar] [CrossRef]
- Tefera, M.L.; Carletti, A.; Altea, L.; Rizzu, M.; Migheli, Q.; Seddaiu, G. Land degradation and the upper hand of sustainable agricultural intensification in sub-Saharan Africa—A systematic review. J. Agric. Rural. Dev. Trop. Subtrop. (JARTS) 2024, 125, 63–83. [Google Scholar] [CrossRef]
- Vamshi, M.; Jagadeesan, R.; Lamani, H.D.; Rout, S.; VijayKumar, R.; Jagadesh, M.; Sachan, K. The Revolutionary Impact of Regenerative Agriculture on Ecosystem Restoration and Land Vitality: A Review. J. Geogr. Environ. Earth Sci. Int. 2024, 28, 1–14. [Google Scholar] [CrossRef]
- Ikanović, J.; Popović, V.; Gavrilović, M.; Ljubičić, N.; Rakašćan, N.; Isakov, M.; Kaiš, K. Agropyrum repens in the function of phytoremediation and soil protection. In Book of Proceedings, 6th IRASA International Scientific Conference-Science, Education, Technology and Innovation (SETI VI 2024), Belgrade, 12 October 2024; International Research Academy of Science and Art (IRASA): Belgrade, Serbia, 2024; pp. 63–83. [Google Scholar] [CrossRef]
- Vidadala, R. Reimagining Agroforestry: Climate-Resilient Landscapes for Regenerative Agriculture. In Agroforestry Solutions for Climate Change and Environmental Restoration; Kumar, S., Alam, B., Taria, S., Singh, P., Yadov, A., Arunachalam, A., Eds.; Springer Nature: Singapore, 2024; pp. 171–201. [Google Scholar] [CrossRef]
- Gassner, V.T.; Symeonidis, D.; Koukaras, K. Harvesting of Agricultural Nutrient Runoff with Algae, to Produce New Soil Amendments for Urban and Peri-urban Olive Tree Agroforestry Systems in Southern Europe. In Nature-Based Solutions for Circular Management of Urban Water. Circular Economy and Sustainability; Stefanakis, A., Oral, H.V., Calheiros, C., Carvalho, P., Eds.; Springer: Cham, Switzerland, 2024; pp. 405–441. [Google Scholar] [CrossRef]
- Beattie, G.A.; Edlund, A.; Esiobu, N.; Gilbert, J.; Nicolaisen, M.H.; Jansson, J.K.; Jensen, P.; van der Meer, J.R. Soil microbiome interventions for carbon sequestration and climate mitigation. mSystems 2024, 10, e01129-24. [Google Scholar] [CrossRef] [PubMed]
- Uroz, S.; Picard, L.; Turpault, M.P. Recent progress in understanding the ecology and molecular genetics of soil mineral weathering bacteria. Trends Microbiol. 2022, 30, 882–897. [Google Scholar] [CrossRef]
- Maqsood, Q.; Waseem, R.; Sumrin, A.; Wajid, A.; Tariq, M.R.; Ali, S.W.; Mahnoor, M. Recent trends in bioremediation and bioaugmentation strategies for mitigation of marine based pollutants: Current perspectives and future outlook. Discov. Sustain. 2024, 5, 524. [Google Scholar] [CrossRef]
- Nookongbut, P.; Thiravetyan, P.; Salsabila, S.; Widiana, A.; Krobthong, S.; Yingchutrakul, Y.; Treesubsuntorn, C. Application of Acinetobacter indicus to promote cigarette smoke particulate matter phytoremediation: Removal efficiency and plant–microbe interactions. Environ. Sci. Pollut. Res. 2024, 31, 52352–52370. [Google Scholar] [CrossRef]
- Gill, R.; Gupta, V.; Patel, M. Utilization of genetically modified weed plants against industrial contaminants: A promising tool of phytoremediation. In Bioremediation of Emerging Contaminants from Soils; Kumar, P., Chaudhary, V., van Hullebusch, E.D., Busquets, R., Srivastav, A.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 611–634. [Google Scholar] [CrossRef]
- Gomes, M.P. Nanophytoremediation: Advancing phytoremediation efficiency through nanotechnology integration. Discov. Plants 2025, 2, 8. [Google Scholar] [CrossRef]
- Gulzar, A.B.M.; Mazumder, P.B. Helping plants to deal with heavy metal stress: The role of nanotechnology and plant growth promoting rhizobacteria in the process of phytoremediation. Environ. Sci. Pollut. Res. 2022, 29, 40319–40341. [Google Scholar] [CrossRef]
- Yasin, M.U.; Haider, Z.; Munir, R.; Zulfiqar, U.; Rehman, M.; Javaid, M.H.; Ahmad, I.; Nana, C.; Saeed, M.S.; Ali, B.; et al. The synergistic potential of biochar and nanoparticles in phytoremediation and enhancing cadmium tolerance in plants. Chemosphere 2024, 354, 141672. [Google Scholar] [CrossRef]
- Raja, V.; Singh, K.; Qadir, S.U.; Singh, J.; Kim, K.H. Alleviation of cadmium-induced oxidative damage through application of zinc oxide nanoparticles and strigolactones in Solanum lycopersicum L. Environ. Sci. Nano 2024, 11, 2633–2654. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).