Improving the Biomass Energy Yield of Cocksfoot Cultivated on Degraded Soil Amended with Organic–Mineral Fertilizer
Abstract
1. Introduction
2. Materials and Methods
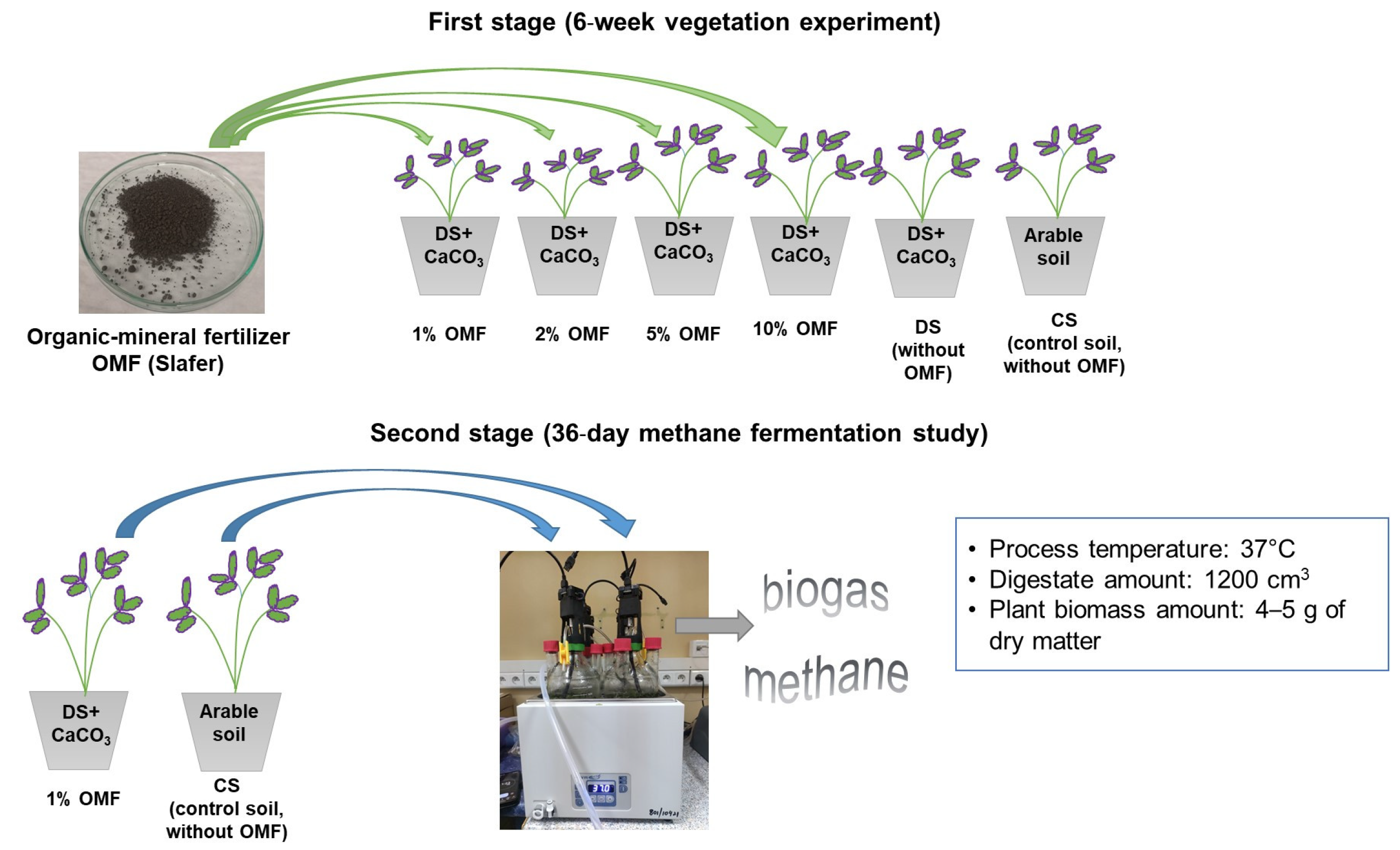
2.1. Experiment Design
2.2. Vegetation Experiment Design
2.2.1. Soil and Mineral-Organic Waste Characteristics
2.2.2. Preparation of Pot Experiment and Plant Growth Condition
2.2.3. Soil Preparation and Analysis of Soil Properties
2.2.4. Plant Preparation and Analysis of Plant Properties
2.3. Methane Fermentation Study
2.3.1. Analysis of Plant Biomass as a Feedstock to Fermentation
2.3.2. Batch Experiment Design
2.3.3. Biogas Production Analysis
2.4. Data Analysis
3. Results
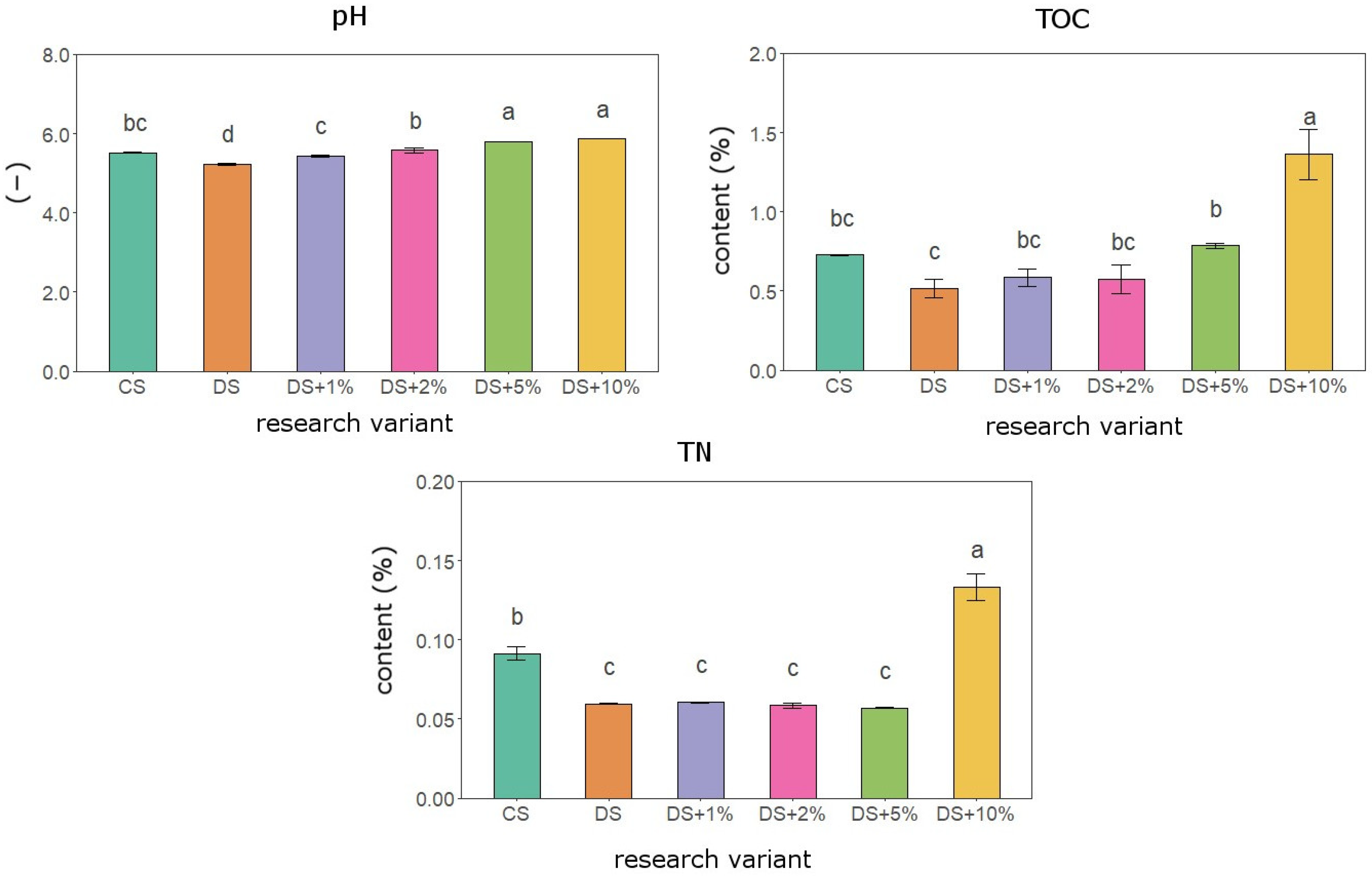
3.1. Physicochemical Soil Properties in Vegetation Experiment
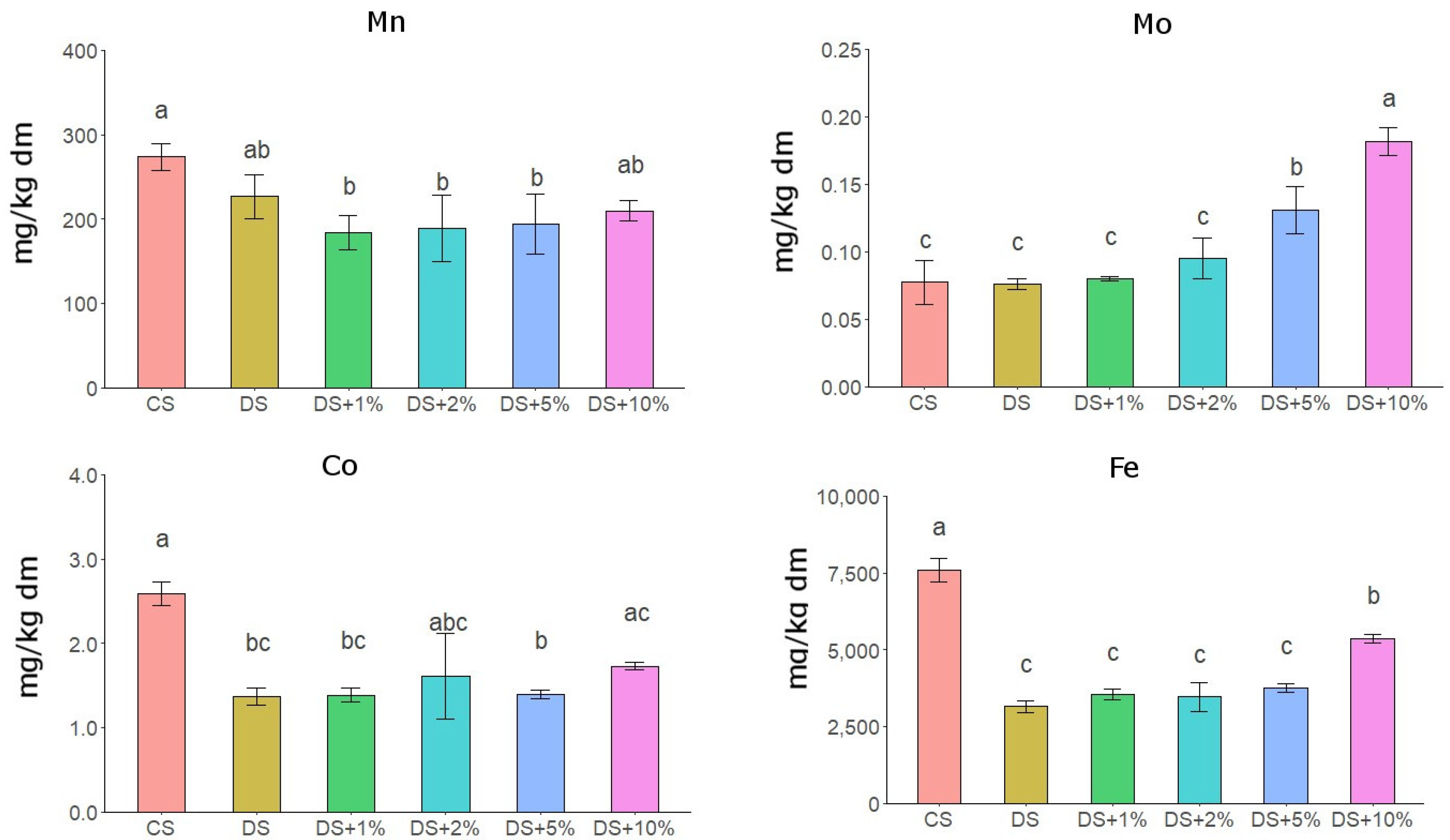
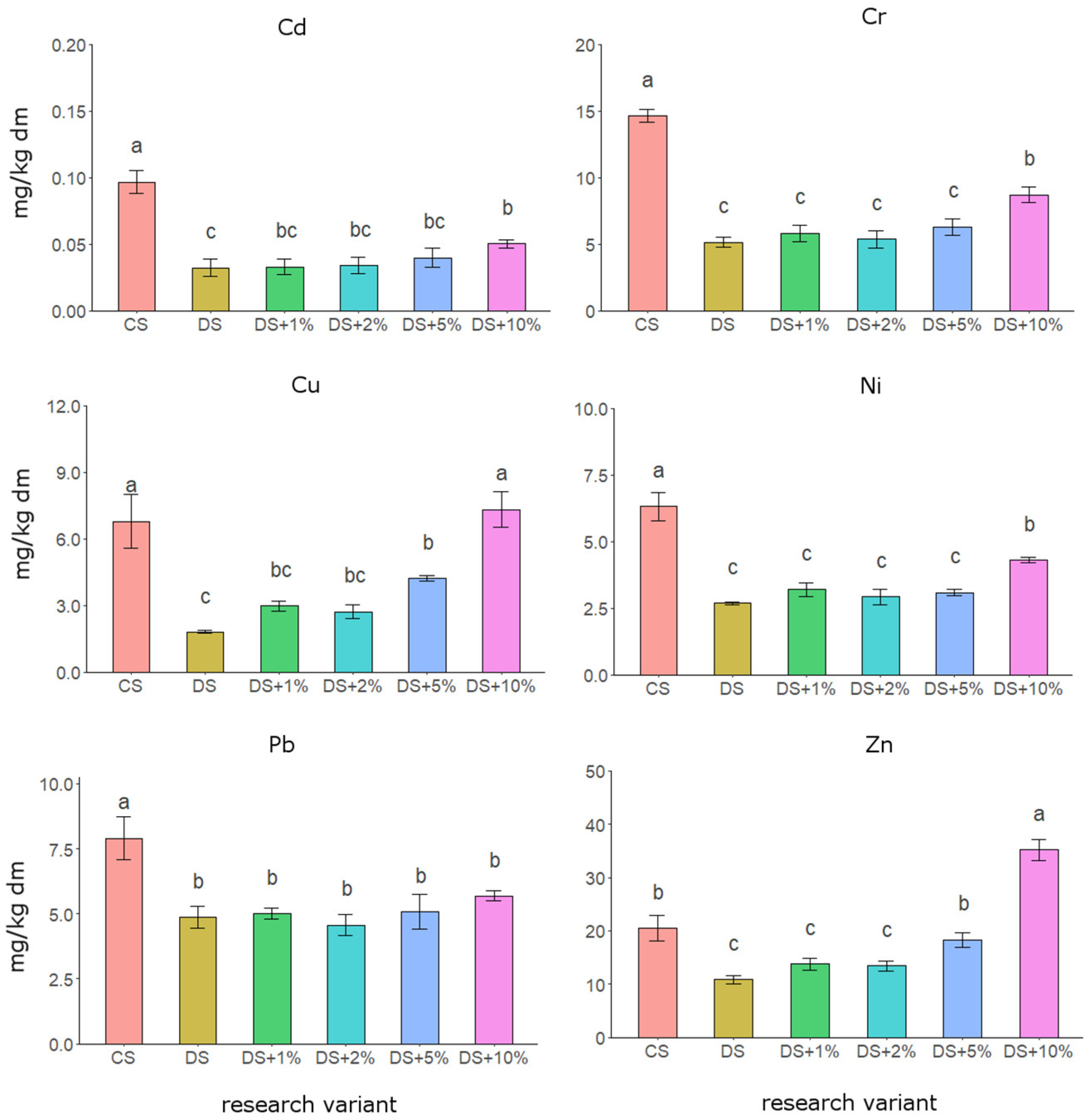
3.2. Content of Micronutrients and Heavy Metals in Soil
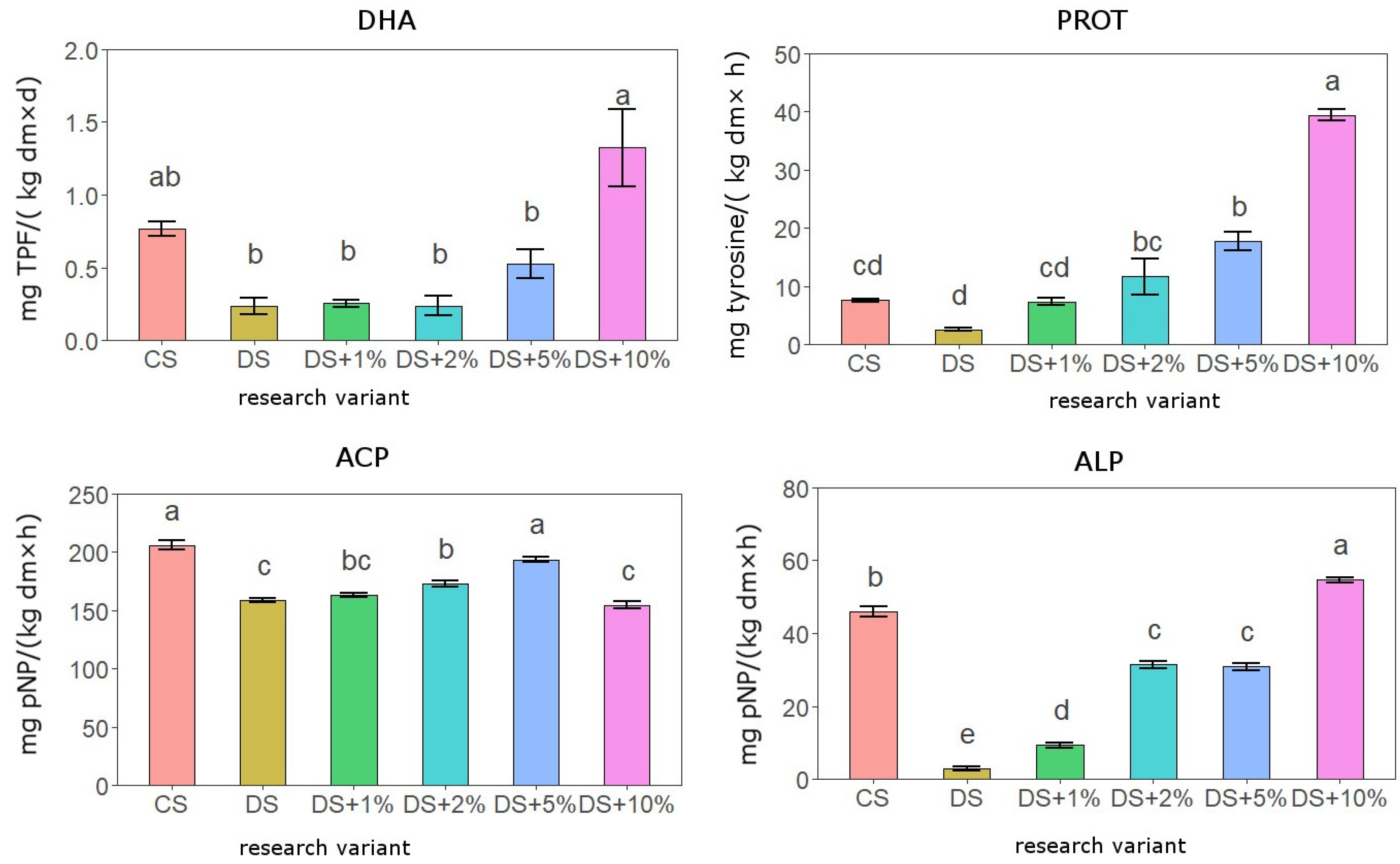
3.3. Activity of Soil Enzymes
3.4. Plant Properties in Vegetation Experiment
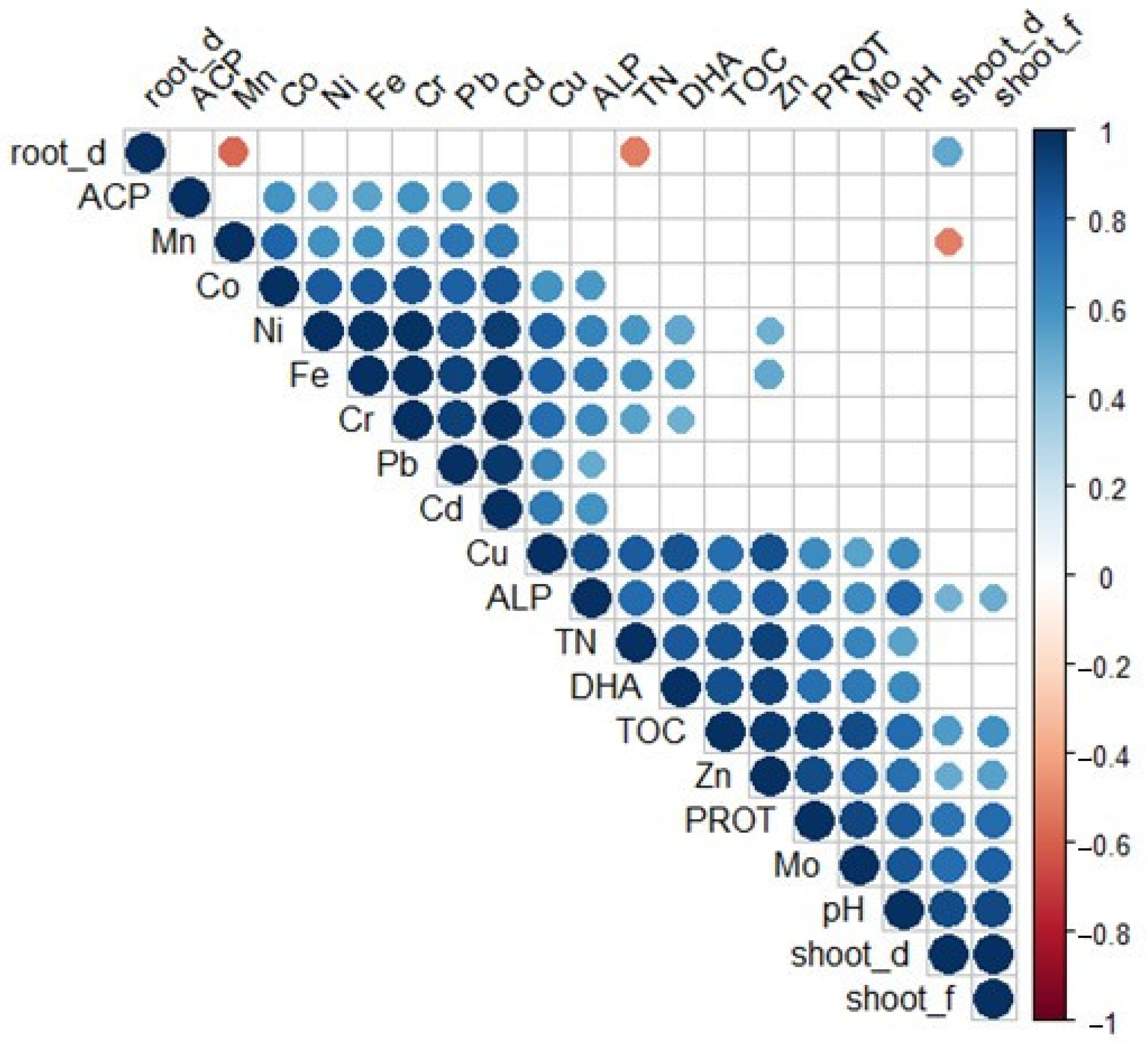
3.5. Correlation Analysis of Studied Parameters in Vegetation Experiment
3.6. Characteristic of Plant Biomass as a Feedstock in Batch Experiment
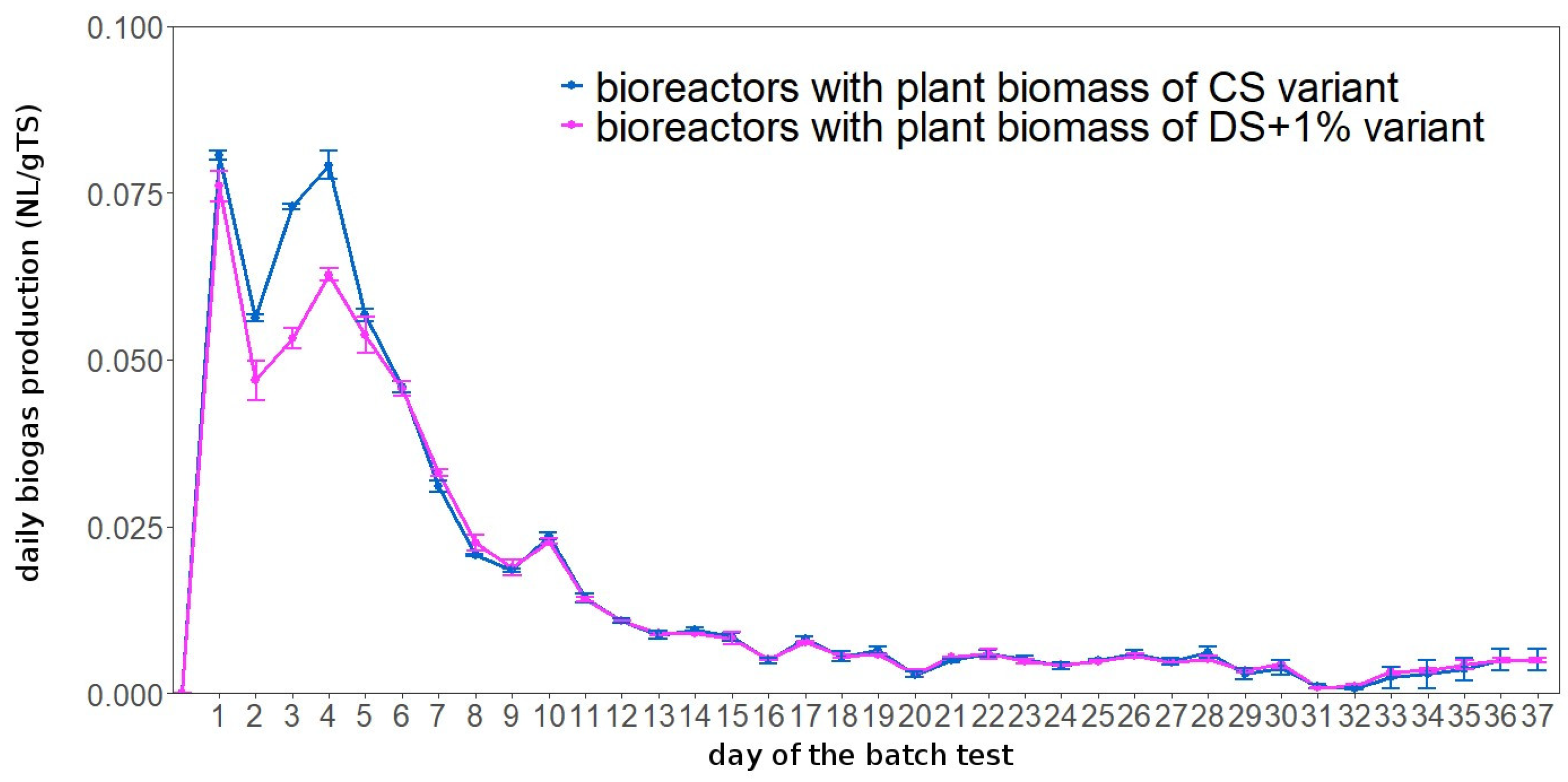
3.7. Biogas and Methane Yield from Cocksfoot Biomass
4. Discussion
4.1. Organic–Mineral Waste as a Substrate to Improve Soil Quality
4.2. Organic–Mineral Waste as a Substrate to Increase Biomass Production
4.3. Effect of Organic–Mineral Fertilizer on Biogas Production
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, S.; Anjum, R.; Raza, S.T.; Ahmed Bazai, N.; Ihtisham, M. Technologies for Municipal Solid Waste Management: Current Status, Challenges, and Future Perspectives. Chemosphere 2022, 288, 132403. [Google Scholar] [CrossRef]
- Przydatek, G.; Wota, A.K. Analysis of the Comprehensive Management of Sewage Sludge in Poland. J. Mater. Cycles Waste Manag. 2020, 22, 80–88. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. Solid Waste Issue: Sources, Composition, Disposal, Recycling, and Valorization. Egypt. J. Pet. 2018, 27, 1275–1290. [Google Scholar] [CrossRef]
- Waste Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Waste_statistics (accessed on 14 February 2025).
- Development of a Gangue-Based Fertilizer and Soil Improver Using Beneficial Microorganisms (in Polish). Mapa dotacji. Available online: https://mapadotacji.gov.pl/projekty/744288/ (accessed on 14 February 2025).
- Song, W.; Zhang, J.; Li, M.; Yan, H.; Zhou, N.; Yao, Y.; Guo, Y. Underground Disposal of Coal Gangue Backfill in China. Appl. Sci. 2022, 12, 12060. [Google Scholar] [CrossRef]
- Hao, Y.; Guo, X.; Yao, X.; Han, R.; Li, L.; Zhang, M. Using Chinese Coal Gangue as an Ecological Aggregate and Its Modification: A Review. Materials 2022, 15, 4495. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Di, Z.; Cao, W.; He, S. Feasibility Analysis of Bacterial-Treated Coal Gangue for Soil Improvement: Growth-Promoting Effects of Alfalfa. Minerals 2024, 14, 676. [Google Scholar] [CrossRef]
- Nan, Y.-C.; Yang, Y.-G.; Wang, Z.-Q.; Zhou, Y.; Su, Q.-M. Effects of Coal Gangue on Soil Property and Plant Growth in Mining Area. Ying Yong Sheng Tai Xue Bao 2023, 34, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Review of Coal Gangue Characteristics and Ecological Restoration Management Technology. IOP Conf. Ser. Earth Environ. Sci. 2021, 781, 032033. [Google Scholar] [CrossRef]
- Du, T.; Wang, D.; Bai, Y.; Zhang, Z. Optimizing the Formulation of Coal Gangue Planting Substrate Using Wastes: The Sustainability of Coal Mine Ecological Restoration. Ecol. Eng. 2020, 143, 105669. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, T.; Gao, X. Incorporation of Self-Ignited Coal Gangue in Steam Cured Precast Concrete. J. Clean. Prod. 2021, 292, 126004. [Google Scholar] [CrossRef]
- Yang, F.; Lv, J.; Zheng, Y.; Cui, J.; Huang, Y.; Cao, X.; Liu, H.; Zhao, L. Enhancement of Coal Gangue Performance by Surface Micro-Crystalline Glaze Derived from Mineral Powder. Sci. Total Environ. 2023, 858, 159986. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Li, S.; Ren, P.; Yan, F.; Wang, L.; Guo, B.; Zhao, Y.; Yang, Y.; Sun, J.; Gao, P.; et al. Enhancing the Carbon Content of Coal Gangue for Composting Through Sludge Amendment: A Feasibility Study. Environ. Pollut. 2024, 348, 123439. [Google Scholar] [CrossRef] [PubMed]
- Muylle, H.; Van Hulle, S.; De Vliegher, A.; Baert, J.; Van Bockstaele, E.; Roldán-Ruiz, I. Yield and Energy Balance of Annual and Perennial Lignocellulosic Crops for Bio-Refinery Use: A 4-Year Field Experiment in Belgium. Eur. J. Agron. 2015, 63, 62–70. [Google Scholar] [CrossRef]
- Tilvikiene, V.; Kadziuliene, Z.; Dabkevicius, Z.; Venslauskas, K.; Navickas, K. Feasibility of Tall Fescue, Cocksfoot and Reed Canary Grass for Anaerobic Digestion: Analysis of Productivity and Energy Potential. Ind. Crops Prod. 2016, 84, 87–96. [Google Scholar] [CrossRef]
- Butkutė, B.; Lemežienė, N.; Kanapeckas, J.; Navickas, K.; Dabkevičius, Z.; Venslauskas, K. Cocksfoot, Tall Fescue and Reed Canary Grass: Dry Matter Yield, Chemical Composition and Biomass Convertibility to Methane. Biomass Bioenergy 2014, 66, 1–11. [Google Scholar] [CrossRef]
- Tang, T.; Wang, Z.; Chen, L.; Wu, S.; Liu, Y. Opportunities, Challenges and Modification Methods of Coal Gangue as a Sustainable Soil Conditioner—A review. Environ. Sci. Pollut. Res. 2024, 31, 58231–58251. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Jia, H.; Pino, V.; Wu, H.; Cheng, F. A Si-K-Based Amendment Prepared by Coal Gangue and Plant Ash Could Improve the Growth of Maize Plants in Saline Soils. J. Soil. Sci. Plant Nutr. 2024, 24, 761–774. [Google Scholar] [CrossRef]
- Lv, X.; Yang, S.; Deng, J.; Lei, J.; Shu, Z. Formulation of Ferric/Phosphorus Composite Coating on Coal Gangue as a Novel Fertilizer for Enhancing Slow-Release of Silicon and Implication of As, Cr and Pb. J. Environ. Manag. 2024, 354, 120347. [Google Scholar] [CrossRef]
- Lu, Y.; Song, C.; Yan, C.; Jin, Z.; Li, Y.; Lai, C.; Wang, D. The Use of Coal Gangue as a Planting Substrate in Arid Mining Areas. Glob. Ecol. Conserv. 2024, 56, e03328. [Google Scholar] [CrossRef]
- Zhu, Q.; Ruan, M.; Hu, Z.; Miao, K.; Ye, C. The Relationship between Acid Production and the Microbial Community of Newly Produced Coal Gangue in the Early Oxidation Stage. Microorganisms 2023, 11, 2626. [Google Scholar] [CrossRef] [PubMed]
- Kominko, H.; Gorazda, K.; Wzorek, Z. Potentiality of Sewage Sludge-Based Organo-Mineral Fertilizer Production in Poland Considering Nutrient Value, Heavy Metal Content and Phytotoxicity for Rapeseed Crops. J. Environ. Manag. 2019, 248, 109283. [Google Scholar] [CrossRef] [PubMed]
- Wydro, U.; Jabłońska-Trypuć, A.; Hawrylik, E.; Butarewicz, A.; Rodziewicz, J.; Janczukowicz, W.; Wołejko, E. Heavy Metals Behavior in Soil/Plant System after Sewage Sludge Application. Energies 2021, 14, 1584. [Google Scholar] [CrossRef]
- Brausmann, A.; Bretschger, L. Economic Development on a Finite Planet with Stochastic Soil Degradation. Eur. Econ. Rev. 2018, 108, 1–19. [Google Scholar] [CrossRef]
- Shanmugavel, D.; Rusyn, I.; Solorza-Feria, O.; Kamaraj, S.-K. Sustainable SMART Fertilizers in Agriculture Systems: A Review on Fundamentals to In-Field Applications. Sci. Total Environ. 2023, 904, 166729. [Google Scholar] [CrossRef]
- Wong, M.H. Ecological Restoration of Mine Degraded Soils, with Emphasis on Metal Contaminated Soils. Chemosphere 2003, 50, 775–780. [Google Scholar] [CrossRef]
- Tahat, M.M.; Alananbeh, K.M.; Othman, Y.A.; Leskovar, D.I. Soil Health and Sustainable Agriculture. Sustainability 2020, 12, 4859. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Energy Crops and Their Implications on Soil and Environment. Agron. J. 2010, 102, 403–419. [Google Scholar] [CrossRef]
- Monforti, F.; Lugato, E.; Motola, V.; Bodis, K.; Scarlat, N.; Dallemand, J.-F. Optimal Energy Use of Agricultural Crop Residues Preserving Soil Organic Carbon Stocks in Europe. Renew. Sustain. Energy Rev. 2015, 44, 519–529. [Google Scholar] [CrossRef]
- El Hafidi, E.M.; Mortadi, A.; Chahid, E.G.; Laasri, S. Optimization of Domestic Wastewater Treatment Using Ferric Chloride Coagulant: Physicochemical Analysis and Impedance Spectroscopy Studies. Water Air Soil. Pollut. 2024, 235, 68. [Google Scholar] [CrossRef]
- El Hafidi, E.M.; Mortadi, A.; Chahid, E.G.; Laasri, S. Monitoring of Domestic Wastewater Treatment Via Infiltration Percolation Using Impedance Spectroscopy. Environ. Technol. Innov. 2023, 32, 103421. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Rural Development (Ministerstwo Rolnictwa i Rozwoju Wsi). Regulation of the Polish Minister of Agriculture and Rural Development of 18 June 2008 on the Implementation of Certain Provisions of the Act on Fertilisers and Fertilisation; No. 119 Item 765; Ministry of Agriculture and Rural Development (Ministerstwo Rolnictwa i Rozwoju Wsi): Warsaw, Poland, 2008.
- Ministry of Agriculture and Rural Development (Ministerstwo Rolnictwa i Rozwoju Wsi). Regulation of the Polish Minister of the Environment of 6 February 2015 on the Use of Municipal Sewage Sludge; Item 257; Ministry of Agriculture and Rural Development (Ministerstwo Rolnictwa i Rozwoju Wsi): Warsaw, Poland, 2015.
- Ostrowska, A. Methods for Analysis and Evaluation of Soil and Plant Properties; Dział Wydawnictw IOŚ: Warsaw, Poland, 1991. [Google Scholar]
- Zur Methodik der Bestimmung der Dehydrogenaseaktivität im Boden mittels Triphenyltetrazoliumchlorid (TTC)—ScienceOpen. Available online: https://www.scienceopen.com/document?vid=c8eab917-02b4-492d-81f1-da36da3a3458 (accessed on 23 December 2024).
- Ladd, J.N.; Butler, J.H.A. Short-Term Assays of Soil Proteolytic Enzyme Activities Using Proteins and Dipeptide Derivatives as Substrates. Soil. Biol. Biochem. 1972, 4, 19–30. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-Nitrophenyl Phosphate for Assay Of Soil Phosphatase Activity. Soil. Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Chomczyńska, M.; Pawłowska, M.; Szczepaniak, O.; Duma, E. Biogas Generation from Maize and Cocksfoot Growing in Degraded Soil Enriched with New Zeolite Substrate. Energies 2022, 15, 377. [Google Scholar] [CrossRef]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 28 November 2024).
- Singh, R.P.; Agrawal, M. Potential Benefits and Risks of Land Application of Sewage Sludge. Waste Manag. 2008, 28, 347–358. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Qiang, X.; Yu, Z.; Li, H.; Sun, Z.; Li, Q.; He, J.; Han, L.; Zhao, N. Improving Saline–Alkaline Soil and Ryegrass Growth with Coal Gangue Treatments. Plants 2024, 13, 3419. [Google Scholar] [CrossRef] [PubMed]
- Achkir, A.; Aouragh, A.; El Mahi, M.; Lotfi, E.M.; Labjar, N.; EL Bouch, M.; Ouahidi, M.L.; Badza, T.; Farhane, H.; EL Moussaoui, T. Implication of Sewage Sludge Increased Application Rates on Soil Fertility and Heavy Metals Contamination Risk. Emerg. Contam. 2023, 9, 100200. [Google Scholar] [CrossRef]
- Nyamangara, J.; Mzezewa, J. Effect of Long-Term Application of Sewage Sludge to a Grazed Grass Pasture on Organic Carbon and Nutrients of a Clay Soil in Zimbabwe. Nutr. Cycl. Agroecosyst. 2001, 59, 13–18. [Google Scholar] [CrossRef]
- Deviatkin, I.; Lyu, L.; Chen, S.; Havukainen, J.; Wang, F.; Horttanainen, M.; Mänttäri, M. Technical Implications and Global Warming Potential of Recovering Nitrogen Released During Continuous Thermal Drying of Sewage Sludge. Waste Manag. 2019, 90, 132–140. [Google Scholar] [CrossRef]
- Joniec, J. Enzymatic Activity as An Indicator of Regeneration Processes in Degraded Soil Reclaimed with Various Types of Waste. Int. J. Environ. Sci. Technol. 2018, 15, 2241–2252. [Google Scholar] [CrossRef]
- Wydro, U.; Jankowska, M.; Wołejko, E.; Kondzior, P.; Łozowicka, B.; Kaczyński, P.; Rodziewicz, J.; Janczukowicz, W.; Pietryczuk, A.; Cudowski, A.; et al. Changes in Soil Biological Properties after Sewage Sludge and Pesticide Application in Wheat Cultivation. Appl. Sci. 2022, 12, 11452. [Google Scholar] [CrossRef]
- Wieczorek, J.; Gambuś, F.; Baran, A. Heavy Metal Content and Yielding of Italian Ryegrass Cultivated in the Soil Intensively Fertilized with Municipal Sewage Sludges. E3S Web Conf. 2013, 1, 15005. [Google Scholar] [CrossRef]
- Farsang, A.; Babcsányi, I.; Ladányi, Z.; Perei, K.; Bodor, A.; Csányi, K.T.; Barta, K. Evaluating the Effects of Sewage Sludge Compost Applications on the Microbial Activity, the Nutrient and Heavy Metal Content of a Chernozem Soil in a Field Survey. Arab. J. Geosci. 2020, 13, 982. [Google Scholar] [CrossRef]
- Lakhdar, A.; Scelza, R.; Scotti, R.; Rao, M.A.; Jedidi, N.; Gianfreda, L.; Abdelly, C. The Effect of Compost and Sewage Sludge on Soil Biologic Activities in Salt Affected Soil. Rev. Cienc. Suelo Y Nutr. Veg. 2010, 10, 40–47. [Google Scholar] [CrossRef]
- Bo, H.; Li, Z.; Wang, W.; Zhang, R.; Wang, H.; Jin, D.; Xu, M.; Zhang, Q. Combining Organic and Inorganic Fertilization Enhances Soil Enzyme Activity, the Bacterial Community, and Molecular Ecological Network Complexity in Coal Mine Reclamation Areas. Agronomy 2024, 14, 1427. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, X.; Liu, X.; Liang, X.; Chen, X. Influence of Root Components of Celery on Pyrene Bioaccessibility, Soil Enzymes and Microbial Communities in Pyrene and Pyrene-Diesel Spiked Soils. Sci. Total Environ. 2017, 599–600, 50–57. [Google Scholar] [CrossRef]
- Tilvikiene, V.; Venslauskas, K.; Povilaitis, V.; Navickas, K.; Zuperka, V.; Kadziuliene, Z. The Effect of Digestate and Mineral Fertilisation of Cocksfoot Grass on Greenhouse Gas Emissions in a Cocksfoot-Based Biogas Production System. Energy Sustain. Soc. 2020, 10, 13. [Google Scholar] [CrossRef]
- Meehan, P.; Burke, B.; Doyle, D.; Barth, S.; Finnan, J. Exploring the potential of grass feedstock from marginal land in Ireland: Does marginal mean lower yield? Biomass Bioenergy 2017, 107, 361–369. [Google Scholar] [CrossRef]
- Motesharezadeh, B.; Ahmadiyan, E.; Alikhani, H.A.; Azarnivand, H. The Use of Coal Gangue as a Cultivation Bed Conditioner in Forage Maize Inoculated with Arbuscular Mycorrhizal Fungi. Commun. Soil. Sci. Plant Anal. 2017, 48, 1266–1279. [Google Scholar] [CrossRef]
- Cuiyun, Y.; Yihang, L.; Jing, Y.; Hongyou, Z.; Zhaoyou, D.; Deying, T.; Oo, A.K.; Lixia, Z. Correlation between Soil Environment and Yield and Quality of SHAREN (Amomi Fructus) Under Different Planting Patterns. Digit. Chin. Med. 2023, 6, 221–233. [Google Scholar] [CrossRef]
- Azam, F. Studies on Organic Matter Dynamics and Nitrogen Availability Using 14C and 15N. J. Agron. 2001, 1, 20–24. [Google Scholar] [CrossRef]
- Wang, B.; Ma, Y.; Lee, X.; Wu, P.; Liu, F.; Zhang, X.; Li, L.; Chen, M. Environmental-Friendly Coal Gangue-Biochar Composites Reclaiming Phosphate from Water as a Slow-Release Fertilizer. Sci. Total Environ. 2021, 758, 143664. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Xiao, L.; Liu, R. Response of Arbuscular Mycorrhizal Fungi and Phosphorus Solubilizing Bacteria to Remediation Abandoned Solid Waste of Coal Mine. Int. J. Coal Sci. Technol. 2019, 6, 603–610. [Google Scholar] [CrossRef]
- Bulak, P.; Proc, K.; Pawłowska, M.; Kasprzycka, A.; Berus, W.; Bieganowski, A. Biogas Generation from Insects Breeding Post Production Wastes. J. Clean. Prod. 2020, 244, 118777. [Google Scholar] [CrossRef]
- Abbasi, T.; Tauseef, S.M.; Abbasi, S.A. Biogas Energy; Springer: New York, NY, USA, 2012; ISBN 978-1-4614-1039-3. [Google Scholar]
- Seppälä, M.; Paavola, T.; Lehtomäki, A.; Rintala, J. Biogas Production from Boreal Herbaceous Grasses—Specific Methane Yield and Methane Yield Per Hectare. Bioresour. Technol. 2009, 100, 2952–2958. [Google Scholar] [CrossRef] [PubMed]
- McEniry, J.; O’Kiely, P. Anaerobic Methane Production from Five Common Grassland Species at Sequential Stages of Maturity. Bioresour. Technol. 2013, 127, 143–150. [Google Scholar] [CrossRef]
- Rodríguez, B.C.; Zuazo, V.H.D.; Rodríguez, M.S.; García-Tejero, I.F.; Ruiz, B.G.; De Torres, M.A.R.-R.; Ordóñez-Fernández, R.; Carbonell-Bojollo, R.M.; Tavira, S.C. Legumes Protect the Soil Erosion and Ecosystem Services. In Advances in Legumes for Sustainable Intensification; Elsevier: Amsterdam, The Netherlands, 2022; pp. 247–266. ISBN 978-0-323-85797-0. [Google Scholar]
- Blanco-Canqui, H. Growing Dedicated Energy Crops on Marginal Lands and Ecosystem Services. Soil. Sci. Soc. Amer J. 2016, 80, 845–858. [Google Scholar] [CrossRef]
- Bužinskienė, R.; Miceikienė, A.; Venslauskas, K.; Navickas, K. Assessment of Energy–Economy and Environmental Performance of Perennial Crops in Terms of Biogas Production. Agronomy 2023, 13, 1291. [Google Scholar] [CrossRef]

| Parameter | Unit | CS | DS | OMF |
|---|---|---|---|---|
| pH (in 1 M KCl) | - | 5.42 | 4.22 | 6.8 |
| N-NH4 | mg/kg dm | 2.43 | 3.08 | 630.07 |
| N-NO3 | mg/kg dm | 12.16 | 3.87 | 1461.2 |
| P2O5 | mg/100 g dm | 23.00 | 6.45 | 155.40 |
| K2O | mg/100 g dm | 20.71 | 2.77 | 54.18 |
| Mg | mg/100 g dm | 8.63 | 4.01 | 85.74 |
| TOC | % | - | - | 15.08 |
| Cd | mg/kg dm | - | - | 0.25 |
| Cr | mg/kg dm | - | - | 34.97 |
| Cu | mg/kg dm | - | - | 64.25 |
| Ni | mg/kg dm | - | - | 21.49 |
| Pb | mg/kg dm | - | - | 18.12 |
| Zn | mg/kg dm | - | - | 317.46 |
| Hg | mg/kg dm | - | - | 0.56 |
| Variant | VS (% of TS) | TN (% of TS) | TOC (% of TS) | Protein (% of TS) | C/N (−) | NDF (g/kg TS) | ADF (g/kg TS) | ADL (g/kg TS) | CE (g/kg TS) | HCE (g/kg TS) | MS (% of TS) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CS | 86.5 ± 0.3 a | 2.0 ± 0.0 a | 47.3 ± 0.7 a | 12.3 ± 0.2 a | 23.9 ± 0.2 b | 712.9 ± 20.5 a | 350.4 ± 35.4 a | 79.6 ± 6.2 a | 270.8 ± 29.8 a | 362.2 ± 33.5 a | 6.6 ± 0.3 a |
| DS + 1% | 89.2 ± 1.5 b | 2.4 ± 0.1 b | 48.3 ± 0.5 a | 15.1 ± 0.4 b | 20.0 ± 0.7 a | 720.7 ± 4.0 a | 319.3 ± 5.3 a | 70.2 ± 18.8 a | 249.4 ± 21.7 a | 401.1. ±8.8 a | 10.0 ± 0.4 b |
| Variant | BGP NL/(gTS) | BMP NL/(gTS) | SE GJ/(MgTS) | CH4 % | BGP NL/(gVS) | BMP NL/(gVS) | SE GJ/(MgVS) |
|---|---|---|---|---|---|---|---|
| CS | 0.629 ± 0.017 a | 0.360 ± 0.011 a | 12.90± 0.41 a | 57.27 ± 0.242 a | 0.726 ± 0.020 a | 0.416 ± 0.013 a | 14.90 ± 0.47 a |
| DS + 1% | 0.580 ± 0.010 b | 0.328 ± 0.004 b | 11.752 ± 0.14 b | 56.63 0.273 a | 0.650 ± 0.011 b | 0.368 ± 0.005 b | 13.19 ± 0.16 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wydro, U.; Wołejko, E.; Joniec, J.; Bober, A.; Chomczyńska, M. Improving the Biomass Energy Yield of Cocksfoot Cultivated on Degraded Soil Amended with Organic–Mineral Fertilizer. Energies 2025, 18, 1165. https://doi.org/10.3390/en18051165
Wydro U, Wołejko E, Joniec J, Bober A, Chomczyńska M. Improving the Biomass Energy Yield of Cocksfoot Cultivated on Degraded Soil Amended with Organic–Mineral Fertilizer. Energies. 2025; 18(5):1165. https://doi.org/10.3390/en18051165
Chicago/Turabian StyleWydro, Urszula, Elżbieta Wołejko, Jolanta Joniec, Agata Bober, and Mariola Chomczyńska. 2025. "Improving the Biomass Energy Yield of Cocksfoot Cultivated on Degraded Soil Amended with Organic–Mineral Fertilizer" Energies 18, no. 5: 1165. https://doi.org/10.3390/en18051165
APA StyleWydro, U., Wołejko, E., Joniec, J., Bober, A., & Chomczyńska, M. (2025). Improving the Biomass Energy Yield of Cocksfoot Cultivated on Degraded Soil Amended with Organic–Mineral Fertilizer. Energies, 18(5), 1165. https://doi.org/10.3390/en18051165








